Abstract
Previous studies of cortisol concentrations in hair have concluded that it is not possible to measure more than 6 months retrospectively. This study shows for the first time that it is possible to analyze hair cortisol concentrations month-by-month for a retrospective period of 24 months. In addition, we have determined whether cortisol concentration decreases with time. The study population was 48 women in the age range 20–51 years, all with hair of length of 24 cm or longer. The participants completed a questionnaire that examined exposure to life stressors and potential confounders. Competitive radioimmunoassay was used to extract and analyze cortisol levels in hair. The overall intraclass correlation for the participants was substantial (ICC = 0.38, 95% CI 0.29, 0.49), indicating a strong within-person correlation during the growth period. The median levels of cortisol were reasonably stable. Wash-out effects were small, even for those who reported that they washed their hair every day.
We conclude that it is possible to detect hair cortisol concentrations every month at least two years back in time. Changes in hair cortisol concentration are more likely to be related to life stressors than changes due to time since growth.
Introduction
Stress is one of the main factors with a negative effect on health in our society today, and disorders related to stress have become an increasing global public health problem (World Health Organization, Citation2001). This results in an increased incidence of several illnesses, such as diabetes type 2, chronic pain, mental problems, obesity, and cardiovascular diseases (Everson-Rose and Lewis Citation2005; VanUum et al. Citation2008; Pereg et al. Citation2011; Dettenborn et al. Citation2012; Manenschijn et al. Citation2013; Faresjö et al. Citation2020). The human body can handle stress, but when the stress-response system mobilizes resources to deal with extreme, repeated, or constant threats without sufficient periods of recovery, this system may cause harm to the body (Selye Citation1985).
The neuroendocrine hypothalamic–pituitary–adrenal (HPA) axis is a well-studied pathway that, together with the sympathetic-adrenomedullary (SAM) system, is a key factor in physiological stress (Van de Kar and Blair Citation1999). Cortisol is secreted when the HPA axis is activated and plays a crucial role in the body’s response to stressors (Selye Citation1985; Koolhaas Citation2011). Stress expressed as cortisol production has traditionally been measured by determining the concentration of cortisol in saliva, blood, or urine (Stalder and Kirsbaum Citation2012). A general drawback of these methods is that they do not reflect long-term stress exposure, only momentary stress (Stalder and Kirsbaum Citation2012). Biomarkers used in medicine give in general only a time-point indication of the current health situation. However, there are exceptions of a biomarker, such as HbA1C, that yield an indication of health history of diabetes (Glei, Goldman, Rodriguez and Weinstein, Citation2015). On the other hand, cortisol concentrations in hair (HCC), can be used to analyze retrospectively the mean value of the activity of the HPA axis (Raul et al. Citation2004; Webb Citation2010; Karlén et al. Citation2013). Hair grows approximately 1 cm per month, and thus a hair sample of length 3 centimeters can be used to determine the mean cortisol concentration during the preceding 3 months. HCC is stable and reliable and may give important information about the role of chronic stress in a variety of diseases (Abell et al. Citation2016). It may also have a potential for clinical application (Sauvè et al. Citation2007; Gow et al. Citation2010; Wester and van Rossum Citation2015). Previous research has shown that cortisol levels can be measured approximately up to 3 months, and possibly 6 months, back in time, that is general knowledge so far (van Uum et al. Citation2008; Webb Citation2010). However, in another study hair segments of 9 cm in length were analyzed from pregnant women cut near the scalp at the time of delivery. This segment allowed them to measure HCC in each of the three trimesters of the pregnancy. The HCC of the mother increased as the due date approached, and peaked during the third trimester (Karlén et al. Citation2013). Even archaeological hair samples from juveniles from in the Nasca Region, Peru, were used to assess isotope compositions and cortisol levels. Stable isotopic data were used in that study to investigate dietary change and nitrogen metabolism, and cortisol levels are used to infer exposure to stress, and the researchers concluded that they were able to assess morbidity, diet, and stress using isotopic and cortisol data from mummified hair (Webb et al. Citation2015). Against this background, if the hair has been stored for a very long time and not a total decline in HCC levels during 9 months occurred, we designed a study to measure hair cortisol concentrations of individuals with long hair equivalent to two years of hair growth. The aim was to determine whether it is possible to determine the monthly cortisol level in hair for longer periods back in time. Further, we designed the study to analyze whether a decline in cortisol concentration occurs with time due to, for example, intensive hair washing.
Materials and methods
Participants
The participants in this study were volunteers from students and university employees at Linköping University, with a few persons from the general population. All participants were in the age range of 20–51 years. One inclusion criterion was to have hair 24 cm or longer, which was met by N = 48 female volunteers. All participants were asked to complete a questionnaire and provide a hair sample.
Questionnaire
The questionnaire included demographic information such as year of birth, gender, original hair colour, presence of chronic diseases, whether the participant was undergoing medical treatment involving inhalation, spray, ointments, and tablets containing steroids, and the frequency of washing hair with shampoo. The frequency asked for were: every day, every other day, and twice a week. Information about the occurrence of life stressors (such as deaths in the family, divorce, and serious illness) was also requested in the questionnaire. The period during which information about stressors was requested was the most recent 2 years, and the dates of such occurrences were specified in three-month periods. The questionnaire also asked: How stressful is your everyday life now? Where a Likert Scale from 1 = not stressful at all to 6 = very stressful was used. Another question was: How stressful do you experience your everyday life now if you compare it to how it was a year ago? Answer options were: less stress than a year ago, same as earlier, must more stress than a year ago.
Analyzing cortisol levels
Hair of a minimum length of 24 cm, was cut with hair scissors from the vertex posterior of the scalp as close to the skin as possible by trained staff, as recommended by the Society of Hair Testing (SoHT) (Wenning, Citation2000). Hair in this area has a regular rate of growth (Villain et al. Citation2004; Thomson et al. Citation2010; Cooper et al. Citation2012). The hair sample was taped to a piece of A4 paper and stored in a plastic folder at room temperature. A competitive radioimmunoassay (RIA) was used to extract and analyze cortisol levels, as described by Yang et al. (Yang et al. Citation1998; Pragst and Balikova, Citation2006). Briefly, the hair sample was cut into 24 pieces each of length one centimeter, measured with a ruler starting from the root. From each one-centimeter long bundle, a sample of weight 5–7 mg was cut into small pieces, frozen for 2 min in liquid nitrogen, and minced together with a steel ball in a Retch Tissue Lyser II for 2 min. Methanol (1 ml) was added to each tube and the samples were extracted overnight on a moving board. Then 0.8 ml of the methanol supernatant was pipetted off and lyophilized using a Savant Speed Vac Plus SC210A. The samples were dissolved in radioimmunoassay buffer and analyzed as described by Mörelius et al. (Mörelius et al. Citation2004). The primary antibody used was a polyclonal antibody suitable for RIA (rabbit cortisol 3 polyclonal antibody). This antibody has an affinity for cortisol and the epitope C-peptide (MyBioSource, San Diego, USA). The secondary antibody Sac-Cel was anti-rabbit (Sac-Cel AA-Sac1, ImmunoDiagnostic Systems Ltd, Boldon, England).
Previous work has shown that hair samples of 5 mg or more are needed to achieve an inter-assay coefficient of variation below 8% for hair extraction and the measurement of cortisol (Mörelius, Nelson and Theodorsson Citation2004). The intra-assay coefficient of variation for the radioimmunoassay was 7% at 10 nmol/L.
Statistical analysis
All data were stored in a common database and statistically analyzed using the SPSS version 24.0 program (SPSS Inc., Chicago, IL, USA). A special syntaxis was written to manage the month-by-month analyses with questions from the questionnaire in different models. Changes in hair cortisol were modeled to test a linear trend with time, a non-linear trend with time, and the correlation between the level and stressful periods in the individual’s life. The models were multilevel models to account for the repeated measurements over the 24 months. The distribution of hair cortisol values was substantially non-normal, and thus conclusions from statistical analysis are based on a non-parametric bootstrap using 1000 bootstrap samples. The overall intraclass correlation was estimated from the null model, while the probability of random slopes was evaluated by calculating the proportion of variance that could be attributed to random effects corresponding to each term in the model. The values were very low, which gives little or no support for random slopes. Four separate models were fitted: Model 1 modeled a linear change in hair cortisol with time, and Model 2 modeled a non-linear trend (chosen to be quadratic, after inspection of Figure ). Periods of stress were defined as the three months around a serious life stressor reported by the participant. If a participant reported several three-month periods in a year, the complete year was classified as a period of life stress. Any bias introduced by this approach will be towards the null hypothesis for stress since non-stressful periods would be misclassified. Further models (Table and ) combined these factors and evaluated the moderation of any linear time trend by hair colour and the frequency of hair washing. The presence of moderation would indicate that the time trend is altered (made more positive or negative) by different values of the moderating factor. A descriptive analysis (Figure ) shows the distribution of original cortisol levels month-by-month for 10 randomly selected individuals.
Figure 1. The repeated measurements of hair cortisol over the 24-months sampling period. Mean and SE and outliers are shown.
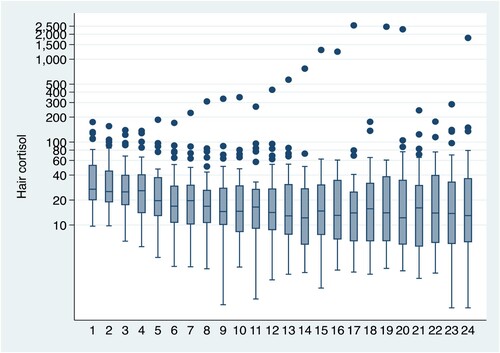
Figure 2. Frequency of hair washing, those who reported they washed their hair every day had a significant negative slope whereas less frequent washing was associated with less negative slopes.
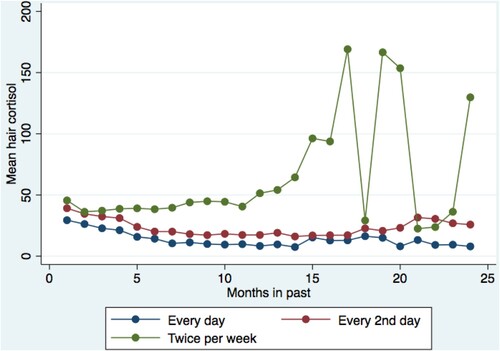
Figure 3. Illustrate original cortisol levels measurement 24 months retrospective for 10 random individuals of the total 48 participants.
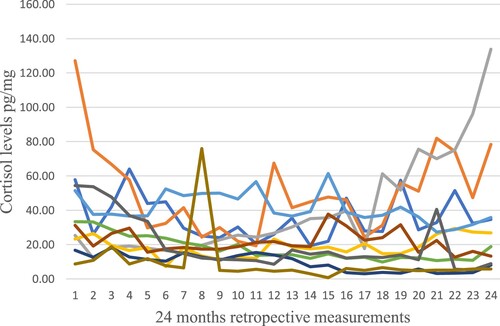
Table 1. Characteristics of the study population.
Table 2. Models of time trends and their moderation.
Ethical approval
This study was approved by the Regional Ethic Research Committee at Linköping University, Ref. No. 2017/123-31. All methods were performed following the relevant guidelines and regulations. Written informed consent was supplied by All participants gave their informed consent to participate.
Results
Characteristics of the included participants
Forty-eight females participated in the study. The mean age of the participants was 31.0 years (SE = 1.45). Over 90% of the participants had either blonde or brown hair. The average frequency of hair washing was once every other day (Table ). Most participants (81.3%) reported not having any chronic disease, while the reported chronic diseases were migraine, hay fever, psoriasis, asthma, polycystic ovary syndrome, endometriosis, hypothyroidism, allergy, epilepsy, and coeliac disease. Only six participants used steroid-based medicine.
Analysis of cortisol concentrations over time
The intraclass correlation (ICC) of cortisol concentration was substantial (ICC = 0.38, 95% confidence interval 0.29-0.49), which shows that the within-person correlation during the growth period was considerable (Figure ). Hair cortisol concentration did not decrease with time, linearly or non-linearly (Table ). However, average hair cortisol values were affected during periods of life stress.
The effect of life stressors remained statistically significant after controlling for the time trend (Table ). Those who reported they washed their hair every day had a significant negative slope (b = −0.532, SE = 0.154, p = 0.001), whereas those who used less frequent washing had less-negative slopes (0.008) (Figure and Table ). Hair colour did not moderate the linear trend in hair cortisol with time (Table ).
Figure shows the distribution of cortisol levels months-by-month for two years for 10 randomly selected individuals from the participants in this study. HCC varies throughout these two years. The retrospective HCC patterns are similar for most participants.
Discussion
This is the first study, to our knowledge, that has retrospectively investigated hair cortisol concentrations month-by-month two years back in time. We have shown that it is quite possible to measure hair cortisol concentrations as far back in time as two years. The level fluctuates, but the median level of cortisol in an individual remains reasonably stable. Overall, the data suggest that changes in hair cortisol are more likely to be related to life stressors than the passage of time since growth, which also provides some degree of validation of the analysis method. Minor wash-out effects of cortisol in hair occur, but peaks of HCC can even so be identified on occasions up to 24 months previously. We saw a decline in HCC in some of the participants that reported very frequent hair washing, but it is hard to make a consensus about the possible wash-out effect because fluctuations were seen.
Research in the field of HCC has intensified in recent years, but few studies have taken a long-term approach. It is generally believed that the measurement of cortisol levels becomes less reliable with time. Measurements for approximately 3 months back in time, or possibly 6 months, are generally acknowledged (Van Uum et al. Citation2008; Webb, Citation2010). It is previously thought that a decline of HCC occurs from the first to the second proximal 3 cm segment of hair (Stalder et al Citation2017), but HCC did not decline in this way in the present study. In a study of pregnancy and child health, Karlén et al. analyzed hair segments of length 9 cm from pregnant women cut near the scalp at the time of delivery. This segment allowed them to measure HCC in the three trimesters of the pregnancy (Karlén et al. Citation2013). The HCC of the mother increased as the due date approached, and peaked during the third trimester. Wester et al. showed that HCC increased in two patients, 3 and 6 months respectively, before Cushing’s syndrome was diagnosed (Wester et al. Citation2017).
HCC tends to increase with age (Wester and van Rossum Citation2015), but this effect was small in the present study, where the mean age of the participants was 31 years. Very few participants reported long-term use of steroids, either as an ointment, by inhalation, or in tablet form, which makes it difficult to draw any conclusions about possible interference from steroids. A question that arises is whether there is a seasonal variation in HCC that explains the peaks during the preceding 2 years. It has been reported that salivary cortisol levels are highest during February-April and lowest during July and August (Persson et al. Citation2008). Unpublished data from our study population showed the same seasonal pattern concerning HCC. However, more studies of the potential seasonal influence on HCC are needed.
Studies of HCC exclude those with insufficient hair length and those who are bald. This limitation affects almost exclusively men and may influence the results if baldness or having short hair is related to chronic stress. There is no evidence that this is the case. HCC was analyzed in this study with an established competitive radioimmunoassay method (RIA). The primary reason for using this method is its very low detection limit, which enables levels of cortisol in samples of hair of weight 5–7 mg to be determined. Such samples can be taken with no cosmetic inconvenience for the participants, and one hair sample can be cut into monthly sections for analysis. Furthermore, the gamma radiation used for detection in the RIA is not influenced by the pigment in the hair that is extracted in the methanol together with the cortisol. This phenomenon may be a confounder when colorimetric methods are used to measure cortisol.
All diseases have specific natural courses that may extend over weeks, months, or years. We have shown here that cortisol levels can be measured for a period extending two years into the past, which may be useful if stress is involved in the etiology of a disease. During the two years, trigger points or breaking points may have occurred, during which increased cortisol levels interact with the disease process. Further research is needed to investigate this.
In conclusion, this study shows that cortisol levels in hair can be retrospectively detected for up to two years. Average hair cortisol concentrations are affected by life stressors more than by the passage of time. The effect of frequent hair washing on cortisol concentration is very small. The present study adds to knowledge and fills a gap in the literature concerning the long-term measurement of HCC. It will be easier to conduct studies with this approach in the future. Further research may investigate whether HCC peaks can be retrospectively related to specific serious and stressful life events or the onset of the disease.
Authors’ contributions
All authors have contributed, agree to be accountable for all aspects of the work, and approved the final version of the article to be published. ÅF, ALT, ET, and OJO were involved in the conception and design of this study. ÅF, ALT, TF, MJ, ET, and OJO were involved in the analysis and interpretation of the data. ÅF, ALT, TF, and MJ were drafting the paper, and ÅF, ALT, TF, MJ, ET, and OJO were revising it critically for intellectual content.
Acknowledgment
We are very grateful to the participating women in this study. We also thank the staff at our research laboratory who cut and analyzed the hair samples.
Data availability statement
The datasets generated and analyzed during the study are available at https://doi.org/10.48360/zw8d-qr94
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abell JG, Stalder T, Ferrie JE, Shipley MJ, Kirchbaum C, Kivismaki M, Kumari M. 2016. Assessing cortisol from hair samples in a large observational cohort: the whitehall II study. Psychoneuroendocrinology. 73:148–156. doi:10.1016/j.psyneuen.2016.07.214.
- Cooper GA, Kronstrand R, Kintz P. 2012. Society of hair testing guidelines for drug testing in hair. Forensic Sci Int. 218:20–24. doi:10.1016/j.forsciint.2011.10.024.
- Dettenborn L, Muhtzc C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, Kirschbaum C, Otte C. 2012. Introducing a novel method to assess cumulative steroid concentrations: increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress. 15:348–353. doi:10.3109/10253890.2011.619239.
- Everson-Rose SA, Lewis TT. 2005. Psychosocial factors and cardiovascular diseases. Ann Rev Public Health. 26:469–500. doi:10.1146/annurev.publhealth.26.021304.144542.
- Faresjö T, Strömberg S, Jones M, Stomby A, Karlsson JE, Östgren CJ, Faresjö Å, Theodorsson E. 2020. Elevated levels of cortisol in hair precede acute myocardial infarction. Sci Rep. 10(1):22456. doi:10.1038/s41598-020-80559-9.
- Glei DA, Goldman N, Rodriguez G, Weinstein M. 2015. Beyond self-reports: changes in biomarkers as predictors of mortality. Popul Dev Rev. 40:331–360.
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G. 2010. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 19:32–37. doi:10.1016/j.forsciint.2009.12.040.
- Karlén J, Frostell A, Theodorsson E, Faresjö T, Ludvigsson J. 2013. Maternal influence on child HPA axis: a prospective study of cortisol levels in hair. J Pediatr. 132:e1333–e1340. doi:10.1542/peds.2013-1178.
- Koolhaas JM, Bartolomucci A, Buwalda B, DeBoer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Planza P, et al. 2011. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav. R. 35:1291–1301. doi:10.1016/j.neubiorev.2011.02.003.
- Manenschijn L, Schaap L, Van Schoor NM, Van der Pas S, Peeters GM, Lips P, Koper JW, Van Rossum EFC. 2013. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocriol. Metab. 98:2078–2083. doi:10.1210/jc.2012-3663.
- Mörelius E, Nelson N, Theodorsson E. 2004. Salivary cortisol and administration of concentrated oral glucose in newborn infants: improved detection limit and smaller sample volumes without glucose interference. Scand J Clin Lab Invest. 64:113–118. doi:10.1080/00365510410004452.
- Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. 2011. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress. 14(1):73–81. doi:10.3109/10253890.2010.511352.
- Persson R, Garde AH, Hansen AM, Ostberg K, Larsson B, Orbäck P, Karlsson B. 2008. Seasonal variation in human salivary cortisol concentrations. Chronobiol Int. 25:923–937. doi:10.1080/07420520802553648.
- Pragst F, Balikova MA. 2006. State-of-the-art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. 370:17–49. doi:10.1016/j.cca.2006.02.019.
- Raul JS, Cirimele V, Ludes B, Kintz P. 2004. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 37:1105–1111. doi:10.1016/j.clinbiochem.2004.02.010.
- Sauvè B, Koren G, Walsh G, Tokmakejians S, Van Uum SH. 2007. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 30:E183–E191. doi:10.25011/cim.v30i5.2894.
- Selye H. 1985. The nature of stress. Basal Facts. 7:3–11.
- Stalder T, Kirschbaum C. 2012. Analysis of cortisol in hair - state of the art and future directions. Brain Behav Immun. 26:1019–1029. doi:10.1016/j.bbi.2012.02.002.
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichman S, Kirschbaum C, Miller R. 2017. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 77:261–274. doi:10.1016/j.psyneuen.2016.12.017.
- Thomson S, Koren G, Frazer LA, Rieder M, Friedman TC, VanUum SH. 2010. Hair analysis provides a historical record of cortisol levels in cushing’s syndrome. Exp Clin Endocrinol Diabetes. 118:133–138. doi:10.1055/s-0029-1220771.
- Van de Kar LD, Blair ML. 1999. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 20:1–48. doi:10.1006/frne.1998.0172.
- Van Uum SH, Sauvè B, Fraser LA, Morley-Foster P, Paul TL, Koren G. 2008. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 11:483–488. doi:10.1080/10253890801887388.
- Villain M, Cirimele V, Kintz P. 2004. Hair analysis in toxicology. Clin Chem Lab Med. 42:1265–1272. doi:10.1515/CCLM.2004.247.
- Webb E. 2010. Assessing individual systemic stress through cortisol analysis of archaeological hair. J Archaol Sci. 37:807–812.
- Webb EC, White CD, Van Uum SH, Longstaffe FJ. 2015. Integrating cortisol and isotopic analyses of archaeological hair: elucidating juvenile ante-mortem stress and behavior. Int J Paleopathol. 9:28–37. doi:10.1016/j.ijpp.2014.1.
- Wennig R. 2000. Potential problems with the interpretation of hair analysis results. Forensic Sci Int. 107:5–12. doi:10.1016/s0379-0738(99)00146-2.
- Wester VL, Reincke M, Koper JW, Van der Akker ELT, Manenschijn L, Berr CM, Fazel J, de Rijke YB, Feelders RA, van Rossum EFC. 2017. Scalp hair cortisol for diagnosis of cushing`s syndrome. Eur J Endocrinol. 176:695–703. doi:10.1530/EJE-16-0873.
- Wester VL, van Rossum EFC. 2015. Clinical applications of cortisol measurements in hair. Eur J Endocrinol. 173:M1–M10. doi:10.1530/EJE-15-0313.
- World Health Organization. 2001. Mental health in Europe. Copenhagen: World Health Organisation.
- Yang HZ, Lan J, Yan JM, Xue JW, Dail WH. 1998. A preliminary study of steroid reproductive hormones human hair. J Steroid Biochem Mol Biol. 67:447–450.
