Abstract
We analyzed the clinical characteristics and prognosis of early pregnancy-associated breast cancer (PABC). In this matched case-control study, the PABC patients and non-PABC patients were recruited from Hebei Breast Disease Treatment Center in a ratio of 1:2, involving 40 PABC patients (10 pregnant women, 30 postpartum women) diagnosed between January 2011 and December 2017, and 80 non-PABC patients with matched tumor staging, age (±3 years), and year of diagnosis (± 2 years). The pathological characteristics, disease-free survival (DFS), and overall survival (OS) were compared between the groups. The first gravidity age in PABC patients was significantly older than that of non-PABC patients (26.8 years vs. 23.5 years, P < 0.05). Categorized by receptor status, the ratio of hormone receptor (HR)-positive breast cancer was lower in PABC patients than in non-PABC patients (57.5% vs. 70.0%, P = 0.221), while the incidences of HER2− (42.5% vs. 37.5%, P = 0.692) and triple-negative breast cancer (20% vs. 12.5%, P = 0.578) were higher in PABC patients than in non-PABC patients, although no significant differences were detected. Compared with non-PABC patients, PABC patients delivered the first gravidity at an older age, but showed no differences in pathological characteristics, DFS, and OS.
Introduction
Pregnancy-associated breast cancer (PABC) is the most common primary malignant tumor diagnosed during pregnancy, lactation or one year after delivery (Lyons et al. Citation2009; Fidler et al. Citation2017; Liao et al. Citation2022; Matar et al. Citation2022). PABC accounts for approximately 4% of breast cancer cases in women aged <45 years (Cottreau et al. Citation2019). As women postpone giving birth to their first child, the global incidence of PABC is on the rise. In Sweden, the national data showed that the incidence and mortality of PABC are 2.3% and 4.3% over a period from 1970 to 2018, respectively, and the prognosis of PABC is worse than that of non-PABC (Johansson et al. Citation2021). In China, with the postponement of childbearing and implementation of the two-child policy, a sharp increase in PABC incidence has been observed (Han et al. Citation2020). Due to physiological changes in the breast caused by pregnancy and lactation, PABC may be easily misdiagnosed, and as a result, delayed treatment and advanced stages may worsen the prognosis. Whether there are differences in pathological characteristics and prognosis between PABC and non-PABC remains controversial. Previous studies have suggested an inferior 5-year overall survival (OS) rate in PABC cohort, and different biologic features of PABC from non-PABC (Bae et al. Citation2018b; Prior et al. Citation2021). Another research has shown no significant difference in the prognosis between PABC and non-PABC (Bae et al. Citation2018a; Bajpai et al. Citation2021; Zhang et al. Citation2021). The poorer prognosis observed in PABC appears to be largely explained by more adverse tumor characteristics at diagnosis (Baulies et al. Citation2015; Johansson et al. Citation2018). This matched case-control study was designed to evaluate the clinical characteristics and prognosis of early PABC in a Chinese population.
Participants and methods
Participants
A retrospective cohort study was designed, involving patients aged less than 40 years with stage I-III disease admitted at Hebei Cancer Hospital from January 2011 to December 2017 (n = 1822). PABC was defined as primary breast cancer diagnosed during pregnancy or within one year after delivery. The inclusion criteria were: (1) pathological confirmation of breast cancer (7th edition of the TNM classification for breast cancer), (2) ages 20–40 years, (3) complete data about pathological characteristics, status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression and Ki-67 index. The exclusion criteria comprised: (1) stage IV or recurrent breast cancer, (2) incomplete follow-up data.
Finally, 40 patients with early PABC were selected as the study subjects. In a ratio of 1:2, 80 non-PABC patients with matched AJCC staging, age (± 3 years), and year of diagnosis (± 2 years) were recruited as the control group. Pathological characteristics, disease-free survival (DFS), and overall survival (OS) were compared between the groups. This was a retrospective, non-interventional study that did not require ethical review.
Data collection
Data included age at diagnosis, age at menarche, age at pregnancy, duration of disease, family history, pathological subtype, tumor staging, tumor size, lymph node metastasis, histological grade, hormone receptor status, HER2 status, Ki-67 expression, therapeutic strategy (surgery, chemotherapy, radiotherapy, endocrine therapy), time of first recurrence, DFS, and OS.
Assessment of receptor status
Two experienced chief physicians reviewed the results of the pathological examinations. Estrogen receptor (ER)- and/or progesterone receptor (PR)-positive breast cancer was defined as a minimum of 1% of breast cancer cells stained positive ER and/or PR. The HER2 expression in breast cancer was scaled to 0–3+ points based on the integrity of membrane staining: 0–1+, HER2−; 2+, uncertainty; and 3+, HER2+. Uncertain staining of HER2 (2+) was further validated using the PathVysion HER2 DNA Probe Kit via fluorescence in situ hybridization (FISH). Breast cancer cases were categorized as HR+/HER2−, HER2+, and triple-negative based on immunohistochemistry results.
DFS was defined as the time from the initial diagnosis to local or systemic recurrence or death. In the absence of recurrence, the DFS was defined as the time from the initial diagnosis to the last follow-up. OS was defined as the time from the initial diagnosis to death or the last follow-up. Considering the long disease-free survival and overall survival with early-stage breast cancer, we only analyzed the DFS and OS in stage II-III patients.
Statistical analyses
Statistical analyses were performed using the SPSS 19.0. Differences in pathological characteristics between the PABC and non-PABC patients were elucidated using the chi-squared test or Fisher’s exact test. The DFS and OS were assessed using the Kaplan-Meier method. Statistical significance was set at P < 0.05.
Results
Baseline characteristics
A total of 40 female patients with early PABC were recruited, including 10 diagnosed during pregnancy and 30 diagnosed within one year after delivery. Among the 80 non-PABC patients, 7 had no childbirth history, and 72 were diagnosed with breast cancer more than 1 year after delivery. The median ages at diagnosis of PABC and non-PABC patients were 29.0 (25–39) years and 29.0 (25–36) years, respectively. Their median ages at menarche were 14.0 (11–17) and 14.0 (11–16) years, respectively. The median duration of disease onset in the PABC patients was longer than that in the non-PABC patients (3.5 [0.1–24] months vs. 2.0 [0.1–36] months, P = 0.625), indicating a later visit in the former. The first gravidity age was significantly higher in the PABC patients than in the non-PABC patients (26.8 years vs. 23.5 years, P < 0.05). Two PABC patients and four non-PABC patients reported a family history of breast cancer.
Invasive ductal carcinoma was the most common histopathological subtype in both groups, with an incidence of 85%. The major tumor stage was stage II breast cancer, which accounted for 57.5% of the patients in both groups. The incidence of HR+ breast cancer was lower in the PABC patients than in the non-PABC patients (57.5% vs. 70%, P > 0.05), whereas the incidence of HER2 (42.5% vs. 37.5%, P > 0.05) was higher in the former. No significant differences were identified in tumor size, lymph node metastasis, histological grade, or Ki-67 expression between the PABC and non-PABC patients (Table ).
Table 1. Description of the study group.
Treatment options
In total, 42.5% of PABC patients and 15% of non-PABC patients received preoperative chemotherapy (mainly taxane-based chemotherapy containing anthracyclines). Among the 10 patients diagnosed with breast cancer during pregnancy, 8 received anthracycline chemotherapy before delivery, and 2 had to terminate their pregnancy (due to their concerns about fetal safety). Approximately 20% of patients in each group underwent breast-conserving surgery or breast reconstruction (Table ). According to the National Comprehensive Cancer Network (NCCN) guideline, all patients were postoperatively treated with chemotherapy, radiotherapy, molecular targeted therapy, and adjuvant endocrine therapy, according to biological parameters (NCCN Citation2011).
Table 2. Treatment of PABC.
DFS and OS
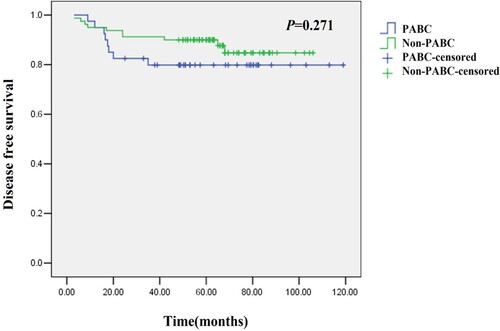
The mean DFS was 54.2 (12.2–112.5) months in the PABC patients and 63.5 (9.4–98.1) months in the non-PABC patients. Recurrence within 20 months after diagnosis was higher in the PABC patients than in the non-PABC patients, although a significant difference was not detected (P = 0.271, Figure ). Among the 8 patients with recurrent PABC, one (12.5%) had local recurrence and seven (87.5%) had distant metastases (the most common sites of metastasis were liver, lungs, bones, and brain). There were 11 recurrent cases in the non-PABC patients, including five (45.5%) with local recurrence and six (55.5%) with distant metastases. Among the 10 PABC patients diagnosed during pregnancy, 3 had distant metastases, including 1 with brain metastasis, 1 with multi-bone metastases, and 1 with liver metastasis.
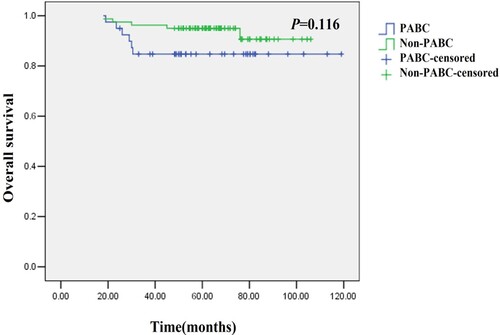
The mean OS in the PABC patients was comparable with that in the non-PABC patients (54.2 months vs. 65.4 months, P = 0.116, shown in Figure ).
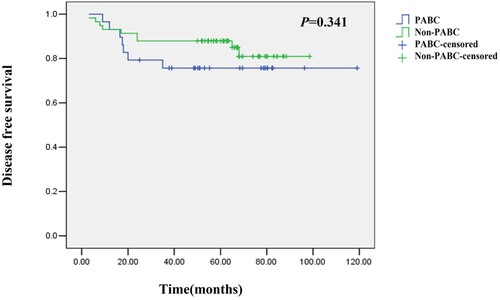
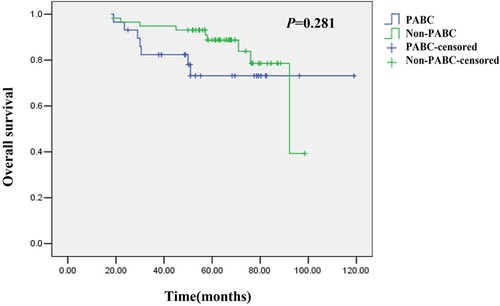
Stratification analysis showed no significant differences in the mean DFS (51.0 months vs. 51.0 months, P = 0.341) and OS (65.0 months vs. 65.9 months, P = 0.281) between stage II-III PABC patients and non-PABC patients (a total of 11 cases of stage I breast cancer patients were excluded) (Figures and ).
Discussion
PABC is a leading pregnancy-related cancer worldwide. Danish research has shown an incidence of pregnancy-associated cancer having increased from 5.4–8.3% over a 30-year period (Eibye et al. Citation2013). The incidence of PABC has also increased with the postponement of childbearing, and its diagnosis and treatment need multidisciplinary cooperation between surgeons, oncologists, gynecologists, and obstetricians (Loibl et al. Citation2015). Most previous studies on PABC are retrospective, rather than prospective, because of its similarity to other cancers that occur during pregnancy (Suleman et al. Citation2019; Wang et al. Citation2019; Han et al. Citation2020). In these studies, whether the prognosis of early PABC is different from that of other non-PABC has not been reported.
In the present study, PABC was defined as breast cancer diagnosed within one year after delivery, and the non-PABC group included breast cancer patients diagnosed more than one year after delivery and non-partum women. A previous study has reported that PABC patients usually have a higher stage of breast cancer (Genin et al. Citation2012; Hartman and Eslick Citation2016). Another study has suggested that the poorer prognosis observed within PABC group may not be due to pregnancy, but a delay in diagnosis and tumor subtype (Baulies et al. Citation2015). Our results did not identify significant differences in tumor staging, tumor size, and lymph node metastasis between PABC and non-PABC patients, but the time from finding breast abnormalities to seeking treatment in PABC patients was longer than that in non-PABC patients, which may be attributed to the increased number, volume, and density of breast glands during pregnancy and lactation. A retrospective clinical study of PABC among multiple centers in China (Jin et al. Citation2021) has shown that invasive ductal carcinoma is the major pathological subtype of PABC, which is consistent with our findings (85%). Other subtypes of PABC include mucinous adenocarcinoma, papillary carcinoma, medullary carcinoma, and ductal carcinoma in situ. It has been reported that the positive rates of ER and PR are relatively low in patients (Ploquin et al. Citation2018; Suelmann et al. Citation2021). In the present study, the incidence of HR+ breast cancer patients was lower in the PABC group than in the non-PABC group. Besides, a consistent result was yielded that a higher ratio of HER2+ breast cancer patients was detected in the PABC group than in the non-PABC group (Loibl et al. Citation2015; Boudy et al. Citation2020). It is unclear whether a high ratio of HER2+ breast cancers is correlated with pregnancy. Previous evidence has pointed out that the postpartum hormone level determines the positive rate of HER2 breast cancer (Cruz et al. Citation2013). Additionally, no significant differences in histological grade and Ki-67 expression were detected between PABC and non-PABC patients.
The pathogenesis of PABC is complex. Family history and BRCA have been reported to be closely associated with PABC. For the women carrying BRCA1/2 mutations, those who have already given birth face a higher risk of breast cancer than those who are nulliparous (Michieletto et al. Citation2014; Zografos et al. Citation2021). In this study, the proportion of patients with a family history of breast cancer was relatively low. We did not detect BRCA mutations, and its effect requires further investigation. Age at first pregnancy is the main risk factor for PABC. Monteiro et al. (Citation2019) have revealed that the risk of PABC increases by 27% for each additional year of the mother’s age at first pregnancy (P < 0.02). Nguyen et al. (Nguyen et al. Citation2019) have shown that genomic alterations of breast cancer are associated with age at first pregnancy but not with parity status alone. According to a report from Kim et al. (Kim et al. Citation2017), early menarche, late age at first childbirth, and BMI ≥23 kg/m2 are more associated with PABC than non-PABC. Our results showed that the age at first gravidity in PABC patients was older than that in non-PABC patients, suggesting that it may be a risk factor for PABC. Age at menarche is also a risk factor for breast cancer, although we did not detect a significant difference between PABC and non-PABC patients.
After analyzing 117 articles on PABC, Psyrri concluded that the prognosis of PABC patients was similar to that of non-pregnant women in the same stage. There is no definitive evidence that pregnancy increases the risk of recurrence (Psyrri and Burtness Citation2005). The national data in Sweden in 1970–2018 have revealed that the survival rate of PABC patients does not significantly decrease over time, while the risk of PABC diagnosed one year after delivery has increased (Johansson et al. Citation2021). In the present study, the matched analyses based on tumor staging and age did not show significant differences in DFS and OS between PABC and non-PABC patients, which is consistent with previous findings.
The prognosis of PABC is correlated with the biological characteristics and stage of breast cancer (Johansson et al. Citation2018; Wang et al. Citation2019). A matched case-control study has shown that pregnancy and postpartum status are independent impacts of DFS (P < 0.05) (Muñoz-Montaño et al. Citation2021). Another case-matching study has shown that DFS and OS are similar between patients with PABC and non-PABC (Zhang et al. Citation2021). Our stratified analysis did not detect significant differences in DFS or OS between patients with early PABC and those with non-PABC. First, it is believed that the influence of pregnancy on breast cancer prognosis may last 5–10 years or even longer. However, the PABC patients in this study included breast cancer patients diagnosed during pregnancy (10 patients) and within one year of delivery (30 patients), which was unlikely to determine the long-term influence of pregnancy on prognosis. Patients diagnosed with postpartum breast cancer (pooled hazard ratio [PHR]: 1.84; 95% confidence interval [CI]; 1.28–2.65) had a worse prognosis than those diagnosed during pregnancy (PHR: 1.29; 95% CI: 0.74–2.24). The DFS data showed that the risk of recurrence associated with PABC was also significantly increased (PHR: 1.60 [1.19–2.16]) (Azim et al. Citation2012). A study from Taiwan of China has reported that patients with breast cancer diagnosed in the first year postpartum have a poor prognosis (Li et al. Citation2020). Breast cancer during pregnancy and within one year after delivery, which was not distinguished in the present study, may belong to two different prognostic categories. The definition of PABC should be refreshed (Lee et al. Citation2017; Amant et al. Citation2021). Second, patients who were initially diagnosed with stage IV breast cancer, which may have caused potential bias, were not included in the present study. Additionally, the small sample size, low ratio of breast cancer patients diagnosed during pregnancy, and retrospective study design might have influenced our results.
Conclusion
Early-stage PABC patients present similar pathological characteristics, OS, and DFS as tumor staging and age-matched non-PABC patients. The age at first gravidity may be a risk factor for PABC. However, this conclusion needs to be supported by larger-sample studies.
Author contributions
Zhifen Yang: Conceptualization, visualization, investigation, writing - review and editing, project administration. Yanshou Zhang and Chunyang Wang: Data curation, writing – original draft, conceptualization, and project administration. Lijia Du and Yingru Liu: Data curation.
Ethic approval
This was a retrospective, non-interventional study that did not require ethical review. This research was exempted from ethics commission approval as treatment was administered as a part of the services offered by the hospital.
Acknowledgement
We would like to acknowledge Yunjiang Liu and Huixin Zhang for their assistance and guidance.
Data availability statement
The data supporting the findings of this study are available at: [DOI:10.7910/DVN/SR06ED].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amant F, Lefrère H, Borges VF, Cardonick E, Lambertini M, Loibl S, Peccatori F, Partridge A, Schedin P. 2021. The definition of pregnancy-associated breast cancer is outdated and should no longer be used. Lancet Oncol. 22(6):753–754.
- Azim HA Jr, Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA. 2012. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev. 38(7):834–842.
- Bae SY, Kim KS, Kim JS, Lee SB, Park BW, Lee SW, Lee HJ, Kim HK, You JY, Jung SP, et al. 2018a. Neoadjuvant chemotherapy and prognosis of pregnancy-associated breast cancer: a time-trends study of the Korean Breast Cancer Registry database. J Breast Cancer. 21(4):425–432.
- Bae SY, Kim SJ, Lee J, Lee ES, Kim EK, Park HY, Suh YJ, Kim HK, You JY, Jung SP. 2018b. Clinical subtypes and prognosis of pregnancy-associated breast cancer: results from the Korean Breast Cancer Society Registry database. Breast Cancer Res Treat. 172:113–121.
- Bajpai J, Simha V, Shylasree TS, Sarin R, Pathak R, Popat P, Mokal S, Dandekar S, Bhansal V, Ghosh J, et al. 2021. Pregnancy associated breast cancer (PABC): report from a gestational cancer registry from a tertiary cancer care centre, India. Breast. 56:88–95.
- Baulies S, Cusidó M, Tresserra F, Fábregas R, Rodríguez I, Úbeda B, Ara C, Fábregas R. 2015. Biological and pathological features in pregnancy-associated breast cancer: a matched case-control study. Eur J Gynaecol Oncol. 36(4):420–433.
- Boudy AS, Ferrier C, Selleret L, Zilberman S, Arfi A, Sussfeld J, Gligorov J, Richard S, Bendifallah S, Chabbert-Buffet N, et al. 2020. Prognosis of HER2-positive pregnancy-associated breast cancer: analysis from the French CALG (Cancer Associé à La Grossesse) network. Breast. 54:311–318.
- Cottreau CM, Dashevsky I, Andrade SE, Li DK, Nekhlyudov L, Ritzwoller DP, Raebel MA, Partridge AH, Pawloski PA, Toh S. 2019. Pregnancy-associated cancer: a U.S. population-based study. J Womens Health (Larchmt). 28(2):250–257.
- Cruz GI, Martínez ME, Natarajan L, Wertheim BC, Gago-Dominguez M, Bondy M, Yao YJ, Zhu W, Wu KJ. 2013. Hypothesized role of pregnancy hormones on HER2+ breast tumor development. Breast Cancer Res Treat. 137(1):237–246.
- Eibye S, Kjaer SK, Mellemkjaer L. 2013. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet Gynecol. 122(3):608–617.
- Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. 2017. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol. 18(12):1579–1589.
- Genin AS, Lesieur B, Gligorov J, Antoine M, Selleret L, Rouzier R. 2012. Pregnancy-associated breast cancers: do they differ from other breast cancers in young women? Breast. 21(4):550–555.
- Han BY, Li XG, Zhao HY, Hu X, Ling H. 2020. Clinical features and survival of pregnancy-associated can breast cer: a retrospective study of 203 cases in China. BMC Cancer. 20(1):244.
- Hartman EK, Eslick GD. 2016. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat. 160(2):347–360.
- Jin YC, Du JX, Fu SM, Chen Q, Qiu YR, Pei A, Yao YJ, Zhu W, Wu KJ. 2021. A retrospective clinical study of patients with pregnancy-associated breast cancer among multiple centers in China (CSBrS-008). Chin Med J (Engl). 134(18):2186–2195.
- Johansson ALV, Andersson TM, Hsieh CC, Jirström K, Cnattingius S, Fredriksson I, Dickman PW, Lambe M. 2018. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int J Cancer. 142(7):1343–1354.
- Johansson ALV, Fredriksson I, Mellemkjaer L, Stensheim H, Lähteenmäki P, Winther JF, Ullenhag GJ, Lundberg FE. 2021. Cancer survival in women diagnosed with pregnancy-associated cancer: An overview using nationwide registry data in Sweden 1970-2018. Eur J Cancer. 155:106–115.
- Kim YG, Jeon YW, Ko BK, Sohn G, Kim EK, Moon BI, Youn HJ, Kim HA, Korean Breast Cancer Society. 2017. Clinicopathologic characteristics of pregnancy-associated breast cancer: results of analysis of a nationwide breast cancer registry database. J Breast Cancer. 20(3):264–269.
- Lee GE, Mayer EL, Partridge A. 2017. Prognosis of pregnancy-associated breast cancer. Breast Cancer Res Treat. 163:417–421.
- Li SS, Hsu YT, Yen CC, Chen YW, Wu PY, Chang KC, Li CY, Chen TY. 2020. Maternal survival of patients with pregnancy-associated cancers in Taiwan-A national population-based study. Cancer Med. 9(24):9431–9444.
- Liao Q, Deng D, Xie Q, Gong X, Meng X, Xia Y, Ai J, Li K. 2022. Clinical characteristics, pregnancy outcomes and ovarian function of pregnancy-associated breast cancer patients: a retrospective age-matched study. BMC Cancer. 22(1):152.
- Loibl S, Schmidt A, Gentilini O, Kaufman B, Kuhl C, Denkert C, von Minckwitz G, Parokonnaya A, Stensheim H, Thomssen C, et al. 2015. Breast cancer diagnosed during pregnancy: adapting recent advances in breast cancer care for pregnant patient. JAMA Oncol. 1(8):1145–1153.
- Lyons T, Schedin P, Borges V. 2009. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 14(2):87–98.
- Matar R, Crown A, Sevilimedu V, Goldfarb SB, Gemignani ML. 2022. Timing of presentation and outcomes of women with stage IV pregnancy-associated breast cancer (PABC). Ann Surg Oncol. 29(3):1695–1702.
- Michieletto S, Saibene T, Evangelista L, Barbazza F, Grigoletto R, Rossi G, Bozza F. 2014. Preliminary monocentric results of biological characteristics of pregnancy associated breast cancer. Breast. 23(1):19–25.
- Monteiro DLM, Nunes CL, Rodrigues NCP, Antunes CA, Almeida EM, Barmpas DBS, Trajano AJB. 2019. Factors associated with gestational breast cancer: case-control study. Cien Saude Colet. 24(6):2361–2369.
- Muñoz-Montaño WR, Cabrera-Galeana P, De la Garza-Ramos C, Azim HA, Tabares A, Perez V, Porras Reyes F, Sanchez Benitez D, Alvarado-Miranda A, Lara-Medina F. 2021. Prognosis of breast cancer diagnosed during pregnancy and early postpartum according to immunohistochemical subtype: a matched case-control study. Breast Cancer Res Treat. 188(2):489–500.
- NCCN. 2011 Jul 29. The NCCN soft tissue sarcoma clinical practice guidelines in oncology (version 1.2011) [EB/OL]. Fort Washington: NCCN. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- Nguyen B, Venet D, Lambertini M, Desmedt C, Salgado R, Horlings HM, Rothé F, Sotiriou C. 2019. Imprint of parity and age at first pregnancy on the genomic landscape of subsequent breast cancer. Breast Cancer Res. 21(1):25.
- Ploquin A, Pistilli B, Tresch E, Frenel JS, Lerebours F, Lesur A, Loustalot C, Bachelot T, Provansal M, Ferrero JM, et al. 2018. 5-year overall survival after early breast cancer diagnosed during pregnancy: A retrospective case-control multicentre French study. Eur J Cancer. 95:30–37.
- Prior L, O’Dwyer R, Farooq AR, Greally M, Ward C, O’Leary C, Aslam R, Darwish W, Ahmed N, Othman EC, et al. 2021. Pregnancy-associated breast cancer: evaluating maternal and foetal outcomes. A national study. Breast Cancer Res Treat. 189:269–283.
- Psyrri A, Burtness B. 2005. Pregnancy-associated breast cancer. Cancer J. 11(2):83–95.
- Suelmann BBM, van Dooijeweert C, van der Wall E, Linn S, van Diest PJ. 2021. Pregnancy-associated breast cancer: nationwide Dutch study confirms a discriminatory aggressive histopathologic profile. Breast Cancer Res Treat. 186(3):699–704.
- Suleman K, Osmani AH, Al Hashem H, Al Twegieri T, Ajarim D, Jastaniyah N, Al Khayal W, Al Malik O, Al Sayed A. 2019. Behavior and outcomes of pregnancy associated breast cancer. Asian Pac J Cancer Prev. 20(1):135–138.
- Wang B, Yang Y, Jiang Z, Zhao J, Mao Y, Liu J, Zhang J. 2019. Clinicopathological characteristics, diagnosis, and prognosis of pregnancy-associated breast cancer. Thorac Cancer. 10(5):1060–1068.
- Zhang R, Liu X, Huang W, Shao B, Yan Y, Liang X, Ran R, Song G, Di L, Jiang H, et al. 2021. Clinicopathological features and prognosis of patients with pregnancy-associated breast cancer: A matched case control study. Asia Pac J Clin Oncol. 17(4):396–402.
- Zografos E, Korakiti AM, Andrikopoulou A, Rellias I, Dimitrakakis C, Marinopoulos S, Giannos A, Keramopoulos A, Bredakis N, Dimopoulos MA, et al. 2021. Germline mutations in a clinic-based series of pregnancy associated breast cancer patients. BMC Cancer. 21(1):572.