Abstract
Lung cancer is the most common malignant cancer in the world, and non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancer cases. Presently, chemotherapy is the main treatment, but the therapeutic effect is not satisfactory due to drug resistance. Feiyanning decoction (FYN), a widely used Chinese Tradition Medicine (TCM), shows a good effect on the treatment of lung cancer. However, the underlying mechanism seems unclear. In this study, FYN was applied to cisplatin-resistant A549 cell line. Cell viability and apoptosis were detected by cell counting kit 8 (CCK-8) and Annexin V/PI kit. Xenograft mouse model was constructed to analyze the tumor growth. The results showed that FYN inhibited cell viability and promoted cell apoptosis in a dose-dependent manner. Downregulated of Bcl-2 and upregulated of HEATR1, TAp73, p53 and Bax were observed after treatment with FYN. Interestingly, all these effects induced by FYN were alleviated by HEATR1 overexpression in A549/DDP cells. Furthermore, FYN played a synergistic effect with cisplatin on the increase of cell apoptosis in vitro and the inhibition of tumor growth in vivo. In conclusion, FYN played a synergistic effect with cisplatin on the inhibition of cell viability in NSCLC via negatively regulating HEATR1.
KEYWORDS:
Introduction
Lung cancer, with the highest morbidity and mortality, is considered as the most common malignant cancer in the world. According to World Health Organization, approximately 2.1 million new cases are diagnosed each year, with 1.8 million deaths(Bray et al. Citation2018). Non-small cell lung cancer (NSCLC) represents the major pathological subtype of lung cancer that accounts for 85% of lung cancer cases(Gridelli et al. Citation2015). At present, chemotherapy is still one of the important treatments for the patients with advanced lung cancer. Platinum-based chemotherapy, especially cisplatin, is the first-line treatment for NSCLC (Nguyen et al. Citation2019). Unfortunately, due to the resistance rate of NSCLC to chemotherapy drugs, the outcome remains unsatisfactory (Wakelee et al. Citation2014). Therefore, understanding the mechanism of drug resistance and enhancing the effect of chemotherapy drugs are crucial.
Feiyanning decoction (FYN), a Chinese Tradition Medicine (TCM), has a good effect on the treatment of lung cancer. Qintong Prescription consists of Astragalus mongholicus Bunge [shenghuangqi], Polygonatum sibiricum F.Delaroche [huangjing], Salvia chinensis Benth [shijianchuan], Amorphophallus sinensis Belval [sheliugu], Iphigenia indica Kunth [shancigu], Polistes olivaceous DeGeer [fengfang], Epimedium sagittatum Sieb. et Zucc [xianlingpi], and Ganoderma Lucidum Karst [lingzhi] at a ratio of 10:10:10:10:5:3:5:5 in dry weight. Astragalus root is a Chinese herb with a wide range of uses and plays a role in various biological functions (Fu et al. Citation2014). It has been found to exhibit immunopotentiation and anticancer activity, and the main contribution is to counteract the side effects of chemotherapeutic drugs (Shao et al. Citation2004; Zhao et al. Citation2011). However, the mechanism by which FYN plays a role in the treatment of lung cancer remains unclear.
HEAT repeat-containing protein 1 (HEATR1) has one HEAT repeat at its c-terminal end which is also found in other proteins, including the PR65/A subunit of protein phosphatase 2A, elongation factor 3, and huntingtin(He et al. Citation2019). Previous studies report that HEATR1 plays a role in the regulation of cytotoxic T lymphocytes and rRNA synthesis (Wu et al. Citation2014). Recently, abnormal expression of HEATR-1 has been found in tumors. Some studies have shown that HEATR-1 is highly expressed in glioma cell lines, suggesting that it may be a potential therapeutic target for glioma (Wu et al. Citation2014). Furthermore, our team discovered that HEATR1 modulated cell survival via activation of the p53/PUMA signaling pathway in NSCLC (He et al. Citation2019). In this study, we aim to explore whether HEATR1 contributes to sensitize NSCLC to cisplatin, which may help to search for a new therapeutic target.
Materials and methods
Cell culture and treatments
The human NSCLC cisplatin-resistant A549 cell line (A549/DDP) was purchased from JRDUN Biotech. (Shanghai, China) and cultured in F-12 K medium (Gibco, Carlsbad, CA, USA) containing 10% (v/v) fetal bovine serum (Gibco) in a cell incubator (5% CO2, 37°C).
A549/DDP cells were treated with FYN (0, 0.05, 0.1, 0.2, 0.5, and 1 mg/mL) and/or 50 μM cisplatin (Sigma, St. Louis, MO, USA) for 0, 12, 24, 48, and 72 h.
Preparation of FYN
Stir-fried Astragalus mongholicus, Polygonatum sibiricum, Salvia chinensis, Amorphophallus sinensis, Iphigenia indica, Polistes olivaceous, Epimedium sagittatum, and Ganoderma Lucidum were decocted at a ratio of 10:10:10:10:5:3:5:5 in dry weight. Then the complex was filtrated and concentrated to 100% (1 g/mL) 1.
Cell viability analysis
Cell viability was measured using a Cell counting kit-8 (CCK-8) assay (SAB, College Park, MD, USA). Briefly, A549/DDP cells (2 × 103) were seeded into each well of a 96-well plate and cultured overnight. Then, different treatments were applied to cells. Finally, 100 μL of CCK-8 working solution containing 10 μL of CCK-8 and 90 μL culture medium was added to each well and cultured for 1 h, followed by measuring the optical density at 450 nm.
Cell apoptosis analysis
Cell apoptosis was measured using a TACS Annexin V-FITC Apoptosis Detection Kit (R&D Systems, Minneapolis, MN, USA). Treated cells were collected by centrifugation at approximately 300 x g for 10 minutes at room temperature. Wash cells once by resuspending them in 500 μL of cold 1X PBS and then pelleting by centrifugation. Gently resuspend cells in 100 μL of the Annexin V Incubation Reagent containing 1 μL TACS Annexin V-FITC and 10 μL Propidium lodide. Incubate in the dark for 15 minutes at room temperature. Cells were collected using a Flow cytometer (BD Biosciences, San Jose, CA, USA).
Clone formation assay
A549/DDP cells (5 × 103) were seeded into each well of a 6-well plate and cultured overnight. Then, the cells were treated with FYN (0, 0.1, 0.2, and 0.5 mg/mL) for 2 weeks. The culture medium was replaced in every 3 days. At the end of the incubation, the cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet or 1% Coomassie Brilliant Blue. The colonies were counted under a dissecting microscope.
Quantitative real-time PCR (qRT-PCR)
The relative mRNA expression levels were measured by qRT-PCR. A Reliance Select cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to reverse transcribed RNA samples to cDNA, which was amplified with the SYBR Green qPCR Master Mixes (Thermo Fisher, Rockford, IL, USA) according to the manufacturers’ instructions on an ABI7500 amplifier (Foster City, CA, USA). The relative mRNA levels were analyzed using the 2−△△Ct method after normalized to GAPDH. The primer sequences were listed:
Western blot assay
Cells were collected using RIPA buffer (Bio-Rad Laboratories, Inc.), and the protein concentration was quantified using a bicinchoninic acidprotein assay kit (Bio-Rad). Cell lysates were separated by electrophoresis and then transferred to polyvinylidene fluoride membrane (Millipore Corp., Bedford, MA, USA). The bands were incubated with optimally diluted primary antibodies overnight at 4°C and second antibodies for 1 h at room temperature sequentially. Signals were detected with a chemiluminescent imaging system (Bio-Rad Laboratories, Inc.). Anti-HEATR1 (ab200693), anti-Bcl2 (ab194583), and anti-Bax (ab32503) were purchased from Abcam (St. Louis, MO, USA), anti-p53 (#2524) and anti-GAPDH (#5174) were purchased from Cell Signaling Technology, (Danvers, MA, USA), anti-TAp73 (N100-56069) was purchased from Novus (Centennial, CO, USA). Ladders were purchased from Bio-Rad (Cat. No. 1610374S) and Thermo Fisher (Cat. No. 26616).
Lentivirus information
Lentiviruses for human HEATR1 overexpression and knockdown as well as the corresponding lentiviral vectors were purchased from Genechem company (shanghai, CHINA). The knock-down sequences were listed:
shHEATR1-1: 5’- CCACGAGACAAGAATATTT-3’;
shHEATR1-2: 5’- GCTGCAACATACATGATAA -3’;
shHEATR1-3: 5’- GCTCCTGTCTTCCAATAAT -3’;
Xenograft mouse model
24 male BALB/c nude mice (6 weeks old) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All experiments for mice were approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine. Author WX was aware of the group allocation at the different stages of the experiment. Mice were anesthetized using isoflurane (3.5% induction for four minutes and 1.5–2.0% maintenance) and injected with A549/DDP cells (100 μL, 2 × 106 cells) in the subdermal space. After 2 weeks, the mice were randomly divided into 4 groups (6 mice per group) using online random number generators (https://www.graphpad.com/quickcalcs/randomize1/), no controlled confounders: control, FYN, DDP, and FYN + DDP groups.
Control group: mice were intraperitoneally injected with equal volume of saline; FYN group: mice were administered intragastrically 200 mg/kg FYN; DDP group: mice were intraperitoneally injected 20 mg/kg DDP; FYN + DDP group: mice were administered intragastrically 200 mg/kg FYN and intraperitoneally injected 20 mg/kg DDP. Tumor size was measured using a caliper every 3 days. The tumor volume was calculated using the formula: L x W2×0.52 (L represents the longest diameter and W represents the shortest diameter). 24 mice were euthanized via CO2 narcosis on the 33 days after cell injection. 24 tumor samples were immediately excised and fixed with 4% paraformaldehyde for TUNEL experiment or snap-frozen at −80°C for western blot. 3 samples were randomly chosen per group for the TUNEL and western blot assay.
TUNEL assay
Tumor samples from different groups (3 samples per group) were fixed with formaldehyde for 24 h. After embedding, the samples were cut into 4 µm sections. Sections of tumor tissues were stained using a TUNEL kit (R&D Systems) according to the instructions.
Statistical analysis
Date was showed as mean value ± SD. All the experiments were performed at least three repetitive. GraphPad Prism 7.0 software (San Diego, CA, USA) was used for statistical analysis. The two-tailed Student’s t-test was used to evaluate statistical differences between the two groups. p < 0.05 was regarded as statistically significant.
Results
FYN inhibited cell viability and increased cell apoptosis in A549/DDP cells
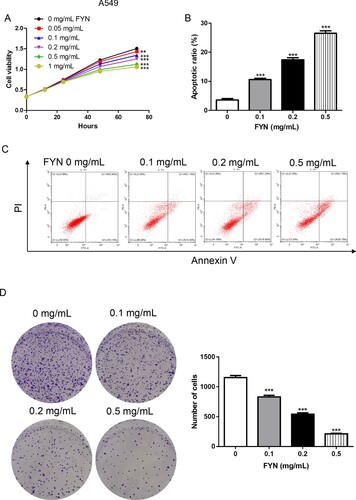
To explore the functions of FYN in cisplatin-resistant human lung cancer cells, different concentrations (0, 0.05, 0.1, 0.2, 0.5, and 1 mg/mL) of FYN were subjected to A549/DDP cells. As shown in Figure (A), FYN inhibited cell viability in a dose-dependent manner. Cell apoptosis results displayed that FYN (0.1, 0.2, and 0.5 mg/mL) significantly increased cell apoptosis rates in A549/DDP cells (Figure (B,C)). Furthermore, we found that FYN decreased the ability of clone formation in A549/DDP cells (Figure (D)). These results indicated that FYN inhibited cell viability and promoted cell apoptosis in A549/DDP cells.
Figure 1. FYN inhibited cell viability and increased cell apoptosis in A549/DDP cells. (A) Cell viability was measured after treatments with different concentrations (0, 0.05, 0.1, 0.2, 0.5, and 1 mg/mL) of FYN. (B, C) Cell apoptosis was detected after treatments with different concentrations (0, 0.1, 0.2, and 0.5 mg/mL) of FYN. (D) Clone formation assay was performed in FYN treated cells. **P < 0.01; ***P < 0.001 VS 0 mg/mL of FYN.
FYN promoted cell apoptosis via down-regulating HEATR1
Based on our previous study, HEATR1 modulates cell survival in NSCLC via activation of the p53/PUMA signaling pathway(He et al. Citation2019). We tried to identify whether HEATR1 played a role in FYN induced cell apoptosis. As expected, FYN notably depressed the mRNA and protein levels of HEATR1 in a dose-dependent manner (Figure (A)), suggesting HEATR1 was involved. Furthermore, FYN increased p53 and Bax protein levels but decreased Bcl-2 level in A549/DDP cells (Figure (B)). The microarray results(He et al. Citation2019) indicated that TAp73, a member of p53 family, was upregulated after silencing HEATR1. We detected the protein level of TAp73 after treatment with FYN and found that FYN significantly promoted TAp73 protein level in A549/DDP cells (Figure (B)).
Figure 2. FYN promoted cell apoptosis via down-regulating HEATR1. (A) Real-time PCR and western bolt assays were used to measure HEATR1 level upon treatment with FYN. (B) TAp73, p53, Bcl-2, and Bax protein levels were analyzed. **P < 0.01; ***P < 0.001 VS 0 mg/mL of FYN. (C) mRNA and protein levels of HEATR1 were measured after transduction with HEATR1 overexpression lentivirus. ***P < 0.001 VS vector. (D, E) Cell apoptosis was detected after treatments with 0.5 mg/mL of FYN and HEATR1 overexpression lentivirus. (F) TAp73, p53, Bcl-2, and Bax protein levels were analyzed. ***P < 0.001 VS vector; ###P < 0.001 VS HEATR1 + FYN. (G) mRNA and protein levels of HEATR1 were measured after transduction with HEATR1 knock down lentivirus. (H, I) Cell apoptosis was detected after transduction with HEATR1 knock down lentivirus. (J) TAp73, p53, Bcl-2, and Bax protein levels were analyzed. ***P < 0.001 VS shNC.
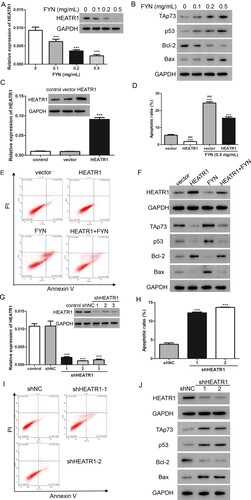
HEATR1 overexpressing lentivirus was applied to overexpress the expression level of HEATR1. Figure (C) showed that, compared with vector, HEATR1 transduction obviously increased HEATR1 expression. Furthermore, A549/DDP cells transduced with HEATR1 lentivirus were exposed to FYN (0.5 mg/mL), and cell apoptosis was assessed (Figure (D,E)). FYN significantly induced cell apoptosis but HEATR1 overexpression alleviated this effect. Western blot assay showed that the upregulations of TAp73, p53, and Bax, and the downregulation of Bcl-2 induced by FYN could be reversed by HEATR1 overexpression (Figure (F)).
It is noticeable that HEATR1 overexpression significantly decreased the cell apoptotic rate in A549/DDP cells. Next, HEATR1 expression was markedly decreased upon transduction with shHEATR1 lentiviruses (Figure (G)). Furthermore, silencing of HEATR1 markedly increased the cell apoptotic rate (Figure (H,I)), the protein levels of TAp73, p53, and Bax, however, decreased the Bcl-2 level in A549/DDP cells (Figure (J)). Taken together, HEATR1 played a role in the increase of cell apoptosis induced by FYN.
Synergistic effects of FYN and cisplatin on the inhibition of cell viability
To investigate the relationship between FYN and cisplatin, FYN and cisplatin were subjected to A549/DDP cells. As shown in Figure (A), 50 μM cisplatin significantly inhibited cell viability in comparison with control. Interestingly, treatments with cisplatin plus FYN (0.1, 0.2, and 0.5 mg/mL) obviously decreased cell viability as compared to DDP alone. In addition, cisplatin significantly increased apoptotic rates, and combined treatment with cisplatin and FYN had a greater effect than cisplatin (Figure (B,C)). These results indicated that FYN had a synergistic effect with cisplatin on the inhibition of cell viability.
Figure 3. Synergistic effects of FYN and cisplatin on the inhibition of cell viability. (A) Cell viability was measured after treatments with 50 μM cisplatin and different concentrations (0.1, 0.2, and 0.5 mg/mL) of FYN. (B, C) Cell apoptosis was detected. (D) Cell apoptosis was detected after treatments with 50 μM cisplatin and shHEATR1. ***P < 0.01 VS control; ##P < 0.01, ###P < 0.001 VS DDP.
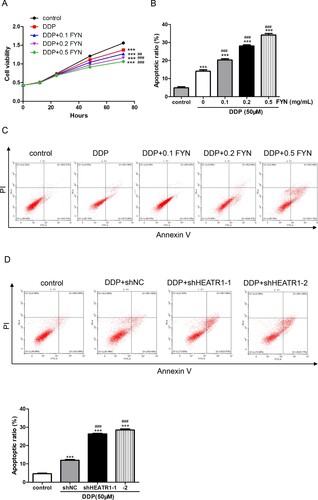
Based on the results in Figure , HEATR1 contributed to the function of FYN in A549/DDP cells. Cisplatin was subjected to A549/DDP cells transduced with HEATR1 knockdown lentiviruses. Cell apoptosis results exhibited that cisplatin significantly induced apoptosis, and combined treatment with cisplatin and shHEATR1 showed higher apoptotic rates than cisplatin (Figure (D)). Taken together, FYN had a synergistic effect with cisplatin on the inhibition of cell viability via regulating HEATR1.
FYN showed synergistic effect with cisplatin on the inhibition of tumor growth
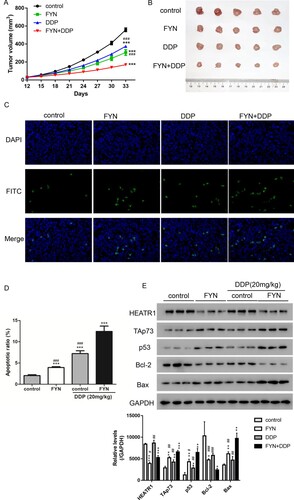
To validate the function of FYN on cisplatin resistance in vivo, a xenograft mouse model was performed with A549/DDP cells subcutaneously injected. As shown in Figure (A), treatments with FYN and/or cisplatin exhibited slower tumor growth rates in comparison with the control group. Interestingly, the slowest tumor growth rate was found in the group treated with both FYN and cisplatin. The tumor size showed the similar results that single treatment with FYN or cisplatin exhibited smaller tumor as compared to the control group, and FYN + DDP group showed the smallest tumor (Figure (B)). Furthermore, cell apoptosis was significantly increased after treatments with FYN or cisplatin, and treatments with both FYN and cisplatin showed the highest level of cell apoptosis according to TUNEL results (Figure (C)). In addition, the upregulated levels of TAp73, p53, and Bax, as well as the downregulated levels of Bcl-2 were displayed in the group treated with cisplatin or FYN. Treatment with both FYN and cisplatin showed synergistic effect. Taken together, treatment with single FNY or cisplatin inhibited tumor growth in vivo, and treatment with both FNY and DDP displayed synergistic effect.
Figure 4. FYN showed synergistic effect with cisplatin in the inhibition of tumor growth. (A) Tumor volume was estimated every 3 days. (B) The tumors in each group. (C and D) TUNEL assay was performed to estimate apoptotic rates. (E) Western blot was used to measure TAp73, p53, Bcl-2, and Bax protein levels. ***P < 0.01 VS control; ###P < 0.001 VS DDP + FYN.
Discussion
NSCLC, accounts for 85% of lung cancer, is a locally advanced or metastatic disease with a poor overall 5-year survival rate of 17% (Kawano et al. Citation2013). Different improved strategies have been applied to the stepwise increase the survival rates but with significant side effects and complications (Ang et al. Citation2015). The TCM has been proposed to decrease the incidence of side effects and increase the efficiency of therapy in cancer treatment. In the present study, we demonstrated FYN, a TCM, exhibited a synergistic effect with cisplatin in the chemotherapy of NSCLC.
FYN contains many TCM supplements including Astragalus, Polygonatum sibiricum, and Salvia chinensis. Astragalus, one popular used health-promoted herbs in TCM, has been widely used as an effective anticancer drug. Astragalus shows synergistic efficacy to platinum-based chemotherapy patients with NSCLC (Cao et al. Citation2019). Astragaloside IV, a major active component of Astragalus, sensitizes NSCLC cells to gefitinib via regulation of SIRT6 (Dai et al. Citation2017). Furthermore, Astragaloside IV has been demonstrated to enhance sensibility of NSCLC to cisplatin through inhibiting autophagy and ER stress (Lai et al. Citation2020). Polygonatum sibiricum has many biological activities, such as anti-cancer, anti-inflammatory effect, antioxidant activity, immunity enhancement effect, and so on (Cui et al. Citation2018). In addition, Polygonatum sibiricum plays a protective role in acute lung injury by inhibiting inflammation (Liu et al. Citation2020). Salvia chinensis has been reported to prominently inhibit hepatocellular carcinoma and repress the lung metastasis (Wang et al. Citation2017). In the current study, we demonstrated that FYN increased cell apoptosis in A549/DDP cells and exhibited a synergistic effect with cisplatin on the chemotherapy of NSCLC.
Human HEATR1, a poorly studied protein, plays a role in ribosome biosynthesis by regulating RNA polymerase I (Bursac et al. Citation2018). We are the first to report that HEATR1 is upregulated and plays a role in NSCLC tumor survival and progression in our previous study (He et al. Citation2019). In the current study, silencing of HEATR1 induced cell apoptosis in A549/DDP cells (Figure ). FYN decreased HEATR1 level and HEATR1 overexpression alleviated the effects induced by FYN (Figures and ), indicating FYN increased cell apoptosis by downregulating HEATR1. Furthermore, combined treatment with FYN and shHEATR1 showed a greater effect on the induction of cell apoptosis (Figure ). FYN was also demonstrated to play a synergistic effect with cisplatin on the suppression of tumor growth (Figure ). Taken together, FYN played a synergistic effect with cisplatin on the chemotherapy of NSCLC via the regulation of HEATR1. Silencing of HEATR1 has been indicated to promote proliferation and gemcitabine resistance in pancreatic cancer (Zhou et al. Citation2020), indicating HEATR1 may play different roles in different cancers.
TAp73 belongs to p53 family and has been demonstrated to play important roles in preventing tumor development by inducing cell apoptosis and cell cycle arrest (Liu et al. Citation2013; Flores Citation2016). In the current study, we found that FYN decreased HEATR1 level but increased TAp73 level in A549/DDP cells (Figure ). Furthermore, HEATR1 overexpression downregulated TAp73 and silencing of HEATR1 upregulated TAp73 protein level (Figure ). These results suggested that FYN contributed to the suppression of NSCLC via negatively regulation of HEATR1, which decreased TAp73 level. In consistent with our results, knock down TAp73 has been reported to enhance cell proliferation in several cancers including colorectal cancer (Ji et al. Citation2019), lung cancer (Amelio et al. Citation2015), and skin squamous cell carcinoma (Amelio et al. Citation2015). In addition, several studies demonstrate that TAp73 contributes to improvement of chemosensitivity, such as improvement of doxorubicin in neuroblastoma cells (Peirce and Findley Citation2009), gemcitabine in pancreatic cancer cells(Nakamura et al. Citation2016), and cisplatin in ovarian cancer cells (Zhang et al. Citation2012). Therefore, TAp73 may participate in the synergistic effect of FYN and cisplatin on the chemotherapy of NSCLC, however, the further mechanism needs to be clarified.
In conclusion, FYN played a synergistic effect with cisplatin on the chemotherapy of NSCLC via negatively regulating HEATR1.
Acknowledegements
Menghan Wang contributed to the conception and design; Zhongchao Mai contributed to the analysis and interpretation of the data in the original version, and contributed to the design of the work, the acquisition, analysis, and interpretation of data in revision and the final approval of the version to be published; Guoyu Wang contributed to the acquisition, analysis, and interpretation of the data and the drafting of the paper; Xing Ma contributed to the revision, including the design of the work, the acquisition, analysis, and interpretation of data in revision; Borong Zhou contributed to the acquisition, analysis, and interpretation of the data; Xinlin Yang contributed to the revision, including the acquisition, analysis, and interpretation of data in revision; Wei Xia contributed to the conception and design of the work, the interpretation of data, modification of the manuscript, and the final approval of the version to be published. Wei Xia and Zhongchao Mai contributed to; and that all authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in ‘figshare’ [https://figshare.com/] at http://doi.org/10.6084/m9.figshare.14803416; http://doi.org/10.6084/m9.figshare.14803437; http://doi.org/10.6084/m9.figshare.14803491; http://doi.org/10.6084/m9.figshare.14803497.
Additional information
Funding
References
- Amelio I, Inoue S, Markert EK, Levine AJ, Knight RA, Mak TW, Melino G. 2015. TAp73 opposes tumor angiogenesis by promoting hypoxia-inducible factor 1alpha degradation. Proc Natl Acad Sci USA. 112:226–231. Epub 2014/12/24.
- Ang YL, Tan HL, Soo RA. 2015. Best practice in the treatment of advanced squamous cell lung cancer. Ther Adv Respir Dis. 9:224–235. Epub 2015/04/24.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. Epub 2018/09/13.
- Bursac S, Jurada D, Volarevic S. 2018. New insights into HEATR1 functions. Cell Cycle. 17:143–144. Epub 2017/12/14.
- Cao A, He H, Wang Q, Li L, An Y, Zhou X. 2019. Evidence of Astragalus injection combined platinum-based chemotherapy in advanced nonsmall cell lung cancer patients: A systematic review and meta-analysis. Medicine (Baltimore). 98:e14798. Epub 2019/03/19.
- Cui X, Wang S, Cao H, Guo H, Li Y, Xu F, Zheng M, Xi X, Han C. 2018. A review: The bioactivities and pharmacological applications of polygonatum sibiricum polysaccharides. Molecules. 23. Epub 2018/05/15.
- Dai PC, Liu DL, Zhang L, Ye J, Wang Q, Zhang HW, Lin XH, Lai GX. 2017. Astragaloside IV sensitizes non-small cell lung cancer cells to gefitinib potentially via regulation of SIRT6. Tumour Biol J Intern Soc Oncodevelop Biol Med. 39:1010428317697555. Epub 2017/04/27.
- Flores ER. 2016. Commentary on “apoptosis, p53, and tumor cell sensitivity to anticancer agents”. Cancer Res. 1(76):6763–6764. Epub 2016/12/03.
- Fu J, Wang Z, Huang L, Zheng S, Wang D, Chen S, Zhang H, Yang S. 2014. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (huangqi). Phytotherapy Res. 28:1275–1283. Epub 2014/08/05.
- Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, Petrella F, Spaggiari L, Rosell R. 2015. Non-small-cell lung cancer. Nat Rev Dis Prim. 21(1):15009. Epub 2015/01/01.
- He S, Ma X, Ye Y, Zhang M, Zhuang J, Song Y, Xia W. 2019. HEATR1 modulates cell survival in non-small cell lung cancer via activation of the p53/PUMA signaling pathway. OncoTarg Ther. 12:4001–4011. Epub 2019/06/14.
- Ji H, Huang C, Wu S, Kasim V. 2019. XBP1-s promotes colorectal cancer cell proliferation by inhibiting TAp73 transcriptional activity. Biochem Biophys Res Commun. 508:203–209. Epub 2018/11/27.
- Kawano Y, Ohyanagi F, Yanagitani N, Kudo K, Horiike A, Tanimoto A, Nishizawa H, Ichikawa A, Sakatani T, Nakatomi K, et al. 2013. Pemetrexed and cisplatin for advanced non-squamous non-small cell lung cancer in Japanese patients: phase II study. Anticancer Res. 33:3327–3333. Epub 2013/07/31.
- Lai ST, Wang Y, Peng F. 2020. Astragaloside IV sensitizes non-small cell lung cancer cells to cisplatin by suppressing endoplasmic reticulum stress and autophagy. J Thor Dis. 12:3715–3724. Epub 2020/08/18.
- Liu T, Roh SE, Woo JA, Ryu H, Kang DE. 2013. Cooperative role of RanBP9 and P73 in mitochondria-mediated apoptosis. Cell Death Dis. 4:e476. Epub 2013/01/26.
- Liu TY, Zhao LL, Chen SB, Hou BC, Huang J, Hong X, Qing L, Fang Y, Tao Z. 2020. Polygonatum sibiricum polysaccharides prevent LPS-induced acute lung injury by inhibiting inflammation via the TLR4/Myd88/NF-kappaB pathway. Exper Therap Med. 3733–3739. Epub 2020/08/29.
- Nakamura M, Sugimoto H, Ogata T, Hiraoka K, Yoda H, Sang M, Sang M, Zhu Y, Yu M, Shimozato O, et al. 2016. Improvement of gemcitabine sensitivity of p53-mutated pancreatic cancer MiaPaCa-2 cells by RUNX2 depletion-mediated augmentation of TAp73-dependent cell death. Oncogenesis. 5:e233. Epub 2016/06/15.
- Nguyen CTT, Petrelli F, Scuri S, Nguyen BT, Grappasonni I. 2019. A systematic review of pharmacoeconomic evaluations of erlotinib in the first-line treatment of advanced non-small cell lung cancer. Eur J Health Econ. 20:763–777. Epub 2019/03/07.
- Peirce SK, Findley HW. 2009. The MDM2 antagonist nutlin-3 sensitizes p53-null neuroblastoma cells to doxorubicin via E2F1 and TAp73. Intern J Oncol. 34:1395–1402. Epub 2009/04/11.
- Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. 2004. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 320:1103–1111. Epub 2004/07/14.
- Wakelee H, Kelly K, Edelman MJ. 2014. 50 Years of progress in the systemic therapy of non-small cell lung cancer. In: Am Soc clin oncol educ book. p. 177–189. Epub 2014/05/27.
- Wang N, Tan HY, Chan YT, Guo W, Li S, Feng Y. 2017. Identification of WT1 as determinant of heptatocellular carcinoma and its inhibition by Chinese herbal medicine Salvia chinensis Benth and its active ingredient protocatechualdehyde. Oncotarget. 8:105848–105859. Epub 2017/12/30.
- Wu ZB, Qiu C, Zhang AL, Cai L, Lin SJ, Yao Y, Tang QS, Xu M, Hua W, Chu YW, et al. 2014. Glioma-associated antigen HEATR1 induces functional cytotoxic T lymphocytes in patients with glioma. J Immunol Res. 131494. Epub 2014/08/16.
- Zhang P, Liu SS, Ngan HY. 2012. TAp73-mediated the activation of c-Jun N-terminal kinase enhances cellular chemosensitivity to cisplatin in ovarian cancer cells. PloS One. 7:e42985. Epub 2012/08/18.
- Zhao LH, Ma ZX, Zhu J, Yu XH, Weng DP. 2011. Characterization of polysaccharide from Astragalus radix as the macrophage stimulator. Cell Immunol. 271:329–334. Epub 2011/09/23.
- Zhou Y, Wang K, Zhou Y, Li T, Yang M, Wang R, Chen Y, Cao M, Hu R. 2020. HEATR1 deficiency promotes pancreatic cancer proliferation and gemcitabine resistance by up-regulating Nrf2 signaling. Redox Biol. 29:101390. Epub 2019/12/01.
