Abstract
Bu-Shen-Yi-Qi granule (BSYQG), a Chinese herbal formula, has been used for the treatment of ischemic stroke with proven efficacy. The purpose of this study is to evaluate the therapeutic efficacy of BSYQG in neurovascular unit (NVU) and its mechanism of action on the thrombin expression in the cerebral ischemic/reperfusion (I/R) model. In the study, a rat model of I/R was established using middle cerebral artery occlusion operation (MCAO). Adult male Sprague–Dawley rats were divided into four groups (n = 36): sham-operation; I/R; I/R + BSYQG(6 g/kg/day), I/R + Argatroban (0.56 mg/ml). Apoptotic cells in the NVU were detected by doubling-labeling using TUNEL assay and immunofluorescence. The dynamics of thrombin expression was detected using immunofluorescence on both sides after I/R injury. Compared with I/R group the numbers and rates of neuronal apoptosis in BSYQG groups significantly decreased (P < 0.05). Furthermore, in the ischemic cortex the thrombin expression at 4 h was significantly higher than that at 1 h (P < 0.01) and 2 h (P < 0.01) after I/R. BSYQG treatment significantly reduced thrombin expression both at 4 h (P < 0.001) and 6 h (P < 0.05) after I/R. Therefore, BSYQG may exert its protective effects in NVU after I/R injury by inhibiting thrombin expression. The results provided the foundation for further investigation in the treatment of ischemic stroke.
Introduction
Stroke is the leading neurological disorder according to the global burden of diseases from 1990–2016 (GBD 2016 Neurology Collaborators Citation2019). Despite effective recanalization in stroke therapy, many stroke patients remain profoundly disabled even after extremely successful reperfusion therapies (Albers et al. Citation2018; Goyal et al. Citation2016), highlighting an unmet need for supplementary neuroprotective treatments for reperfusion injury (Stegner et al. Citation2019). In an ischemia/reperfusion (I/R) injury model, infarcts can grow despite successful reperfusion (Amani et al. Citation2019). However, the mechanism of reperfusion injury is less evident in the human brain compared to experimental models (Gauberti et al. Citation2018).
Because platelet degranulation and activated platelet receptors may also lead to subsequent infarct growth (Prévost et al. Citation2005; Stegner and Nieswandt Citation2011), enhanced platelet activation may play an essential role in the development of ischemic stroke (Lieschke et al. Citation2020). Therefore, understanding the mechanism of platelet's contribution to cerebral ischemic injury may contribute to the development of a potential strategy in translation medicine.
Acute ischemic stroke has remained one of the leading causes of disability and mortality around the world. Medical science has made a breakthrough in stroke management with the success of thrombolytic clinical trials (National Institute of Neurologucal Disorders and Stroke rt-PA Stroke Study Group Citation1995). Thrombin regulates the hemostatic process by activating platelets and, through the activation of cellular protease-activated receptor (PAR), plays a vital role in ischemic stroke (Brailoiu et al. Citation2017). In ischemic stroke, thrombin is increased following blood–brain barrier (BBB) disruption (Ye et al. Citation2021). Subsequently, intravascular activation of thrombin causes brain edema and increases the ischemia region by acting on PARs, which are expressed in neuron and glial cells (Chen et al. Citation2010; Stegner et al. Citation2019; van Nieuw Amerongen et al. Citation2000).
Thrombin may serve as a potential therapeutic target for the treatment of ischemic stroke. Argatroban is a direct thrombin inhibitor recently approved for the treatment of ischemic stroke (Hou et al. Citation2021; Lv et al. Citation2022). According to emerging evidence, argatroban treatment for ischemic stroke is safe and effective (Barreto et al. Citation2017; Berekashvili et al. Citation2018; Yang et al. Citation2020). In addition, argatroban does not enhance the risk of bleeding in the acute phase of cerebral infarction, according to the results of a meta-analysis (Lv et al. Citation2022). Argatroban's efficacy in non-cardioembolic stroke is similar when compared to that in cardioembolic stroke (Oguro et al. Citation2018; Wada et al. Citation2016).
Our previous studies have shown that Bu-Shen-Yi-Qi granule (BSYQG), a three-herb complex traditional Chinese herbal medicine, has anti-inflammatory and neuroprotective effects (Luo et al. Citation2014; Qin et al. Citation2022; Wei et al. Citation2015). BSYQG, an empirical Traditional Chinese Medicine (TCM) prescription for ischemic stroke treatment at our hospital, significantly improved neurobehavioral dysfunction, alleviated neuronal damage, and inhibited neuronal pyroptosis (Liu, Han et al. Citation2022; Luo et al. Citation2014; Qin et al. Citation2022; Wei et al. Citation2015). High-dose treatment with 6 g/kg Bu-Shen-Yi-Qi granule (BSYQG), but not 0.6 or 3 g/kg BSYQG, showed the strongest impact in our previous study (Liu, Sun et al. Citation2022). Furthermore, our earlier studies have shown that raw Radix rehmanniae, the primary component of BSYQG, can protect nerve cells in vitro by inhibiting thrombin (Liu and Li Citation2009; Liu et al. Citation2008; Zhou et al. Citation2012). Similarly, Epimedium brevicornu Maxim (EB-W), another component of BSYQG, may be a potential alternative therapy for ischemic stroke. EB-W can inhibit apoptosis in neurons after cerebral ischemia-reperfusion (Mo et al. Citation2020). Relaxation-evoked EB-W is significantly reduced in intact endothelial preparations, although not abolished, after endothelial removal in the presence of a nitric oxide (NO) synthase inhibitor L-NAME (Liu et al. Citation2018). Furthermore, glibenclamide, an ATP-selective K+ channel blocker, counteracts EB-W evoked relaxation (Dhein et al. Citation2000). Based on these data, this BSYQG formula has been demonstrated to be an excellent treatment for ischemic stroke and is very popular among patients.
In this current work, BSYQG was administered in a rat I/R model. The purpose of this study is to evaluate the therapeutic efficacy of BSYQG in the neurovascular unit (NVU) and its mechanism of action on the thrombin expression after ischemic/reperfusion injury.
Materials and methods
Drugs and reagents
BSYQG comprises three traditional Chinese herbal medicines: Astragalus membranaceus (Huangqi), Epimedium brevicornu Maxim (Yinyanghuo), and Rehmannia glutinosa (Dihuang), all of which were purchased as herbal granules from Jiangyin Tianjiang Pharmaceutical Co. Ltd. (Jiangyin, China). In brief the granule was prepared as follows: dried Astragali membranaceus (3 kg), Epimedium brevicornu Maxim (2 kg), and Rehamannia glutinosa(1.5 kg) were separately immersed in a tenfold mass of water for 2 h and then boiled for 1 h. After filtration, granules were separately prepared by spray-drying process until the density reached 1.11-1.15 (60%). Three granules were then mixed for 30 min with a ratio of 3:2:1.5(w/w/w) and made into 18–40 mesh particles. Finally, the granules were stored at 4°C and dissolved in distilled water at the desired concentrations before use.
Argatroban, a direct thrombin inhibitor that lowers brain damage following ischemic stroke, was used as a positive control (Chen et al. Citation2010). Rats were administrated with IV argatroban injection (10 mg/kg dose dissolved in 0.9% saline at 0.56 mg/ml concentration) at 0.5, 1, 2, 4, and 6 h after ischemic/reperfusion (Singh et al. Citation2016).
Animals and middle cerebral artery occlusion/reperfusion (MCAO/R) model
A total of 36 adult male Sprague–Dawley rats (body weight 240∼270g) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). The rats were randomly divided into four groups: sham operation, ischemia/reperfusion (I/R), I/R + BSYQG-treatment (6 g/kg/day), and I/R + Argatroban (0.56 mg/ml).
The rat I/R model was developed using a method of reversible regional I/R injury (Liu et al. Citation2008). Rats were anaesthetized intraperitoneally with 10% chloral hydrate (0.35mL/100g body weight) prior to operation. During the neck incision, the left common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) were isolated and exposed. The embolus created from the nylon filament was then used to occlude the MCA through a tiny incision in the internal carotid artery. After 90 min, a filament was removed to allow for 24 h of reperfusion (Figure (A)). The rats in the I/R, BSYQG, and argatroban groups were given brain ischemia and reperfusion, whereas the sham-operated animals were given an identical treatment but without artery ligation.
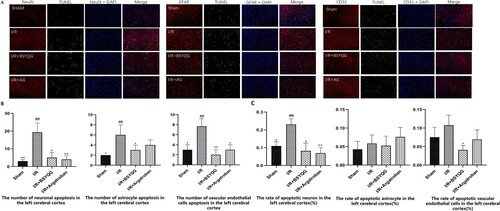
Figure 1. The expression of apoptotic cells in the neurovascular unit of different groups after ischemia/reperfusion. (A) TUNEL and immunofluorescence double staining in neurovascular unit subjected to ischemia/reperfusion in different groups. NeuN/GFAP/CD31 (red), TUNEL (green), DAPI (blue). Scale Bar = 50μm. (B) The number of apoptotic cells in neurovascular unit after ischemia/reperfusion in the left cerebral cortex. (C) The ratio of apoptotic cells in neurovascular unit after ischemia/reperfusion in the left cerebral cortex. Data were expressed as the mean ± SD, n = 3; # P < 0.05 vs the sham group, ## P < 0.001 vs the sham group, *P < 0.05 vs the I/R group, **P < 0.001 vs the I/R group.
The effectiveness of the I/R model in rats was validated by neurobehavioral scores. During the surgery, the rat's body temperature and respiration rate were monitored.
After the ischemic rats were prepared the rats in I/R + BSYQG-treatment were administrated orally with BSYQG (6 g/kg/day) for 7 days. The rats in I/R + Argatroban treatment were administered with 0.56 mg/ml argatroban 12 h and 1 h before ischemia induction. The rats in the sham and ischemia/reperfusion group were administrated orally with the same double-distilled water (0.1ml/kg/day) for 7 days.
Determination of cell apoptosis in the NVU
Cell apoptosis in the neurovascular unit was detected using terminal doxynucleotidyl transferase dUTP nick and labeling (TUNEL) and immunofluorescence double staining. Anti-NeuN (Abcam, Ab104224), anti-GFAP (Proteintech, 16825-1-AP), and anti-CD31 (Proteintech, 11265-1-AP) antibodies were utilized to stain neurons, astrocytes, and vascular endothelial cells respectively with a TUNEL kit (Roche Diagnostics GmbH, 11684817910, Penzberg, Germany). Brain slices were first blocked with goat serum for 1 h before being treated with the primary antibody overnight at 4°C.
After being rinsed with PBS for 5 min 3 times, the slices were incubated with secondary antibodies for 60 min at 37°C in a light-shielded environment. For TUNEL staining, the slices were incubated in a dark humidified chamber at 37°C for 1 h with TUNEL reaction mixture, followed by a final wash for 10 min 3 with PBS. To stain nuclei, 4’,6-diamidino-2-phenylindole (DAPI, Beyotime, C1002, China) was applied for 15 min.
The expression of NeuN-positive, GFAP-positive, or CD31-positive cells were detected by red fluorescence in the cytoplasm and nucleus. TUNEL-positive cells contained green dots while nuclei were stained blue by DAPI. The number of nuclei stained with DAPI was counted on the same slice of ischemic penumbra. Counting was performed by a researcher blinded to the treatment conditions in five different fields of view per five adjacent brain sections from each mouse via light microscopy (NIKON, ECLIPSE Ni, Japan). The number of triple positive cells with blue nuclei compared to all cells was calculated as the rate of cell apoptosis.
Immunofluorescence
The expression of thrombin in each group was observed dynamically by immunofluorescence at 0.5h, 1h, 2h, 4h, 6h after I/R. Two paraffin sections were taken from the brain tissue at each time point from each rat, baked in a constant temperature oven at 65 °C for 30 min, immersed in xylene I and II for 15 min, and soaked for 5 min in various concentrations of alcohol (100%, 95%, 85%, 75%) before being rinsed for 10 min with tap water. After natural cooling and antigen unmasking with high pressure in 0.01M sodium citrate buffer solution (pH 6.0) for 15 min, primary antibody against thrombin (Santa Cruz, sc-271449) was added dropwise and incubated at 4°C overnight. Fluorescent secondary antibody (Alexa Fluor 555 labeled donkey anti-mouse IgG(H + L), Beyotime) (Shanghai, China) (1:200) was then added dropwise, followed by incubation at room temperature for 1 h. After washing with 0.02M PBS three times (3 min each), the slides were stained with DAPI diluted 1:500 in anti-quenching mounting medium, mounted, and stored at −20°C.
To ensure the reliability of the results, at least five randomly selected fields per five adjacent brain sections from each mouse were counted in a blinded manner, using ImagePro Plus 6 Software.
Statistical analysis
To compare different groups, one-way ANOVA followed by an LSD comparison test or unpaired Student's t-tests using IBM SPSS version 11.5 for Windows (Chicago, IL, USA). A statistically significant difference was concluded if P<0.05.
Statement
The experiments were carried out in accordance with the guidelines issued by the Institutional Animal Care and Use Committee (IACUC) at Fudan University (No.20220104(46)).
Results
BSYQG treatment reduced I/R-mediated cell apoptosis in the NVU
The number of NeuN-positive, GFAP-positive or CD31-positive cells (red) with TUNEL-positive cells (green) were calculated to measure apoptosis (Figure (A)). A small number of apoptotic cells in the neurovascular unit were observed in the brain cortex of the rats in the sham-operated group (Figure (A,B)). After cerebral ischemia-reperfusion, the number of apoptotic cells in the neurovascular unit was significantly increased in the ischemic cortex of the I/R group compared with those in the sham-operated group (P < 0.001) (Figure (B)). A higher rate of neuronal apoptosis was detected in the I/R group compared with that in the sham-operated group (P < 0.001) (Figure (C)). There was a significant reduction in the number of apoptotic in neurons (P < 0.05) and vascular endothelial cells (P < 0.001) in argatroban treatment group compared with those in I/R group. However, BSYQG treatment group showed significant reduction cell apoptosis of neuron (P < 0.05), astrocyte (P < 0.05) and vascular endothelial cells (P < 0.001) compared with I/R group (Figure (B)). Furthermore, compared with I/R group the rates of neuronal apoptosis in all the treatment groups significantly decreased (P < 0.05) (Figure (C)). A significant effect of BSYQG inhibition on the rate of the vascular endothelial apoptosis was detected (P < 0.001) only in the BSYQG group (Figure (C)).
Thrombin was upregulated after I/R injury
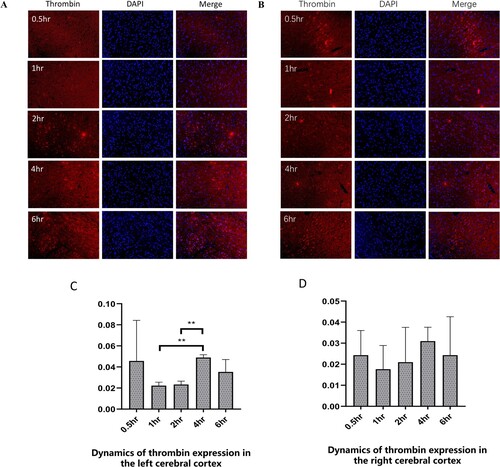
The number of thrombin (red) and DAPI (blue) dual positive were calculated to measure the expression of thrombin (Figure (A,B)). Thrombin expression decreased at 0.5-2h and then increased and peaked at 4h in the both brain cortex of the rats after I/R (Figure (A,B)). In the ischemic cortex, the expression of thrombin at 4h was significantly higher than that at 1h (P < 0.01) and 2h (P < 0.01) (Figure (C)). However, in the left cortex, there was no significant difference at different time-points after I/R injury (Figure (D)).
Figure 2. The dynamics of thrombin expression in the cerebral cortex of ischemia/reperfusion rats. (A-B) The immunofluorescence of thrombin in the left ischemic cerebral cortex (A) and the right cerebral cortex (B) subjected to ischemia/reperfusion at different times. Thrombin (red), DAPI (blue). Scale Bar = 50μm. (C-D) Dynamics of thrombin expression in the left ischemic cerebral cortex (C) and the right cerebral cortex (D). Values were expressed as mean ± standard error of the mean (n = 3) in the left cerebral hemisphere. **P < 0.01.
BSYQG treatment reduced thrombin after I/R injury
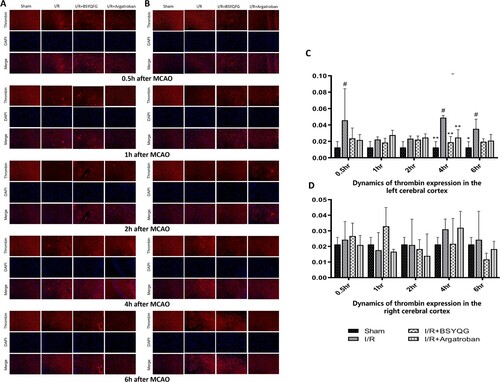
Thrombin expression was measured by the number of cells labeled with thrombin (red) and DAPI (blue) dual positivity at 0.5h, 1h, 2h, 4h and 6h in both cerebral cortex after MCAO ( Figure (A,B)). Compared with the sham-operated group, the I/R group had higher thrombin expression at 2h (P < 0.05), 4h (P < 0.001) and 6h (P < 0.001) in the left ischemic cortex (Figure (C)). Argatroban treatment significantly decreased thrombin expression at 4h (P < 0.01) after I/R compared with the I/R group. Furthermore, BSYQG treatment significantly reduced thrombin expression both at 4h (P < 0.001) and 6h (P < 0.05) after I/R compared with I/R group (Figure (C)). As shown in Figure (B,D), there was no significant difference among the groups in the right cerebral cortex.
Figure 3. Effects of BSYQG on thrombin expression. (A-B) The immunofluorescence of thrombin in the left ischemic cerebral cortex (A) and the right cerebral cortex (B) subjected to ischemia/reperfusion for different times in different groups. Thrombin (red), DAPI (blue). Scale Bar = 50μm. (C-D) Effects of BSYQG on thrombin expression at different times in the left (C) and right (D) cerebral cortex. The values were expressed as mean ± standard error of the mean (n = 3). #P < 0.05, ##P < 0.001 vs Sham group; *P < 0.05, **P < 0.01 vs I/R group.
Discussion
In the present study, we demonstrated that BSYQG has protective effects in a rat model of I/R. BSYQG ameliorated cell apoptosis and protected the NVU. Specifically, I/R increased the levels of thrombin, peaking at 4 h after I/R, and BSYQG treatment reduced these levels. Furthermore, the effect of BSYQG treatment on thrombin was significant at 4 and 6 h after the I/R. These findings may underpin the mechanism of BSYQG treatment.
Reperfusion of ischemic brain tissue may result in the breakdown of BBB as well as neurovascular injury and neuronal death (Jung et al. Citation2010). The NVU is a complex multi-cellular structure with neurons, endothelial cells, astrocytes as the main components. These cells are closely linked to each other and form the BBB (Kugler et al. Citation2021). These components of the NVU are considered key factors in brain protection, as dysfunction of the NVU can be characterized by dysregulation of neurovascular coupling and neuronal apoptosis (Zhào et al. Citation2021). In the present study, ischemic damages induced neuronal death, upregulated astrocytic apoptosis, and significantly reduced the number of endothelial cells in the I/R area. Furthermore, the number and rates of apoptotic cells were significantly ameliorated by BSYQG treatment in the penumbra of the I/R injury area.
In our previous studies, we demonstrated that BSYQG may have anti-inflammatory and neuroprotective effects (Luo et al. Citation2014; Qin et al. Citation2022; Wei et al. Citation2015). BSYQG treatment improves neurological function, ameliorates the pathological abnormalities, and significantly reverses the reduction of cortical infarct volume compared with the I/R rats in a dose-effective manner (Liu, Sun et al. Citation2022).
Thrombin is involved in stroke pathology via multiple pathways, including neuronal apoptosis, vascular endothelial cell damage, and inflammatory response (Chen Citation2012). Thrombin may also play an important role in the primary hemostatic process and increase the ischemic area after BBB disruption (Ye et al. Citation2021). Furthermore, increased thrombin levels induce cell apoptosis, disrupt the tight junctions of the endothelial cells, and damage the NVU after I/R injury (Krenzlin et al. Citation2016; Ye et al. Citation2021). Therefore, thrombin may potentially be a valuable therapeutic target in treating ischemic stroke.
Although previous studies have mainly focused on the detrimental effects of thrombin to neuronal death(Krenzlin et al. Citation2020; Pleşeru and Mihailă Citation2018), thrombin exhibits concentration-dependent effects after I/R injury (Ye et al. Citation2021). Low concentration thrombin may have beneficial effects on neurons and the brain, while high concentrations may cause brain edema and neuronal death in ischemic brain disease (Sokolova and Reiser Citation2008) and hemostasis (Delvaeye and Conway Citation2009; Fenton Citation1986; Petzold and Massberg Citation2019; Rajput et al. Citation2020). These results may explain why we found that thrombin expression showed an inverted U effect as it decreased at 0.5-2 h and then peaked at 4 h after I/R injury in our study.
In our previous study, the primary active chemical compounds in BSYQG were determined by HPLC (Nurahmat et al. Citation2014). The highest concentrations were Icariin and epimedin C, followed by catalpol, epimedin B, astragaloside IV, and baohuoside-I (Nurahmat et al. Citation2014). Icarlin is a key active ingredient of Epimedium grandiflorum, a plant used in TCM. Icarlin has been shown to have a neuroprotective effect, preventing brain damage caused by ischemia/reperfusion (He et al. Citation2020; Xiong et al. Citation2016). Catapol, the main component of Rehmannia glutinosa, can ameliorate impaired neurovascular units in the ischemic region and promote angiogenesis to replenish lost vessels and neurons (Wang et al. Citation2022). Astragaloside IV, a primary bioactive compound of Radix Astragali, decreases the neurological score, reduces the brain's infarct volume, and alleviates cerebral injury after cerebral injury (Zhang et al. Citation2019). Therefore, the protective effect of BSYQG on I/R injury in rats may be closely related to these compounds, which can be further studied in future studies.
In conclusion, the present study suggested that BSYQG showed the protective effects in the NVU via decreased thrombin expression after I/R injury. The results presented in this study provide a novel approach and lay the foundation for further investigation in the treatment of ischemic stroke.
Author’s contribution statement
Aihua Liu and Zhenxiang Han are the principal investigators of the study, overseeing study design, data collection and manuscript approval. Jing Sun initiated the study. Hongling Wang and Dongxu Tang checked the data and contributed to the interpretation of data. John Wong and Sagun Tiwari are the coinvestigators who drafted and critically revised the manuscript for important intellectual content. All authors contributed to the refinement of the study and approved the final manuscript. Note: Preprint link of this manuscript is https://doi.org/10.21203/rs.3.rs-1553761/v1
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on the figshare website at https://doi.org/10.6084/m9.figshare.19730092.v9
Additional information
Funding
References
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, et al. 2018 Feb 12. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 378:708–718. Epub 2018/01/25.
- Amani H, Mostafavi E, Alebouyeh MR, Arzaghi H, Akbarzadeh A, Pazoki-Toroudi H, Webster TJ. 2019. Would colloidal gold nanocarriers present an effective diagnosis or treatment for ischemic stroke? Int J Nanomedicine. 14:8013–8031. Epub 2019/10/22.
- Barreto AD, Ford GA, Shen L, Pedroza C, Tyson J, Cai C, Rahbar MH, Grotta JC. 2017 Jun. Randomized, multicenter trial of ARTSS-2 (argatroban with recombinant tissue plasminogen activator for acute stroke). Stroke. 48:1608–1616. Epub 2017/05/17.
- Berekashvili K, Soomro J, Shen L, Misra V, Chen PR, Blackburn S, Dannenbaum M, Grotta JC, Barreto AD. 2018 Dec. Safety and feasibility of argatroban, recombinant tissue plasminogen activator, and intra-arterial therapy in stroke (ARTSS-IA study). J Stroke Cerebrovasc Dis. 27:3647–3651. Epub 2018/09/27.
- Brailoiu E, Shipsky MM, Yan G, Abood ME, Brailoiu GC. 2017 Feb 15. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 1657:167–175. Epub 2016/12/22.
- Chen B. 2012. Thrombin in ischemic stroke targeting. In: Lapchak P, Zhang J, editors. Translational stroke research. Springer series in translational stroke research. New York (NY): Springer. https://doi.org/10.1007/978-1-4419-9530-8_9.
- Chen B, Cheng Q, Yang K, Lyden PD. 2010 Oct. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 41:2348–2352. Epub 2010/08/14.
- Delvaeye M, Conway EM. 2009 Sep 17. Coagulation and innate immune responses: can we view them separately? Blood 114:2367–2374. Epub 2009/07/09.
- Dhein S, Pejman P, Krüsemann K. 2000 Jun. Effects of the I(K.ATP) blockers glibenclamide and HMR1883 on cardiac electrophysiology during ischemia and reperfusion. Eur J Pharmacol.. 398:273–284. Epub 2000/06/16.
- Fenton JW 2nd. 1986. Thrombin. Ann N Y Acad Sci. 485:5-15. Epub 1986/01/01.
- Gauberti M, Lapergue B, Martinez de Lizarrondo S, Vivien D, Richard S, Bracard S, Piotin M, Gory B. 2018 Dec. Ischemia-Reperfusion Injury After Endovascular Thrombectomy for Ischemic Stroke. Stroke. 49:3071–3074. Epub 2018/12/21.
- Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. 2019 May. Lancet Neurol. 18:459–480. Epub 2019/03/19
- Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. 2016 Apr 23. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 387:1723–1731. Epub 2016/02/24.
- He C, Wang Z, Shi J. 2020. Pharmacological effects of icariin. Adv Pharmacol. 87:179–203. Epub 2020/02/25.
- Hou X, Jin C, Pan C, Wang X, Xue J, Yang Z, Qi D. 2021 Aug. Effects of argatroban therapy for stroke patients: a meta-analysis. J Clin Neurosci. 90:225–232. Epub 2021/07/20.
- Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, et al. 2010 Jun. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 41:172–179. Epub 2010/02/17.
- Krenzlin H, Frenz C, Schmitt J, Masomi-Bornwasser J, Wesp D, Kalasauskas D, Kerz T, Lotz J, Alessandri B, Ringel F, et al. 2020. High CSF thrombin concentration and activity is associated with an unfavorable outcome in patients with intracerebral hemorrhage. PLoS One. 15:e0241565. Epub 2020/11/12.
- Krenzlin H, Lorenz V, Danckwardt S, Kempski O, Alessandri B. 2016 Jan 11. The importance of thrombin in cerebral injury and disease. Int J Mol Sci. 17(1):84.
- Kugler EC, Greenwood J, MacDonald RB. 2021. The “neuro-glial-vascular” unit: the role of glia in neurovascular unit formation and dysfunction. Front Cell Dev Biol. 9:Article 732820. Epub 2021/10/15.
- Lieschke F, Zheng Y, Schaefer JH, van Leyen K, Foerch C. 2020. Measurement of platelet function in an experimental stroke model with aspirin and clopidogrel treatment. Front Neurol. 11:85 . https://doi.org/10.3389/fneur.2020.00085.
- Liu A, Han Z, Zhao X, Dong J. 2022. Prevention and treatment strategies to stroke by tonifying kidney and invigorating qi based on theories of traditional chinese medicine constitution. Zhōngyī wénxiàn zázhì. 01:27-29+38 .
- Liu A, Li H. 2009. Experimental study of the protective effects of Shengdi injection on the neuron of stroke model. Journal of Tongji University (Medical Science). 30:52–54.
- Liu A, Sun J, Kong L, Han Z, Dong J. 2022. Effects of Bushen Yiqi prescription on PI3K/AKT pathway in rats with middle cerebral artery occlusion. Zhōnghuá zhōng yīyào zázhì. 37(9):5329–5333.
- Liu A YX, Wang Y, Wei J. 2008. An experimental study of protective effects of Shengdi injection on neuron injured by thrombin. Chin J TCM WM Crit Care. 03:155–158.
- Liu H, Xiong Y, Wang H, Yang L, Wang C, Liu X, Wu Z, Li X, Ou L, Zhang R, et al. 2018 Jul 15. Effects of water extract from epimedium on neuropeptide signaling in an ovariectomized osteoporosis rat model. J Ethnopharmacol. 221:126–136. Epub 2018/05/01.
- Luo QL, Nurahmat M, Li MH, Sun J, Chen MX, Liu F, Wei Y, Dong JC. 2014 Sep 25. Pharmacological investigation of a HPLC/MS standardized three herbal extracts containing formulae (Bu-Shen-Yi-Qi-Tang) on airway inflammation and hypothalamic-pituitary-adrenal axis activity in asthmatic mice. Phytomedicine. 21:1439–1450. Epub 2014/07/16.
- Lv B, Guo FF, Lin JC, Jing F. 2022 Jan 14. Efficacy and safety of argatroban in treatment of acute ischemic stroke: A meta-analysis. World J Clin Cases. 10:585–593. Epub 2022/02/01.
- Mo ZT, Liao YL, Zheng J, Li WN. 2020 Aug 15. Icariin protects neurons from endoplasmic reticulum stress-induced apoptosis after OGD/R injury via suppressing IRE1α-XBP1 signaling pathway. Life Sci. 255:Article 117847. Epub 2020/05/30.
- Nurahmat M, Chen M, Luo Q, Ling Y, Dong J, Huang C. 2014 Dec. Rapid characterization and determination of multiple components in Bu-Shen-Yi-Qi-Fang by high-performance liquid chromatography coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry. J Sep Sci. 37:3509–3517. Epub 2014/09/13.
- Oguro H, Mitaki S, Takayoshi H, Abe S, Onoda K, Yamaguchi S. 2018 Aug. retrospective analysis of argatroban in 353 patients with acute noncardioembolic stroke. J Stroke Cerebrovasc Dis. 27:2175–2181. Epub 2018/05/01.
- Petzold T, Massberg S. 2019 Apr 16. Thrombin: a gas pedal driving innate immunity. Immunity.. 50:1024–1026. Epub 2019/04/18.
- Pleşeru AM, Mihailă RG. 2018 Oct. The role of thrombin in central nervous system activity and stroke. Clujul Med. 91:368–371. Epub 2018/12/20.
- Prévost N, Woulfe DS, Jiang H, Stalker TJ, Marchese P, Ruggeri ZM, Brass LF. 2005 Jul 12. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc Natl Acad Sci U S A. 102:9820–9825. Epub 2005/07/05.
- Qin J, Wuniqiemu T, Wei Y, Teng F, Cui J, Sun J, Yi L, Tang W, Zhu X, Xu W, et al. 2022 Jan. Proteomics analysis reveals suppression of IL-17 signaling pathways contributed to the therapeutic effects of Jia-Wei Bu-Shen-Yi-Qi formula in a murine asthma model. Phytomedicine. 95:Article 153803. Epub 2021/11/18.
- Rajput PS, Lamb J, Kothari S, Pereira B, Soetkamp D, Wang Y, Tang J, Van Eyk JE, Mullins ES, Lyden PD. 2020 Feb. Neuron-generated thrombin induces a protective astrocyte response via protease activated receptors. Glia. 68:246–262. Epub 2019/08/28.
- Singh S, Houng AK, Wang D, Reed GL. 2016 Sep. Physiologic variations in blood plasminogen levels affect outcomes after acute cerebral thromboembolism in mice: a pathophysiologic role for microvascular thrombosis. J Thromb Haemost. 14:1822–1832. Epub 2016/06/21.
- Sokolova E, Reiser G. 2008 Oct. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemost. 100:576–581. Epub 2008/10/09.
- Stegner D, Klaus V, Nieswandt B. 2019. Platelets as modulators of cerebral ischemia/reperfusion injury. Front Immunol.10:2505. Epub 2019/11/19.
- Stegner D, Nieswandt B. 2011 Feb. Platelet receptor signaling in thrombus formation. J Mol Med (Berl). 89:109–121. Epub 2010/11/09.
- Tissue plasminogen activator for acute ischemic stroke. 1995 Dec 14. Platelet receptor. N Engl J Med. 333:1581–1587. Epub 1995/12/14.
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. 2000 Aug 18. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 87:335–340. Epub 2000/08/19.
- Wada T, Yasunaga H, Horiguchi H, Matsubara T, Fushimi K, Nakajima S, Yahagi N. 2016 Feb. Outcomes of argatroban treatment in patients with atherothrombotic stroke: observational nationwide study in Japan. Stroke. 47:471–476. Epub 2015/12/17.
- Wang HJ, Ran HF, Yin Y, Xu XG, Jiang BX, Yu SQ, Chen YJ, Ren HJ, Feng S, Zhang JF, et al. 2022 Jul. Catalpol improves impaired neurovascular unit in ischemic stroke rats via enhancing VEGF-PI3 K/AKT and VEGF-MEK1/2/ERK1/2 signaling. Acta Pharmacol Sin. 43(7):1670–1685.
- Wei Y, Luo QL, Sun J, Chen MX, Liu F, Dong JC. 2015 Apr 22. Bu-Shen-Yi-Qi formulae suppress chronic airway inflammation and regulate Th17/Treg imbalance in the murine ovalbumin asthma model. J Ethnopharmacol. 164:368–377. Epub 2015/01/28.
- Xiong D, Deng Y, Huang B, Yin C, Liu B, Shi J, Gong Q. 2016 Jan. Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by NF-κB, PPARα and PPARγ in rats. Int Immunopharmacol. 30:157–162. Epub 2015/12/19.
- Yang Y, Zhou Z, Pan Y, Chen H, Wang Y. 2020 Jul. Randomized trial of argatroban plus recombinant tissue-type plasminogen activator for acute ischemic stroke (ARAIS): Rationale and design. Am Heart J. 225:38–43. Epub 2020/06/03.
- Ye F, Garton HJL, Hua Y, Keep RF, Xi G. 2021 Jun. The role of thrombin in brain injury after hemorrhagic and ischemic stroke. Transl Stroke Res. 12:496–511. Epub 2020/09/30.
- Zhang Y, Zhang Y, Jin XF, Zhou XH, Dong XH, Yu WT, Gao WJ. 2019 May 13. The role of astragaloside IV against cerebral ischemia/reperfusion injury: suppression of apoptosis via promotion of P62-LC3-autophagy. Molecules. 24(9):1838.
- Zhào H, Wang R, Zhang Y, Liu Y, Huang Y. 2021 Mar. Neuroprotective effects of troxerutin and cerebroprotein hydrolysate injection on the neurovascular unit in a rat model of middle cerebral artery occlusion. Int J Neurosci. 131:264–278. Epub 2020/03/04.
- Zhou X WZ, Li W, Wei J. 2012. Experimental study on apoptosis of astrocytes induced by antithrombin of Shengji Injection. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease. 10:1098–1099.
