Abstract
Background: Prunetin (Pru), a bioactive flavonoid present in Caulis spatholobi, has been reported to possess a variety of pharmacological effects. However, the therapeutic effect and underlying mechanisms of Pru in inflammatory bowel disease have not been previously investigated. This study aimed to explore the protective action of Pru on dextran sulfate sodium (DSS)-induced colitis by network pharmacology and experimental validation.
Methods: The corresponding genes of Pru were predicted using the Comparative Toxicogenomics Database (CTD) and SwissTargetPrediction. Differentially expressed genes (DEGs) between the control rats and DSS-induced colitis rats were identified from the microarray profile GSE54005. Cytoscape software was used to visualize the protein–protein interaction (PPI) networks of overlapped targets. Besides, an animal experiment was performed to verify the therapeutic effect of Pru on colitis.
Results: 21 potential targets related to colitis treated by Pru were identified. The topological analysis revealed that Tnf, Il6, and Il1b were the key genes. The KEGG and GO enrichment analyses showed that key targets were enriched in the inflammatory bowel disease, IL-17 signaling pathway, TNF signaling pathway, regulation of inflammatory response, and acute inflammatory response. The in vivo experiment revealed that Pru improves pathological injury and alleviated colitis symptoms via the regulation of Tnf, Il6, and Il1b, which were involved in the acute inflammatory response.
Conclusion: Our findings demonstrated that Pru alleviates colitis symptoms through the regulation of inflammatory response, which provides a scientific basis for Pru in preventing and treating colitis.
Introduction
Ulcerative colitis is a relapsing, chronic, and immune-mediated inflammatory bowel disease with typical clinical symptoms of weight loss, gastrointestinal bleeding, diarrhea, and abdominal pain (Szigethy et al. Citation2010). The prevalence of ulcerative colitis is on the rise, which seriously affects the quality of life and health of patients (Ng et al. Citation2017). Although our understanding of colitis has improved in recent years, the detailed pathogenesis of colitis is still unknown and may be related to a variety of factors. Accumulated evidence has revealed that food factors, childhood immunological events, psychological stress, genetic factors, and smoking are involved in the pathogenesis of ulcerative colitis (Packey and Sartor Citation2008). Recent reports have also proved that inflammatory cytokines and inflammation contribute to the initiation and progression of ulcerative colitis (Tatiya-Aphiradee et al. Citation2018; Yao et al. Citation2019). It has been reported that nutrients or natural compounds such as vitamins, dietary fiber, polysaccharides, prebiotics, polyphenols, and flavonoids participate in the maintenance of intestinal environmental stability through complex interactions with the immune system and microbiome (Statovci et al. Citation2017). These ingredients appeared to reduce inflammatory response and cytokines and maintain intestinal homeostasis through a variety of mechanisms (Ogita et al. Citation2011; Hung and Suzuki Citation2016; Murakami et al. Citation2016; Mayangsari and Suzuki Citation2018).
Prunetin (Pru) belongs to the group of isoflavones and is isolated from Caulis spatholobi with a variety of pharmacological effects, including anti-cancer activity, osteoinductive activity, anti-adipogenic effect, and anti-inflammatory effect (Mansoor et al. Citation2011; Ahn et al. Citation2013; Yang et al. Citation2013; Khan et al. Citation2015). Recently, Hu reported that Pru suppresses lipopolysaccharide (LPS)-induced inflammatory response via inhibiting the myeloid differentiation factor 88/toll-like receptor 4 (MyD88/TLR4) signaling pathways in human nasal epithelial cells (Hu and Li Citation2018). Pru was found to be efficacious and exert an anti-proliferative effect on gastric cancer cells (Vetrivel et al. Citation2020). Moreover, Pru inhibited the pro-inflammatory cytokine-induced decrease in barrier integrity via the inactivation of the nuclear factor kappa B (NF-kB) pathway, implying the intestinal barrier-modulating effects of Pru also in vivo (Piegholdt et al. Citation2014). However, to the best of our knowledge, no studies have reported the protective effects of Pru on DSS-induced colitis in rats.
Network pharmacology is a novel strategy integrating systems biology, omics, and pharmacological data, which improves the clinical efficacy and understanding of the side effects of drugs. This approach is accepted as the next model of drug development, which updates the current research model of ‘single drug target’ into a novel model of ‘drug-disease multiple targets and multiple signaling pathways’ (Huang et al. Citation2014). In the present study, network pharmacology was performed to predict the therapeutic genes and related signaling pathways of Pru in the treatment of colitis. Moreover, we established a rat model of colitis to verify the therapeutic effect and underlying mechanism of Pru.
Materials and methods
Prediction of pru-related targets
The 2D chemical structure of Pru was downloaded from the PubChem Database (https://pubchem.ncbi.nlm.nih.gov/). Pru-related targets were obtained from SwissTargetPrediction database (http://www.swisstargetprediction.ch/) and Comparative Toxicogenomics Database (CTD) (http://ctdbase.org/). In the SwissTargetPrediction database, targets with a probability value ≥0.1 were selected as Pru-related targets. In the CTD database, targets with interaction ≥1 were selected as Pru-related targets.
Acquisition of differentially expressed genes (DEGs)
The microarray profile GSE54005 was obtained from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). Then, the DEGs between normal group samples and DSS-treated group samples were identified using a GEO2R tool by setting p < 0.05 and ∣logFC∣ ≥ 0.5 (Liu et al. Citation2019; Aihaiti et al. Citation2021). Finally, the Venny online tool was used to visualize the intersection of DEGs and Pru-related targets.
Construction of the protein–protein interaction (PPI) network
The overlapped genes were introduced into the String database (https://www.string-db.org/), the species was chosen as Rattus norvegicus and the confidence score of >0.9 was set to generate the TSV format file. The file was imported into Cytoscape software (3.8.0). The PPI network was visualized and key genes were screened by the CytoHubba plug.
Kyoto encyclopedia of genes and genomes (KEGG) and gene ontology (GO) enrichment analysis
The overlapped genes were introduced into the database (http://metascape.org/gp/#/main/step1) to collect KEGG data and GO biological function data. Then, the KEGG pathway analysis and GO enrichment analysis were visualized using a free online platform (http://www.bioinformatics.com.cn/).
Animals and induction of ulcerative colitis model
Animal experimental procedures were approved by the Guangzhou University of Chinese Medicine (approval number: 2021056A). Male Wistar rats (180–200 g) were obtained from the Experimental Animal Center of Guangdong Province and kept under a controlled environment (humidity at 45%−55% and room temperature at 20–24°C) with a 12 h dark/light cycle. All rats were provided with water and food ad libitum. After one week of adaptive feeding, the ulcerative colitis model was induced by administration of 4% (wt/vol) dextran sulfate sodium (DSS) in tap water for seven days (Liu et al. Citation2021).
Animal experimental protocol
The rats were divided into three groups (n = 8 in each group): in the control group, rats received 2 mL tap water by oral gavage once a day for eight days; in the DSS group, ulcerative colitis rats received 2 mL tap water by oral gavage once a day for eight days; in the DSS + Pru group, ulcerative colitis rats received 2 mL Pru solution (1 mg/kg) by oral gavage once a day for eight days. The effective dose of Pru was determined by performing a pilot experiment and a previous study (Khan et al. Citation2015). All animals were monitored daily (diarrhea, body weight, and rectal bleeding) and disease activity index scores were calculated based on previous reports to evaluate the degree of ulcerative colitis (Gong et al. Citation2019). At the end of the experiment, rats were anesthetized and sacrificed. Then, colon tissues were collected. Part of the colon tissue was fixed with 4% paraformaldehyde, and the other colon samples were immediately stored at −80°C for further assay.
Histopathological evaluation
The colon tissue samples were embedded in paraffin and cut into 5 µm thickness, and then the sections were stained using Hematoxylin and Eosin reagents. The images were observed under a light microscope. The histological scoring system was performed based on the following parameters: 0–4 for the crypt damage, 0–3 for the depth of the damage, and 0–3 for the severity of inflammation.
Assay of inflammatory cytokines
Colon tissues were homogenized in cold 0.9% saline (w/v: 1/9), and the homogenate was centrifuged at 5000 g for 10 min. Then, the supernatant was collected for analysis. The bicinchoninic acid protein kit was used to measure the total protein levels of the supernatant. Levels of tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), IL-1β, and IL-10 in the colon tissues were measured by commercially available kits (Jian Cheng Bioengineering Institute, China) based on the protocols.
Quantitative real-time polymerase chain reaction qPCR
Total RNA was extracted from colon tissue using TRIzol Reagent (Invitrogen, CA) based on the manufacturer’s protocols. 2 µg of purified RNA was reverse-transcribed into cDNA using reverse transcriptase reagents (Thermo Fisher, USA) following the manufacturer’s protocols. Then, the qPCR reaction was carried out by the SYBR GREEN Master Mix (Bio-Rad) in a 7500 real-time PCR system. The 2−ΔΔCt method was used to quantify the mRNA expression levels. Primers were presented in Table .
Table 1. Sequences of primers used quantitative real-time PCR.
Statistical analyses
The statistical analysis was performed by a one-way analysis of variance followed by Dunnett’s Multiple Comparison Test using the GraphPad Prism 5.0 software. Experimental data were presented as mean ± standard deviation (SD). P < 0.05 was indicated as statistically significant.
Results
Prediction of potential targets in Pru and colitis
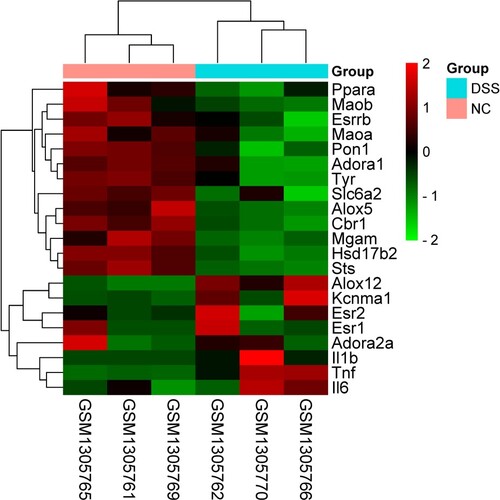
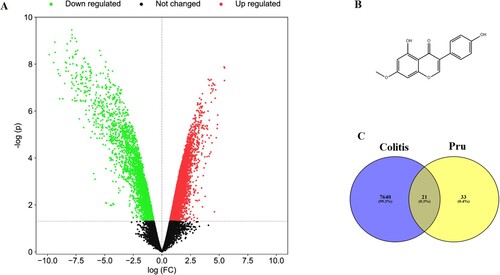
A total of 54 Pru-related genes were screened from the SwissTargetPrediction database and CTD database. A total of 7661 DEGs were identified from the microarray profile GSE54005 (Figure A). The chemical structure of Pru was shown in Figure B. After matching the DEGs with the targets of Pru, 21 overlapped genes were identified as potential targets in the therapeutic effect of Pru against colitis (Figure C). In addition, the heat map of these 21 common genes was presented in Figure .
Figure 1. The potential target genes of Pru and colitis were screened. (A) The gene volcano diagram shows the gene distribution in the microarray profile GSE54005. Downregulated genes were marked in green and upregulated genes were marked in red. (B) Chemical structure of Pru. (C) Venn diagram showing the collective targets between Pru and DEGs.
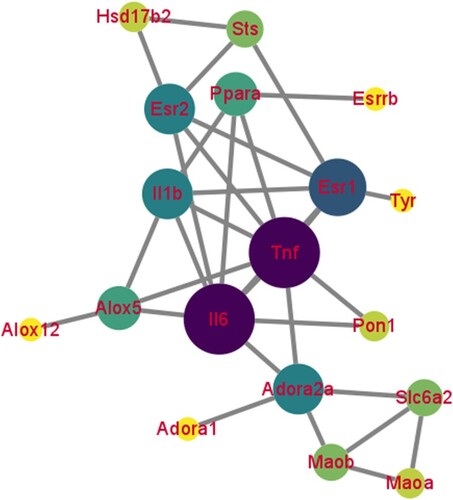
PPI network analysis.
The PPI network of 21 common genes was constructed by STRING and visualized by the Cytoscape software. There were 18 nodes and 32 edges in the PPI network (Figure ). The top 10 key genes were Tnf, Il6, estrogen receptor alpha 1 (Esr1), adenosine receptor 2a (Adora2a), Il1b, estrogen receptor alpha 2 (Esr2), arachidonate 5-lipoxygenase (Alox5), peroxisomal proliferation-activated receptor alpha (Ppara), norepinephrine transporter gene (Slc6a2), and monoamine oxidase B (Maob), which were screened as hub targets of Pru against colitis. Among them, Tnf, Il-6, and Il-1b were inflammation-related genes. Our findings showed that the inflammatory response may be a primary pathway for Pru in the treatment of colitis.
KEGG and GO enrichment analysis
The 21 common genes were uploaded into the Metascape database to further investigate the multiple mechanisms of Pru at a systematic level. We screened 51 signaling pathways with a P < 0.05. And the results of the top 16 signaling pathways were visualized as a bubble map (Figure A). In addition, the KEGG enrichment analysis revealed that the mechanisms of Pru in the treatment of colitis were mainly enriched in the inflammation-related signaling pathways, such as inflammatory bowel disease, IL-17 signaling pathway, and TNF signaling pathway.
Figure 4. The enrichment analysis of 21 collective genes. (A) The KEGG enrichment analysis of the top 16 signaling pathways. (B) The GO enrichment analysis of the top 16 biological processes.
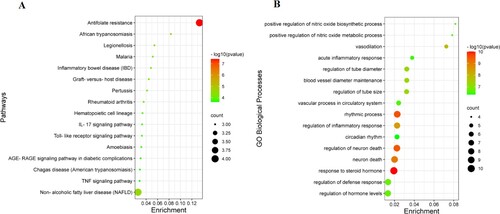
Besides, our GO analysis results indicated that these common genes were involved in multiple biological processes. There were 425 GO biological processes with a P < 0.05 that were screened. The results of the top 16 GO biological processes were visualized as a bubble map (Figure B). In addition, the GO enrichment analysis indicated that the mechanisms of Pru against colitis were mainly concentrated in the inflammation-related biological processes, such as acute inflammatory response and regulation of inflammatory response.
Pru alleviated the symptoms of DSS induced-colitis
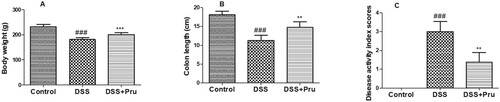
As shown in Figure , after administration of DSS, colitis rats suffered weight loss, shorter colon length, and higher disease activity index scores, compared to those in the control group. However, these colitis symptoms were alleviated by treatment with Pru, indicating that Pru exerted the therapeutic effect against DSS-induced colitis.
Figure 5. Effect of Pru on the rat model of colitis. (A) Bodyweight in different groups was measured at the end of the experiment. (B) Colon length in different groups was recorded. (C) Disease activity index scores. Experimental data were presented as mean ± SD. Significance: ###P < 0.001 in comparison with the control group; ***P < 0.001, **P < 0.01 in comparison with the DSS group.
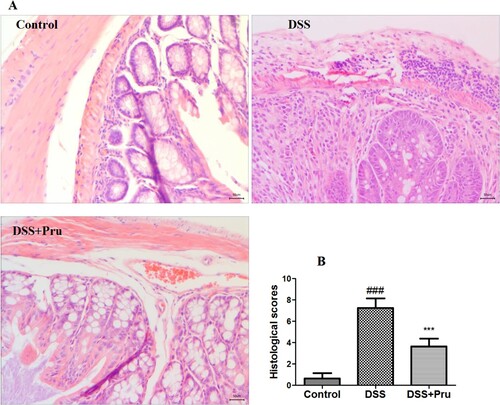
As shown in Figure A, the colon tissues of rats from the control group exhibited normal and healthy histological appearance. Administration of DSS induced abnormalities in the colonic architecture and higher histological scores (Figure B), which were characterized by extensive inflammatory cell infiltration, alteration of crypt structure, and severe lesions of the mucosa. Pru administration alleviated these histological changes and reduced the histological scores compared to those in the DSS group.
Figure 6. Pru ameliorated DSS-induced colon injury in rats. (A) Representative H&E-stained images in different groups (original magnification × 200). (B) Histological scores in different groups. Experimental data were presented as mean ± SD. Significance: ###P < 0.001 in comparison with the control group; ***P < 0.001 in comparison with the DSS group.
Pru ameliorated DSS-induced colonic inflammation in rats
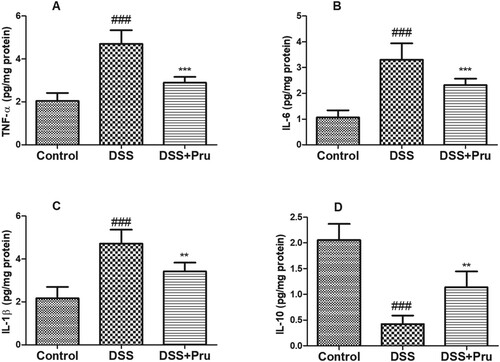
As shown in Figure , after the administration of DSS, the levels of TNF-α, IL-6, and IL-1β were increased while the levels of IL-10 were decreased in the DSS group compared to those in the control group. Pru administration increased the levels of IL-10 and decreased the levels of TNF-α, IL-6, and IL-1β.
Figure 7. Pru inhibited the generation of inflammatory cytokines in a rat model of colitis. The levels of TNF-α (A), IL-6 (B), IL-1β (C), and IL-10 (D) were measured by ELISA kits in colon tissues. Experimental data were presented as mean ± SD. Significance: ###P < 0.001 in comparison with the control group; ***P < 0.001, **P < 0.01 in comparison with the DSS group.
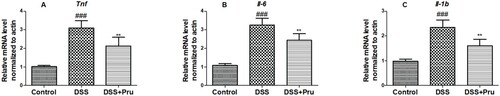
Moreover, as shown in Figure , prominent up-regulation in the mRNA expression of Tnf, Il-6, and Il-1b in the DSS group compared with the control group. After treatment with Pru, the expressions of Tnf, Il-6, and Il-1b displayed a significant downtrend in the DSS group. Taken together, our findings showed that Pru efficiently inhibited colonic inflammation in a colitis rat model.
Figure 8. Pru down-regulated the inflammation-related gene expressions in a rat model of colitis. Gene expression levels of Tnf (A), Il6 (B), and Il1b (C) were measured by qRT-PCR in colon tissues. Experimental data were presented as mean ± SD. Significance: ###P < 0.001 in comparison with the control group; **P < 0.01 in comparison with the DSS group.
Discussion
Colitis is an inflammatory bowel disease, characterized by ulceration of the colon tissue and recurring inflammation. This disease often occurs in the large intestine, and its pathogenesis remains unclear. Pru is an O-methylated isoflavone isolated from Caulis spatholobi. Previous reports have shown that Pru inhibited NF-κB-dependent inflammatory response in a mouse model of endotoxic shock (Yang et al. Citation2013). Moreover, Pru improved intestinal epithelial barrier function in vitro (Piegholdt et al. Citation2014). However, there were no studies on the anti-colitis effects of Pru in vivo. In the present study, network pharmacology analysis was used to investigate the therapeutic effects of Pru in colitis. These results indicated that the complicated mechanism actions for the Pru treatment of colitis involved the interactions of multiple targets and signaling pathways. Besides, inflammation-related targets (Tnf, Il6, and Il1b) were screened as the key genes. And inflammatory bowel disease, IL-17 signaling pathway, TNF signaling pathway, acute inflammatory response, and regulation of inflammatory response were found in the main pathways and biological processes, which were associated with the inflammatory response. Based on these findings, it was speculated that Pru might inhibit the secretion of inflammatory cytokines via regulation of inflammatory response, thus suppressing the TNF signaling pathway and ultimately alleviating the symptoms of colitis. The previous report demonstrated that Pru could down-regulate the expressions of TNF-α, IL-1β, and IL-6 in mice challenged with LPS via the inactivation of the NF-kB pathway (Yang et al. Citation2013). Similarly, Pru was found to suppress LPS-evoked pro-inflammatory cytokine secretion via inactivation of the TLR4/MyD88 pathway (Hu and Li Citation2018). These findings were consistent with our hub genes, KEGG, and GO enrichment analysis results. Moreover, the results of the animal experiment revealed that Pru alleviated the symptoms of DSS induced-colitis, which was associated with inflammatory response and was involved in the regulation of Tnf, Il6, and Il1b expression and TNF signaling pathway. The results of the animal experiment were consistent with the network pharmacology findings. A previous study revealed that Pru could improve epithelial barrier function in vitro via down-regulation of the NF-kB pathway, implying intestinal barrier-modulating functions of Pru also in vivo (Piegholdt et al. Citation2014). This result was coherent with our animal experimental results.
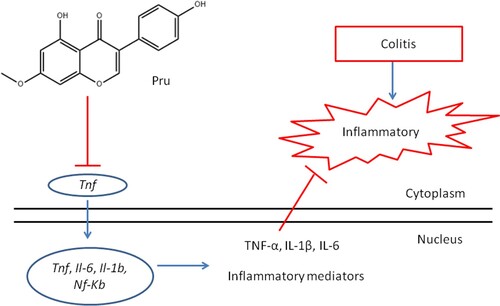
Colitis is an idiopathic chronic inflammatory disease of colonic mucosa. Inflammatory cytokines and persistent inflammation of the colon play an important role in the initiation and progression of colitis (Yao et al. Citation2019). Among pro-inflammatory cytokines, TNFs are the main mediator of inflammation and play a particularly important role. The previous report indicated that DSS administration up-regulated TNF-α mRNA expression in mice with chronic colitis (Popivanova et al. Citation2008). Besides, the clinical use of anti-TNF-α monoclonal antibodies has been demonstrated to be highly effective in patients with inflammatory bowel disease (Griffiths et al. Citation2020). These reports confirmed the important role of TNF-α in the progression of colitis and suggested that targeting TNF-α may be an effective therapy in the treatment of colitis. IL-6 is a multifunctional NF-κB-mediated cytokine. Increased generation of IL-6 was found in the colonic tissue of DSS-treated mice (Xiao et al. Citation2016). Previous studies have shown that serum IL-6 is a clinically relevant parameter of colitis that is closely related to the inflammatory activity of inflammatory bowel disease (Takac et al. Citation2014). These studies showed that IL-6 may be an important inflammatory cytokine during the development of colitis. In the present study, we identified Tnf, Il6, and Il1b as the hub genes in TNF signaling pathway, inflammatory bowel disease, and IL-17 signaling pathway. In addition, a rat model of colitis was established to investigate whether Pru could down-regulate the mRNA expressions of Tnf, Il6, and Il1b. Interestingly, we not only observed Pru alleviated the symptoms of DSS induced-colitis but also found Pru down-regulated the mRNA expressions of Tnf, Il6, and Il1b, ultimately inhibiting the secretion of inflammatory factors (IL-1β, IL-6, and TNF-α) in DSS-treated rats. Pru regulated the level of the inflammatory cytokine that may be associated with the inactivation of the NF-kB signaling pathway, and Figure illustrated the potential mechanism process in detail. Previous studies have supported this hypothesis, for example, Pru could induce apoptosis via regulation of TNF-α gene expression (Köksal Karayildirim et al. Citation2021). Pru could decrease LPS-induced TNF-α production and mRNA expression in the RAW 264.7 macrophage cells (Yang et al. Citation2013). Pru suppressed TNF-α-induced degradation of IkB and translocation of NF-kB p65 (Ryu et al. Citation2013). However, more experiments are needed to verify the specific mechanism of Pru against colitis. Therefore, these findings demonstrated that Tnf, Il6, and Il1b are the potential targets of Pru in the treatment of colitis. Combined with the findings of network pharmacology and animal experiment verification, the inflammatory response is the primary mechanism of action of Pru in the treatment of colitis, especially the TNF signaling pathway.
Although our research methods offered some advantages: the network pharmacology combined with experimental verification not only provides a novel approach to explore the potential therapeutic targets of natural products but also predicts their potential toxic side effects before actual clinical trials, thereby decreasing the risk and promoting the development of new drugs. There were still some limitations in our study. The major limitation of the present research was that we only focused on the inflammation-related genes and pathways in our verification experiment. Other identified genes still need to be confirmed, such as Adora2a, Esr1, Esr2, Ppara, Alox5, etc. And toll-like receptor signaling pathway, which was involved in the anti-colitis effects of Pru, should also be verified in further study.
Conclusions
Overall, the therapeutic effects and mechanism actions of Pru against colitis were investigated through network pharmacology and animal experimental confirmation. Our findings demonstrated that Pru suppressed inflammatory cytokines generation in DSS-treated rats via regulation of inflammatory response, providing a novel approach to explore the potential therapeutic strategy for inflammatory bowel disease.
Ethics declarations
All experimental protocols involving the use of animals were approved by the Ethics Committee of the Guangzhou University of Chinese Medicine (approval number: 2021056A).
Author contributions
Xiaobo Yang designed the experiments and wrote the manuscript. Ludi Fan carried out the experiments and analyzed the data. Jinglong Shi contributed to the conception of the work and interpretation of data for the research. All authors approved the final version for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The data for network pharmacology is available publicly from the GSE54005 dataset at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE54005. Raw data for the animal experiment are available at http://doi.org/10.57760/sciencedb.01824.
References
- Ahn TG, Yang G, Lee HM, Kim MD, Choi HY, Park KS, Lee SD, Kook YB, An HJ. 2013. Molecular mechanisms underlying the anti-obesity potential of prunetin, an O-methylated isoflavone. Biochem Pharmacol. 85(10):1525–1533. doi:10.1016/j.bcp.2013.02.020.
- Aihaiti Y, Song Cai Y, Tuerhong X, Ni Yang Y, Ma Y, Shi Zheng H, Xu K, Xu P. 2021. Therapeutic effects of naringin in rheumatoid arthritis: network pharmacology and experimental validation. Front Pharmacol. 12:672054.
- Gong Y, Niu W, Tang Y, Zhang Q, Liu S, Liu X, Wang X, Xu Y. 2019. Aggravated mucosal and immune damage in a mouse model of ulcerative colitis with stress. Exp Ther Med. 17(3):2341–2348.
- Griffiths OR, Landon J, Coxon RE, Morris K, James P, Adams R. 2020. Advances in protein chemistry and structural biology. Adv Protein Chem Struct Biol. 119:157–198. doi:10.1016/bs.apcsb.2019.08.009.
- Hu H, Li H. 2018. Prunetin inhibits lipopolysaccharide-induced inflammatory cytokine production and MUC5AC expression by inactivating the TLR4/MyD88 pathway in human nasal epithelial cells. Biomed Pharmacother. 106:1469–1477. doi:10.1016/j.biopha.2018.07.093.
- Huang C, Zheng C, Li Y, Wang Y, Lu A, Yang L. 2014. Systems pharmacology in drug discovery and therapeutic insight for herbal medicines. Brief Bioinform. 15(5):710–733. doi:10.1093/bib/bbt035.
- Hung TV, Suzuki T. 2016. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J Nutr. 146(10):1970–1979. doi:10.3945/jn.116.232538.
- Khan K, Pal S, Yadav M, Maurya R, Trivedi AK, Sanyal S, Chattopadhyay N. 2015. Prunetin signals via G-protein-coupled receptor, GPR30(GPER1): stimulation of adenylyl cyclase and cAMP-mediated activation of MAPK signaling induces Runx2 expression in osteoblasts to promote bone regeneration. J Nutr Biochem. 26(12):1491–1501.
- Köksal Karayildirim Ç, Nalbantsoy A, Karabay Yavaşoğlu N. 2021. Prunetin inhibits nitric oxide activity and induces apoptosis in urinary bladder cancer cells via CASP3 and TNF-α genes. Mol Biol Rep. 48(11):7251–7259. doi:10.1007/s11033-021-06719-w.
- Liu F, Yao Y, Lu Z, Zhang Q, Liu C, Zhu C, Lin C. 2021. 5-Hydroxy-4-methoxycanthin-6-one alleviates dextran sodium sulfate-induced colitis in rats via regulation of metabolic profiling and suppression of NF-κB/p65 signaling pathway. Phy Int J Phy Phyt. 82:153438.
- Liu W, Ye J, Yan H. 2019. Investigation of Key genes and pathways in inhibition of oxycodone on vincristine-induced microglia activation by using bioinformatics analysis. Dis Markers. 2019:3521746.
- Mansoor TA, Ramalho RM, Luo X, Ramalhete C, Rodrigues CM, Ferreira MJ. 2011. Isoflavones as apoptosis inducers in human hepatoma HuH-7 cells. Phytother Res. 25(12):1819–1824. doi:10.1002/ptr.3498.
- Mayangsari Y, Suzuki T. 2018. Resveratrol ameliorates intestinal barrier defects and inflammation in colitic mice and intestinal cells. J Agric Food Chem. 66(48):12666–12674. doi:10.1021/acs.jafc.8b04138.
- Murakami Y, Tanabe S, Suzuki T. 2016. High-fat diet-induced intestinal hyperpermeability is associated with increased bile acids in the large intestine of mice. J Food Sci. 81(1):H216–H222. doi:10.1111/1750-3841.13166.
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, et al. 2017. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London, England). 390(10114):2769–2778.
- Ogita T, Tanii Y, Morita H, Suzuki T, Tanabe S. 2011. Suppression of Th17 response by streptococcus thermophilus ST28 through induction of IFN-γ. Int J Mol Med. 28(5):817–822.
- Packey CD, Sartor RB. 2008. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 263(6):597–606. doi:10.1111/j.1365-2796.2008.01962.x.
- Piegholdt S, Pallauf K, Esatbeyoglu T, Speck N, Reiss K, Ruddigkeit L, Stocker A, Huebbe P, Rimbach G. 2014. Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-κB, and tyrosine phosphorylation. Free Radical Biol Med. 70:255–264. doi:10.1016/j.freeradbiomed.2014.02.025.
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. 2008. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 118(2):560–570.
- Ryu J, Lee HJ, Park SH, Sikder MA, Kim JO, Hong JH, Seok JH, Lee CJ. 2013. Effect of prunetin on TNF-α-induced MUC5AC mucin gene expression, production, degradation of IκB and translocation of NF-κB p65 in human airway epithelial cells. Tuberc Respir Dis (Seoul). 75(5):205–209.
- Statovci D, Aguilera M, MacSharry J, Melgar S. 2017. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 8:838.
- Szigethy E, McLafferty L, Goyal A. 2010. Inflammatory bowel disease. Child Adolesc Psychiatr Clin N Am. 19(2):301–318. ix.
- Takac B, Mihaljević S, Stefanić M, Glavas-Obrovac L, Kibel A, Samardzija M. 2014. Importance of interleukin 6 in pathogenesis of inflammatory bowel disease. Coll Antropol. 38(2):659–664.
- Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. 2018. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol. 30(1):1–10. doi:10.1515/jbcpp-2018-0036.
- Vetrivel P, Kim SM, Ha SE, Kim HH, Bhosale PB, Senthil K, Kim GS. 2020. Compound prunetin induces cell death in gastric cancer cell with potent anti-proliferative properties: In vitro assay, molecular docking, dynamics, and ADMET studies. Biomolecules. 10(7):1086. doi:10.3390/biom10071086.
- Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. 2016. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine. 83:189–192. doi:10.1016/j.cyto.2016.04.012.
- Yang G, Ham I, Choi HY. 2013. Anti-inflammatory effect of prunetin via the suppression of NF-κB pathway. Food Chem Toxicol. 58:124–132. doi:10.1016/j.fct.2013.03.039.
- Yao D, Dong M, Dai C, Wu S. 2019. Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and Its associated cancer. Inflamm Bowel Dis. 25(10):1595–1602. doi:10.1093/ibd/izz149.