?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This work evaluated the potential of Camellia pollen extracts as an antioxidant and lipase activity inhibitor from the pollen composition and the extraction solvent. The lipase inhibitory activity, the scavenging radical’s ability (ABTS and DPPH) and the ferric-reducing ability of the seven extracts were examined. The fatty acid profile of the extracts with excellent antioxidant and lipase inhibitory activity was also determined by GC-MS. The ethyl acetate (IC50 = 2.07 ± 0.20 mg/mL) and the acetone extracts (IC50 = 1.19 ± 0.07 mg/mL) showed better lipase inhibitory activity. For the antioxidant activity, the methanol (IC50 = 2.55 ± 0.34 mg/mL), ethanol (IC50 = 2.87 ± 0.23 mg/mL) and the water (IC50 = 2.62 ± 0.05 mg/mL) extracts had superior ABTS radical-scavenging ability. Greater DPPH radical-scavenging ability was seen in the ethanol (IC50 = 2.92 ± 0.04 mg/mL) and acetone extracts (IC50 = 3.09 ± 0.12 mg/mL). The ethyl acetate and petroleum ether extracts manifested better FRAP. The fatty acid composition of the methanol and ethyl acetate extracts belonged to the unsaturated fatty acids. This study indicated that the lipase inhibitory activity and the antioxidant activity found in Camellia pollen may exert dual benefits for preventing and treating obesity.
Introduction
As an important part of traditional medicine, bee pollen is a fine, powder-like material produced by flowering plants and gathered by bees. It contains nearly all nutrients such as proteins, amino acids, lipids, phenolic compounds, vitamins or minerals required by humans (Ares et al. Citation2018) and thus used as a dietary supplement (C. K. Roberts and K. K. Sindhu Citation2009) and considered as a functional food (Lia et al. Citation2018). Bertoncelj et al. (Citation2018) pointed out that pollen has an evident antioxidant ability due to the phenolic compounds, flavonoids and other kinds of phytochemicals. A recent review pointed out bee pollen exerted strong antimicrobial activities on several bacterial strains of fungi and yeasts, shows anticarcinogenic, antiallergic and immune responses activity and could enhance the hepatic detoxified functions (Qiang-Qiang Li et al. Citation2018). The bee pollen was also proposed as a protective and therapeutic strategy in the case of MeHg neurotoxicity (Al-Osaimi et al. Citation2018). Rape bee pollen can be used for the treatment of benign prostatic hyperplasia (BPH) (Chen et al. Citation2018).
Obesity is considered to be a strong risk factor for cardiovascular diseases, diabetes, cancer (Kocot, et al. Citation2018), (Arts et al. Citation2004), hypertension, hyperlipidemia and arteriosclerosis. Lipase inhibition is an effective way to reduce fat absorption, orlistat is one effective pancreatic lipase inhibitor, which can effectively treat human obesity (Ballinger and Peikin Citation2002), but with certain unpleasant gastrointestinal side effects. Many efforts have been in the search for more effective lipase agents with fewer side effects. To our knowledge, no research focused on the lipase inhibition activity from bee pollen.
The bioactivity and composition are different from the origin of the bee pollen (Gardana et al. Citation2018). Tea plant [Camellia sinensis (L.) O. Kuntze] is one of the most important economic crops in many countries, such as China, India, Sri Lanka and Japan (Chen et al. Citation2006). Camellia pollen, produced by flowering Camellia sinensis and gathered by bees, is one of the most important bee pollens extensively consumed in China (Yang et al. Citation2016).
Thus, the present work was carried out (1) to determine the bio-compounds, antioxidant and lipase inhibitory effects of seven solvent extracts of Camellia pollen; (2) to determine the fatty acid composition of the methanol and ethyl acetate pollen extracts; (3) analyze the correlation between the bioactivity and the content of the total phenols, total proteins, total flavonoids, and the total reducing sugar of pollen extracts.
Materials and methods
Camellia pollen was provided by the Beijing Apiculture Company (BAC).
Reagents and standards
2,4,6-tri-(2-pyridyl)-1,3,5-triazine (TPTZ) was purchased from TCI (Shanghai Development Co., Ltd. Shanghaicity Chemical Industrial Zone Pudong road NO96, China); 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium Salt (ABTS diammonium Salt, High Purity Grade) and lipase (200-400 U/mg), p-nitrophenylbutyrate (p-NPB) were purchased from Sigma-Aldrich Co. (Sigma-Aldrich, 3050 Spruce street, Saint Louis, mo 63103, UAS). 4-Morpholinepropanesulfonic acid (MOPS) (Ultra Pure Grade) was purchased from Amresco 10870 (Amresco SCO Inc. 30175 Solon Industrial Parkway, USA). Methanol and n-hexane were of HPLC analytical grade. All other reagents were of analytical grade.
Sample preparation
Camellia pollen (10 g) (Shattered and passed through a 50 mesh screen) magnetic stirrer extracted with seven solvents (methanol, ethanol, water, ethyl acetate, acetone, n-butyl alcohol, and petroleum ether) at a ratio of 1:10 (w/v) for 10 min (100 rpm). The supernatant of the three times extraction was collected and combined. After evaporation to dryness, each crude extract was redissolved in 25 mL dimethyl sulfoxide (DMSO) and stored at −20°C for later use.
The total phenol content
The total phenol content (TPC) was estimated using the Folin–Ciocalteu method according to previous researchers (Sawczuk et al. Citation2021). Briefly, 1.0 mL of each solvent extract was added into 5.0 mL of 10% Folin–Ciocalteu reagent, after shaking for 30 s and reacting for 8 min, 4.0 mL of 7.5% Na2CO3 solution was added. 60 min later in the dark, the absorbance was read at 765 nm. 1 mL DMSO acted as a blank. The TPC value was expressed as milligrams of gallic acid equivalents (GAE) per gram of the sample.
The total protein content
The Folin–Ciocalteu method was used to determine the protein of each solvent extracts (Jianhua et al. Citation2017). Briefly, an appropriate volume of pollen extracts was put in 10 mL glass tubes and added solvent to 1 mL. 5 mL of alkaline copper sulfate reagent was added, reacting for 10 min at room temperature, 0.5 mL of Folin-Ciocalteu’s reagent was added. 30 min later the absorbance was read at 650 nm. DMSO was used as a blank and the protein content was expressed as milligrams of casein per gram of the sample. The alkaline copper sulfate reagent was freshly prepared.
The total flavonoid content (TFC)
The aluminum nitrate method (Chinese Standard GB/T 20574-2006) was used to determine the total flavonoid content. 500 µL of approximately diluted pollen extracts was put in a 25 mL graduated tube. Then 7 mL DMSO, 0.5 mL 10% aluminum nitrate, and 0.5 mL 9.8 g/L potassium acetate were added, finally added distilled water to 25 mL. After the reaction at room temperature for 1 h, the absorbance was 415 nm. The TFC was expressed as micrograms of rutin equivalents (RE) per gram sample.
The total reducing sugar (TRS)
3, 5-dinitrosalicylic acid (DNS) Bernfeld method (José R Ayala et al. Citation2021) was used to determine the protein content. An appropriate amount of each pollen extract was put in 25 mL glass tubes. 1.5 mL of the DNS reagent was added and mixed thoroughly for 15 min. After the reaction at 100°C for 5 min, it was cooled and diluted to 25 mL. The absorbance was read at 540 nm. The TRS was expressed as milligrams of glucose equivalents (GE) per gram of the sample.
Lipid content
The lipid content of pollen and the residue after solvent extraction were determined by the Soxhlet extraction method. The lipid content of the pollen solvent extract was calculated using the equation below:
where A1 is the percentage of lipid in the Camellia pollen, and A2 is the percentage of lipid in the pollen residue after solvent extraction.
GC-MS analysis of the fatty acid composition
Fatty acids were derivatized to their corresponding methyl esters using boron trifluoride in a methanol (BF3-MeOH) solution. 100 µL of pollen extract and 1 mL of 0.5 N NaOH-MeOH were put in a 10 mL glass tube in nitrogen, saponified at 90°C for 30 min and cooled at room temperature. 1 mL of 14% BF3-MeOH was added to the mixture for esterification at 60°C for 5 min. After cooling, 2 mL of n-hexane was added, after centrifuging at 13,000 rpm for 10 min and analyzed for the fatty acid profile using GC-MS.
The fatty acid methyl esters in n-hexane were injected into an Rtx-5MS capillary column coated with 95% dimethyl-5% diphenyl polysiloxane (60, 0.32 mm inner diameter, 0.25 m film thickness, Resek company) installed in GC-MS QP2010 (Shimadzu, Tokyo 101-8448, Japan) equipped with quadrupole mass spectrometers. The sample (1 µL) was injected with a split ratio of 10:1 and the inlet temperature was set at 250°C. The initial oven temperature was set at 50°C for 4 min. Then, it was raised gradually to 250°C at a rate of 4°C/min and kept for 50 min. The carrier gas was nitrogen with a flow rate of 5.0 mL/min. MS analyzes were made in the electron impact (EI+) mode at 70 eV, the mass range was m/z 29–500 and the chromatogram was acquired in total ion current (TIC). The components were quantitated by the relative percent peak area of TIC from the MS signal and identified by comparing their mass fragmentation patterns with the patterns stored in the spectrometer database NIST (National Institute of Standards and Technology).
ABTS radical-scavenging ability
The ABTS radical-scavenging ability was measured according to the method described by Sandra et al. (Citation2021) with some modifications. The ABTS·+ cation radical was freshly prepared by reacting 5 mL of 7 mM ABTS solution with 88 µL of 140 mM potassium persulphate solution in the dark at room temperature for 16 h. The solution was stable for 3 days. Before use, the ABTS·+ solution was diluted with ethanol to get an absorbance of 0.70 ± 0.02 at 734 nm. The resulting solution (2 mL) was mixed with 100 µL of the diluted sample and after 10 min reaction, the absorbance was 734 nm, Vc was a positive control). The inhibition rate of each sample was calculated using the equation below:
where Abss is the absorbance value of the sample (pollen extract and the ABTS·+ solution), and Absb is the absorbance value of the blank (pollen extract and the corresponding extract solution). Absc is the absorbance value of the control (ABTS·+ solution and the corresponding extract solution).
Ferric-reducing antioxidant power (FRAP)
The ferric-reducing antioxidant power (FRAP) assay was according to Fogliano et al. (Citation1999). The FRAP solution was freshly prepared before use. 450 µL of FRAP solution and 735 µL of water were gradually added to 20 µL of the pollen extracts in a 2 mL centrifugal tube (DMSO as a blank). 100 µL of the mixture was transferred into a 96 well-flat bottom micro-plate (Costar, Cambridge, MA), sealed and incubated in a dark environment at 37°C for 30 min. The absorbance was at 595 nm using a microplate reader (OPTIMA, Germany). The reported values are expressed in terms of the amount, in mM, of the ferrous form of [Fe2+] produced from a standard curve plot of ferrous ascorbate. Vc was the positive control.
DPPH radical-scavenging ability
The 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) radical-scavenging ability was evaluated by a slightly modified method of Joseph R. Hyatt, et al. (Citation2021). Briefly, 40 µL of an appropriately diluted extract of the bee pollen was mixed with a 200 µL of 0.2 mM DPPH solution. The reaction was kept in the dark at room temperature for 30 min and then the absorbance was read at 517 nm, using a microplate reader (OPTIMA, Germany). Vc was a positive control, and IC50 is defined as the concentration of the extract required to scavenge 50% of the DPPH. The DPPH radical-scavenging rate was calculated using the following equation:
where Abss is the absorbance value of the sample (pollen extract and DPPH solution) and Absb is the absorbance value of the blank (pollen extract and corresponding extract solution). Absc is the absorbance value of the control (DPPH solution and corresponding extract solution).
Lipase inhibitory assay
The lipase inhibitory ability of the pollen extracts was assessed using the previous method with a minor modification (Kim et al. Citation2007). An enzyme buffer was prepared by the addition of 30 µL (10 units) of a solution of porcine pancreatic lipase (Sigma, St. Louis, MO) in 10 mM MOPS (4-morpholinepropanesulfonic acid), and 1 mM EDTA, pH 6.8, to 850 µL of Tris-buffer (100 mM Tris-HCl and 5 mM CaCl2, pH 7.0). Then, 100 µL of the pollen extract or Orlistat (Roche, Switzerland) was mixed with 880 µL of the enzyme buffer and incubated at 37°C for 15 min. Then 20 µL of the substrate solution [10 mM p-NPB (p-nitrophenylbutyrate) in DMSO] was added and the enzymatic reaction was allowed to proceed for 15 min at 37°C. Pancreatic lipase activity was determined by measuring the hydrolysis of p-NPB to p-nitrophenol at 405 nm using a microplate reader (OPTIMA, Germany). Lipase inhibition activity was expressed as the percentage decrease in the OD when porcine pancreatic lipase was incubated with the test sample. Orlistat acted as a positive control and IC50 is defined as the concentration of the extract required to inhibit 50% lipase enzyme activity.
Statistical analysis
Data were shown using three analysis (± standard deviations, SD). Differences between means were considered significant at p < 0.05 according to Duncan’s multiple range test. Statistical analysis was performed by SPSS 17.0. Comparisons between groups were done with the one-way analysis of variance (ANOVA). IC50 values were obtained from the least-squares regression line of the plots of the Napierian Logarithm of sample concentration (ln) versus the antioxidant scavenging and lipase inhibitory activity.
Results
Total phenol content
The total phenol content of the Camellia pollen extracts was different according to the kind of extraction solvent. The phenol content of pollen extracts varied significantly (p < 0.05) between different extraction solvents (Table ). The total phenols extracted the most in the methanol solvent with a value of 15.98 ± 0.66 GAE/g dry pollen, followed by the ethanol extraction (10.29 ± 0.66 GAE/g dry pollen) and water extraction (9.08 ± 0.40 GAE/g dry pollen). For the acetone, ethyl acetate, n-butylalchol and petroleum ether solvent, the total phenols contents were not significantly different (p > 0.05). Solvent extraction is effectively used for the isolation of biological compounds which possess different polarities. Methanol and methanol in water are usually preferred for the recovery of phenolic compounds. In this study, the content of total phenols of Camellia pollen methanol extracts was similar to the previous results obtained by Mărghitaş (Mărghitaş et al. Citation2009). While the total phenols content of the flowers of Salix spp. and Taraxacum officinale Web were 16.4 and 16.2 mg GAE/g, respectively. The previous study also pointed out that the total phenol content ranged from 1.7 to 2.2% (Negri et al. Citation2011).
Table 1. The biocompound content of the Camellia pollen extracts.
Total protein content
The antioxidant capacity of pollen is widely regarded the combined activity of a wide range of compounds, including phenolic compounds, organic acids as well as small peptides and enzymes. The protein content varied between the solvents (Table ) (p < 0.05). In the methanol the protein content was the most (571.05 ± 4.91 mg casein/g dry pollen), followed by the water extract (495.60 ± 1.99 mg casein g−1 dry pollen) and then the ethanol extract (258.79 ± 2.50 mg casein/g dry pollen). While not so much protein content is shown in the lower polarity solvents such as acetone, petroleum, ethyl acetate, and n-butyl alcohol.
Total flavonoid content (TFC)
The flavonoid content of the pollen extracts varied according to the solvents. The ethanol and methanol extracts contained higher levels of flavonoids (5.62 ± 0.28 and 4.19 ± 0.20 mg RE/g dry pollen respectively) compared with the other solvent extracts (p < 0.05). In water extracts the flavonoid content was the lowest (0.44 ± 0.01 mg RE/g dry pollen).
Total reducing sugar (TRS)
Bee Pollen contains carbohydrates, lipids, fats and vitamins, which contribute to the health effects. The total reducing sugar content of the seven solvent extracts were shown in Table . The total reducing sugar from water, methanol and ethanol extracts were 343.36 ± 1.91, 319.41 ± 5.18 and 181.69 ± 3.27 mg glucose/g dry pollen, respectively. The other solvent extracts with lower reducing sugar content.
Oil content and GC-MS analysis of the fatty acid composition
The oil content of the Camellia pollen was 4.38%. The results were lower than the previous study, oil the oil content of lotus bee pollen by Soxhlet extraction was 4.90% (Xu et al. Citation2011). This variation might be the different origins of the pollens. The oil content and fatty acid composition of the methanol and ethyl acetate pollen extracts were various with the extraction ability, the oil content of the ethyl acetate pollen extract was higher than the methanol extract. The unsaturated fatty acids (USFA) represented the main fatty acids of the total fatty acids in two extracts, accounting for 63.80% and 53.14%, respectively.
Linolenic acid was the main fatty acid in the Camellia pollen extracts from the two extracts (57.98 and 49.02%, respectively), followed by oleic acid (30.39% and 31.95%, respectively), and then linoleic acid (5.82 and 4.12%, respectively) and stearic acid (1.27 and 2.06%, respectively). These results were similar to the previous. The oil of the rape bee pollen was rich in linoleic acid and linolenic acid (Xu et al. Citation2011). However, the fatty acid composition of the Camellia pollen in this study was different from the lotus (Nelumbo nucifera Gaertn) bee pollen (Xu et al. Citation2011), in which fatty acid extracted by supercritical CO2 were palmitic acid (16:0), margaric acid (17:0), stearic acid (18:0), oleic acid (18:l), linoleic acid (18:2) and linolenic acid (18:3). Saturated fatty acids (SFA) accounting for more than 52% of the total fatty acids. The different composition may be the different extraction methods.
The antioxidant properties
To fully evaluate the biological activity of antioxidants and compare with the results from other kinds of literature, different antioxidant assay methods were used in this study. The solvent effect is an essential parameter of the chemical behavior of antioxidant compounds (Celik et al. Citation2010). The seven solvent extracts were chosen to compare the antioxidant by the ABTS, DPPH and FRAP assay methods.
ABTS radical-scavenging activity
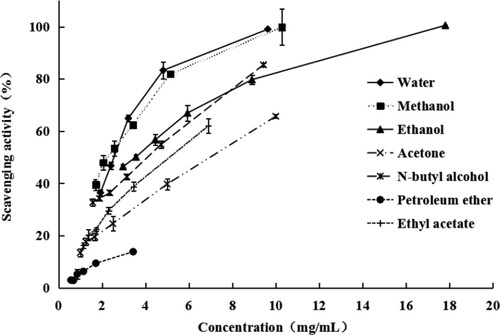
In ABTS·+ decolorization method, all the tested extracts of Camellia pollen possessed potent and distinct ABTS radical-scavenging activity and even exhibited a dose-dependent tendency. The ABTS radical-scavenging ability is shown in Figure and the IC50 values are shown in Table . The water, methanol and ethanol extracts showed better scavenging ability than the other solvent extracts, with IC50 values of 2.617 ± 0.05, 2.554 ± 0.34 and 2.869 ± 0.23 mg/mL, respectively. Higher ABTS radical scavenging was obtained in some herbal medicine, IC50s of the ethanol and water extracts of D. loureiroi and the ethanol extract of T. grandis as well as the water extract of S. pinata (carbonized) and E. rheedii (carbonized) ranged from 8 to 10 µg/mL compared to the positive controls: Trolox = 4.71 ± 0.04 µg/mL and BHT = 5.66 ± 0.26 µg/mL (Bhanuz et al. Citation2021).
Table 2. Lipase inhibitory ability and antioxidant activity of the Camellia pollen extracts.
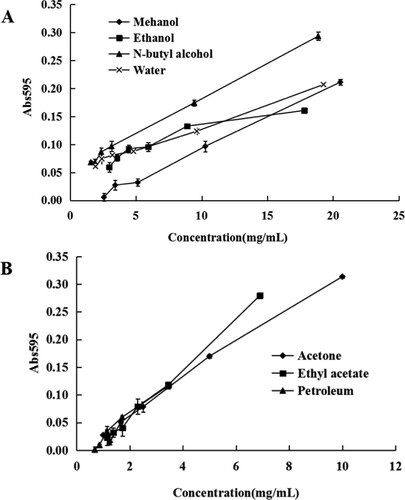
Ferric-reducing antioxidant power (FRAP)
All the tested extracts of Camellia pollen possessed potent and distinct FRAP values. Figure shows the ferric-reducing antioxidant power, all the extracts were dose-dependent. The FRAP IC50 values of the seven extracts differed significantly (p < 0.05). The methanol and the ethyl acetate extracts showed FRAP IC50 values of 10.65 ± 0.03 and 0.90 ± 0.12 mg/mL (Table ). The ethyl acetate extract displayed a higher ferric-reducing ability than the methanol extracts.
DPPH radical-scavenging ability
The results of the DPPH radical-scavenging activity of the different Camellia pollen extracts are presented in Figure and Table . The DPPH radical-scavenging activity of the seven extracts was dose-dependent. The ethanol extracts showed the best DPPH radical-scavenging ability (IC50 value of 2.920 ± 0.04 mg/mL) compared with the other solvent extracts. Moreover, the methanol extracts (IC50 = 5.61 mg/mL) showed better DPPH radical scavenging than the ethyl acetate extract, but lower than the previous results. Silvaa et al. (Citation2006) found that the DPPH IC50 values of the crude ethanol extracts of the yellow and brown pollen were 104.5 ± 0.5 and 106.1 ± 1.3 µg/mL, for the ethyl acetate extracts the IC50 values were 41.9 ± 0.2 and 43.7 ± 0.3 µg/mL, respectively.
Figure 3. DPPH radical-scavenging ability of the Camellia pollen extracts. (A) DPPH radical-scavenging ability of Methanol, ethanol, N-butyl alcohol, and water extracts. (B) DPPH radical-scavenging ability of acetone, ethyl acetate, and petroleum ether extracts.
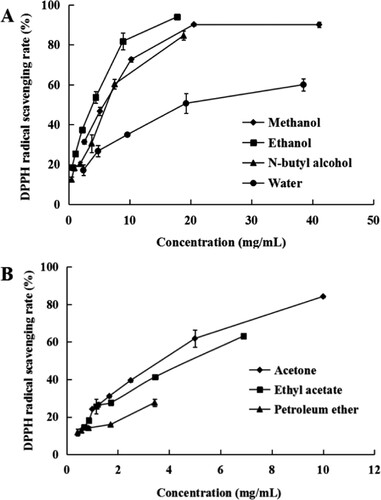
In other medicinal plants, such as the Bidens pilosa Linn. Radiata, the DPPH radical-scavenging ability of the leaves and flowers was 61 and 172 µg/mL, respectively (Deba et al. Citation2008). But for the fruits Momordica Charantia L. (leaf, stem, green fruit and ripe fruit), DPPH IC50 of the water extracts (leaf, stem, green fruit and ripe fruit) was IC50 = 9.72 ± 0.25 (leaf), 17.8 ± 0.66 (stem), 11 ± 0.76 (green fruit) and 27.6 ± 0.23 (ripe fruit) mg/mL, respectively. The main compounds in them were gallic acid, tannic acid, catechin, caffeic acid, p-coumaric acid, ferulic acid and benzoic acid (Kubola and Siriamornpun Citation2008). The ethanol extract of S. pinata (carbonized) showed the highest activity with IC50 of 5.72 ± 0.90 µg/mL compared to 14.87 ± 0.94 (BHT) and 5.02 ± 0.20 (Bhanuz et al. Citation2021).
Lipase inhibitory analysis
The inhibitory activities of the Camellia pollen extracts on lipase are shown in Table and Figure . The lipase inhibitory effect of the water, ethanol, methanol, acetone and ethyl acetate pollen extracts was dose-dependent (Table ). The lipase inhibitory activities of the n-butyl alcohol and the petroleum ether extracts were both lower than 20% at all concentrations. The lipase inhibition of the methanol and ethyl acetate extracts increased with the concentration from 11 to 75% and from 7.2 to 66.8%, respectively. In addition, the acetone and the ethyl acetate extracts showed better lipase inhibitory activity, with IC50 of 1.19 ± 0.07 mg/mL and 2.07 ± 0.20 mg/mL (Table ). The IC50 values of lipase inhibitory activity of the methanol, ethanol, and water extracts were 43.01 ± 0.91, 24.56 ± 0.94 and 78.09 ± 9.28 mg/mL, respectively.
Figure 4. Lipase inhibitory activity of the Camellia pollen extracts. (A) Lipase inhibitory activity of the Methanol, ethanol, and water extracts. (B) Lipase inhibitory activity of Acetone, N-butyl alcohol, ethyl acetate, and petroleum ether extracts.
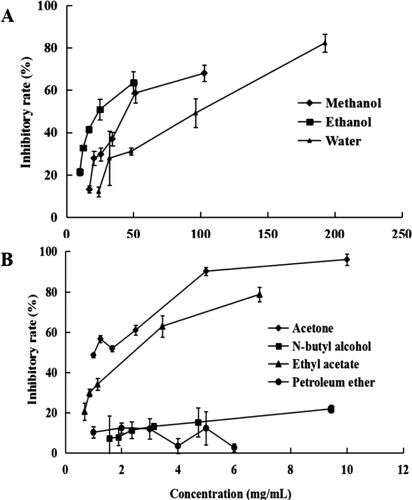
Table 3. The correlation coefficient between the components’ content of pollen extracts and the antioxidant activity.
Many identified phytochemicals obtained from traditional medicinal plants present an exciting opportunity for the development of newer therapeutics. The methanol extracts of Dioscorea nipponica root showed strong lipase inhibitory activity (IC50 = 20 µg/mL). The derivatives of the Dioscin showed strong inhibitory activity against porcine pancreatic lipase, with respective IC50 values of 28.0 µg/mL (Diosgenin), 28.9 µg/mL (gracillin),1.8 µg/mL (prosapogenin), 42.2 µg/mL (prosapogenin C) (Kwon et al. Citation2003). The aforementioned purified compounds showed better lipase inhibition than in this study. The inhibitory activity of the compounds 1(1,3-di-O-trans-feruloyl quinic acid) and 2 (p-hydroxybenzaldehyde) is purified by the invasive plant S. anglica with the IC50 value of 131.72 and 86.71 µg/mL (241.91 and 710.02 µM), respectively (Geum et al. Citation2021). Though the lipase inhibitory activity from the bee pollen extracts is weaker than in some plants, the purification of the compounds needs more work in the future.
Correlation analysis
Pearson’s analysis was conducted between the contents of the biocompounds and the antioxidant activity, the antioxidant activity and the lipase inhibitory activity. Results are shown in Tables and . From Table , the flavonoid content was negatively (−0.869) (p < 0.05) correlated with the DPPH radical-scavenging ability. The protein content was negatively (−0.829) (p < 0.05) correlated with the ABTS radical-scavenging ability. The lipase inhibitory activity was not significantly correlated with the content of the phenolics, flavonoids, reducing sugar, carotenoid and protein. However, the content of the flavonoid was negatively correlated with lipase inhibition (p > 0.05).
Table 4. The correlation coefficient between the components’ content of pollen extracts and the lipase inhibitory activity.
Discussion
Oxidative stress is thought to play a major role in the pathogenesis of hyperlipidemia (Sharma et al. Citation2005). Natural antioxidants such as tocopherols, ascorbic acid, and flavonoids have gained much attention for their obvious antioxidant, antitumor, anti-lipase, anti-diabetes, anti-mutagenic, and anti-carcinogenic activities. Phenolic compounds especially from the plant (Aviram et al. Citation2000) may contribute directly to the antioxidant action. The redox properties they possess made them reducing agents, hydrogen donors, and even singlet oxygen quenchers (Negri et al. Citation2011). Several research papers have mentioned the natural sources of antioxidant molecules in food, beverages and cosmetics (Moon and Shibamoto Citation2009).
Bee pollen could decrease the level of lipid oxidation in erythrocytes that showed its antioxidant capacity (Negri et al. Citation2011). Flavonoids and phenolic acids found in bee pollen may be accounted for their ability to scavenge free radicals. In addition, studies pointed out that antioxidant capacities were different for each floral species and were not correlated with the total phenol content in the case of all bee pollen samples (Mărghitaş et al. Citation2009). A lower correlation coefficient between the total phenols content and the free radical-scavenging ability of Camellia pollen was observed, which proved that the direct correlation was questionable in some species (Leja et al. Citation2007). Accordingly, the methanol extract, which had higher contents of phenolic, flavonoid, proteins and reducing sugars, showed relatively higher ABTS and DPPH radical-scavenging ability than the ethyl acetate extract. The higher antioxidant activity of the methanol pollen extract may be a result of a synergistic effect of all those compounds. Flavonoids, as a kind of phenolic compounds, are poorly absorbed in the body, but they become active as an antioxidant or antiradical agent, preventing free radical toxicity, oxidative stress and pathophysiology of various diseases (Šarić et al. Citation2009).
Although the phenolic compounds were found in high amounts in the methanol pollen extracts (Table ), higher lipase inhibitory activity was not observed. Interestingly, the total flavonoid content of the ethyl acetate extract was higher than that of the methanol extracts (Table ). In addition, the oil content of the ethyl acetate extract was higher than the methanol extract expressed as a rutin/g dry sample (Table ). This means that the profile of ethyl acetate extracts was different from the methanol extracts, the compounds in ethyl acetate extracts may play a key role in lipase inhibition.
Bee pollen as a healthy apicultural product considered to be a first-rate reservoir of antioxidants such as sterols, vitamins, fatty acids, phenolic compounds and other physiologically active substances. The composition of fatty acids has been studied recently (Xu et al. Citation2009; Xu et al. Citation2011). The oil content of the ethyl acetate extracts was higher than that of the methanol extracts. Four main fatty acids (unsaturated fatty acids) were identified in the methanol and ethyl acetate extracts (Table ). The ω−3 polyunsaturated fatty acids such as linolenic acid can reduce the risk of cardiovascular diseases. Fatty acids possess antioxidant, hypotensive, hypolipidemic, anticancer, and anti-inflammatory effects. Many unsaturated fatty acids are inhibitory to the lipase activity of Pseudomonas fragi; salts of shorter chain fatty acids (C2-C10) were soluble, but not inhibitory (Smith and Alford Citation1966). Despite their higher relative content of polyunsaturated fatty acids (Table ), the ethyl acetate and the methanol extracts exhibit differently for the lipase inhibitory activity. Lipase inhibition activity was higher in ethyl acetate extracts than in the methanol extracts. More studies on the content of the oil composition and the lipase inhibition activity of each composition are needed. Currently, synthetic drugs are used intensively to control oxidative stress and to inhibit hyperlipidemia key enzymes but with certain unpleasant gastrointestinal side effects. Therefore, the inhibitory activities against lipase along with the antioxidant activities found in Camellia pollen may provide a possible strategy for preventing and auxiliary treating obesity.
Table 5. Oil content and fatty acid content of Camellia pollen extracts.
Conclusion
In this study, the seven solvents showed multiple antioxidants and lipase inhibitory activities. Methanol, ethanol and water extract showed better ABTS radical-scavenging ability. Ethanol and acetone extracts showed better DPPH-scavenging ability. Ethyl acetate and petroleum ether extracts manifest better FRAP. Ethyl acetate and acetone extracts showed better lipase inhibitory activity, while methanol, ethanol and water extract with lower lipase inhibitory activity. The flavonoid content was negatively correlated with the DPPH radical-scavenging ability (p < 0.05); the protein content was negatively corrected with ABTS radical-scavenging ability (p < 0.05). The flavonoid content was negatively correlated with the lipase inhibitory activity (p > 0.05). The lipase inhibitory activity of the ethyl acetate and acetone extracts may correlate with the flavonoid and the composition of the oil.
These findings showed that bee pollen could be possibly used as part of a dietary strategy for managing hyperlipidemia. However, further investigations are necessary to verify the antioxidant and anti-lipase activities of the pollen extracts under in vivo conditions. Moreover, the synergistic effect of different compounds needs more work, and the content of each fatty acid in the extract was needed.
Author contributions statement
Yuanfan Yang: conception and design, Reviewing & Editing, and Resources; Yating Dong, Jiayu Fu & Xiaoyan Lai: Experimental design, Investigation, Methodology, Data analysis and interpretation, and Writing-Original draft; Cunshan Zhou, Haile Ma: Conceptualization, Supervision, Reviewing, and Editing; Abu El-Gasim A. Yagoub: Reviewing & Editing; Wenjun Peng: Conceptualization. Hui Ni: Conception and design, resources, and the final approval of the version to be published.
Acknowledgments
Thanks to the Beijing Apiculture Company (BAC) for providing the Camellia bee pollen.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in the Figshare repository at https://doi.org/10.6084/m9.figshare.20544090.v1 and https://doi.org/10.6084/m9.figshare.20218853.v3.
References
- Al-Osaimi M, El-Ansary A, Al-Daihan S, Bhat RS, Ben Bacha A. 2018. Therapeutic and protective potency of bee pollen against neurotoxic effects induced by prenatal exposure of rats to methyl mercury. J Mol Neurosci. 65:327–335.
- Ares AM, Valverde S, Bernal JL, Nozal MJ, Bernal J. 2018. Extraction and determination of bioactive compounds from bee pollen. J Pharmaceut Biomed. 147:110–124.
- Arts Mariken JTJ, Sebastiaan Dallinga J, Haenen Guido RMM, Bast Aalt. 2004. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 88(4):567–570.
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B. 2000. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 71:1062–1076.
- Ballinger A, Peikin SR. 2002. Orlistat its current status as an anti-obesity drug. Eur J Pharmacol. 440:109–117.
- Bertoncelj J, Polak T, Pucihar T, Lilek N, Kandolf Borovšak A, Korošec M. 2018. Carbohydrate composition of slovenian bee pollens. Int J Food Sci Tech. 53:1880–1888.
- Bhanuz D, Pathompong P, Jitpisute C, Thana J, Onmanee P, Kitrawee J. 2021. Antibacterial, anti-inflammatory and antioxidant activities of mahanintangtong and its constituent herbs, a formula used in Thai traditional medicine for treating pharyngitis. BMC Complement Med. 21:1–12.
- Celik SE, Ozyurek M, Guclu K, Apak R. 2010. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta. 81:1300–1309.
- Chen L, Zhou ZX, Yang YJ. 2006. Genetic improvement and breeding of tea plant (Camellia sinensis) in China: from individual selection to hybridization and molecular breeding. Euphytica. 154:239–248.
- Chen X, Wu RZ, Zhu YQ, Ren Z, Tong YL, Yang F, Dai GH. 2018. Study on the inhibition of Mfn1 by plant-derived miR5338 mediating the treatment of BPH with rape bee pollen. BMC Complemnt Alternative Med. 18:38.
- Deba F, Xuan TD, Yasuda M, Tawata S. 2008. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control. 19:346–352.
- Fogliano V, Verde V, Randazzo G, Ritieni A. 1999. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agr Food Chem. 47:1035–1040.
- Gardana C, Del Bo’ C, Quicazán MC, Corrrea AR, Simonetti P. 2018. Nutrients, phytochemicals and botanical origin of commercial bee pollen from different geographical areas. J Food Compos Anal. 73:29–38.
- Geum JK, Songhee P, Eonmi K, Hyukbean K, Hae-Jin P, Joo-Won N, Seong-Soo R, Hyukjae C. 2021. Antioxidant, pancreatic lipase inhibitory, and tyrosinase inhibitory activities of extracts of the invasive plant Spartina anglica (cord-grass). Antioxidants. 10:242.
- Hyatt JR, Zhang SY, Akoh CC. 2021. Comparison of antioxidant activities of selected phenolic compounds in O/ W emulsions and bulk oil. Food Chem. 349:129037.
- Jianhua He, Lin Lu, Chunjun Shao, Guilian Xu. 2017. Comparison of protein content determination respectively by folin-ciocalteu method and coomassie brilliant blue binding method for mannatide oral solution. China Pharmacist. 20:1861–1863.
- José R Ayala Gisela, Montero Marcos A, Coronado Conrado, García Mario A, José A, León Carlos A, Sagaste Daniela G. 2021. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules. 26:1348.
- Kim JH, Kim HJ, Park HW, Youn SH, Choi DY, Shin CS. 2007. Development of inhibitors against lipase and alpha-glucosidase from derivatives of monascus pigment. Fems Microbiol Lett. 276:93–98.
- Kocot J, Kielczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. 2018. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 7074209.
- Kubola J, Siriamornpun S. 2008. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit extracts in vitro. Food Chem. 110:881–890.
- Kwon CS, Sohn HY, Kim SH, Kim JH, Son KH, Lee JS, Lim JK, Kim JS. 2003. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotech Bioch. 67:1451–1456.
- Leja M, Mareczek A, Wyżgolik G, Klepacz-Baniak J, Czekońska K. 2007. Antioxidative properties of bee pollen in selected plant species. Food Chem. 100:237–240.
- Li QQ, Wang K, Sawaya Achf, Hu L, Wu LM, Hu FL. 2018. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. Journal of Functional Foods. 49:472–484.
- Lia QQ, Wang K, Marcucci MC, Sawaya ACHF, Hud L, Xue XF, Wu LM, Hue FL. 2018. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J Funct Foods. 49:472–484.
- Mărghitaş LA, Stanciu OG, Dezmirean DS, Bobiş O, Popescu O, Bogdanov S, Campos MG. 2009. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 115(3):878–883.
- Moon JK, Shibamoto T. 2009. Antioxidant assays for plant and food components. J Agr Food Chem. 57:1655–1666.
- Negri G, Teixeira EW, Alves ML, Moreti AC, Otsuk IP, Borguini RG, Salatino A. 2011. Hydroxycinnamic acid amide derivatives, phenolic compounds and antioxidant activities of extracts of pollen samples from southeast Brazil. J Agr Food Chem. 59:5516–5522.
- Roberts CK, Sindhu KK. 2009. Oxidative stress and metabolic syndrome. Life Sciences. 84:705–712.
- Sandra Zapata Bustamante, Gil Jesús, Sforza Stefano, Tedeschi Tullia. 2021. Bioactivity and peptide profile of whey protein hydrolysates obtained from Colombian double-cream cheese production and their products after gastrointestinal digestionLebensmittel-Wissenschaft und-Technologie. 145:111334.
- Šarić A, Balog T, Sobočanec S, Kušić B, Šverko V, Rusak G, Likić S, Bubalo D, Pinto B, Reali D, Marotti T. 2009. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem Toxicol. 47:547–554.
- Sawczuk R, Karpinska J, Filipowska D, Bajguz A, Hryniewicka M. 2021. Evaluation of total phenols content, anti-DPPH activity and the content of selected antioxidants in the honeybee drone brood homogenate. Food Chem. 368:130745 .
- Sharma P, Mishra S, Ajmera P, Mathur S. 2005. Oxidative stress and metabolic syndrome. Indian J Clin Bioche. 20:145–149.
- Silvaa TMS, Camaraa CA, Silva Lins AC, Barbosa-Filhoa JM, Silva EMS, Freitas BM, Santos FAR. 2006. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J Food Compos Anal. 6:507–511.
- Smith JL, Alford JA. 1966. Inhibition of microbial lipases by fatty acids. Appl Micro. 14:699–705.
- Xu X, Dong J, Mu XF, Sun LP. 2011. Supercritical CO2 extraction of oil, carotenoids, squalene and sterols from lotus (Nelumbo nucifera Gaertn) bee pollen. Food Bioprod Process. 89:47–52.
- Xu X, Sun LP, Dong J, Zhang HC. 2009. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov Food Sci Emerg. 10:42–46.
- Yang YF, Lai XY, Lai GY, Jiang ZD, Ni H, Chen F. 2016. Purification and characterization of a tyrosinase inhibitor from camellia pollen. J Funct Foods. 27:140–149.