ABSTRACT
This study explored the main active ingredients and the underlying mechanism of TwHF in the treatment of ALL by network pharmacology and molecular docking. TCMSP, OMIM and GeneCards databases were used to obtain active ingredients of TwHF to predict related targets of TwHF and ALL. Cytoscape and STRING databases were used to construct Ingredient-target-disease and PPI networks, respectively. Enrichment analysis of GO and KEGG was conducted by Bioconductor database. Molecular docking was performed using the Autodock platform to predict the binding of the active components to key action targets.CCK-8 assay was performed to measure the effects of drugs on the proliferation of ALL cells. The ingredient-target-disease network contained 16 compounds and 43 corresponding targets. Key targets included VEGFA, CASP3, RELA, ESR1, BCL2, and MAPK8. There were 79 GO items in the GO enrichment analysis (p < .05) and 91 signalling pathways (p < .05) in KEGG primarily including tumour necrosis factor signalling pathway and apoptosis pathway. Molecular docking results showed that triptolide, kaempferol and other phytocompounds had a high affinity for the corresponding targets.CCK-8 assay suggested that triptolide and kaempferol have anti-proliferative effects on ALL cells. Triptolide, kaempferol and other active ingredients from TwHF may exert therapeutic effects on ALL through multiple pathways and targets.
Highlights
There are 43 potential action targets of TwHF for ALL treatments, among which VEGFA and CASP3 are the core genes.
16 active ingredients including triptolide and kaempferol play a role in the treatment of leukaemia.
The bioactive components of TwHF can treat the ALL by modulating biological processes such as nuclear receptor activity and biological pathways such as apoptosis pathway and tumour necrosis factor signalling pathway.
Triptolide and kaempferol significantly inhibited the viability of Jurkat, MOLT-4 and Nalm-6 cells in a concentration- and time-dependent manner.
ALL, Acute lymphoblastic leukaemia; BCL2, B lymphocyte tumor-2 gene; CASP3, Caspase-3; CCK-8, cell counting kit 8; DL, Drug-likeness; ESR1, estrogen receptor 1; MAPK8, mitogen-activated protein kinase 8; OB, Oral bioavailability; OD, optical density; PPI, protein–protein interaction; PDGF, platelet-derived growth factor; SD, standard deviation; TwHF, Tripterygium wilfordii Hook F; Triptolide: Kaempferol; TCMSP, Traditional Chinese Medicine Systems Pharmacology; T-ALL, T-cell Acute lymphoblastic leukaemia; TRAIL, Tumor necrosis factor-related apoptosis-inducing ligand; TPL, Triptolide; VEGFA, vascular endothelial growth factor A gene.
Introduction
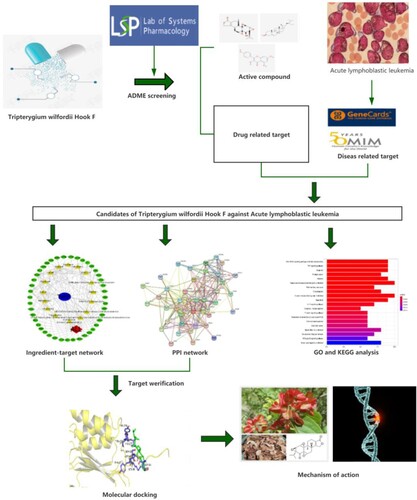
Acute lymphoblastic leukaemia (ALL) is a neoplastic disease originating from B-line or T-line lymphoid progenitor cells that invade the bone marrow, blood, and extramedullary sites (Malard and Mohty Citation2020). ALL is the most common malignant tumour that occurs during childhood. Through effective chemotherapy, the 5-year survival rate of childhood ALL can reach 90%; however, a recurrence rate of 20–30% still exists. Recurrence is one of the difficulties encountered in the current treatment (Hunger and Mullighan Citation2015; Hunger and Raetz Citation2020). In recent years, with an in-depth understanding of ALL pathogenesis, in addition to conventional chemotherapy, several promising treatment methods were discovered, including immunotherapy, using monoclonal antibodies and antibody-drug conjugates (Li and Wang Citation2020). Following years of research, numerous medicines for autoimmune diseases, tumours, and other diseases have been extracted from Chinese herbal medicines. In recent years, the role of traditional Chinese medicine (TCM) extracts for the treatment of leukaemia has received widespread attention. Chinese medicine reduces the toxicity and side effects of chemotherapy, prolongs or prevents the recurrence of leukaemia, reverses its multi-drug resistance, and prevents complications arising from leukaemia (Sun Citation2010; Hao et al. Citation2019; Luo et al. Citation2019; Jammal et al. Citation2020). Tripterygium wilfordii Hook F (TwHF) is an important medicinal plant possessing several bioactive compounds, which confer immunoregulatory, anti-inflammatory, anti-tumour, and anti-bacterial effects (Zhao et al. Citation2020). Several studies have shown that T. wilfordii lactone can treat leukaemia by inducing tumour cell apoptosis, inhibiting tumour cell growth, and enhancing tumour cell sensitivity to chemotherapeutic drugs. However, most studies have focused on myeloid leukaemia, while the pharmacological mechanism of TwHF in the treatment of ALL remains unclear (Liu et al. Citation2013; Shi et al. Citation2021). Network pharmacology explains the development process of diseases by establishing databases and networks, analysing these networks, using experimental verification, providing powerful tools for exploring the mechanism of Chinese medicine and developing its bioactive compounds. Additionally, network pharmacology through the visual presentation of the network composition of “drug-target-disease” effectively highlights important compounds of TCM and predicts its potential mechanism (Wang et al. Citation2008). This study explores the mechanism of TwHF in the treatment of ALL via network pharmacology and provides research and development ideas for the drug treatment of ALL (Figure ).
Materials and methods
Design
The bioactive compounds of the drug and targets for the interaction between the drug and the disease were screened. The regulatory network of the component-disease-target was constructed, GO and KEGG analysis was carried out, and molecular docking verification was performed.CCK-8 assay was performed to measure the effects of drugs on the proliferation of ALL cells.
Time and place
The experiment was performed at Southwest Medical University from February 2021 to June 2022.
Candidate targets
TwHF-related targets and bioactive compounds
Oral bioavailability (OB) is an important indicator for evaluating the absorption efficiency of active drugs into the systemic circulation. Drug-likeness (DL) refers to the similarity between a compound and a known drug. Both are important indicators for new drug development. In this study, the active components and target genes of TwHF were searched in the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php) with OB >30% and drug-like properties >0.18 (Zhang, Yang, Wang, et al. Citation2021). Using Perl scripts, all screened targets were imported into the Uniport database (https://www.uniprot.org/), and all names were corrected to official names.
ALL-related targets
“Acute lymphoblastic leukaemia” was used as the key word to search disease-related targets in GeneCards (https://www.genecards.org/) and OMIM (https://omim.org/) databases. The targets obtained from the two databases were combined and duplicate genes were removed, and finally, the targets related to the disease were retrieved.
Drug-disease common targets
Venn Diagram data from R language 3.6.3 were used to conduct Venn analysis on targets of ALL and the phytocompounds of TwHF. Intersection targets were obtained to preliminarily identify the candidate targets of action of TwHF in the treatment of ALL.
Network construction
Construction of the ingredient-target-disease network
Intersection targets and bioactive compounds were imported into Cytoscape 3.6.1. The nodes represented ALL, TwHF, bioactive compounds, and intersection targets. Edges represented the regulation or membership relationship between nodes, and the regulatory network diagram of TwHF - bioactive compounds - intersection target - ALL was drawn; thus, the regulation relationship was visualised.
Construction of the protein-protein interaction (PPI) network
Obtained intersection targets were imported into the STRING (https://string-db.org/) database, with the species limited as “Homo sapiens”. The highest confidence was set to 0.4, and PPI correspondence was obtained after searching. The obtained file of protein interaction was imported into Cytoscape3.6.1, and the Analyse Network function was used to conduct a topological analysis of the network, with a degree as a parameter. The top 30 targets were selected as core targets, and R was used for visual processing.
GO and KEGG analysis
Using R language and the Bioconductor (https://www.bioconductor.org/) database, with P < 0.05 and Q < 0.05 as screening criteria, GO and KEGG analyses of intersection targets were performed. The top 20 P values were plotted as bar graphs.
Molecular docking verification
To verify the interrelationship between the bioactive compounds of the drug and targets, PPI ranked high, and targets that were well-studied were selected for molecular docking. We downloaded the 2D structure of the compounds in the PubChem (http://pubchem.ncbi.nim.nih.gov/) database and used Chem 3D to convert the 2D structure into a 3D structure, which was downloaded from PDB (https://www.rcsb.Org/). The water molecule and the small molecule ligand of the target protein were removed by PyMOL, and targets were subsequently imported into AutoDocktools 1.5.6 for hydrogenation, charge distribution, and atomic type addition. AutoDock Vina 1.1.2 was used for molecular docking, and PyMOL software was used to optimise the resulting output to obtain a molecular docking diagram.
Experimental validation
Reagents and cell lines
Triptolide (TPL) and Kaempferol (Kae) with purity >98% were purchased from Chengdu PureChem-Standard Co., LTD (Chengdu, China) and Beijing Solarbio Science & Technology Co., Ltd (Beijing, China), respectively. Human T cell acute lymphoblastic leukaemia cell lines (Jurkat and MOLT-4) and human B cell acute lymphoblastic leukaemia cell lines (Nalm-6) were purchased from ZhongqiaoXinzhou Biotechnology CO., LTD (Shanghai, China).
Cell culture
Jurkat, MOLT-4 and Nalm-6 cell lines were cultured in RPMI-1640 (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA), 100 Units/mL penicillin and 100 μg/mL streptomycin (Gibco, USA) at 37°C and 5% CO2.
Cell viability assay
The cell viability was analyzed by cell counting kit 8 (CCK-8) assay (Dojindo, Japan). Jurkat, MOLT-4 and Nalm-6 cells were inoculated into 96-well plates at 20 × 104 cells/mL, and treated with TPL (0, 2, 4, 8, 16, 32 and 64 nM) and Kae (0, 20, 40, 60, 80, 100, 120 and 140 μM), respectively. After incubation for 24 and 48 h, added 10 μL CCK-8 into each well and incubated for 4 h at 37 °C. Then, the optical density (OD) was measured at 450 nm. Cell viability was calculated according to the following formula: cell viability (%) = [(the OD of experimental group − the OD of blank group)/(the OD of control group − the OD of blank group)] × 100%.
Statistical analysis
SPSS 20.0 statistical software was used for data analysis. The data were expressed as mean ± standard deviation (SD). Statistical analyses were analyzed by one-way ANOVA. P < 0.05 was considered statistically significant.
Results
TwHF-related targets and bioactive compounds
A total of 51 bioactive compounds of TwHF, which met the conditions of OB ≥30% and DL ≥0.18, were selected from the TCMSP database (Table ). By matching these ingredients into the TCMSP database and mining its action targets, 63 targets were retrieved.
Table 1. Potential effective ingredients of TwHF.
ALL-related targets
We searched the GeneCards and OMIM databases using “acute lymphoblastic leukaemia” as the keyword. Following the removal of duplicates, 4432 genes related to ALL-related diseases were identified.
Prediction of drug-disease interaction targets
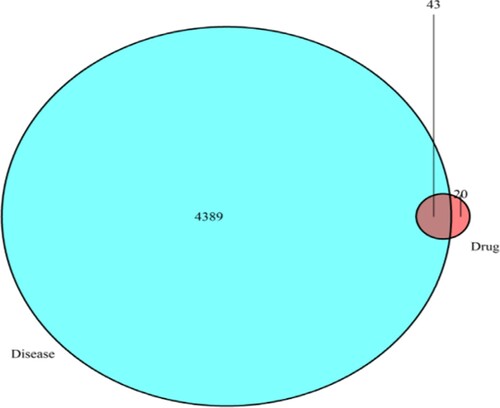
By matching ALL-related targets with drug-related targets, 43 potential action targets of TwHF for ALL treatment were obtained, and a Venn diagram was drawn (Figure ).
Ingredient-target-disease network
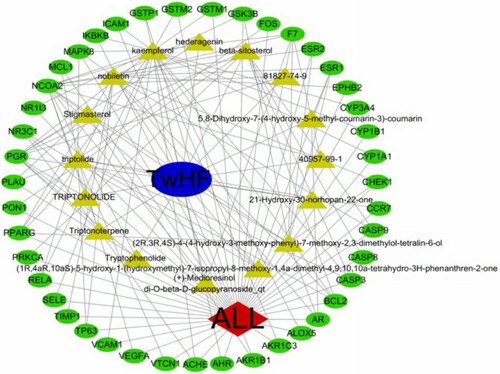
The above data were imported into Cytoscape 3.7.2 to construct a visual network diagram of the ingredients of TwHF and intersection targets. Following the removal of 35 compounds without a target, the network contained 61 nodes (including 16 compounds and 43 genes) and 148 edges (Figure ).
Protein interaction network
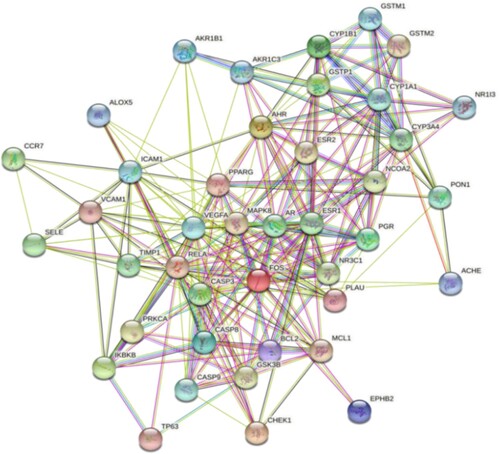
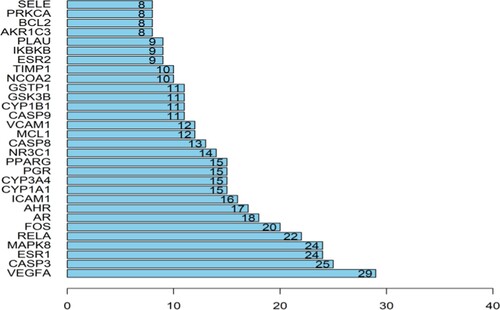
The PPI network was composed of 41 nodes and 243 edges (Figure ). Using Analyse Network topological analysis with Cytoscape 3.6.1, the degree was calculated as a parameter and sorted out, and the top 30 targets were selected as core targets. Vascular endothelial growth factor gene (VEGFA), cysteine protease 3 (CASP3), oestrogen receptor 1 (ESR1), mitogen-activated protein kinase 8 (MAPK8), and RELA ranked high in degree value and were the core targets with more connections (Figure ).
GO and KEGG analysis
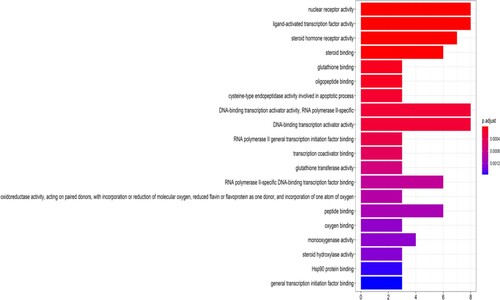
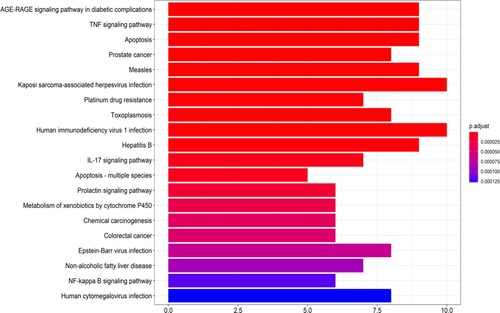
The results of GO and KEGG analyses of intersection targets of TwHF in ALL treatment are shown in Figures and . The 79 GO functions of target genes were primarily associated with nuclear receptor movement, transcription factor activity, steroid binding, glutathione binding, apoptosis involved in cysteine endopeptidase activity, DNA transcriptional activator activity, RNA polymerase II specificity, transcriptional coactivator binding, etc. Targets involved 91 signalling pathways, including the tumour necrosis factor pathway, apoptosis-related pathway, viral infection-related pathway, IL-17 signalling pathway, prolactin-related pathway, tumour-related pathway, and NF-kB signalling pathway.
Molecular docking
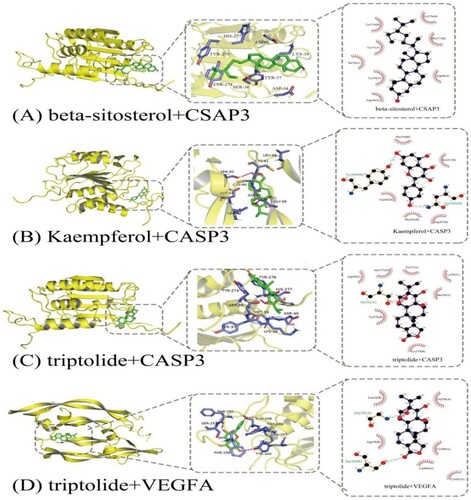
The binding energy is a parameter used to evaluate whether the ligand and receptor can bind.Negative binding energy indicates that ligand and receptor can be combined. It is generally believed that the binding energy of ligand and receptor ≤ −5.0 kcal/mol indicates that the two have good binding activity and the lower the binding energy of the ligand and the receptor, the more stable the binding (Zhang, Yang, Zhao, et al. Citation2021; Zhang, Li, Wang, et al. Citation2021). The core targets ranked at the top in the PPI network and their corresponding ingredients were verified via molecular docking. These results showed that the binding energy between each target and their corresponding ingredient was ≤ −5.0 kcal/mol (Figure and Table ). This indicated that the selected key ingredients of TwHF had good binding activity with the interaction targets, confirming the reliability of the prediction of this study.
Table 2. Molecular docking results of target and active compounds.
Triptolide and Kaempferol inhibited the proliferation of ALL cells
CCK-8 assay was performed to measure the effects of TPL and Kae on the proliferation of ALL cells. The results showed that TPL and Kae significantly inhibited the viability of Jurkat, MOLT-4 and Nalm-6 cells in a concentration- and time-dependent manner (Figures and ). The IC50 values of TPL on Jurkat, MOLT-4 and Nalm-6 cells for 48 h were 12.595 ± 0.659 nM, 7.213 ± 0.095 nM, and 27.863 ± 1.108 nM, respectively. The IC50 values of Kae on Jurkat, MOLT-4 and Nalm-6 cells for 48 h were 63.677 ± 4.736 μM, 37.693 ± 3.186 μM, and 68.930 ± 0.594 μM, respectively (Table ). These results suggested that TPL and Kae have anti-proliferative effects on acute lymphoblastic leukaemia cells.
Figure 9. Triptolide inhibited the proliferation of ALL cells. The cell viability of Jurkat (A), MOLT-4 (B) and Nalm-6 (C) cells treated with different concentrations of triptolide (0, 2, 4, 8, 16, 32 and 64 nM) for 24 and 48 h. *P < 0.05 vs the control group.
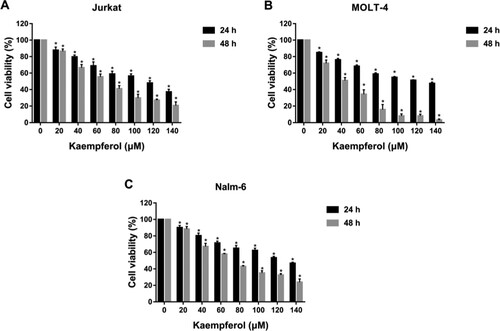
Figure 10. Kaempferol inhibited the proliferation of ALL cells. The cell viability of Jurkat (A), MOLT-4 (B) and Nalm-6 (C) cells treated with different concentrations of kaempferol (0, 20, 40, 60, 80, 100, 120 and 140 μM) for 24 and 48 h. *P < 0.05 vs the control group.
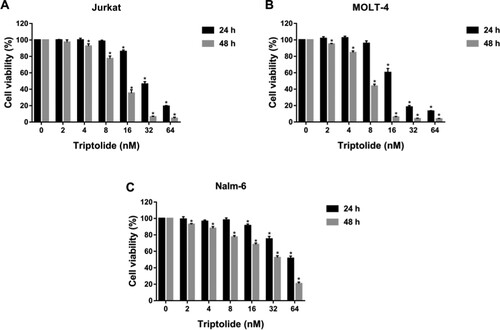
Table 3. The IC50 values of Triptolide and Kaempferol on Jurkat, MOLT-4 and Nalm-6 cells for 48 h.
Discussion
TCM has been used for thousands of years and has been demonstrated to have therapeutic effects on cancer. Network pharmacology is an important research method that constructs a drug-disease-gene regulatory network and acutely explores the effective bioactive compounds, targets, biological processes, and pathways of TCM in the treatment of diseases, and promotes the development of TCM from empirical medicine. The transformation of evidence-based medicine systems provides a theoretical basis for drug development (Shao and Zhang Citation2013).
This study was based on the method of network pharmacology to explore the relevant targets and pathways of TwHF in the treatment of ALL and to clarify its mechanism of action. Through the analysis of the components of TwHF, we obtained 16 bioactive compounds that are effective in the treatment of ALL. It was evident from the ingredient-target-disease network that kaempferol and triptolide, tangerine, β-sitosterol, and isolaricidin matched the largest number of targets and were the main bioactive compounds in the treatment of ALL. Haiying et al. demonstrated that small doses of triptolide can significantly inhibit the abnormal methylation of the p15 gene and inactivate the proliferation of T-cell (T)-ALL Molt4 cells. The mechanism may be to induce demethylation of abnormally methylated p15 in MOLT4 cells and restore its expression, thereby negatively regulating the cell cycle (Fu and Shen Citation2005). Haijun et al. demonstrated via in vitro and in vivo experiments that low triptolide concentrations can reverse drug resistance of drug-resistant cell lines, NALM-6/R and ADM, and can also inhibit the proliferation of primary drug-resistant ALL cells and promote their apoptosis, reducing tumour burden in mice (Zhao Citation2017). Jianghua demonstrated that triptolide can inhibit transcription of the retrovirus HERV-K Np9 gene and regulate its downstream signalling molecules, thereby inducing apoptosis in human T-ALL Jurkat cells (Chen et al. Citation2015). Triptolide can inhibit MDM2 expression at the transcriptional level and induce ALL cell apoptosis by upregulating P53 and downregulating XIPA (Huang et al. Citation2013). Triptolide can also inhibit T-ALL cells by inhibiting the Wnt signalling pathway (Ma Citation2016), resulting in anti-tumour effects. Kaempferol is the most common type of aglycone flavonoid in the form of a glycoside and has a wide range of anti-inflammatory and anti-tumour activities (Kadioglu et al. Citation2015; Imran et al. Citation2019). Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and kaempferol are used in MOLT-4 cells to promote apoptosis of ALL MOLT-4 cells and reverse TRAIL resistance by upregulating DR4/5, inhibiting intracellular anti-apoptotic proteins, and inhibiting the expression of VEGF-β (VEGFB) and FGF-8 (Hassanzadeh et al. Citation2019). Our experimental results indicated that TPL and Kae significantly inhibited the viability of Jurkat, MOLT-4 and Nalm-6 cells in a concentration- and time-dependent manner. Briefly, several studies have shown that triptolide and kaempferol can treat ALL via a variety of methods, which agrees with the results of this study. However, there is still a paucity of information concerning the therapeutic effects of other bioactive compounds of TwHF on ALL, and these compounds have the potential to be targets for the development of new drugs for the future treatment of ALL.
It is evident from the ingredient-target-disease network that there are several common targets between the bioactive compounds, indicating that they may have a synergistic effect in ALL treatment; the PPI network showed that the VEGFA and CASP3 genes ranked at the top, and may be the most crucial targets in ALL treatment. VEGFA belongs to the platelet-derived growth factor superfamily. Studies have shown that VEGFA is highly expressed in haematological malignancies such as acute myeloid leukaemia, ALL, chronic myelogenous leukaemia, and myelodysplastic syndrome (Zhang et al. Citation2005). The VEGF expression in the bone marrow of children with relapsed ALL after complete remission was significantly higher than that in children with newly diagnosed ALL. The increased VEGF expression in children with relapsed ALL indicated poor prognosis and poor sensitivity to chemotherapy drugs and can be used as an independent prognostic factor (Koomagi et al. Citation2001; Wellmann et al. Citation2004). Münch et al. and several other researchers have shown that VEGF was highly expressed in ALL cells of central nervous system origin compared with ALL cells of bone marrow origin (with or without central nervous system leukaemia), and the capture or downregulation of VEGF in vivo can reduce the transendothelial cell transport of leukaemia cells, and this phenomenon has a central nervous system-specific effect (Münch et al. Citation2017). The CASP gene family is divided into priming and effect type genes, and CASP3 is the main effect type gene in the family (Soung et al. Citation2004). The low expression of pro-apoptotic genes such as CASP3 was associated with a poor curative effect on day 7 of induction chemotherapy for childhood ALL, and the high expression of CASP3 was associated with increased disease-free survival in children with CD10+ and B-cell (B)-ALL (Mata et al. Citation2010). FRU can activate CASP3 to enhance the apoptosis of T-ALL cells induced by oxidative stress (Diaz-Aguirre et al. Citation2016), and tetrandrine can induce apoptosis of T-ALL cells by upregulating CASP-3/6/8/9 expression, especially that of CASP3 (Xu et al. Citation2019). Molecular docking analysis showed that VEGFA, CASP3, and their corresponding bioactive compounds showed good binding power, indicating that the bioactive compounds of TwHF may prevent or treat central nervous system leukaemia and induce tumour cell apoptosis by capturing VEGFA or downregulating VEGFA expression and upregulating CASP3 expression.
Although RELA, MAPK8, and ESR1 genes rank high in PPI network analysis, their relationship with the occurrence, treatment, and prognosis of ALL remains unclear. RELA is an important target of the NF-κB pathway, and the encoded ReLA (p65) is an important member of the Rel family (Hayden and Ghosh Citation2012). Studies have shown that the silencer of death domain (SODD) and active P65 protein were highly expressed in childhood ALL bone marrow cells, and their expression was positively correlated. Vinblastine can downregulate the expression of the SODD protein and inhibit the activation of NF-κB-P65, thereby restoring the sensitivity of ALL cells to apoptosis and enhancing the effect of chemotherapy (Tao et al. Citation2007). MDM2 enhances the activity of the p65 promoter in a p53-dependent and non-p53-dependent manner, thereby enhancing the expression of p65 in ALL cells and enhancing the resistance of ALL cells to adriamycin (Gu et al. Citation2002). The MAPK8 gene is also called the c-Jun N-terminal kinase 1 (JNK1) gene because of its specific phosphorylation effect on the nuclear transcription factor c-Jun. Studies have shown that JNK promotes the growth of T-ALL cells through abnormal subcellular localisation, and JNK1 siRNA can inhibit the growth of T-ALL cells and promote apoptosis (Cui et al. Citation2009). ESR1 is closely associated with breast cancer and is primarily involved in oestrogen metabolism (Yang et al. Citation2021). At present, there is no definitive study on the relationship between ESR1 and ALL; molecular docking validation showed that RELA, MAPK8, and ESR1 had good binding force with the corresponding bioactive compounds, which may reverse the resistance of ALL cells to chemotherapy drugs and promote cell apoptosis by downregulating RELA expression and upregulating MAPK8 expression. These may also be candidate targets for the bioactive compounds of TwHF to treat ALL.
The B lymphocyte tumor-2 (BCL2) and cytochrome P450 gene family members (CYP1A1, CYP3A4, CYP1B1) rank lower in the PPI network. Several studies have reported on these genes, with molecular docking verification indicating that they have a good binding force with the corresponding bioactive compounds and may also be candidate targets. BCL2 is a proto-oncogene found at the ectopic breakpoint of the t(14,18) chromosome in follicular B-cell lymphoma. It is a member of the BCL2 family with many highly homologous genes (Craig Citation1995). Presently, the BCL2 gene and protein inhibitors have made progress in the treatment of haematological malignancies (Ruefli-Brasse and Reed Citation2017). BCL2 is highly expressed in immature T-ALL, whereas its expression level in mature T-ALL is low. ABT-199 inhibits the growth of immature T-ALL cells by selectively inhibiting BCL2 expression (Peirs et al. Citation2014). RBP2 regulates BCL2 expression by directly binding to its promoter. The RBP2 deletion inhibits the proliferation of ALL and promotes its apoptosis by downregulating BCL2 expression (Wang et al. Citation2016). High-dose prednisolone may induce apoptosis in CCRF-CEM cells resistant to it by downregulating the expression of BCL2 and upregulating the expression of BAX (Ganbarjeddi et al. Citation2020). CYP1A1, CYP3A4, and CYP1B1 are members of the CYP (cytochrome P450) superfamily, which are primarily involved in the biotransformation process of foreign organisms and drugs (Manikandan and Nagini Citation2018). The CYP1A1*2A gene polymorphism is related to the genetic susceptibility of ALL in Asian and Caucasian children (Zou et al. Citation2015). The CYP3A4 rs2246709 SNP and gender were two interrelated factors that significantly affected the survival rate of children with ALL who received chemotherapy. Among them, male heterozygous mutations have a relatively high survival rate (Gézsi et al. Citation2015). The relative prognosis of patients with ALL with CYP1B1 methylation was poor. CYP1B1 methylation negatively correlated with CYP1B1 gene expression, and downregulation of CYP1B1 gene expression may diminish the effect of glucocorticoids on ALL (DiNardo et al. Citation2013).
GO and KEGG analyses showed that the bioactive compounds of TwHF can modulate different biological processes and pathways to treat ALL. The main biological processes involved were nuclear receptor activity and transcription factor activity. The main biological pathways involved were apoptosis pathway and tumour necrosis factor signalling pathway. These molecular functions and signalling pathways are closely associated with intersection targets; however, the specific regulatory mechanism remains unclear and this is the main direction of future research. Abnormal cell apoptosis is of great importance in the development of several solid tumours and haematological malignancies. Several chemotherapeutic drugs currently used to treat ALL, such as dexamethasone and daunorubicin, can induce the apoptosis of ALL cells through a variety of methods (Laane et al. Citation2007; Du et al. Citation2017). The bioactive compounds found in TwHF such as triptolide and kaempferol also induce ALL cell apoptosis. Among the targets, CASP3, RELA, MAPK8, and BCL2 all play an important role in apoptosis of tumour cells. The apoptotic pathway may be the primary pathway for the bioactive compounds of TwHF in the treatment of ALL.
Conclusion
Triptolide and Kaempferol from Tripterygium wilfordii Hook F are important phytocompounds for the treatment of ALL; VEGFA, CASP3, RELA, BCL2, CYP1A1, CYP4A3, and CYP1B1 are important targets for the treatment of ALL; and most of these active compounds and target genes are related to tumour cell apoptosis. TwHF uses multiple bioactive compounds and target genes to synergistically affect multiple molecular functions and signalling pathways, providing a novel basis for TwHF in the treatment of ALL.
Owing to the limitations of the network pharmacology method and the complexity of drug components in decoction and human metabolic reactions, only network pharmacology analysis was performed on drug components and the mechanism of TwHF, which still requires further animal experiments for verification.
Acknowledgements
WJL, XL, XQ and TTF conceived and designed the research. XL and TTF analysed the data. XQ performed all of the experimental works. XL and XQ made the graphs and prepared the manuscript. WJL revised the manuscript and optimised the graphs. All authors reviewed the manuscript, and all authors read and approved of the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of network pharmacology and molecular docking are available in TCMSP at http://tcmspw.com/tcmsp.php,in Uniport at https://www.uni.protorg/, in GeneCards at https://www.genecards.org/, in OMIM at https://omim.org/, in STRING at https://string-db.org/, in Bioconductor at https:// www.bioconductor.org/, in PubChem at http://pubchem.ncbi.nim.nih.gov/, in PDB at https://www.rcsb.Org/. The data that support the findings of cell viability experiments are available in the figshare repository at https://doi.org/10.6084/m9.figshare.21159064.
Additional information
Funding
References
- Chen JH, Zheng WW, Jiang XD, Lu XY, Xu RZ. 2015. Triptolide induces apoptosis of human acute T lymphocyte leukemia Jurkat cells by inhibiting the transcription of retrovirus HERV-K Np9 gene. Journal of Southern Medical University. 35(05):702–706.
- Craig RW. 1995. The BCL-2gene family. Semin Cancer Biology. 6(1):35–43.
- Cui J, Wang Q, Wang J, Lv M, Zhu N, Li Y, Feng J, Shen B, Zhang J. 2009. Basal c-Jun NH2-terminal protein kinase activity is essential for survival and proliferation of T-cell acute lymphoblastic leukemia cells. Molecular Cancer Therapeutics. 8(12):3214–22.
- Diaz-Aguirre V, Velez-Pardo C, Jimenez-Del-Rio M. 2016. Fructose sensitizes Jurkat cells oxidative stress-induced apoptosis via caspase-dependent and caspase-independent mechanisms. Cell Biology International. 40(11):1162–1173.
- DiNardo CD, Gharibyan V, Yang H, Wei Y, Pierce S, Kantarjian HM, Garcia-Manero G, Rytting M. 2013. Impact of aberrant DNA methylation patterns including CYP1B1 methylation in adolescents and young adults with acute lymphocytic leukemia. American Journal of Hematology. 88(9):784–9.
- Du X, Tong J, Lu H, He C, Du S, Jia P, Zhao W, Xu H, Li J, Shen Z, et al. 2017. Combination of bortezomib and daunorubicin in the induction of apoptosis in T-cell acute lymphoblastic leukemia. Molecular Medicine Reports. 16(1):101–108.
- Fu HY, Shen JZ. 2005. The effect of triptolide on p15 gene expression in Molt4 cell line of acute lymphoblastic leukemia. Chinese Journal of Internal Medicine. 04:301–302.
- Ganbarjeddi S, Azimi A, Zadi Heydarabad M, Hemmatzadeh M, Mohammadi S, Mousavi Ardehaie R, Zamani M, Baharaghdam S, Esmaeili S, Ghasemi A. 2020. Apoptosis induced by prednisolone occurs without altering the promoter methylation of BAX and BCL-2 genes in acute lymphoblastic leukemia cells CCRF-CEM. Asian Pacific Journal of Cancer Prevention. 21(2):523–529.
- Gézsi A, Lautner-Csorba O, Erdélyi DJ, Hullám G, Antal P, Semsei ÁF, Kutszegi N, Hegyi M, Csordás K, Kovács G, et al. 2015. In interaction with gender a common CYP3A4 polymorphism may influence the survival rate of chemotherapy for childhood acute lymphoblastic leukemia. The Pharmacogenomics Journal. 15(3):241–7.
- Gu L, Findley HW, Zhou M. 2002. MDM2 induces NF-κB/p65 expression transcriptionally through Sp1-binding sites: a novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood. 99(9):3367–75.
- Hao JX, Gao ZS, Gao H, Bi S, Wang J, Li ZJ. 2019. Discussion on the mechanism of Tripterygium wilfordii nephrotoxicity based on network pharmacology.Chinese. Journal of Experimental Formulas. 25(16):142–151.
- Hassanzadeh A, Naimi A, Hagh MF, Saraei R, Marofi F, Solali S. 2019. Kaempferol improves TRAIL-mediated apoptosis in leukemia MOLT-4 cells by the inhibition of anti-apoptotic proteins and promotion of death receptors expression. Anti-Cancer Agents in Medicinal Chemistry. 19(15):1835–1845.
- Hayden MS, Ghosh S. 2012. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes & Development. 26(3):203–234.
- Huang M, Zhang HL, Liu T, Tian D, Gu LB, Zhou MX. 2013. Triptolide inhibits MDM2 and induces apoptosis in acute lymphoblastic leukemia cells through a p53-independent pathway. Molecular Cancer Therapeutics. 12(2):184–194.
- Hunger SP, Mullighan CG. 2015. Acute lymphoblastic leukemia in children. New England Journal of Medicine. 373(16):1541–52.
- Hunger SP, Raetz EA. 2020. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 136(16):1803–1812.
- Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, Shahbaz M, Tsouh Fokou PV, Umair Arshad M, Khan H, et al. 2019. Kaempferol: a key emphasis to its anticancer potential. Molecules. 24(12):2277.
- Jammal N, Chew S, Jabbour E, Kantarjian H. 2020. Antibody based therapy in relapsed acute lymphoblastic leukemia. Best Practice & Research Clinical Haematology. 33(4):101225.
- Kadioglu O, Nass J, Saeed ME, Schuler B, Efferth T. 2015. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 35(5):2645–2650.
- Koomagi R, Zintl F, Sauerbrey A, Volm M. 2001. Vascular endothelial growth factor in newly diagnosed and recurrent childhood acute lymphoblastic leukemia as measured by real-time quantitative polymerase chain reaction. Clin Cancer Res. 7(11):3381–4.
- Laane E, Panaretakis T, Pokrovskaja K, Buentke E, Corcoran M, Söderhäll S, Heyman M, Mazur J, Zhivotovsky B, Porwit A, et al. 2007. Dexamethasone-induced apoptosis in acute lymphoblastic leukemia involves differential regulation of Bcl-2 family members. Haematologica. 92(11):1460–9.
- Li L, Wang Y. 2020. Recent updates for antibody therapy for acute lymphoblastic leukemia. Experimental Hematology & Oncology. 9(1):33.
- Liu L, Li G, Li Q, Jin Z, Zhang L, Zhou J, Hu X, Zhou T, Chen J, Gao N. 2013. Triptolide induces apoptosis in human leukemia cells through caspase-3-mediated ROCK1 activation and MLC phosphorylation. Cell Death & Disease. 4(12):e941–e941.
- Luo D, Zuo ZY, Zhao HY, Tan Y, Xiao C. 2019. Immunoregulatory effects of Tripterygium wilfordii Hook F and its extracts in clinical practice. Front MED. 13(5):556–563.
- Ma YN. 2016. The effect of triptolide on the epigenetics of acute T lymphocytic leukemia. Huazhong University of Science and Technology.
- Malard F, Mohty M. 2020. Acute lymphoblastic leukaemia. The Lancet. 395(10230):1146–1162.
- Manikandan P, Nagini S. 2018. Cytochrome P450 structure, function and clinical significance: a review. Current Drug Targets. 19(1):38–54.
- Mata JF, Silveira VS, Mateo EC, Cortez MA, Queiroz RG, Yunes JA, Lee ML, Toledo SR, Petrilli AS, Brandalise SR, et al. 2010. Low mRNA expression of the apoptosis-related genes CASP3, CASP8, and FAS is associated with low induction treatment response in childhood acute lymphoblastic leukemia (ALL). Pediatric Blood & Cancer. 55(1):100–107.
- Münch V, Trentin L, Herzig J, Demir S, Seyfried F, Kraus JM, Kestler HA, Köhler R, Barth TFE, Te Kronnie G, et al. 2017. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood. 130(5):643–654.
- Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, Degryse S, Canté-Barrett K, Briot D, Clappier E, et al. 2014. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 124(25):3738–47.
- Ruefli-Brasse A, Reed JC. 2017. Therapeutics targeting Bcl-2 in hematological malignancies. Biochemical Journal. 474(21):3643–3657.
- Shao LI, Zhang B. 2013. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chinese Journal of Natural Medicines 11(2):110–20.
- Shi P, Zha J, Feng J, Jiang ZW, Zhao HJ, Deng MM, Liao NY, Li P, Jiang YR, Song HH, et al. 2021. Low-dose triptolide enhanced activity of idarubicin against acute myeloid leukemia stem-like cells via inhibiting DNA damage repair response. Stem Cell Reviews and Reports. 17(2):616–627.
- Soung YH, Lee JW, Kim SY, Park WS, Nam SW, Lee JY, Yoo NJ, Lee SH. 2004. Somatic mutations of CASP3 gene in human cancers. Human Genetics. 115(2):112–5.
- Sun LH. 2010. Screening of Chinese medicines for anti-acute lymphoblastic leukemia and study on the mechanism of action ingredients. Peking Union Medical College.
- Tao H, Hu Q, Fang J, Liu A, Liu S, Zhang L, Hu Y. 2007. Expression of SODD and P65 in ALL of children and its relationship with chemotherapeutic drugs. Journal of Huazhong University of Science and Technology. 27(3):326–9.
- Wang X, Wang ZY, Zheng JH, Li S. 2008. A systematic review of acupuncture and moxibustion treatment for chronic fatigue syndrome in china. The American Journal of Chinese Medicine. 36(1):1–24.
- Wang X, Zhou M, Fu Y, Sun T, Chen J, Qin X, Yu Y, Jia J, Chen C. 2016. RBP2 promotes adult acute lymphoblastic leukemia by upregulating BCL2. PLoS One. 11(3):e0152142.
- Wellmann S, Guschmann M, Griethe W, Eckert C, von Stackelberg A, Lottaz C, Moderegger E, Einsiedel HG, Eckardt KU, Henze G, Seeger K. 2004. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 18(5):926–933.
- Xu W, Wang X, Tu Y, Masaki H, Tanaka S, Onda K, Sugiyama K, Yamada H, Hirano T. 2019. Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chemico-Biological Interactions. 310:108726.
- Yang W, He X, He C, Peng L, Xing S, Li D, Wang L, Jin T, Yuan D. 2021. Impact of ESR1 polymorphisms on risk of breast cancer in the Chinese Han population. Clinical Breast Cancer. 21(3):e235–e242.
- Zhang JL, Li H, Wang WJ, Huang SJ, Mang Y, Ding Y, Wen AD. 2021. Investigation on the mechanism of Salviae Miltiorrhizae-Cortex Moutan against cerebral ischemic injury based on network pharmacology. Nat Prod Res Dev. 33:103–113.
- Zhang MX, Yang JW, Zhao XL, Zhao Y, Zhu S. 2021. Network pharmacology and molecular docking study on the active ingredients of qidengmingmu capsule for the treatment of diabetic retinopathy. Scientific Reports. 1(11):7382.
- Zhang Y, Pillai G, Gatter K, Blázquez C, Turley H, Pezzella F, Watt SM. 2005. Expression and cellular localization of vascular endothelial growth factor A and its receptors in acute and chronic leukemias: an immunohistochemical study. Human Pathology. 36(7):797–805.
- Zhang ZM, Yang L, Wang Y, Jiang S, Shang X, Qin DW, Duan JA. 2021. The synergic renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced chronic kidney disease rats based on integrated plasma metabolomics and network pharmacology approach. Life Sciences. 278:119545.
- Zhao HJ. 2017. Low-concentration triptolide reverses the drug resistance of acute lymphoblastic leukemia by interfering with DNA damage repair. Southern Medical University.
- Zhao X, Liu Z, Ren ZY, Wang HG, Wang ZS, Zhai JL, Cao D, Lyu SC, Li LX, Lang R, et al. 2020. Triptolide inhibits pancreatic cancer cell proliferation and migration via down-regulating PLAU based on network pharmacology of Tripterygium wilfordii Hook F. European Journal of Pharmacology. 880:173225.
- Zou ZQ, Yue LJ, Ren YF. 2015. Association between CYP1A1* 2A polymorphism and susceptibility to childhood acute lymphoblastic leukemia: a meta analysis. Zhongguo Dang Dai Er Ke Za Zhi. 17(10):1112–8.