Abstract
Introduction: Measuring soluble interleukin-2 receptor (sIL-2R) might be useful for diagnosis and monitoring of immune-mediated diseases. The study investigated factors associated with serum sIL-2R concentrations in adults.
Methods: Serum sIL-2R concentrations were measured in 1499 randomly selected individuals (44.6% male, median age 52 years, range 18–91 years). Lifestyle factors (alcohol consumption, smoking, and physical activity) were assessed by questionnaire. Body mass index and data defining metabolic syndrome were measured in all participants. Atopy was defined by skin tests to aeroallergens.
Results: Serum sIL-2R concentrations displayed wide variation (2.5–97.5 percentile range, 209–950 U/mL). A total of 230 (15.3%) individuals showed values higher than the standard reference range (158–623 U/mL). After adjusting for covariates, sIL-2R concentrations increased with age, particularly after 65 years. Serum sIL-2R concentrations were higher in males than in females, were higher in smokers than in never smokers, and were higher in atopic than in non-atopic individuals. Serum sIL-2R concentrations were positively associated with metabolic syndrome, particularly with abdominal obesity, and with a history of ischemic heart disease. Light alcohol drinkers showed lower sIL-2R concentrations than abstainers.
Conclusion: Aging, sex, lifestyle factors, atopy, metabolic abnormalities, and ischemic heart disease might influence serum sIL-2R concentrations in adults.
Introduction
The interleukin (IL)−2 – IL2 receptor (IL-2R) pathway plays important roles in human health and disease. The soluble (s) IL-2Rα chain, sIL-2R (CD25) is shed upon immune activation and is abundantly found in the circulation of healthy individuals [Damoiseaux Citation2020; Dik and Heron Citation2020]. Binding of IL-2 to sIL-2R can either reduce or enhance responses depending on the target cell being involved in immunity or self-tolerance [Damoiseaux Citation2020; Dik and Heron Citation2020]. In summary, sIL-2R is a marker of T cell activation [Rubin and Nelson Citation1990]. Increased serum concentrations of sIL-2R are a marker for diagnosing or monitoring immune-mediated diseases, as was recently reviewed [Damoiseaux Citation2020; Dik and Heron Citation2020]. Serum sIL-2R concentrations are a promising biomarker in a number of rare diseases that involve T cell activation, including sarcoidosis [Thi Hong Nguyen et al. Citation2017], common variable immune deficiency-associated granulomatous disease [van Stigt et al. Citation2021], and Kawasaki disease [Teraura et al. Citation2017]. While most of the literature focuses on high sIL-2R levels, it should be noted that low sIl-2R levels predict response to infliximab in rheumatoid arthritis [Kuuliala et al. Citation2006]. Serum sIL-2R are particularly increased in patients with hemophagocytic lymphohistiocytosis (HLH), where they constitute a diagnostic criterion [Lin et al. Citation2017]. Serum sIL-2R was initially used in pediatric HLH trials, but has recently been shown to be a sensitive disease marker in adult HLH as well [Hayden et al. Citation2017]. A recent study has found that a serum ferritin >1000 ng/mL and sIL-2R > 3900 U/mL (the so-called ‘optimized HLH inflammatory index’) are an excellent test for malignancy-associated HLH [Zoref-Lorenz et al. Citation2022]. Serum sIL-2R concentrations have prognostic value not only for HLH but also in lymphomas [Nozaki et al. Citation2020; Morita-Fujita et al. Citation2019]. More recently, sIL-2R concentrations are being used to monitor the immune storm in patients with Coronavirus (COVID-19) disease [Quartuccio et al. Citation2021], although the major cytokine pathway alteration in COVID-19 is not IL-2/IL-2R but the IL-6/IL-6R axis [Alende-Castro et al. Citation2021; Chen et al. Citation2021].
Studies of clinical analytes in the general population could help the interpretation of abnormal laboratory results in the disease, the definition of reference values, and the investigation of the influence of common factors. To the best of our knowledge, similar studies in adults are lacking, since studies investigating the distribution of sIL-2R have included selected samples from blood donors [Rothkrantz-Kos et al. Citation2004] or groups defined by exposures [Tollerud et al. Citation1992], disease [Damoiseaux Citation2020; Dik and Heron Citation2020 Lassalle et al. Citation1992; Lin et al. Citation2017; Matsumoto et al. Citation1991; Quartuccio et al. Citation2021], or age [Durda et al. Citation2015; Manoussakis et al. Citation1990; Miller et al. Citation1996]. The present study was aimed to investigate serum sIL-2R concentrations in a random sample of the adult population, and their potential relationship with demographic factors (sex and age), lifestyle factors (smoking, alcohol consumption, and regular physical exercise), common metabolic abnormalities (obesity and its related metabolic syndrome), and frequent immune disorders, such as atopy.
Methods
Study population and design
This cross-sectional study was conducted in the municipality of A-Estrada (Spain), as reported elsewhere [Alende-Castro et al. Citation2019]. The A-Estrada Glycation and Inflammation Study [AEGIS] outline is available at www.clinicaltrials.gov (code NCT01796184). The study included a random sample of the adult population from the municipality. The municipality had an adult population (>18 years) of 18,474 when the study started in 2012. An age-stratified random sample of the population was drawn from Spain’s National Health System Registry, which covers more than 95% of the population and contains the name, date of birth, and address of every person entitled to primary care. From an initial sample of 3500 individuals, main reasons for exclusion were no response (n = 211), death (n = 84), lack of heath care coverage (n = 19), change of address (n = 134), termination of the recruitment period (n = 428) or incapacitating disease (dementia, mental retardation, cerebrovascular disease, terminal cancer or inability to communicate, n = 394). The remaining 2230 eligible individuals were offered to participate. A total of 714 declined and 1516 (67.9%) signed the informed consent for participation. There were no significant differences in terms of age or residence (rural vs urban) between participants and non-participants. Seventeen individuals were excluded due to specific inflammatory diseases. The remainder (n = 1499; 44.6% men; median age, 52 years; range, 18–91 years) underwent a systematic physical examination, completed an interviewer-administered questionnaire, and had a blood sample drawn for analyte measurements. The study was approved by the regional (Galician) Ethics Committee (code 2010-315).
Lifestyle factors
Lifestyle factors were assessed by questionnaire [Alende-Castro et al. Citation2019]. Alcohol consumption was evaluated as the number of standard units regularly consumed per week, totalling the number of glasses of wine (1 unit, 10 g), bottles of beer (1 unit, 10 g), and spirits (2 units, 20 g) consumed. Participants consuming of 1–13 units/week, 14–27 units/week, and ≥28 units/week were considered light, moderate, and heavy drinkers, respectively. The remainder, comprising abstainers and occasional drinkers, were grouped together.
We considered consumers of at least 1 cigarette per day as smokers. Individuals who had quit smoking during the preceding year were still considered smokers; those who had quit over 1 year prior to the study were considered ex-smokers.
Physical activity was assessed with the International Physical Activity Questionnaire (short version, https://sites.google.com/site/theipaq/home). The questionnaire allows for the stratification of habitual physical activity as low, moderate, or high, as previously described [Alende-Castro et al. Citation2019].
Metabolic abnormalities
We classified the participants according to body mass index as having normal weight (<25 kg/m2), overweight (25–30 kg/m2), or obesity (>30 kg/m2). We considered the participants as having metabolic syndrome if they had at least 3 of the following Adult Treatment Panel III criteria, as previously described [Adult Treatment Panel III Citation2001]: abdominal obesity (waist circumference >102 cm for men and >88 cm for women); hypertriglyceridemia (fasting serum triglycerides ≥150 mg/dL); low high-density lipoprotein (HDL)-cholesterol levels (fasting HDL-cholesterol levels <40 mg/dL for men and <50 mg/dL for women); increased blood pressure (arterial blood pressure ≥130/≥85 mmHg or current anti-hypertensive medication use); and hyperglycemia (fasting serum glucose levels ≥110 mg/dL or current anti-diabetic therapy).
Pre-existing disease (cardiovascular disease and malignant neoplasm)
Data on pre-existing diseases, specifically physician-diagnosis of ischemic heart disease and cancer were obtained from the electronic clinical records of all participants.
Atopy
Participants underwent a panel of skin prick tests with relevant aeroallergens in the area including house dust mites (Dermatophagoides pteronyssinus, Lepidoglyphus destructor), pollens (Phleum pratense, Plantago lanceolata, Betula alba, Parietaria judaica), vegetable panallergens (profilin, peach lipid transfer protein), molds (Alternaria alternate, Aspergillus spp), and animal dander (dog, cat). The control tests included 10 mg/mL histamine and saline solution. Wheals with a mean diameter >3 mm after 15 min were deemed positive. The presence of at least 1 positive skin prick test was considered indicative of allergic sensitization or atopy, as previously described [Alende-Castro et al. Citation2021]. Data were not available for 2 individuals.
Laboratory measurements
All laboratory determinations were performed on fresh samples in the clinical laboratory of the reference institution. Manufacturer instructions were strictly followed. We measured sIL-2R concentrations by means of a commercial chemiluminescent immunoassay (Immulite System, Siemens). The analytical sensitivity for this method is 5 U/mL, and the normal concentration reference values range from 158 to 623 U/mL, according to the manufacturer. The same chemiluminescent immunoassay was used for tumor necrosis factor (TNF)-alpha and IL-6 measurements. The sensitivity for IL-6 is 2.0 pg/mL, and its normal reference values range from undetectable to 5.9 pg/mL. The distribution of IL-6 in this population has been reported elsewhere [Alende-Castro et al. Citation2021]. The sensitivity for TNF-alpha is 1.7 pg/mL, and its normal reference values range from undetectable to 8.1 pg/mL. We measured wide-range C-reactive protein (CRP) concentrations with commercial latex-enhanced immunoturbidimetry in an Advia 2400 Clinical Chemistry System (Siemens) and their results have been reported elsewhere [Alende-Castro et al. Citation2021b]. We measured the erythrocyte sedimentation rate (ESR), employing an automated TEST-1 device (Alifax). The reference ESR values are 0–20 mm/h for men and 0–30 mm/h for women. The ESR was available for 1472 participants and their results in this population have been reported elsewhere [Alende-Castro et al. Citation2019].
Statistical analyses
We used the Mann–Whitney test to compare numerical variables and Spearman’s rank test to assess correlation. All serum samples had detectable sIL-2R concentrations. We attributed an arbitrary value of zero to IL-6 and TNF-alpha concentrations that were below the detection limit. The generalized Akaike Information Criterion was used to select the most appropriate distribution. For multivariable analyses, we considered a Box – Cox t (BCT) distribution in order to capture the kurtosis and heteroscedasticity shown by the sIL-2R data. BCT regression models were performed to investigate sIL-2R concentrations in relation to covariates. The covariates (age, gender, lifestyles [physical activity, alcohol and tobacco consumption], metabolic syndrome components [abdominal obesity, low HDL-cholesterol, hypertriglyceridemia, high blood pressure, hyperglycemia], ischemic heart disease, malignant neoplasm, and atopy) were forced to enter the equation in the regression model. Statistical analyses were performed using R statistical software (version 4.0.4; R Foundation, http://www.r-project.org) with the ‘gamlss’ package.
Results
Serum sIL-2R concentrations showed wide variation in the population. Figure represents the histogram of serum sIL-2R distribution, which was skewed to the right. Only one (0.06%) participant showed values <158 U/mL; however, 230 (15.3%) showed values >623 U/mL, the standard lower and upper reference values, respectively, according to the chemiluminescent immunoassay manufacturer. Serum sIL-2R concentrations were correlated with those of TNF-alpha (Rho, 0.322; P < 0.001), CRP (Rho, 0.219; P < 0.001), and IL-6 (Rho, 0.196; P < 0.001), as well as with the ESR (Rho, 0.177; P < 0.001).
Figure 1. Histogram of sIL-2R concentrations in the general adult population. Cases with sIL-2R concentrations higher than 2000 U/mL (n = 4) are not represented but are included in calculations. IQ, interquartile.
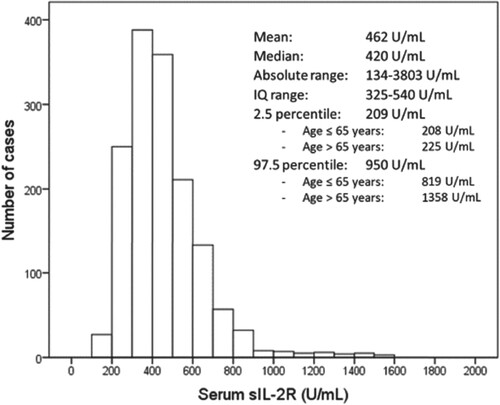
Serum sIL-2R concentrations were higher in men, both in the univariable analysis and after adjusting for potential confounders (Table ). Aging was associated with higher sIL-2R concentrations; however, the effect was only evident among participants >65 years (Table ; Figure ).
Table 1. Serum concentrations of sIL2R in relation to demographic data, lifestyle variables, metabolic abnormalities, and atopy in the studied population. Univariable and multivariable analyses are presented.
Smoking was strongly associated with higher sIL-2R concentrations, independent of covariates (Table ). Heavy drinking was associated with higher sIL-2R concentrations in the univariable analysis, but the association was largely attenuated after adjusting for covariates. Light alcohol consumption was associated with lower serum sIL-2R concentrations in the multivariable analysis. Regular physical exercise was negatively associated with lower sIL-2R concentrations in the univariable analysis; however, this association was attenuated after adjusting for covariates (Table ).
Abdominal obesity was independently associated with higher sIL-2R concentrations (Table ). Accordingly, individuals with metabolic syndrome (n = 308) had significantly higher serum sIL-2R concentrations (median 468 U/mL, interquartile range [IQR] 365–601 U/mL) than those without it (n = 1191; median 408 U/mL, IQR 317–524 U/mL; P < 0.001). Obese individuals (n = 511) showed higher sIL-2R concentrations (median 447 U/mL, IQR 352–562 U/mL) than those with normal-weight (n = 421; median 403 U/mL, IQR 315–525 U/mL; P < 0.001). Serum sIL-2R concentrations in patients with overweight (n = 567) were similar to that of those with normal weight (median 413 U/mL, IQR 314–523 U/mL, P = 0.808). BMI was not included in the multivariable regression model because of collinearity with abdominal obesity. If BMI categories were introduced in the model instead of abdominal obesity, they were not significantly associated with sIL-2R concentrations (data not shown). Conversely, hypertriglyceridemia was negatively associated with sIL-2R measurements (Table ). A history of ischemic heart disease was independently associated with higher serum sIL-2R concentrations (Table ). Conversely, no significant association was observed between a history of malignant neoplasm and serum sIL-2R concentrations (Table ).
Atopy (skin prick test positivity to aeroallergens) was associated with higher serum sIL-2R concentrations (Table ), although the association was only evident after adjusting for covariates, particularly age, because atopy is more common in the young and serum sIL-2R is higher in the elderly. A history of bronchial asthma (as revealed by a positive answer to the question Have you ever been diagnosed as having asthma?) was not significantly associated with sIL-2R concentrations when added to the multivariable model (data not shown).
Discussion
The present study shows that aging, male sex, smoking, metabolic syndrome components (abdominal obesity), a history of ischemic heart disease, and atopy are associated with higher sIL-2R concentrations, whereas light alcohol consumption is associated with lower concentrations in adults. Altogether, these covariates explain little (nearly 10%) of the variation of serum sIL-2R concentrations, but could be taken into consideration when interpreting sIL-2R levels. The observed 2.5–97.5 percentile range was wider that that reported by the manufacturer, and was closer to that observed in a sample of 282 healthy blood donors (241–846 U/mL) [Rothkrantz-Kos et al. Citation2004]. These suggest that both the lower and the upper reference values should be higher than those indicated by the manufacturer, which were established using a sample of 100 apparently healthy blood donors of unspecified age and sex distribution. Of note, there are 2 types of commercial immunoassays to measure sIL-2R concentrations. Chemiluminescent immunoassay results are expressed as U/mL, whereas enzyme-linked immunosorbent assay (ELISA) results are expressed as pg/mL. There is a good correlation between the results of both assays [Dik & Heron, Citation2020], but they offer distinct absolute results, with values of the ELISA approximately 7–8 times higher than those of chemiluminescent immunoassay [Damoiseaux Citation2020; Dik and Heron Citation2020]. Serum sIL-2R concentrations were correlated with those of TNF-alpha, CRP, and IL-6, as well as with the ESR, which agrees with the overall view of sIL-2R as an inflammation marker [Damoiseaux Citation2020; Dik and Heron Citation2020].
Sex and age influenced serum sIL-2R concentrations. A previous study in a smaller sample of blood donors did not observe sex differences [Rothkrantz-Kos et al. Citation2004]. The higher sIL-2R concentrations in men is consistent with results from a sample of individuals >65 years [Durda et al. Citation2015], and with an effect of estrogens suppressing IL-2 and the IL-2 receptor [McMurray et al. Citation2001]. Age relationship was also observed in studies focused on individuals >65 years [Durda et al. Citation2015] and in studies comparing healthy elderly individuals with non-elderly controls [Manoussakis et al. Citation1990]. The effect of age was not observed in blood donors, probably due to their narrower age range [Rothkrantz-Kos et al. Citation2004]. The 97.5 percentile among individuals >65 years in our series was more than twice the recommended reference range, further indicating that the upper reference value should be increased in this age group.
Lifestyle factors (alcohol consumption and smoking) influenced serum sIL-2R concentrations. In agreement with previous reports [Lassalle et al. Citation1992; Tollerud et al. Citation1992], smoking was associated with higher sIL-2R concentrations. This result agrees with an overall proinflammatory effect of tobacco smoking [Tollerud et al. Citation1992]. Light alcohol consumption was independently associated with lower serum sIL-2R concentrations. To the best of our knowledge, this association was not previously reported and is consistent with an anti-inflammatory effect of low-dose alcohol consumption [Alende-Castro et al. Citation2019].
Common metabolic abnormalities (particularly metabolic syndrome components such as abdominal obesity) were associated with higher serum sIL-2R concentrations. Similar associations of IL-2R concentrations with metabolic abnormalities were observed in a selected sample of individuals >65 years [Durda et al. Citation2015] and are consistent with a proinflammatory state in patients with metabolic syndrome [Alende-Castro et al. Citation2019; Citation2021; Citation2021b]. Conversely, hypertriglyceridemia was negatively associated with sIL-2R measurements. This consistent with the finding of inhibition of IL-2R expression by hyperlipemic serum in vitro [Terao and Fukase Citation1991]. In line with the association of sIL-2R with risk factors such as metabolic disorders and smoking, patients with a history of ischemic heart disease showed higher concentrations of sIL-2R than patients without it. Moreover, the association of ischemic heart disease with sIL-2R levels was independent of these risk factors. These results are consistent with a proinflammatory state in patients with ischemic heart disease [Liuzzo and Rizzello Citation2001; Natali et al. Citation2003].
Atopy (skin prick test positivity to aeroallergens) was associated with higher serum sIL-2R concentrations after adjusting for confounders. A similar association between atopy (the most frequent immune disorder in industrialized countries), and sIL-2R has been observed in children [Matsumoto et al. Citation1991; Miller et al. Citation1996] but not in smaller samples of adults [Lassalle et al. Citation1992; Tollerud et al. Citation1992]. The association of sIL-2R with bronchial hyperresponsiveness and asthma is also controversial [Lassalle et al. Citation1992; Tollerud et al. Citation1992]. The potential role of IL-2 and sIL-2R in allergic and non-allergic asthma deserves further study.
Limitations of the study include its cross-sectional design and the universal white ethnicity of the participants, precluding an analysis of that covariate, which was associated with sIL-2R in previous studies [Durda et al. Citation2015]. To the best of our knowledge, however, this is the first study focused on serum sIL-2R concentrations in a random sample of adults that comprehensively investigates the effect of demographic, lifestyle, immunologic, and metabolic covariates. In summary, the study shows that aging, male sex, smoking, metabolic syndrome components, ischemic heart disease, and atopy are associated with higher sIL-2R concentrations, whereas light alcohol consumption is associated with lower concentrations in adults. These factors should be considered when interpreting serum sIL-2R concentrations, which are becoming widely used in clinical practice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Arturo Gonzalez-Quintela and Francisco Gude were responsible for conception and design. Francisco Gude and Manuela Alonso-Sampedro performed the analysis and interpretation of the data. Carmen Fernández-Merino, Bernardo Sopeña, and Vanessa Alende-Castro were involved in the patient management and data collection. Vanessa Alende-Castro, Carmen Vidal and Arturo Gonzalez-Quintela drafted the paper. All authors approved the version to be published and agree to be accountable for all aspects of the work.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Additional information
Funding
References
- Adult Treatment Panel III. 2001. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA: The Journal of the American Medical Association. 285:2486–2497. doi:10.1001/jama.285.19.2486
- Alende-Castro V, et al. 2019. Factors influencing erythrocyte sedimentation rate in adults. Medicine (Baltimore). 98:e16816. doi:10.1097/MD.0000000000016816
- Alende-Castro V, et al. 2021b. C-Reactive protein versus erythrocyte sedimentation rate: implications among patients with no known inflammatory conditions. J Am Board Fam Med. 34:974–983. doi:10.3122/jabfm.2021.05.210072
- Alende-Castro V, et al. 2021. Serum concentrations of interleukin 6 in the general adult population: possible implications for anti–IL-6 therapy in SARS-Cov-2 infection and IL-6–related diseases. J Investig Allergol Clin Immunol. 31:75–78. doi:10.18176/jiaci.0601
- Chen LYC, et al. 2021. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Reports Medicine. 2:100269. doi:10.1016/j.xcrm.2021.100269
- Damoiseaux J. 2020. The IL-2 - IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clin Immunol. 218:108515. doi:10.1016/j.clim.2020.108515
- Dik WA, Heron M. 2020. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth J Med. 78:220–231.
- Durda P, et al. 2015. Plasma levels of soluble interleukin-2 receptor α. Arterioscler Thromb Vasc Biol. 35:2246–2253. doi:10.1161/ATVBAHA.115.305289
- Hayden A, et al. 2017. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 1:2529–2534. doi:10.1182/bloodadvances.2017012310
- Kuuliala A, et al. 2006. Low circulating soluble interleukin 2 receptor level predicts rapid response in patients with refractory rheumatoid arthritis treated with infliximab. Ann Rheum Dis. 65:26–29. doi:10.1136/ard.2004.034728
- Lassalle P, et al. 1992. Levels of soluble IL-2 receptor in plasma from asthmatics. correlations with blood eosinophilia, lung function, and corticosteroid therapy. Clin Exp Immunol. 87:266–271. doi:10.1111/j.1365-2249.1992.tb02986.x
- Lin M, et al. 2017. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann Hematol. 96:1241–1251. doi:10.1007/s00277-017-2993-y
- Liuzzo G, Rizzello V. 2001. C-reactive protein and primary prevention of ischemic heart disease. Clin Chim Acta. 311:45–48. doi:10.1016/S0009-8981(01)00557-5
- Manoussakis MN, et al. 1990. Soluble interleukin-2 receptors and auto antibodies in the serum of healthy elderly individuals. Autoimmunity. 7:129–137. doi:10.3109/08916939008993385
- Matsumoto T, et al. 1991. Serum levels of soluble IL-2 receptor, IL-4 and IgE-binding factors in childhood allergic diseases. Clin Exp Immunol. 85:288–292. doi:10.1111/j.1365-2249.1991.tb05720.x
- McMurray RW, et al. 2001. 17-β-estradiol suppresses IL-2 and IL-2 receptor. Cytokine. 14:324–333. doi:10.1006/cyto.2001.0900
- Miller AL, et al. 1996. Serum levels of the soluble low affinity receptor for IgE and soluble interleukin-2 receptor in childhood, and their relation to age, gender, atopy and allergic disease. Pediatr Allergy Immunol. 7:68–74. doi:10.1111/j.1399-3038.1996.tb00109.x
- Morita-Fujita M, et al. 2019. High level of serum soluble interleukin-2 receptor is associated with poor survival in patients with first relapsed or refractory peripheral T-cell lymphoma, not otherwise specified: A retrospective study. Clin Lymphoma Myeloma Leuk. 19:e337–e342. doi:10.1016/j.clml.2019.03.031
- Natali A, et al. 2003. Erythrocyte sedimentation rate, coronary atherosclerosis, and cardiac mortality. Eur Heart J. 24:639–648. doi:10.1016/S0195-668X(02)00741-8
- Nozaki K, et al. 2020. Pretreatment serum soluble interleukin-2 receptor level predicts survival in patients with newly diagnosed follicular lymphoma. Leuk Lymphoma. 61:2113–2121. doi:10.1080/10428194.2020.1759054
- Quartuccio L, et al. 2021. Interleukin 6, soluble interleukin 2 receptor alpha (CD25), monocyte colony-stimulating factor, and hepatocyte growth factor linked with systemic hyperinflammation, innate immunity hyperactivation, and organ damage in COVID-19 pneumonia. Cytokine. 140:155438. doi:10.1016/j.cyto.2021.155438
- Rothkrantz-Kos S, et al. 2004. Reference values of soluble interleukin-2 receptor on the IMMULITE. Clin Chem Lab Med. 42:976–977.
- Rubin LA, Nelson DL. 1990. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med. 113:619–627. doi:10.7326/0003-4819-113-8-619
- Terao K, Fukase Y. 1991. Effects of hyperlipaemic serum on interleukin-2 (IL-2) production, IL-2 receptor expression, and T cell proliferation induced by IL-2 in cynomolgus monkeys. Jpn J Med Sci Biol. 44:17–28. doi:10.7883/yoken1952.44.17
- Teraura H, et al. 2017. The serum concentration of soluble interleukin-2 receptor in patients with kawasaki disease. Ann Clin Biochem. 54:209–213. doi:10.1177/0004563216677583
- Thi Hong Nguyen C, et al. 2017. Serum soluble interleukin-2 receptor level is more sensitive than angiotensin-converting enzyme or lysozyme for diagnosis of sarcoidosis and may be a marker of multiple organ involvement. J Dermatol. 44:789–797. doi:10.1111/1346-8138.13792
- Tollerud DJ, et al. 1992. Elevated soluble lnterleukin-2 receptors in young healthy cigarette smokers: lack of association with atopy or airways hyperresponsiveness. Int Arch Allergy Appl Immunol. 97:25–30. doi:10.1159/000236091
- van Stigt AC, et al. 2021. Soluble interleukin-2 receptor is a promising serum biomarker for granulomatous disease in common variable immune deficiency. J Clin Immunol. 41:694–697. doi:10.1007/s10875-020-00947-8
- Zoref-Lorenz A, et al. 2022. An improved index for diagnosis and mortality prediction in malignancy-associated hemophagocytic lymphohistiocytosis. Blood. 139:1098–1110. doi:10.1182/blood.2021012764
