Abstract
Pancreatic cancer is one of the most malignant tumors due to its highly aggressive nature, but pancreatic metastases to the colorectum are extremely rare. A 43-year-old male patient complained of increased stool frequency. Colonoscopy revealed a prominence lesion in the rectum and computed tomography (CT) scan confirmed a rectal mass. CT scan also showed retroperitoneal masses in the pancreatic tail involving the spleen and adjacent blood vessels. The patient was initially misdiagnosed as having rectal cancer with pancreatic metastasis. After immunohistochemical staining results became available, the diagnosis was changed to pancreatic ductal adenocarcinoma with rectal metastasis. Elevated CA19-9 also supported the diagnosis. In conclusion, we describe a case of synchronous colonic metastasis from pancreatic cancer. Although rare, the suspicion of pancreatic metastases to the rectum should be excluded when a patient has both rectal and pancreatic masses.
Introduction
According to the latest statistical data, pancreatic cancer is the seventh leading cause of cancer-related mortality (Sung et al. Citation2021). The hallmark of pancreatic cancer is systemic dissemination, which makes it one of the most malignant tumors. The most common metastatic site of pancreatic cancer is the liver, other sites include the lung, bones, abdominal cavity and lymph nodes. Pancreatic metastases to the colorectum are extremely rare. Here we present a rare case of pancreatic cancer metastatic to the rectum.
Case presentation
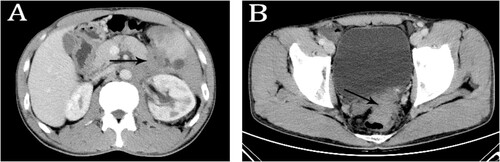
A 43-year-old male patient had been complaining of increased stool frequency from one time per day to 4–6 times per day for one month. The patient went to a local hospital for examination. Colonoscopy revealed a prominence lesion with a size of 3.0 cm × 2.8 cm and an erosive surface in the rectum (5 cm from the anus). Biopsy taken at colonoscopy revealed adenocarcinoma and tumor cells stained positive for villin-3 but negative for CK-20 and CDX2 (Figure S1). Abdominal computed tomography (CT) scan showed a mass in the rectum. Retroperitoneal masses were also evident on the CT scan in the pancreatic tail, involving the spleen and adjacent blood vessels. These were considered to be pancreatic metastases and multiple enlarged lymph nodes surrounding adjacent blood vessels (Figure ). The patient was transferred to the regional hospital for further treatment. To further clarify the diagnosis, pancreatic tissue was biopsied by laparoscopy. Histological examination revealed adenocarcinoma with a few cancer nests in fibrous connective tissues (Figure S2). While results of immunohistochemical stains were pending, the patient was diagnosed as having rectal cancer with pancreatic metastasis based on imaging and pathology results available. He was commenced on Xelox regimen chemotherapy (oxaliplatin with capecitabine) in the Department of General Surgery.
Figure 1. Contrast-enhanced computed tomography (CT) scan. CT scan showed retroperitoneal masses involved in the pancreatic tail surrounding adjacent blood vessels (A) and a mass in rectum (B).
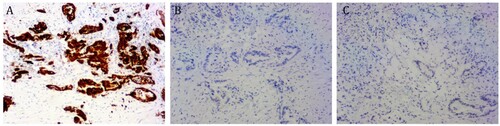
The results of immunohistochemical stains became available after one cycle of Xelox regimen. Immunohistochemical stains of pancreatic tissue showed that cancer cells were strongly positive for cytokeratin 7 (CK-7) but negative for CK-20 and CDX2 (Figure ). Immunohistochemical findings supported the diagnosis of a pancreatic ductal adenocarcinoma with rectal metastasis. The patient was transferred to the Department of Oncology. Additional tests for serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were undertaken. The level of CA19-9 was markedly elevated to 9330 U/ml (normal range: 0–39 U/ml) while CEA was 5.3 ng/ml (normal range: 0–4.7 ng/ml). The chemotherapy regimen was changed to paclitaxel-albumin and gemcitabine. He received this regimen for 8 cycles. An assessment at the end of 8 cycles of this regimen showed that although the tumors did not significantly shrink on CT scan, the level of CA19-9 reduced from 9330 U/ml to 228 U/ml. The main adverse reaction was myelosuppression.
Discussion and conclusions
Here, we report a case of pancreatic cancer metastasizing to the rectum that was originally misdiagnosed as rectal cancer metastasizing to the pancreas.
Pancreatic cancer most commonly spreads to regional lymph nodes, liver and lung. Colorectal metastases from pancreatic cancer are very rare. Only 9 cases of rectal metastasis from pancreatic cancer are reported in the literature so far (Table ). One of them was a rare histological type, pancreatic acinar cell carcinoma (PACC). The other 8 cases were pancreatic ductal adenocarcinomas (PDAC), the same as our case. Prognoses of PACC are better than PDAC (Holen et al. Citation2002). The patient with PACC was a 67-year-old man who received surgical resections without additional chemotherapy. No tumor recurrence was observed after a median follow-up of 40 months (Ohara et al. Citation2018).
Table 1. Reports of pancreatic metastases to colorectum.
Among the other 8 PDAC cases, six were men and two were woman. The age ranged from 45 to 91 years. Overall survival (OS) ranged from 7 months to 14 months. Three cases were metachronous metastasis and five cases were synchronous metastasis. In our case, the patient had synchronous metastasis. Inada et al. reported a case that colonic metastasis occurred 7 years after pancreatoduodenectomy (Inada et al. Citation2013). The mechanism behind this atypical metastatic pattern is not well described. Japanese doctors believed that peritoneal metastasis was the most likely pathway (Inada et al. Citation2013).
Colonic metastasis from pancreatic cancer is often misdiagnosed especially on initial presentation. If masses are found in both pancreas and intestinal tract, they are often presumed as primary colon cancer with pancreatic metastasis because they have similar radiologic features. Although CA19-9 could be increased in many types of gastrointestinal cancers (Perkins et al. Citation2003; Stefan-van Staden et al. Citation2020), CA19-9 functions as a biomarker in pancreatic cancer, with a sensitivity of approximately 80% (Luo et al. Citation2021). The level of CA19-9 may help distinguish between cancer and other disorders in pancreatic masses. In our case, CA19-9 was elevated to 9330 U/ml. Similarly, the level of CA19-9 was also elevated in other reported cases (Inada et al. Citation2013; Kim and Lee Citation2015; Kahl et al. Citation2019).
Immunohistochemistry is the most valuable tool to determine tumor origin. The different expression patterns of CK-7 and CK-20 are commonly used to distinguish colorectal adenocarcinomas. CK-7 is an epithelial marker which often expressed in adenocarcinoma. Though in a few cases, colorectal carcinomas could express CK-7, especially if they are MSI or BRAF mutated cases or for seerated carcinomas (Judge et al. Citation1987), CK-7 is rarely expressed in the vast majority of colorectal carcinomas. CK-20 is expressed in all colorectal cancer cases, 62% of cases of pancreatic cancer and 50% of cases of gastric adenocarcinoma. Most colorectal cancers are CK-7 negative and CK-20 positive. Positive CK-7 and negative CK-20 suggest that the tumor originates from the pancreaticobiliary duct. CDX2 is an intestine-specific transcription factor and a key regulatory protein for intestinal epithelial formation and differentiation. It can be expressed in the small intestine, colon and rectum. So CK-7, CK-20 and CDX2 are good biomarker combinations to distinguish the primary origin of the tumor. Immunohistochemistry in our case showed strongly positive staining of CK-7 but negative staining for CK-20 and CDX2, suggesting that the rectal mass was a metastasis from the patient’s pancreatic primary, which is similar with previous reported cases.
The case is extremely rare, thus is often misdiagnosed. There were still several limitations of our study. Firstly, after laparoscopy, there was a delay in receiving the results of immunohistochemistry, but the patient asked for anti-tumor treatment as soon as possible. Based on the CT scan and pathological findings of colonoscopy, the patient was initially treated as having metastatic colorectal cancer. Moreover, only the pancreatic tumor was biopsied by laparoscopy in our hospital; the rectal tumor was biopsied by colonoscopy at the local hospital.
In conclusion, our report describes a case of synchronous colonic metastasis from pancreatic cancer. Elevated CA19-9 is helpful to distinguish the primary cancer site, while immunohistochemistry of CK-7, CK-20 and CDX2 provides the most important evidence for the identification of the primary site. Although rare, the suspicion of pancreatic metastases to the rectum should be excluded when a patient has both rectal and pancreatic masses.
Supplemental Material
Download Zip (4.2 MB)Acknowledgements
QT designed the work, conceived the idea, analysed the data, wrote the initial draft of the paper and revised the paper. WX collected the data, analysed the data and wrote the initial draft of the paper. WR and LY performed the histological examination of the tumor tissues. PW, ZY and ZC provided the patient data and images. LM and GY are the first author’s advisors. The authors read and approved the final manuscript. This study was approved by the ethics committee of our hospital (Approval No. 2022-QT-10). Written informed consent for this study was obtained from the patient. Written informed consent was obtained from the patient for publication of this case, including all individual details and accompanying images. The consent form is available for review by the editor of the journal upon request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data and materials supporting this study are available, without breaching participant confidentiality, in the figshare repository (https://figshare.com/articles/figure/Figures/21304122).
References
- Bellows C, Gage T, Stark M, McCarty C, Haque S. 2009. Metastatic pancreatic carcinoma presenting as colon carcinoma. South Med J. 102(7):748–750.
- Fukatsu H, Nagahara Y, Ishiki K, Iwamura M, Hamada F. 2009. Pancreatic cancer metastasis to the rectum detected on colonoscopy. Endoscopy. 41(Suppl. 2):E167–E168.
- Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB. 2002. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 20(24):4673–4678.
- Inada K, Shida D, Noda K, Inoue S, Warabi M, Umekita N. 2013. Metachronous colonic metastasis from pancreatic cancer seven years post-pancreatoduodenectomy. World J Gastroenterol. 19(10):1665–1668.
- Judge M, Moss SE, Besley GT, Parker AC. 1987. Lysosomal enzyme abnormalities in preleukaemic Sweet’s disease: case report. J Clin Pathol. 40(1):67–69.
- Kahl R, George K, Patel K, Stawick L. 2019. Pancreatic adenocarcinoma with rare sigmoid colon metastasis. ACG Case Rep J. 6(7):1–3.
- Kim W, Lee Y. 2015. Metachronous colonic metastasis from pancreatic cancer presenting as mechanical obstruction: a case report. Clin Imaging. 39(4):699–701.
- Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, Qian Y, Huang Q, Ni Q, Liu C, Yu X. 2021. Roles of CA19-9 in pancreatic cancer: biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 1875(2):188409.
- Miyasaka M, Noji T, Tanaka K, Nakanishi Y, Asano T, Ebihara Y, Kurashima Y, Nakamura T, Murakami S, Tsuchikawa T, et al. 2018. Oncological emergency surgery for metachronous large and small bowel metastases after pancreaticoduodenectomy for pancreatic cancer: a case report. Surg Case Rep. 4(1):99.
- Nogueira ST, Pinto BL, Silva EF, Garcia HA, Carneiro F. 2018. Pancreatic cancer presenting as colonic disease. A rare case report. Int J Surg Case Rep. 44:4–7.
- Ohara Y, Oda T, Enomoto T, Hisakura K, Akashi Y, Ogawa K, Owada Y, Domoto Y, Miyazaki Y, Shimomura O, et al. 2018. Surgical resection of hepatic and rectal metastases of pancreatic acinar cell carcinoma (PACC): a case report. World J Surg Oncol. 16(1):158.
- Park DY, Krishnamurthi S, Chahal P, Downs-Kelly E, Morris-Stiff G. 2019. Pancreatic metastases to the colon: an unusual cause of colonic obstruction. BMJ Case Rep. 12(11):e228578.
- Perkins GL, Slater ED, Sanders GK, Prichard JG. 2003. Serum tumor markers. Am Fam Physician. 68(6):1075–1082.
- Stefan-van Staden R-I, Ilie-Mihai R-M, Gurzu S. 2020. Simultaneous determination of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and serum protein p53 in biological samples with protoporphyrin IX (PIX) used for recognition by stochastic microsensors. Anal Lett. 53(16):2545–2558.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249.