?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigated the leaf litter decomposition dynamics of Hayat-ul-Mir subtropical scrub forest in Pakistan. Litter bags were incubated for 360 days in the forest to elucidate the effect of elevation and incubation time and for 90 days in microcosms to investigate the effect of projected warming (+2.3°C and +4.5°C) and soil moisture (M15% and M20%) on litter decomposition. Site elevation significantly affected k and T50% while time affected mass loss, residual weight, remaining C and N at p < .01 in field. After 360 days of decomposition, ∼48% residual weight with 11.7 ± 0.4% of C and 1.07 ± 0.06% of N was observed in the litter bags. Higher elevations (850–1020 m) were recorded with low C and N mineralization compared to 600–850 m elevations indicating their potential for long term carbon storage. Warming (+4.5°C) significantly accelerated the pace of decomposition in microcosms with comparatively higher mass loss (+26%), k (+34%), and soil CO2 efflux (+50%) reducing T50% by 77 days than observed under ambient conditions (To). The effect of moisture was insignificant but comparatively, decomposition was high at M20%. The study concludes that future warming will significantly accelerate the pace of nutrient cycling reducing the carbon storage potential of this ecosystem.
Introduction
Litter fall and decomposition play a crucial role in nutrient turnover and in maintaining the carbon balance of terrestrial ecosystems (Romero-Olivares et al. Citation2017). The litter fall mainly constitutes foliage (70%) and small twigs, bark tissues, and propagative structures (30%) which are decomposed by heterotrophs (bacteria and fungi) and insects on the forest floor (Krishna and Mohan Citation2017). The decomposition results in mineralization and leaching of soluble compounds (labile fractions) while lignin and other complex compounds (recalcitrant) tend to degrade slowly leading to the formation of humus and take years in nutrient turnover (Wang et al. Citation2009; Krishna and Mohan Citation2017; Romero-Olivares et al. Citation2017). The process is affected by a number of factors including climate, litter/substrate quality and quantity, soil physico-chemical properties, microbial enzymes, and community composition (Innangi et al. Citation2015; Gregorich et al. Citation2017; Stuble et al. Citation2019). Any disturbance in these factors and acceleration of the rate of decomposition under a warmed climate can result in positive feedback to global warming (Xu et al. Citation2015; Gregorich et al. Citation2017; Romero-Olivares et al. Citation2017). For instance, the decomposition of complex leaf litter results in emitting 20% of CO2 as soil CO2 efflux or soil respiration which might exacerbate under warmed climate (Krishna and Mohan Citation2017). Accelerated decomposition makes organic matter deficient soils leading to loss of fertility and increased susceptibility to erosion (Obalum et al. Citation2017). Hence factors of litter decomposition and their role in maintaining ecosystem processes and productivity are crucial to be investigated considering the current pace of climate change.
Tropical and subtropical forests store a major proportion (550 Gt) of the total carbon (861 Gt) stored in the global terrestrial ecosystems (Pan et al. Citation2011). However, the projected 2.6–4.8°C rise in mean global temperature by the year 2100 might significantly alter the carbon storage potential of these ecosystems due to shifting rates of decomposition (Stuble et al. Citation2019; WMO Citation2019). Climate warming directly, and indirectly affects decomposition by altering the activity of microbial extracellular enzymes and their community composition associated with decaying litter (Stuble et al. Citation2019). Climate warming also affects plant species composition and thereby litter quantity and quality thus changing the rate of decomposition (Petraglia et al. Citation2019).
A number of studies have investigated the role of climatic factors on litter decomposition using the litter bag technique. High rates of decomposition of European beech leaf litter at warmer sites compared to colder sites were reported for Italy by Innangi et al. (Citation2015). Romero-Olivares et al. (Citation2017) reported a decrease in litter recalcitrant fractions due to high microbial enzyme activity under warming in the Alaskan Boreal forest. Warming led to fast nitrogen mineralization and also reduced litter residence time on the forest floor in Hawaii Island (Bothwell et al. Citation2014). Gregorich et al. (Citation2017) also reported a decrease in the litter half life of labile and recalcitrant fractions under warming in Canada. Warming has also been documented for increasing the rate of heterotrophic respiration during an in situ heating experiment in a temperate Sitka spruce plantation in Ireland (Zou et al. Citation2018). The rate of soil CO2 efflux was also higher during a short term microcosm warming (+3°C) experiment (Stuble et al. Citation2019). Changes in soil moisture and precipitation regime also affect soil respiration. Deng et al. (Citation2017) reported an increase in soil CO2 efflux after rain in a Chinese subtropical forest. Chuckran et al. (Citation2020) reported some decrease in the decomposition rate in response to warming induced reduction in litter moisture.
The historical 0.6–1°C rise in temperature in hyper arid and mountain regions and a 25% increase in the annual precipitation during the last 40 years have been observed for Pakistan. The projected 2–5°C rise in temperature might significantly alter the country’s ecosystem functions (e.g. terrestrial carbon cycle) (Chaudhry Citation2017). But no such studies investigating the impact of warming on litter decomposition and carbon turnover have been made in Pakistan. In our recent study, significant decline in productivity and shifts in ecosystem structure and functions have been observed for subtropical scrub broadleaved forests (focus of this study) in response to climate warming (Ghafoor et al. Citation2021). Siddiqui et al. (Citation1999) also reported a decline in cover of scrub forest due to change in precipitation (±3%) and temperature (+1.8°C) by year 2050 compared to average of 1961–1990.
The subtropical broadleaved scrub forests covers an area of 1.3 Mha in Pakistan extending in the foothills and lower slopes of Himalayas, Kala Chitta, Suleman and Salt ranges. The dominant vegetation type comprise of both deciduous and evergreen tree species including Olea ferruginea, Acacia modesta, Pistacia integerima and Tecoma undulata which are native to Pakistan, Afghanistan and India (Sheikh Citation1993). Considering the current pace of global warming and its potential impacts on carbon turnover this study was designed to investigate the (1) rate of leaf litter decomposition in the Hayat-ul-Mir (HM) subtropical scrub forest, Pakistan and (2) to investigate the impact of predicted climate warming and increase in soil moisture on litter decomposition to gain insights into future trends in decomposition dynamics. It was hypothesized that climate warming will accelerate the pace of decomposition and will lead to a reduction in residual weight and carbon content of leaf litter.
Materials and methods
Site description
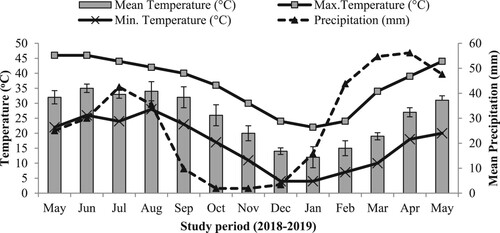
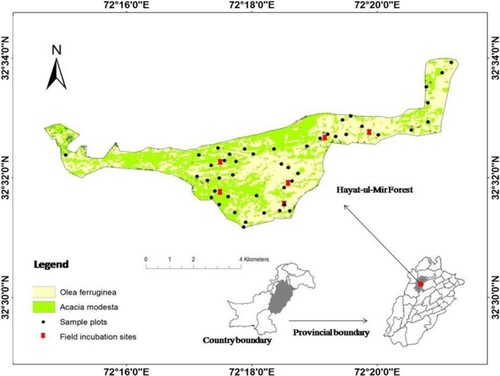
Hayat-ul-Mir (HM) subtropical scrub reserve forest (32.54°N and 72.31°E) is located in the Soan Valley of Punjab Pakistan. The forest expands over 1652 ha with an average elevation of the hills varying between 600 and 1000 m. The climate of the site is BShw (Steppe hot with dry winter) as per the Koppen Gieger Classification System. The mean annual temperature of the site is 24°C. The ambient temperature varied between 12°C (min) and 35°C (max.) with 30.63 ± 5.5 mm/month precipitation and 60% relative humidity was recorded during the study period (May 2018 to May 2019) (Figure ). The soil has a silty-clay loam texture with 1.58 ± 0.1% N, 491 ± 42.9 mg/kg P, 12.53 ± 1.1 mg/kg K and 386 ± 10.8 mg/kg Na. The valley experiences frequent prolonged drought in summer and frost in winter season (Khan et al. Citation2013). The dominant broadleaved vegetation in the forest comprises of bi-climax community of O. ferruginea Royle (evergreen) and A. modesta Linn. (deciduous) including rich shrub cover made by Buxus semipervirens L., Dodonaea viscosa L., Justicia adhatoda L., and Maytinus royleanus Wall. The forest is also invaded at some locations by exotic Prosopis juliflora Swartz (Ghafoor et al. Citation2020).
Sample collection and preparation of litter bags
Freshly fallen mixed species leaf litter of bi-climax community and shrubs was collected randomly after establishing 1 m2 survey plots in the HM forest (Figure ). The leaf litter was air dried for one week and oven dried at 60°C for 48 h until a constant mass was attained. Litter bags of nylon mesh (15 cm × 15 cm) with a pore size of 1.5 mm2 were sealed with 5 g (±0.01) of dried litter for both laboratory and field experiments. To more realistically predict litter decomposition trends, litter bags were constructed with a representative mix of litter from both shrubs and tree species because of mixed plantations in the HM forest. Pore size was kept sufficiently smaller to allow for the soil microbial interaction with litter preventing any losses of smaller leaves and also restricting entry of the mesofauna into litter bags (Innangi et al. Citation2015).
Litter initial carbon (C) and nitrogen (N) content
The sub-samples of leaf litter were analyzed for percentage initial carbon (C) and nitrogen (N) content before starting the experiment. Litter initial C was analyzed through Loss on Ignition method by igniting 1 g of leaf litter at 550°C for 4 h in a muffle furnace (Salehi et al. Citation2011). Initial litter nitrogen (N) content was analyzed through the Kjeldahl method by treating samples with concentrated acid (H2SO4) in the presence of the Kjeldahl catalyst. The acid digested samples were distilled using 10N NaOH, and ammonia was trapped in 0.1N H3BO3 and was titrated with 0.01N H2SO4 (Muñoz-Huerta et al. Citation2013).
Field incubation experiment
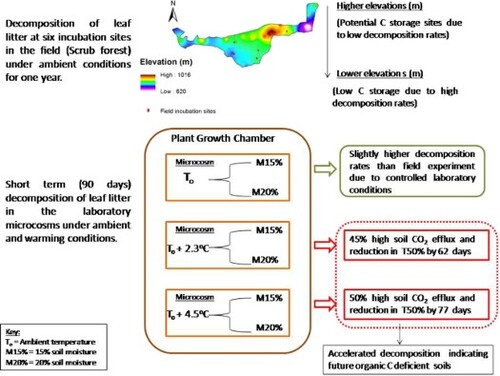
Two elevation ranges, i.e. E (600–850 m) and E (850–1020 m) were selected to assess the rate of litter decomposition in the HM forest (Figure ). Six incubation sites were chosen in these two elevation ranges, i.e. sites with 792, 816, 830, and 844 m elevation in E (600–850 m) range and sites with 905 and 1010 m elevation in E (850–1020 m) range. The selected incubation sites were at a sufficient distance (1.5–2.5 km) from each other and had different aspects, i.e. the southwest and southeast in case of E (600–850 m) and southeast in case of E (850–1020 m). In the forest soil (10–15 cm depth), 102 litter bags containing mixed species leaf litter were incubated and were covered with litter from forest floor to avoid any physical losses or exposure to harsh conditions. Of the six incubation sites, each received 17 replicate litter bags from which three bags per incubation site were taken at each of the four harvest intervals (12 bags in total). The factorial design of the experiment was six sites × three replicate litter bags × four harvest intervals. Extra bags (five per site) were incubated to compensate for the losses due to physical damage during extraction of bags at harvest or others. All the incubation sites were marked and their geographic position was also noted. During the field experiment, mean soil moisture remained 15.16 ± 1.3% at the incubation sites. The field incubation experiment continued between May 2018 to May 2019 and replicate bags were harvested four times after 90, 180, 270 and 360 days of decomposition in the months of August, November 2018 and February, May 2019. Complete geographic and thermal detail of incubation sites is available in Table S1.
Laboratory microcosm incubation experiment
A laboratory microcosm incubation experiment was designed to assess litter decomposition dynamics under projected climate change by the year 2100 for Pakistan (Cruz et al. Citation2007; Chaudhry Citation2017). To construct microcosms, soil was collected (at random) from the HM forest. The composite mixture of soil (un-sieved) was pre-incubated at 24°C and 15% soil moisture to keep microbial communities alive and acclimatize before starting experiment. Microcosms were constructed by taking six plastic boxes (320 × 260 × 100 mm) in which nine replicate litter bags/box were placed on 10 cm thick layer of pre-incubated soil and covered with additional layer of soil and litter material on top to mimic field conditions. The experiment was a factorial design with three temperature and two soil moisture treatments (nine litter bags × three temperature × two soil moisture). The microcosms were kept in the Plant Growth Chamber (WiseCube WGC-450 Daihan Scientific) at ambient (To) HM conditions (24.6°C/18°C day/night) based on five years metrological record (2013–2018) and at two warming treatments, i.e. To + 2.3°C (or 26.9°C/20.3°C) and To + 4.5°C (or 29.1°C/22.5°C) to simulate low (RCP 2.6) and high emission (RCP 8.5) scenarios of IPCC respectively. Two replicate microcosms per temperature treatment (To, To + 2.3°C and To + 4.5°C) were established in the plant growth chamber in a way that one replicate was maintained at M15% (control) and the other at M20% (elevated) of gravimetric soil moisture. Relative humidity during the whole experiment was maintained at 60% and replicate litter bags were harvested after 30, 60 and 90 days of incubation.
CO2 evolution from soil respiration in the microcosms
CO2 evolved from soil respiration during laboratory microcosm experiment was analyzed three times during decomposition (i.e. 30, 60, and 90 days). The CO2 evolved was trapped in 20 ml of 1M NaOH exposed to experimental conditions for 24 h. The carbonates formed in alkali solution (NaOH) were precipitated with 3M BaCl2 and the un-reacted alkali was titrated with 1M HCl using phenolphthalein as an indicator. The amount of soil respiration or CO2 (mg/day) evolved was computed using Equation (1) (Anderson Citation1982).
(1)
(1) where B is the volume of acid used to titrate blank, V is the volume of acid used to titrate un-reacted NaOH after trapping CO2, N is the normality of acid and E is the equivalent weight of CO2, i.e. 22.
Leaf litter decomposition dynamics
Harvested litter bags were carefully opened and excess soil particles were removed with the help of a soft brush. The decaying litter was dried at 75°C to constant mass and percentage mass loss was calculated following Equation (2) (Kurz-Besson et al. Citation2005).
(2)
(2) where lo is initial mass, lt is mass of litter in the bag after decomposition at interval of t time. The percentage residual weight (RW = lo/lt × 100) of decaying litter was calculated following Wang et al. (Citation2009). Percentage of carbon and nitrogen remaining in the decaying litter was determined through Loss on Ignition and Kjeldahl methods and litter C:N was then calculated (Salehi et al. Citation2011; Muñoz-Huerta et al. Citation2013). Decay rate (k day−1) was evaluated (Equation (3)) through a single exponential model of Olson (Citation1963) which suggests a fast nutrient regulated initial decomposition stage linked to the leaching and degradation of easily degradable soluble compounds and carbon sources. The later stages are associated with lignin and nitrogen decomposition until a stable phase is reached. Based on Olson (Citation1963) kinetics, litter half life or time required to decompose 50% (T50% = 0.693/k) of litter was also calculated (Wang et al. Citation2009).
(3)
(3)
Statistical analyses
For data analyses, Statistical Package for Social Sciences (v. 21) was used. Descriptive statistics were used to compute the means of parameters investigated to report litter decomposition dynamics. Bivariate correlation analysis was done to check the effect of elevation on litter decomposition. One way and three way ANOVA with the Tukey HSD test was used to check the main effects of independent variables on litter decomposition in the field and microcosms. Multiple comparisons were made to identify the treatments that were significantly different from each other. Multiple regression analysis was used to analyze the percentage variance in parameters of litter decomposition under the influence of predictor variables (i.e. treatment conditions).
Results
Litter decomposition dynamics in the field
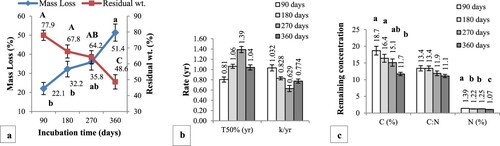
Figure presents a comparatively high rate of litter mass loss (38.9 ± 2.2%) and k (0.931 ± 0.01) with low T50% (0.92 ± 0.02 yr) was recorded at E (600–850 m) compared to E (850–1020 m). The remaining C (14.2 ± 0.6%), N (1.196 ± 0.01%), and C:N (11.8 ± 0.4) were also low at E (600–850 m) compared to E (850–1020 m).
Figure 3. Effect of elevation on litter decomposition dynamics in the field; (a) mass loss and residual weight, (b) half life and decay rate and (c) remaining C, N and C:N.
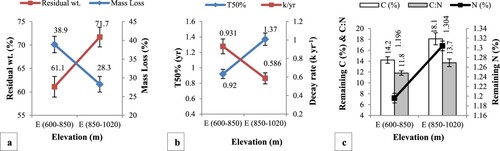
Table presents the Bivariate correlation analysis of the effect of elevation on litter decomposition dynamics in the field. A negatively significant correlation (p < .05) was found between elevation and litter decay rate (k) however the relationship was positively significant (p < .05) between elevation and litter half life (T50%). No significant correlation was found between elevation and mass loss, residual weight, litter C, N, and C: N. Other than elevation, the litter decomposition parameters were also related to each other. A negatively significant correlation was found between mass loss and litter C, N (p < .01) and C:N (p < .05). A significant negative correlation was also observed between decay rate (k) and litter half life (T50%) at p < .01. Multiple regression analysis showed that elevation explained 21% (F = 7.154) and 20% (F = 6.610) variance in decay rate and litter half life at p < .05.
Table 1. Correlation analysis of site elevation with litter decomposition dynamics.
One way ANOVA showed a significant effect of incubation time on litter mass loss, residual weight (F = 9.029, p < .01), remaining C (F = 7.203, p < .01) and N (F = 22.305, p < .001). Significant difference (p < .01) in the mean litter mass loss and residual weight was observed between 90 (22.1 ± 2.1% and 77.9 ± 2.1%) and 360 days (51.48 ± 3.6% and 48.51 ± 3.6%) of incubation. There was no significant difference in the litter decay rate (k yr−1) and T50% between incubation intervals although k was highest (1.032 ± 0.1) after 90 days with lowest T50% (0.81 ± 0.01 yr). The initial litter C and N was 42.2 ± 0.9% and 1.55 ± 0.01% respectively with 27.3 ± 0.4 C:N. Significant (p < .01) fast decline in C (18.7 ± 1.1%) and N (1.39 ± 0.07%) was observed during the first 90 days of field incubation. Due to significantly high rate of mineralization, 11.7 ± 0.4% of C and 1.07 ± 0.06% of N were left after 360 days of decomposition (Figure ). Multiple regression analysis showed that incubation time explained 40% (F = 40.169), 23% (F = 18.909) and 64% (F = 27.978) variance in litter mass loss, remaining C and N at p < .001 respectively.
Litter decomposition dynamics in the laboratory microcosms
Significant effect of incubation temperature and time was observed on litter mass loss and residual weight (p < .01, p < .001), k (p < .01 and p < .05), remaining C and N content and C:N (p < .001) and soil respiration (p < .001). T50% was significantly affected with incubation temperature only (p < .01). Effect of soil moisture and two way and three way interactions of incubation temperature, time and soil moisture also remained insignificant for litter decomposition dynamics except in case of soil respiration (Table ). Multiple regression analysis showed that incubation temperature explained 11% (F = 7.247), 47% (F = 48.729), 65% (F = 65.16), 71% (F = 4.88), 22% (F = 16.009), 20% (F = 13.799) and 73% (F = 94.403) variance at p < .001 in litter mass loss, C and N content, C:N, k, T50% and soil respiration respectively. Incubation time explained 47% (F = 47.479), 28% (F = 21.469), 29% (F = 15.59), 65% (F = 64.76), 11% (F = 7.648), 43% (F = 42.314) variance at p < .001 in mass loss, C and N content, C:N, k and soil respiration respectively while it caused no variance in T50%. Soil moisture also predicted no variance in litter decomposition dynamics.
Table 2. Univariate analysis of variance with effect of temperature (n = 3), soil moisture (n = 2) and time (n = 3) on litter decomposition dynamics in microcosms.
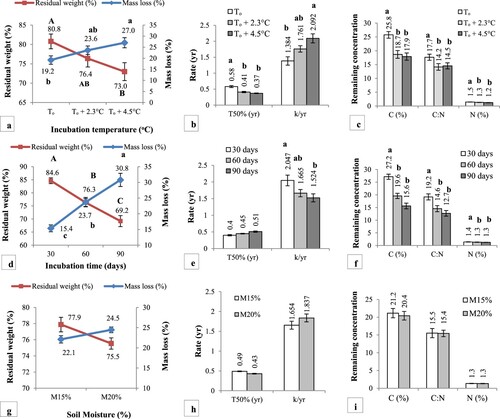
Figure presents the main effects of treatments on litter decomposition during the microcosm experiment. Litter mass loss and residual weight significantly (p < .01) varied between To (ambient temperature) and To + 4.5°C (warming) and the highest mass loss with corresponding low residual weight was recorded at To + 4.5°C (27 ± 2.3% and 73 ± 2.3%). T50% (half life) varied significantly between ambient and warming temperatures (p < .001) with a decrease in T50% from 0.58 ± 0.01 yr (To) to 0.37 ± 0.01 yr (To + 4.5°C). Litter decay rate (k yr−1) was significantly high at To + 4.5°C (2.092 ± 0.14) compared to lowest k (yr−1) at To (1.384 ± 0.13). Litter C (carbon) and N (Nitrogen) varied significantly among incubation temperatures and significantly (p < .01) low remaining C and N was recorded at To + 4.5°C (17.9 ± 1.3% and 1.2 ± 0.01%) than at To due to accelerated pace of decomposition (Figure (a–c)). Litter mass loss and residual weight also varied significantly (p < .001) among incubation intervals and it was highest after 90 days (30.8 ± 2.1% and 69.2 ± 2.1% respectively) of incubation. T50% was highest after 90 days of incubation (0.51 ± 0.02 yr) but the mean difference between incubation intervals remained insignificant. Litter decay (k) significantly varied among incubation intervals (p < .01) and it was significantly high during the initial 30 days (2.047 ± 0.01) but became low after 90 days of decomposition. The effect of incubation intervals remained significant for litter C and N (p < .01) and significantly low remaining C and N (15.6 ± 1.1% and 1.3 ± 0.01% respectively) was recorded after 90 days of decomposition. Litter C:N ratio also followed the same trend (Figure (d–f)). No significant effect of soil moisture was observed on litter mass loss, residual weight, k, T50% C, N, and C: N, although decomposition was high at M20% (Figure (g–i)).
Figure 5. Effect of incubation temperature (a–c), time (d–f) and soil moisture (g–i) on litter decomposition dynamics during microcosm experiment. Bars and lines with different letters indicate significant difference at p < .05 based on Tukey HSD test.
Effect of warming on soil respiration in the microcosms
The rate of soil respiration (mgCO2/day) varied significantly (p < .001) among incubation temperatures and time and it was highest under To + 4.5°C (14.5 ± 0.1 mgCO2/day) compared to To (7.65 ± 0.1 mgCO2/day) and after 90 days of decomposition (12.7 ± 0.1 mgCO2/day) than it was in the beginning (11.1 ± 0.1 mgCO2/day). No significant effect of soil moisture was observed on soil respiration although it was high at M20% (12.1 ± 0.0.8 mgCO2/day) (Figure ).
Figure 6. Main effect of incubation temperature, time and soil moisture on soil respiration. Bars with different letters differ significantly at p < .05 based on Tukey HSD.
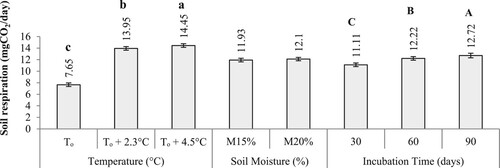
Multiple comparisons of the effect of incubation temperature, time, and moisture on respiration showed that CO2 evolution remained significantly low under To during each incubation interval at both M15% and M20%. Warming accelerated the pace of decomposition in the microcosms resulting in significant release of CO2 as soil respiration and it was highest under To + 4.5°C during each incubation interval, except after 90 days of decomposition with M20% when it was significantly lower than To + 2.3°C (Table ).
Table 3. Multiple comparison of effect of treatments on soil respiration (mgCO2/day) in the microcosms.
Discussion
In this study the rate of mixed species leaf litter decomposition was investigated in the field and under laboratory conditions to investigate the influence of predictor variables on litter decomposition dynamics. During field experiment, low litter decomposition rates at higher elevation sites were recorded which is consistent with the findings of Illig et al. (Citation2008) and Marian et al. (Citation2017). Reasons for the decrease in the decomposition rate are related to different aspects and comparatively low temperature at higher elevations compared to low elevation sites in this study. The southeast facing sites at higher elevations had a slightly lower temperature as those were receiving sunlight during morning only. Whereas, the southwest facing sites at lower elevations were relatively warmer for receiving sunlight during most of the day. The aspect and thermal differences resulted in low decomposition rate and accumulation of litter at higher elevations in the HM forest (Table S1). Low carbon mineralization at higher altitude suggests long term soil carbon sequestration due to unfavorable environmental conditions for decomposer community.
Decrease in the litter residual weight over time was observed during field experiment which is consistent to the findings of Alhamd et al. (Citation2004), Ono et al. (Citation2013), and Naeem et al. (Citation2017) in the subtropical and temperate forests. In the current study, ∼52% of litter mass was decomposed in one year however; Luo et al. (Citation2017) reported comparatively higher (65%) litter mass loss for subtropical forests in China, and the differences are related to sites’ physical characteristics. Naeem et al. (Citation2017) reported comparatively lower litter mass loss (39%) during one year field experiment in subtropical forest in Pakistan. The differences in the estimates of both studies are related to the experimental design (placement of litter bags in soil) and type of litter (mixed or single species) under decomposition. Naeem et al. (Citation2017) placed litter bags (containing single species, i.e. A. modesta) on top of forest soil (un-covered) exposing litter to harsh environmental conditions resulting in low decomposition rate. However, litter bags containing mixed species leaf litter were incubated in the forest soil at a depth of 10–15 cm.
The decomposition rates are related to the priming or fertilization effect induced by litter mixtures where easily decomposable litter might stimulates microbial growth enhancing decay rate. In the current study, the litter mixtures varied in their physical structure with thick waxy cuticles in the leaves of O. ferruginea than a litter of A. modesta and shrub species. It is possible that litter from shrub and deciduous species might had induced fertilization effect and stimulated microbial community enhancing decay of tough waxy leaf litter of O. ferruginea. Similar effect of litter mixtures on decay rate has also been reported for subtropical forests in China by Wang et al. (Citation2007) and Liu et al. (Citation2016).
Litter decomposition in the field is strongly influenced by seasonal variations affecting microbial activity. Field experiment was started in the month of May and was regulated by high summer temperature resulting in higher k rate. The rate was then relatively slowed during monsoon rains (July–September) and winter season (October–January), while it was resumed, although at relatively slow rate, on arrival of spring and next summer. Similar effect of seasonal variation on litter decomposition was also documented by Naeem et al. (Citation2017) reporting reduction in microbial degradation of litter during winter season.
The rate of decomposition is also related to the litter quality. During field experiment, the labile fractions (soluble sugars, cellulose and hemicelluloses) degraded initially at a rapid rate while the recalcitrant fractions (lignin) accumulated towards the end of the experiment with lower k values. Similar trend of litter decomposition has also been reported for evergreen broadleaved forest, subtropical, temperate and boreal forests in China, Japan and Europe (Alhamd et al. Citation2004; Ono et al. Citation2013; Innangi et al. Citation2015; Trogisch et al. Citation2016). The T50% estimated in the current study was initially low due to the fast decay rate of litter which later increased with low k due to the accumulation of recalcitrant fractions towards the end field of experiment.
Litter decomposition is also regulated by litter nutrient concentration. The initial high litter N concentration with a low C:N ratio played a crucial role in accelerating the pace of decomposition in the current study. However, low initial litter N and high C:N are documented for reducing decomposition rates (Wang et al. Citation2007; Wang et al. Citation2009). Litter N mineralized in three phases, i.e. initial leaching, then slight gain, and then loss of nitrogen from decaying litter consistent to Alhamd et al. (Citation2004), Wang et al. (Citation2007), and Innangi et al. (Citation2015). These three phases are linked with microbial demand for nitrogen in the beginning, its translocation from soil to litter by microbes, and then its release to soil during decay.
The rate of decomposition during 90 days microcosm experiment under To was comparatively fast than field decomposition which is consistent with Rinkes et al. (Citation2013) and Innangi et al. (Citation2015). The controlled laboratory conditions accelerated the pace of decomposition while daily changes in the temperature, humidity, and soil moisture influenced decomposer communities in the field. Decomposition in the field is also affected by higher levels of secondary metabolites (e.g. phenolics) exuded from plant roots affecting microbial processes (Wang et al. Citation2009). These conditions, however, remain constant under controlled conditions in microcosms and also exclude the effects of live plant communities.
The effect of warming was significant in accelerating the pace of decomposition leading to higher C mineralization and CO2 evolution indicating potential future organic matter deficient soils in Pakistan. The high amount of CO2 evolution (cumulatively 50%) under warming suggests an accelerated pace of C turnover which can influence atmospheric CO2 levels leading to positive feedback on climate change. It is also expected that the subtropical vegetation in the HM forest might produce leaf litter with high carbon and nitrogen content (Ghafoor et al. Citation2021) in the warm future which might lead to higher CO2 efflux from decomposition. Consistent to the findings of the current study, Salinas et al. (Citation2011), Salah and Scholes (Citation2011), Butenschoen et al. (Citation2011), Butenschoen and Scheu (Citation2014) and Zou et al. (Citation2018) also documented higher litter decomposition rates and soil CO2 efflux under laboratory and field warming experiments. The current study found no effect of soil moisture indicating that changes in precipitation rates in future might not significantly alter the rates of decomposition as also reported by Salinas et al. (Citation2011).
High litter decomposition rate under warming also depicts a decrease in litter residence time on forest floor. A reduction of 59 and 77 days in T50% was observed under +2.3°C and +4.5°C warming respectively showing the temperature sensitivity of decomposing leaf litter. Bothwell et al. (Citation2014) reported a decrease of 31 days in litter residence time in tropical forest of Hawaii with +1°C warming. Gregorich et al. (Citation2017) also documented faster decomposition rate with decrease in T50% of litter exposed to RCP 4.5 and RCP 8.5 climate scenarios with minimal effect of soil parameters (including moisture) on litter decomposition.
Along with the effects of temperature and moisture changes, rising atmospheric CO2 concentration at a rate of 2.5 ppm/year (expected 530–650 ppm in 2040 for Asia) can also significantly alter ecosystem productivity, litter biochemistry and decomposition rates. Cha et al. (Citation2017) and Amani et al. (Citation2019) reported decrease in litter decomposition rate due to low foliar N concentration in leaves exposed to elevated CO2 concentration. Current study has examined the effect of future warming and moisture changes only and not of CO2 concentration hence the decomposition rates may vary under combined effect of these variables in future.
Conclusion
The investigation on decomposition of mixed species leaf litter in the HM forest showed that incubation time was the main driving factor in reducing litter mass by half in the field. Decomposition trajectory followed a two stage model with initial fast and later slow rate overtime due to inherent litter quality and seasonal variations in the field. The effect of elevation was insignificant because of smaller difference in the range. But low rate of carbon and nitrogen mineralization at higher elevations suggest greater potential to hold these elements for longer time. The laboratory microcosm experiments showed that although decomposition rate was slow in the field but when the soil of HM forest was incubated in the laboratory under ambient (favorable) conditions it promoted decomposition. The short term climatic warming facilitated microbial activity and significantly accelerated the pace of litter decomposition. Higher C and N mineralization rate also reduced litter half life. The soil CO2 efflux was higher due to increase in moisture and warming highlighting the interaction between both climatic variables. The study suggests that a future warmer climate with increase in precipitation will lead to positive feedback to climate change emitting more CO2 from soil of HM forest than it would be stored. Although a faster nutrient cycling might facilitate plant growth but soil respiration may outcompete the net carbon stored in the soil reducing carbon sequestration potential of the forest in future.
Supplemental Material
Download MS Word (27.5 KB)Acknowledgements
Authors are thankful to the officials from Forest department Khushab Pakistan for their support during field experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethical approval
There are no ethical issues in the publication of this manuscript.
Data availability statement
The data supporting the findings of this study are available Mendeley Data, V1, DOI: 10.17632/tkddkcv6sf.1 (https://data.mendeley.com/datasets/tkddkcv6sf/1).
Additional information
Funding
References
- Alhamd L, Arakaki S, Hagihara A. 2004. Decomposition of leaf litter of four tree species in a subtropical evergreen broad-leaved forest, Okinawa Island. Japan Forest Ecol Manag. 202(1–3):1–11.
- Amani M, Graça MA, Ferreira V. 2019. Effects of elevated atmospheric CO2 concentration and temperature on litter decomposition in streams: a meta-analysis. Int Review Hydrobiol. 104(1–2):14–25.
- Anderson JPE. 1982. Soil respiration. In: Miller RH, Keeney DR, editor. Methods of soil analysis, part 2. Chemical and microbiological properties. 2nd ed. Madison (Wl): American Society of Agonomy; p. 831–871.
- Bothwell LD, Selmants PC, Giardina CP, Litton CM. 2014. Leaf litter decomposition rates increase with rising mean annual temperature in Hawaiian tropical montane wet forests. PeerJ. 2:e685.
- Butenschoen O, Scheu S. 2014. Climate change triggers effects of fungal pathogens and insect herbivores on litter decomposition. Acta Oecol. 60:49–56.
- Butenschoen O, Scheu S, Eisenhauer N. 2011. Interactive effects of warming, soil humidity and plant diversity on litter decomposition and microbial activity. Soil Biol Biochem. 43(9):1902–1907.
- Cha S, Chae HM, Lee SH, Shim JK. 2017. Effect of elevated atmospheric CO2 concentration on growth and leaf litter decomposition of Quercus acutissima and Fraxinus rhynchophylla. PloS One. 12(2):e0171197.
- Chaudhry QUZ. 2017. Climate change profile of Pakistan. Mandaluyong City, Manila, Philippines: Asian Development Bank.
- Chuckran PF, Reibold R, Throop HL, Reed SC. 2020. Multiple mechanisms determine the effect of warming on plant litter decomposition in a dryland. Soil Bio Biochem. 145:107799.
- Cruz RV, Harasawa H, Lal M, Wu S, Anokhin Y, Punsalmaa B, Honda Y, Jafari M, Li C, Huu Ninh N. 2007. Asia. Climate Change 2007: impacts, adaptation and vulnerability. In: Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; p. 469–506.
- Deng Q, Hui D, Chu G, Han X, Zhang Q. 2017. Rain-induced changes in soil CO2 flux and microbial community composition in a tropical forest of China. Sci Rep. 7(1):1–9.
- Ghafoor GZ, Sharif F, Khan AU, Shahid MG, Siddiq Z, Shahzad L. 2021. Effect of climate warming on seedling growth and biomass accumulation of Acacia modesta and Olea ferruginea in a subtropical scrub forest of Pakistan. Ecoscience. (In Press). doi:10.1080/11956860.2021.1958536.
- Ghafoor GZ, Sharif F, Khan AU, Shahzad L, Hayyat MU. 2020. Assessment of tree biomass carbon stock of subtropical scrub forest, Soan Valley Pakistan. App Ecol Env Res. 18(2):2231–2245.
- Gregorich EG, Janzen H, Ellert BH, Helgason BL, Qian B, Zebarth BJ, Anger DA, Beyaert RP, Drury CF, Duguid SD, et al. 2017. Litter decay controlled by temperature, not soil properties, affecting future soil carbon. Glob Change Bio. 23(4):1725–1734.
- Illig J, Schatz H, Scheu S, Maraun M. 2008. Decomposition and colonization by micro-arthropods of two litter types in a tropical montane rain forest in southern Ecuador. J Trop Ecol. 24:157–167.
- Innangi M, Schenk MK, d’Alessandro F, Pinto S, Menta C, Papa S, Fioretto A. 2015. Field and microcosms decomposition dynamics of European beech leaf litter: influence of climate, plant material and soil with focus on N and Mn. App Soil Ecol. 93:88–97.
- Khan AU, Ahmad F, Sharif F. 2013. Rapid ranking method for prioritizing restoration by evaluating human influences on the status of scrub forest: a case study. Pak J Bot. 45(1):11–16.
- Krishna MP, Mohan M. 2017. Litter decomposition in forest ecosystems: a review. Energ Ecol Env. 2(4):236–249.
- Kurz-Besson C, Coûteaux MM, Thiéry JM, Berg B, Remacle J. 2005. A comparison of litterbag and direct observation methods of Scots pine needle decomposition measurement. Soil Biol Biochem. 37(12):2315–2318.
- Liu C, Liu Y, Guo KE, Zhao H, Qiao X, Wang S, Zhang L, Cai X. 2016. Mixing litter from deciduous and evergreen trees enhances decomposition in a subtropical karst forest in southwestern China. Soil Biol Biochem. 101:44–54.
- Luo D, Cheng R, Shi Z, Wang W. 2017. Decomposition of leaves and fine roots in three subtropical plantations in China affected by litter substrate quality and soil microbial community. Forests. 8(11):412.
- Marian F, Sandmann D, Krashevska V, Maraun M, Scheu S. 2017. Leaf and root litter decomposition is discontinued at high altitude tropical montane rainforests contributing to carbon sequestration. Ecol Evol. 7(16):6432–6443.
- Muñoz-Huerta RF, Guevara-Gonzalez RG, Contreras-Medina LM, Torres-Pacheco I, Prado-Olivarez J, Ocampo-Velazquez RV. 2013. A review of methods for sensing the nitrogen status in plants: advantages, disadvantages and recent advances. Sensors. 13(8):10823–10843.
- Naeem I, Ansari L, Nizami SM, Asif T, Tariq H, Mahmood T. 2017. Investigating the rate of litterfall and decomposition in Phulai dominated forest of Pakistan: a nutrient cycling perspective. Int J Biosci. 10(1):42–51.
- Obalum SE, Chibuike GU, Peth S, Ouyang Y. 2017. Soil organic matter as sole indicator of soil degradation. Env Monit Assess. 189(4):176.
- Olson JS. 1963. Energy storage and balance of producers and decomposers in ecological systems. Ecology. 44:322–331. doi:10.2307/1932179.
- Ono K, Hiradate S, Morita S, Hirai K. 2013. Fate of organic carbon during decomposition of different litter types in Japan. Biogeochemistry. 112(1-3):7–21.
- Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurtz WA, Phillips OC, Shvidenko A, Lewis SL, Canadell JG, et al. 2011. A large and persistent carbon sink in the world’s forests. Science. 333:988–993.
- Petraglia A, Cacciatori C, Chelli S, Fenu G, Calderisi G, Gargano D, Abeli T, Orsenigo S, Carbognani M. 2019. Litter decomposition: effects of temperature driven by soil moisture and vegetation type. Plant Soil. 435(1–2):187–200.
- Rinkes ZL, Sinsabaugh RL, Moorhead DL, Grandy AS, Weintraub MN. 2013. Field and lab conditions alter microbial enzyme and biomass dynamics driving decomposition of the same leaf litter. Front Microbiol. 4:260.
- Romero-Olivares AL, Allison SD, Treseder KK. 2017. Decomposition of recalcitrant carbon under experimental warming in boreal forest. PloS One. 12(6):e0179674.
- Salah YM, Scholes MC. 2011. Effect of temperature and litter quality on decomposition rate of Pinus patula needle litter. Procedia Environ Sci. 6:180–193.
- Salehi MH, Beni OH, Harchegani HB, Borujeni IE, Motaghian HR. 2011. Refining soil organic matter determination by loss-on-ignition. Pedosphere. 21(4):473–482.
- Salinas N, Malhi Y, Meir P, Silman M, Roman CR, Huaman J, Salinas D, Huaman V, Gibaja A, Mamani M, Farfan F. 2011. The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol. 189(4):967–977.
- Sheikh MI. 1993. Trees of Pakistan (Vol. 110). Islamabad: Pictorial Printers.
- Siddiqui KM, Mohammad I, Ayaz M. 1999. Forest ecosystem climate change impact assessment and adaptation strategies for Pakistan. Clim Res. 12(2-3):195–203.
- Stuble KL, Ma S, Liang J, Luo Y, Classen AT, Souza L. 2019. Long-term impacts of warming drive decomposition and accelerate the turnover of labile, not recalcitrant, carbon. Ecosphere. 10(5):e02715.
- Trogisch S, He JS, Hector A, Scherer-Lorenzen M. 2016. Impact of species diversity, stand age and environmental factors on leaf litter decomposition in subtropical forests in China. Plant Soil. 400(1–2):337–350.
- Wang Q, Wang S, Fan B, Yu X. 2007. Litter production, leaf litter decomposition and nutrient return in Cunninghamia lanceolata plantations in south China: effect of planting conifers with broadleaved species. Plant Soil. 297(1–2):201–211.
- Wang Q, Wang S, Huang Y. 2009. Leaf litter decomposition in the pure and mixed plantations of Cunninghamia lanceolata and Michelia macclurei in subtropical China. Biol Fert Soils. 45(4):371–377.
- WMO. 2019. WMO statement on the state of the global climate in 2019. Geneva, Switzerland: World Meteorological Organization.
- Xu Z, Zhao C, Yin H, Liu Q. 2015. Warming and forest management interactively affect the decomposition of subalpine forests on the eastern Tibetan Plateau: a four-year experiment. Geoderma. 239:223–228.
- Zou J, Tobin B, Luo Y, Osborne B. 2018. Response of soil respiration and its components to experimental warming and water addition in a temperate Sitka spruce forest ecosystem. Agri For Meteorol. 260:204–215.