Abstract
Long non-coding RNAs (lncRNAs) are more than 200 bp in length and do not translate into functional proteins. They are involved in inhibiting cell growth, migration, and invasion, and in promoting apoptosis. It was found that cancer-related LINC00961 is downregulated in a variety of malignant tumors, including lung cancer, renal cell carcinoma, and hepatocellular carcinoma. LINC00961 either inhibits or promotes the expression of many genes to affect cancer progression via microRNAs such as miR-5581-3p, miR-367, miR-223-3p, miR-19-3p, and miR-125b-5p, epithelial–mesenchymal transition, and Wnt/β-catenin signaling pathway. Thus, LINC00961 may be a viable biomarker or therapeutic target for human cancers. In this review, we summarize the current evidence of the biological functions of LINC00961 in tumor development, to provide new insights and ideas for molecular targeted therapy for patients with cancer.
Abbreviations: CRLS1, cardiolipin synthase 1; EMT, epithelial–mesenchymal transformation; HCC, hepatocellular carcinoma; LncRNA, long non-coding RNA; LUAD, lung adenocarcinoma; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; PCAN, proliferating cell nuclear antigen; PTEN, phosphatase and tensin homologue; RCC, renal cell carcinoma; SPAR, small regulatory polypeptide of amino acid response; TSCC, tongue squamous cell carcinoma; VSMCs, vascular smooth muscle cell.
Introduction
In 2020, there were 1,806,590 new cancer cases, and 606,520 cancer-associated deaths in the United States. Cancer-associated morbidity and mortality rates have continued to remain high throughout the year, resulting in large social burden (Siegel et al. Citation2020). Therefore, an urgent need to develop new treatments to reduce the adverse effects of cancer has arisen. In recent years, targeted therapy has brought new hope for patients with cancer and is expected to improve the prognosis of cancer (Ghafoor et al. Citation2018; Hsu et al. Citation2019; McCoach et al. Citation2017; Lee et al. Citation2018; Bhattacharjee and Nandi Citation2017). Long non-coding RNAs (lncRNAs) can be categorized as sense, antisense, bidirectional, intronic, or intergenic, and can participate in various biological processes, such as gene expression regulation, histone modification, transcriptional activation, nuclear transport regulation, and cell cycle regulation, depending on their subcellular localization (Wang et al. Citation2018a). Additionally, lncRNAs may be involved in regulating gene expression through microRNAs (miRNAs), thereby affecting cancer cell growth and migration (Zheng et al. Citation2019; Guo et al. Citation2021; Liu et al. Citation2022). For example, lncRNA RAET1K is upregulated in lung adenocarcinoma (LUAD) tissues and is associated with poor prognosis in LUAD patients. Overexpression of RAET1K increases the expression level of CCNE1 by sponging miR-135a-5p, thereby promoting LUAD cell growth (Zheng et al. Citation2019).
Recently, lncRNA small regulatory polypeptide of amino acid response (SPAR) (GenBank accession ID: 158376), also called LINC00961, which is localized at 9p13.3 and is more than 200 bp in length, was discovered as a tumor suppressor in renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), non-small cell lung cancer (NSCLC), and other cancers (Yin et al. Citation2019; Jiang et al. Citation2018; Huang et al. Citation2018; Chen et al. Citation2019; Sui et al. Citation2022). For example, LINC00961 was downregulated in NSCLC tissues. Overexpression of LINC00961 reduced the expression of miR-19a-3p, miR-19b-3p, and miR-125b-5p, inhibited cell proliferation, migration, and invasion, and promoted apoptosis. Overexpression of miR-19a-3p, miR-19b-3p, and miR-125b-5p reversed the effects of LINC00961 on A549 cell function. LINC00961 may act as a tumor suppressor by affecting the PI3K-AKT/MAPK/mTOR signaling pathway (Sui et al. Citation2022). Here, we describe the biological effects and signaling pathways of LINC00961 in tumor growth and migration and discuss the potential clinical applications of LINC00961 as a prognostic marker and therapeutic target.
Biological function of LINC00961
Cell proliferation and apoptosis are closely related to cell cycle regulation and play an important role in tumor growth. Abnormal cell cycle can lead to the development of tumors (Wang et al. Citation2018b; Zhang et al. Citation2019a). Metastasis is the most important step in tumor progression. Metastasis to the lymph node or blood can lead to poor long-term prognosis of patients with early-stage cancer (Takai et al. Citation2014; Yan et al. Citation2019; Cao et al. Citation2020; Yi et al. Citation2018). Increasing or decreasing the expression of lncRNAs could inhibit the growth and migration of cancer (Zhang et al. Citation2020; Shang et al. Citation2019; Parolia et al. Citation2019; Wang et al. Citation2019), which can be employed to reduce the rate of early metastasis and improve the treatment and prognosis of cancer patients. LINC00961 is critical in the growth and migration of tumor cells, and could inhibit the development of cancer (Table and Table ).
Table 1. Functional characterization of lncRNA LINC00961 in the growth of cancers.
Table 2. Functional characterization of lncRNA LINC00961 in the metastasis of cancers.
The roles of LINC00961 in cell growth
LINC00961 inhibits the proliferation and cell cycle transition of cancer cells and promotes apoptosis (Table ). For example, expression of LINC00961 is reduced in HCC, and LINC00961 overexpression significantly inhibits the proliferation, clone formation, and cell cycle transition of HCC cells and promotes their apoptosis (Yin et al. Citation2019). Huang et al. reported that LINC00961 overexpression promotes apoptosis and inhibits proliferation of NSCLC cells (Huang et al. Citation2018). Adverse consequences were observed when the expression of LINC00961 was disturbed in NSCLC (Jiang et al. Citation2018; Sui et al. Citation2022). LINC00961 overexpression has no effect on the proliferation of NSCLC cells (Jiang et al. Citation2018). LINC00961 expression is significantly decreased in RCC. The proliferation activity, cell cycle transformation, and tumor forming ability in nude mice are inhibited when LINC00961 is overexpressed, and the rate of apoptosis increases (Chen et al. Citation2019). Mu et al. found that the expression of LINC00961 is decreased in skin melanoma, and overexpression of LINC00961 could inhibit proliferation and promote apoptosis in skin melanoma cells. Tumor formation experiments in nude mice have shown that LINC00961 overexpression can inhibit the tumorigenicity of melanoma cells and reduce the expression of the apoptosis-related protein Ki-67 (Mu et al. Citation2019). Furthermore, Pan et al. reported that LINC00961 expression is reduced in oral squamous cell carcinoma (OSCC) and that increasing the expression of LINC00961 inhibits the proliferation and cell cycle transition and promoted apoptosis in OSCC cells. Contrasting results were observed in OSCC cells if LINC00961 was disrupted. The tumor formation experiments in nude mice have showed similar results. LINC00961 overexpression reduces the tumor volume and weight of OSCC cells (Pan et al. 2019). The expression of LINC00961 in proliferative tongue squamous cell carcinoma (TSCC) is decreased, and LINC00961 overexpression inhibits the proliferation of TSCC cells (Zhang et al. Citation2019b). Increasing the expression of LINC00961 inhibits the proliferation of vascular smooth muscle cells (VSMCs), and interfering with the expression of LINC00961 promotes the proliferation of VSMCs (Wu et al. Citation2019).
The roles of LINC00961 in cell metastasis
LINC00961 has the ability to inhibit cancer cell migration during tumor metastasis. The overexpression of LINC00961 significantly inhibits the migration and invasion of HCC cells (Yin et al. Citation2019). LINC00961 overexpression could inhibit the invasion and migration of NSCLC cells. The number of metastatic nodules in the lungs of nude mice injected with LINC00961, which was overexpressed in A549 cells, through the tail vein, was significantly lower than that in the control group at 8 weeks post injection (Jiang et al., Citation2018; Huang et al. Citation2018; Sui et al. Citation2022). This suggests that LINC00961 overexpression inhibits NSCLC metastasis. Moreover, Chen et al. reported that LINC00961 overexpression could inhibit the migration and invasion of RCC cells (Chen et al. Citation2019). Mu et al. reported that increasing LINC00961 expression in cutaneous melanoma could inhibit melanoma invasion and migration (Mu et al. Citation2019). In addition, Lu et al. found that the upregulation of LINC00961 can inhibit the malignant properties of glioma, colon cancer, and TSCC cells (Lu et al., Citation2018; Zhang et al., Citation2019b; Wu et al. Citation2020). These data indicate that LINC00961 acts as a tumor suppressor in HCC, lung cancer, RCC, and other tumors (Table ). Therefore, the upregulation of LINC00961 is expected to delay tumor metastasis, which could improve the prognosis of cancer patients.
The signaling mechanisms of LINC00961 involved in cell growth and migration
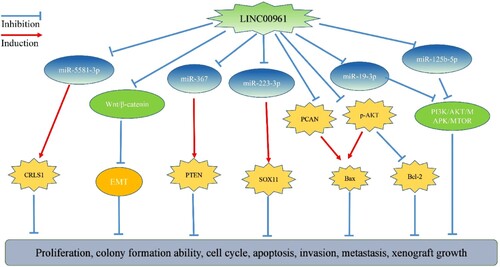
LINC00961 has similar effects as tumor suppressor genes during the progression of malignant tumors. It is well known that lncRNAs can inhibit the proliferation, migration, and invasion of cancer cells and promote their apoptosis by acting on target miRNAs (Xu et al. Citation2020; Zhou et al. Citation2020; Shen et al. Citation2020). LINC00961 regulates gene expression both directly and through miRNAs, thereby affecting the growth and migration of tumor cells (Figure ). For example, Yin et al. found that miR-5581-3p promotes the growth and migration of HCC cells and confirmed that LINC00961 reduces miR-5581-3p expression. Increasing the expression of miR-5581-3p in HCC cells that overexpress LINC00961 reverses the effects of LINC00961 on cancer cell proliferation, clone formation, apoptosis, migration, and invasion. Moreover, miR-5581-3p specifically inhibits the expression of cardiolipin synthase 1 (CRLS1) in HCC cells that overexpresses LINC00961, thereby enhancing the effect of LINC00961 (Yin et al. Citation2019). Mu et al. confirmed that LINC00961 inhibits the expression of miR-367, thereby inhibiting the proliferation, migration, and invasion of skin melanoma cells and promoting apoptosis. In skin melanoma cells overexpressing LINC00961, overexpression of miR-367 reverses the effects of LINC00961. In addition, miR-367 specifically regulates the expression of the phosphatase and tensin homolog (PTEN) gene, and the effect of LINC00961 is reversed in the skin melanoma cells when PTEN is inhibited (Mu et al. Citation2019). Wu et al. confirmed that miR-223-3p promotes the migration and invasion of colon cancer cells, whereas LINC00961 inhibits the expression of miR-223-3p, thereby inhibiting the migration and invasion of colon cancer cells. SRY-related high-mobility-group box 11 (SOX11) gene is the a direct target of miR-223-3p, and the function of SOX11 in colon cancer is the opposite of that of miR-223-3p. LINC00961 inhibits the expression of SOX11 and therefore, colon cancer by binding to miR-223-3p (Wang et al. Citation2019).
LINC00961 regulates the epithelial–mesenchymal transformation (EMT) of tumor cells. Chen et al. reported that of LINC00961 overexpression in nude mice inhibits the expression of SLUG and N-cadherin during the EMT process in RCC cells (Chen et al. Citation2019). In TSCC cells, overexpression of LINC00961 inhibits the expression of Snail, vimentin, and N-cadherin, and promotes the expression of E-cadherins. In addition, LINC00961 inhibits β-catenin expression. XAV-939, an inhibitor of Wnt/β-catenin pathway, reverses the effect of LINC00961 on the proliferation and invasion of SCC1 cells (Zhang et al. Citation2019b). Jiang et al. found that increasing LINC00961 reduced the expression of β-catenin protein (Jiang et al. Citation2018; Huang et al. Citation2018), indicating that LINC00961 is an important anti-metastatic lncRNA.
In addition, LINC00961 modulates tumor cell growth by regulating gene expression. Huang et al. showed that elevated LINC00961 levels could inhibit the expression of proliferating cell nuclear antigen (PCAN) and increase B cell leukemia/lymphoma 2 (Bcl2)-associated X (Bax). In contrast, LINC00961 induces the expression of PCAN and lowers the level of Bax using western blot analysis, if LINC00961 is disturbed (Huang et al. Citation2018). Pan et al. found that LINC00961 overexpression inhibits the expression of p-AKT and Bcl-2 proteins and promotes the expression of the Bax protein. The opposite effect occurs when LINC00961 expression is disturbed (Pan et al. 2019). The tumor suppressing effect of LINC00961 are through regulation of the expression of tumor-related genes through direct or indirect signaling pathways. Decreased expression of LINC00961 plays an important role in the progression of malignant tumors, inducing cancer cell proliferation, migration, invasion, and apoptosis.
LINC00961 expression affects the prognosis of patients
Not only is LINC00961 downregulated in lung cancer, liver cancer, RCC, and some other tumors, it also leads to poor prognosis of patients with cancer (Table ). The decreased expression of LINC00961 in patients with HCC is associated with tumor enlargement, lymph node metastasis (LNM), tumor node metastasis, and poor prognosis. Multivariate correlation analysis has shown that the level of LINC00961 is an independent risk factor for poor prognosis in HCC patients (Yin et al. Citation2019). The decreased expression of LINC00961 in patients with lung cancer is associated with clinical stage LNM and reduced overall survival (OS) and disease-free survival. The decreased expression of LINC00961 in cutaneous melanoma is associated with poor prognosis (Mu et al. Citation2019). LINC00961 expression is reduced in gliomas and is related to the WHO stage, Karnofsky performance score, and shorter OS (Lu et al. Citation2018). In addition, Chen et al. found decreased LINC00961 expression in tumor tissues of RCC, OSCC, and TSCC (Table ). The decreased expression of LINC00961 in human tumor tissues is related to poor prognosis of cancer patients, which indicates that LINC00961 can be a potential biomarker of cancer prognosis and a target for anti-tumor therapy.
Table 3. Expression of lncRNA LINC00961 in various cancers and its clinical significance.
Conclusions
Available data indicate that LINC00961 is a tumor suppressor, which is involved in the regulation of cell proliferation, apoptosis, migration, and invasion through regulation of the EMT process and Wnt/β-catenin signaling pathway, and interaction with genes coding for PCAN and p-AKT, and microRNAs, including miR-5581-3p, miR-367, miR-223-3p, miR-19a-3p, miR-19b-3p, and miR-125b-5p (Figure ). From the perspective of clinical application, insights into the heterogeneity of LINC00961 expression and its effects in different cancers are still emerging; however, its downregulation is closely related to poor prognosis of cancer. LINC00961 can therefore be used as a potential biomarker for cancer prognosis and as a target for cancer treatment. The expression level of LINC00961 in the serum and other biological samples from patients with tumors is yet to be investigated. Molecular targeted therapy shows stronger tumor specificity and lower systemic toxicity than traditional cytotoxic chemotherapy, and LINC00961, which is now known to be involved in proliferation, migration, invasion, and apoptosis of cancer cells, can be a novel target for cancer treatment. However, research on LINC00961 is still in its infancy, and the role of LINC00961 in cancer needs to be further investigated to provide a strong basis for the clinical application of LINC00961 in anti-tumor therapy. In general, LINC00961-directed interventions may enhance treatment outcomes in cancer patients.
Disclosure of interest
The authors report no conflicts of interest regarding the publication of this paper.
Authors’ contributions
Qiang Guo and Jia-Long Guo designed the plan. Kai Li and Yan-Mei Ji collected the data and wrote the manuscript. Jia-Long Guo, and Yan-Mei Ji provided guidance and corrections. The authors have approved the final manuscript and agreed to be accountable for all aspects of the work.
Availability of data and materials
Data sharing is not applicable to this article, as no new data were obtained or analyzed in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bhattacharjee S, Nandi S. 2017. Synthetic lethality in DNA repair network: A novel avenue in targeted cancer therapy and combination therapeutics. Iubmb Life. 69:929–937.
- Cao R, Yuan L, Ma B, Wang G, Qiu W, Tian Y. 2020. An EMT-related gene signature for the prognosis of human bladder cancer. J Cell Mol Med. 24:605–617.
- Chen D, Zhu M, Su H, Chen J, Xu X, Cao C. 2019. LINC00961 restrains cancer progression via modulating epithelial-mesenchymal transition in renal cell carcinoma. J Cell Physiol. 234:7257–7265.
- Chen WJ, Tang RX, He RQ, Li DY, Liang L, Zeng JH, Hu XH, Ma J, Li SK, Chen G. 2017. Clinical roles of the aberrantly expressed lncRNAs in lung squamous cell carcinoma: a study based on RNA-sequencing and microarray data mining. Oncotarget. 8:61282–61304.
- Ghafoor Q, Baijal S, Taniere P, O'Sullivan B, Evans M, Middleton G. 2018. Epidermal Growth Factor Receptor (EGFR) Kinase Inhibitors and Non-Small Cell Lung Cancer (NSCLC) -Advances in Molecular Diagnostic Techniques to Facilitate Targeted Therapy. Pathol Oncol Res. 24:723–731.
- Guo Q, Li D, Luo X, Yuan Y, Li T, Liu H, Wang X. 2021. The Regulatory Network and Potential Role of LINC00973-miRNA-mRNA ceRNA in the Progression of Non-Small-Cell Lung Cancer. Front Immunol. 12:684807.
- Hsu PC, Jablons DM, Yang CT, You L. 2019. Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC). Int J Mol Sci. 20(15):3821–3839.
- Huang Z, Lei W, Tan J, Hu HB. 2018. Long noncoding RNA LINC00961 inhibits cell proliferation and induces cell apoptosis in human non-small cell lung cancer. J Cell Biochem. 119:9072–9080.
- Jiang B, Liu J, Zhang YH, Shen D, Liu S, Lin F, Su J, Lin QF, Yan S, Li Y, et al. 2018. Long noncoding RNA LINC00961 inhibits cell invasion and metastasis in human non-small cell lung cancer. Biomed Pharmacother. 97:1311–1318.
- Lee YT, Tan YJ, Oon CE. 2018. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 834:188–196.
- Liu X, Shen X, Zhang J. 2022. Long non-coding RNA LINC00514 promotes the proliferation and invasion through the miR-708-5p/HOXB3 axis in cervical squamous cell carcinoma. Environ Toxicol. 37(1):161–170.
- Lu XW, Xu N, Zheng YG, Li QX, Shi JS. 2018. Increased expression of long noncoding RNA LINC00961 suppresses glioma metastasis and correlates with favorable prognosis. Eur Rev Med Pharmacol Sci. 22:4917–4924.
- McCoach CE, Blumenthal GM, Zhang L, Myers A, Tang S, Sridhara R, Keegan P, Pazdur R, Doebele RC, Kazandjian D. 2017. Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann Oncol. 28:2707–2714.
- Mehrpour LS, Arabpour M, Esmaeili R, Esmaeili R, Naghizadeh MM, Tavakkoly Bazzaz J, Shakoori A. 2020. Evaluation of the potential role of long non-coding RNA LINC00961 in luminal breast cancer: a case-control and systems biology study. Cancer Cell Int. 20:478.
- Mu X, Mou KH, Ge R, Han D, Zhou Y, Wang LJ. 2019. Linc00961 inhibits the proliferation and invasion of skin melanoma by targeting the miR-367/PTEN axis. Int J Oncol. 55:708–720.
- Pan LN, Sun YR. 2019. LINC00961 suppresses cell proliferation and induces cell apoptosis in oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 23:3358–3365.
- Parolia A, Venalainen E, Xue H, Mather R, Lin D, Wu R, Pucci P, Rogalski J, Evans JR, Feng F, et al. 2019. The long noncoding RNA HORAS5 mediates castration-resistant prostate cancer survival by activating the androgen receptor transcriptional program. Mol Oncol. 13:1121–1136.
- Shang AQ, Wang WW, Yang YB, Gu CZ, Ji P, Chen C, Zeng BJ, Wu JL, Lu WY, Sun ZJ, Li D. 2019. Knockdown of long noncoding RNA PVT1 suppresses cell proliferation and invasion of colorectal cancer via upregulation of microRNA-214-3p. Am J Physiol Gastrointest Liver Physiol. 317:G222–G232.
- Shen S, Li K, Liu Y, Liu B, Ba Y, Xing W. 2020. Silencing lncRNA AGAP2-AS1 Upregulates miR-195-5p to Repress Migration and Invasion of EC Cells via the Decrease of FOSL1 Expression. Mol Ther Nucleic Acids. 20:331–344.
- Siegel RL, Miller KD, Jemal A. 2020. Cancer statistics, 2020. CA-Cancer J Clin. 70(1):7–30.
- Sui J, Zhao Q, Zhang Y, Liang G. 2022. Dysregulated LINC00961 Contributes to the Vitality and Migration of NSCLC Via miR-19a-3p/miR-19b-3p/miR-125b-5p. DNA Cell Biol. 41(3):319–329.
- Takai M, Terai Y, Kawaguchi H, Ashihara K, Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M, et al. 2014. The EMT (epithelial-mesenchymal- transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. 7:76–83.
- Wang J, Su Z, Lu S, Fu W, Liu Z, Jiang X, Tai S. 2018a. LncRNA HOXA-AS2 and its molecular mechanisms in human cancer. Clin Chim Acta. 485:229–233.
- Wang J, Zhou J, Jiang C, Zheng J, Namba H, Chi P, Asakawa T. 2019. LNRRIL6, a novel long noncoding RNA, protects colorectal cancer cells by activating the IL-6-STAT3 pathway. Mol Oncol. 13:2344–2360.
- Wang L, Kong W, Liu B, Zhang X. 2018b. Proliferating cell nuclear antigen promotes cell proliferation and tumorigenesis by up-regulating STAT3 in non-small cell lung cancer. Biomed Pharmacother. 104:595–602.
- Wu CT, Liu S, Tang M. 2019. Downregulation of linc00961 contributes to promote proliferation and inhibit apoptosis of vascular smooth muscle cell by sponging miR-367 in patients with coronary heart disease. Eur Rev Med Pharmacol Sci. 23:8540–8550.
- Wu H, Dai Y, Zhang D, Zhang X, He Z, Xie X, Cai C. 2020. LINC00961 inhibits the migration and invasion of colon cancer cells by sponging miR-223-3p and targeting SOX11. Cancer Med. 9:2514–2523.
- Xu J, Yang B, Wang L, Zhu Y, Zhu X, Xia Z, Zhao Z, Xu L. 2020. LncRNA BBOX1-AS1 upregulates HOXC6 expression through miR-361-3p and HuR to drive cervical cancer progression. Cell Prolif. 53:e12823.
- Yan H, Li H, Silva MA, Guan Y, Yang L, Zhu L, Zhang Z, Li G, Ren C. 2019. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J Exp Clin Cancer Res. 38:356–368.
- Yi C, Wan X, Zhang Y, Fu F, Zhao C, Qin R, Wu H, Li Y, Huang Y. 2018. SNORA42 enhances prostate cancer cell viability, migration and EMT and is correlated with prostate cancer poor prognosis. Int J Biochem Cell Biol. 102:138–150.
- Yin J, Liu Q, Chen C, Liu W. 2019. Small regulatory polypeptide of amino acid response negatively relates to poor prognosis and controls hepatocellular carcinoma progression via regulating microRNA-5581-3p/human cardiolipin synthase 1. J Cell Physiol. 234:17589–17599.
- Zhang L, Shao L, Hu Y. 2019b. Long noncoding RNA LINC00961 inhibited cell proliferation and invasion through regulating the Wnt/β-catenin signaling pathway in tongue squamous cell carcinoma. J Cell Biochem. 120:12429–12435.
- Zhang Y, Zhang J, Mao L, Li X. 2020. Long noncoding RNA HCG11 inhibited growth and invasion in cervical cancer by sponging miR-942-5p and targeting GFI1. Cancer Med. 9(19):7062–7071.
- Zhang Z, Shao L, Wang Y, Luo X. 2019a. MicroRNA-501-3p restricts prostate cancer growth through regulating cell cycle-related and expression-elevated protein in tumor/cyclin D1 signaling. Biochem Biophys Res Commun. 509:746–752.
- Zheng C, Li X, Ren Y, Yin Z, Zhou B. 2019. Long Noncoding RNA RAET1K Enhances CCNE1 Expression and Cell Cycle Arrest of Lung Adenocarcinoma Cell by Sponging miRNA-135a-5p. Front Genet. 10:1348.
- Zhou Q, Guo J, Huang W, Yu X, Xu C, Long X. 2020. Linc-ROR promotes the progression of breast cancer and decreases the sensitivity to rapamycin through miR-194-3p targeting MECP2. Mol Oncol. 14:2231–2250.