?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The study aimed to investigate the effect of fermented n-hexane extract of Pentaclethra macrophylla seeds (FPMSE) in an experimental model of glaucoma. Eighteen rabbits (1-1.8 kg body weight) were allocated into six groups of three rabbits (six eyes per group). Group 1 was the normal control. Ocular hypertension was induced with 1% prednisolone acetate ophthalmic solution in groups 2-6, twice daily for 21 days. Group 2 received no treatment, while groups 3–6 received 5 mg/kg b.w. acetazolamide, 100, 200, and 300 mg/kg b.w. extract, respectively. The intraocular pressures and other biochemical parameters were evaluated. The existence of bioactive compounds was determined via phytochemical analysis. Compared to group 2, the administration of FPMSE resulted in a significant decrease in IOP in the treated groups. Malondialdehyde concentrations in the treated groups’ aqueous humor and plasma were considerably lower (p < 0.05) than in group 2. Compared to group 2, glutathione, nitric oxide, and antioxidant levels improved significantly (p < 0.05). C-reactive protein and glutamate levels in group 2 were comparable (p > 0.05). FPMSE substantially reduced phospholipase A2 activity. Histological assessment of the ciliary body showed adequate pigmentations in the treatment groups compared to group 2. Hence, FPMSE possesses ocular hypotensive effects.
Introduction
Glaucoma affects roughly 60 million persons worldwide and is the next leading cause of blindness after cataract (Debjit et al. Citation2012; Natalie et al. Citation2015). Glaucoma is mainly due to injury to the optic nerve which conducts visual information from the eye to the brain (Debjit et al. Citation2012). The injury to the optic nerve is typically ascribed to elevated pressure in the eye. Intraocular pressure (IOP) increases due to fluctuations in aqueous humor dynamics caused by variations in the trabecular meshwork, which causes damage of the aqueous humor drainage system. Since a rise in IOP adds to retinal ganglion cells (RGC) death, lowering IOP can aid in halting the onset of glaucoma (Renu et al. Citation2009). Due to multifaceted interaction of factors, the malfunction and loss of RGC in glaucomatous eyes lead to irremediable visual loss (Kaushik et al. Citation2003).
Risk factors associated with glaucoma include elevated IOP, oxidative stress, inflammation processes, metabolic irregularities, blood flow dysfunction, excess glutamate levels, altered nitric oxide metabolism and vascular alterations. The ocular tissue is rich in reduced glutathione (GSH) and superoxide dismutase-catalase system, which supplies it with an effective antioxidant defense mechanism (Renu et al. Citation2009). The antioxidant potential in the aqueous humor of glaucoma patients has been found to be severely decreased (Ferreira et al. Citation2004).
Plants have been shown to possess bioactive compounds with medicinal potentials; some of these plants’ derived secondary metabolites have been shown to have fewer side effects compared to synthetic drugs (El-Shemy et al. Citation2007). Bioactive components derived from plants may be the remedy and the best approach for reducing the consumption of unhealthy items (Russell and Duthie Citation2011). Because of its rich bioactive compounds, Pentaclethra macrophylla Benth, is a key source of raw materials for the nutrition and pharmaceutical sectors (Osabor et al. Citation2017). The African oil bean tree (Pentaclethra macrophylla Benth) is a tropical tree crop that thrives in the wild in West Africa's southern rainforest zone. The hard seeds, coated with smooth flat brown coats, are contained in a long-flattened green pod. Oil bean seeds become edible after processing and fermentation (Cangao Citation2011). In Southeastern Nigeria, the seeds are boiled, decoated, cut into small pieces, and fermented to make Ugba, which is used to make a variety of delicious African dishes such as African salad, soups, and sausages. African oil bean is also known as ‘Congo acacia’ in Congo, ‘Duala Kombola’ in Cameroon, Apara in Yoruba, Ugba or Ukpaka in the Southeastern part of Nigeria (Enujiugha and Akanbi Citation2005; Nwanjo et al. Citation2006).
Research has shown that the African oil bean has antimicrobial, wound-healing, antidiarrhoea (Alinnor and Oze Citation2011) and anti-coccidiosis potential (Cedric et al. Citation2018). According to Anioke (Citation2019), African oil bean seeds have protective potential against atherosclerosis. Ethnomedicinal practitioners in Southeastern Nigeria apply a decoction of the plant for treating peptic ulcers (Ugbogu et al. Citation2017). In high-fat diet and streptozotocin-induced diabetic rats, treatment with Pentaclethra macrophylla seeds exhibited hypolipidemic potential (Nwosu et al. Citation2017). The seed extract may possess hematopoietic and immunity-boosting effects and could be developed for future clinical use in managing blood-related conditions (Ufelle et al. Citation2015). There is an ethnomedicinal record in Amaeze Okpanku in Aninri Local Government Area of Enugu State, Nigeria that fermented Pentaclethra macrophylla seeds aid vision. However, this claim has not been scientifically validated; hence, investigating the effects of the seeds on IOP, a strong risk factor of glaucoma, is warranted.
Materials
Plant materials
Newly harvested seeds of Pentaclethra macrophylla were acquired from Ogige market in Nsukka, Enugu State, Nigeria. Mr. Alfred Ozioko of the Bioresources Development and Conservation Program (BDCP), a research center in Nsukka, Enugu State, validated the seeds. The seeds were processed traditionally before being air-dried and then pulverized into powdered form. A voucher specimen, Intercedd/1572, has been deposited at the BDCP's herbarium ().
Animals
Eighteen experimental rabbits with a weight range between 1000 g and 1800g were obtained from the Animal Household of the Faculty of Veterinary Medicine, University of Nigeria, Nsukka. The rabbits were allowed to acclimatize for a week prior to the start of the experimentation with a 12 h bright and dim cycle, and were kept on a regular feed (standard pellet) and water ad libitum. The study protocol received the approval of the Faculty Committee on Ethics and Biosafety, Faculty of Biological Sciences, University of Nigeria (Ethical clearance number: UNN/FBS/EC/1071).
Chemicals and reagents
All chemicals and reagents used in this research were diagnostic grades (Sigma Aldrich in the United States; British Drug House (BDH) in England; Burgoyne in India; Harkin and Williams in England; Qualikems in India; Fluka in Germany; and May and Baker in England). Commercial kits used for biochemical assay were products of Randox and Teco diagnostics (TC) in the United States. Prednisolone acetate (Scott-Edil Pharmacia Ltd, India) and acetazolamide (Wyeth Lederie, India) were obtained from a well-known pharmaceutical store in Nsukka, Nigeria.
Methods
Traditional production of Ugba
The traditional method of Ugba preparation previously described by Obeta (Citation1983) was slightly modified. The oil bean seeds were thoroughly sorted, washed, and boiled at 100 0C for 2 hr to soften the coat and enhance dehulling. The boiled seeds were dehauled after cooling and the cotyledons were sliced longitudinally into 3.5-5.0 cm x 1.0-1.1 cm pieces, washed, and boiled in water at 1000C for 1 hr. The slices were then drained and soaked in water for 8 hr, washed severally in potable water, and allowed to drain in a sieve. The slices were fermented in an air-tight bucket for two days. After that, the fermented seeds were allowed to dry on an aerated basket at room temperature for 23 days and pulverized.
The pulverized seeds (4 kg) were macerated in n-hexane (7.5 L) for three days and filtered through a mesh and then with Whatman No. 1 filter paper (to remove fine particles). The solvent (hexane) in the filtrate was removed using a rotary evaporator at 450C to obtain an oily extract subsequently referred to as fermented Pentaclethra macrophylla seed extract (FPMSE).
Determination of the percentage yield of FPMSE
The weight of the dried powdered plant before maceration and the weight of the crude extract after concentration were used to calculate the percentage yield of the n-hexane extract using the formula below;
Phytochemical analysis of the FPMSE
The presence of bioactive molecules in the extract was confirmed via phytochemical analysis of FPMSE. Trease and Evans (Citation1989) and Harborne’s (Citation1973) guidelines were followed.
Determination of mineral and vitamin compositions of the FPMSE
The mineral compositions (Fe, Zn, Ca, K, Na and nitrates) and vitamin contents of both fermented and pulverized seed and its n-hexane extract were determined as described by Association of Official Analytical Chemists (AOAC Citation1990).
Acute toxicity study of the FPMSE
The n-hexane extract's acute toxicity study or median lethal dosage (LD50) of FPMSE was determined as described by Lorke (Citation1983). Eighteen male albino mice were used to determine the acute toxicity of FPMSE. The test involved two phases: in the first phase, nine mice were divided into three groups of three mice each and were treated with FPMSE at doses of 10, 100, and 1000 mg/kg body weight respectively. The mice were observed for 24 h for obvious toxic symptoms, morbidity, and mortality. In the second phase, nine mice were grouped into three groups of three mice each and were also treated with FPMSE at doses of 1600, 2900, and 5000 mg/kg body weight respectively. The administration of the extract was done orally using an oral intubation tube. The animals were also observed for 24 h for the signs reported previously. The LD50 was determined using the formula:
Where D0 is the highest dose that recorded no mortality while D100 is the lowest dose that produced mortality.
Experimental design for the anti-glaucoma study
Eighteen rabbits were randomized into six groups of three rabbits (6 eyes per group) and were treated as follows:
Group 1: normal control (received standard feed and water)
Group 2: untreated control (prednisolone acetate-induced glaucoma rabbits without treatment)
Group 3: standard control (prednisolone acetate-induced glaucoma rabbits + 5 mg/kg b.w. of acetazolamide)
Group 4: low-dose test treatment (prednisolone acetate-induced glaucoma rabbits + 100 mg/kg b.w of FPMSE)
Group 5: mid-dose test treatment (prednisolone acetate-induced glaucoma rabbits + 200 mg/kg b.w. of FPMSE)
Group 6: high-dose test treatment (prednisolone acetate-induced glaucoma rabbits + 300 mg/kg b.w. of FPSME)
Determination of intraocular pressure (IOP)
Prior to the commencement of the induction of ocular hypertension/glaucoma, the IOP of all the rabbits were taken using Biro Schoitze Tonometer (CE0483 Model, Germany) as described by Melena et al. (Citation1998). Before taking the IOP measurements, each rabbit was anesthetized with drops of lignocaine injection (BP 2%) and held adequately such that the cornea was widely exposed. The Schoitze tonometer was calibrated to zero level before using 5.5 g weight. Two weights (7.5 and 10 g) were used to ensure accurate measurement. The tonometer was placed gently on the cornea while carefully raising the nictating membrane. The readings obtained were converted to mmHg using the conversion charts, and the average values for both weights (7.5 and 10 g) were recorded. The reduction in IOP was calculated using the formula:
Where: IOPt0 = IOP before treatment; IOPt = IOP at different times; IOP = intraocular pressure.
Induction of ocular hypertension
Induction of ocular hypertension/glaucoma was carried out as described by Melena et al. (Citation1998). The rabbits were randomly grouped into six, as shown above. Group one served as the normal control while groups 2–6 were induced with ocular hypertension/glaucoma topically using 1% prednisolone acetate drops (2 drops, ∼ 40 µL each) twice daily between 9 am and 5 pm for 21 days. Following confirmation of IOP increase, group 3 was given 5 mg/kg b.w. acetazolamide (reference drug), whereas groups 4–6 were given n-hexane extract at 100 mg/kg b.w., 200 mg/kg b.w., and 300 mg/kg b.w. respectively. The therapy lasted two weeks, with IOP measurements obtained every three days. To eliminate diurnal variations in IOP, all tonometer readings were carried out at the same time each day, approximately 8 am. At the end of the treatment, the rabbits were anesthetized, and blood was collected from each rabbit's marginal ears and eyes for biochemical analyses. Aqueous humor was carefully drawn from the eyes, and after which the eyes were harvested for histological assessment.
Determination of biochemical parameters
The concentrations of nitric oxide (NO), C-reactive protein (CRP), glutamate, glutathione, vitamins (A, C, and E), sodium ion (Na+), potassium ion (K+), and chloride ion (Cl-) were determined using the methods of Bryan and Grisham (Citation2007), Andersen and McCarthy (Citation1950), Bernt and Bergmeyer (Citation1965), King and Wootton (Citation1959), Pearson (Citation1976), Trinder (Citation1951) and Maruna (Citation1958), Terri and Sesin (Citation1985), and Tietz (Citation1976), respectively. The lipid peroxidation status was determined by measuring the malondialdehyde (MDA) level using Wallin et al. (Citation1993) method, while the activities of superoxide dismutase (SOD) and catalase were assayed according to the methods of Xin et al. (Citation1991) and Aebi (Citation1983) respectively. The inhibitory phospholipase A2 activity was assayed using Iwueke et al. (Citation2006) method.
Histological assessment
Histological assessment was carried out as described by Bancroft and Stevens (Citation1977). The animals’ enucleated eyeballs were fixed in 10% neutral buffered formalin and embedded in paraffin for histological analysis. After that, sections were made and stained with hematoxylin and eosin. Images were captured with a 12.6 MP Motic camera attached to an Olympus BX-51 microscope (China).
Statistical analysis
The data were analyzed with Statistical Product for Service Solutions (SPSS) version 20 and reported as means and standard deviations (SD). Both one-way and two-way analysis of variance (ANOVA) (IOP analysis) and t-test (comparing raw sample and extract) were used to determine statistical significance; p values < 0.05 were considered statistically significant.
Results
Bioactive constituents
Four thousand grams (4000 g) of the powdered fermented Pentaclethra macrophylla seeds extracted with 100% n-hexane yielded 837 g of the n-hexane extract which constituted 20.93% of the total weight of dried plant sample used. The preliminary qualitative and quantitative analysis of the FPMSE revealed high amounts of terpenoids and alkaloids. At the same time, flavonoids, total phenolics and volatile oil were found at moderate levels. Carotenoids, saponins, tannins, and cardiac glycosides were found in relatively small amounts, while glycosides were not detected. (Table )
Table 1. Phytochemical composition of FPMSE.
Acute toxicity of FPMSE
In an acute toxicity test between dosages of 10–5000 mg/kg body weight, the n-hexane extract of FPMSE demonstrated no fatality or an adverse response. This demonstrated the extract's relative safety or non-toxic nature at that concentration range.
Mineral composition of pulverized fermented Pentaclethra macrophylla seeds (raw) and FPMSE
Mineral composition of both raw and extract of Pentaclethra macrophylla Benth revealed the presence of some minerals in this order: nitrates > Na > Fe > K> Zn > Ca for the sample and Na > K > nitrates >Zn > Fe > Ca for n-hexane extract (Table ).
Table 2. Mineral composition of pulverized fermented Pentaclethra macrophylla seeds (raw) and FPMSE.
Vitamin composition of pulverized fermented Pentaclethra macrophylla seeds (raw) and FPMSE
Table reveals the vitamin compositions of both raw and extract of FPMSE. Vitamin C content in the raw sample was found to be significantly (p < 0.05) higher than the extract, while vitamin E content in the extract was significantly (p < 0.05) higher compared to the raw sample. There was no significant (p > 0.05) difference between the concentration of vitamin A in both samples.
Table 3. Vitamin composition of pulverized fermented Pentaclethra macrophylla seeds (raw) and FPMSE.
Effect of FPMSE on the intraocular pressures of steroid-induced glaucoma rabbits
Table below shows the effect of FPMSE on the IOP of prednisolone-induced glaucoma in rabbits. From the result, there was a significant (p < 0.05) reduction in the group treated with the n-hexane extract compared to the untreated (group 2). Group 6 (300 mg/kg b.w. of extract) was found to have the highest (p < 0.05) percentage reduction followed by standard group 3 (5 mg/kg b.w. of acetazolamide). Group 4 (100 mg/kg b.w. of extract) and group 5 (200 mg/kg b.w. of extract) were found to be significantly (p < 0.05) higher than the untreated group (group 2) (Table ).
Table 4. Effect of FPMSE on the intraocular pressures of steroid-induced glaucoma rabbits.
Effect of FPMSE on both aqueous humor and serum/plasma concentrations of lipid peroxidation marker, MDA, and some antioxidant parameters of steroid-induced glaucoma rabbits
The effects of FPMSE on both aqueous humor and plasma MDA concentrations revealed a significant (p < 0.05) reduction in the treated groups compared to group 2 (untreated animals) (Table ). Serum GSH, SOD, and catalase were found to be higher though not significant (p > 0.05) compared to the untreated animals (Table ). Aqueous humor concentration of GSH was found to be significantly (p < 0.05) higher at 200 mg/kg b.w (group 5) compared to the untreated group (group 2). Meanwhile, there was no significant (p > 0.05) difference in GSH concentrations among other groups.
Table 5. Effect of FPMSE on both aqueous humor and serum/plasma concentrations of lipid peroxidation marker, MDA, and some antioxidant parameters of steroid-induced glaucoma rabbits.
Effect of FPMSE on the plasma vitamin concentrations of steroid-induced glaucoma rabbits
Table below shows the effect of the FPMSE on the antioxidant vitamins A, C, and E. From the result, there was a significant (p < 0.05) increase in the concentration of serum vitamins A, C, and E compared to the untreated group (group 2).
Table 6. Effect of FPMSE on the plasma vitamin concentrations of steroid-induced glaucoma rabbits.
Effect of FPMSE on both serum and aqueous humor concentrations of NO, glutamate and CRP of steroid-induced glaucoma rabbits
Table below shows the effect of FPMSE on both aqueous humor and serum NO, glutamate and CRP concentrations of prednisolone-induced glaucoma rabbits. From the table, the NO concentrations of the treatment groups in both aqueous humor and serum were found to be significantly (p < 0.05) higher than the untreated group (Group 2). There was no significant (p > 0.05) difference within the glutamate and CRP concentrations in both aqueous humor and serum (Table ).
Table 7. Effect of FPMSE on both serum and aqueous humor concentrations of NO, glutamate and CRP of steroid-induced glaucoma rabbits.
Effect of FPMSE on blood glucose and electrolyte levels of both aqueous humor and serum of steroid-induced glaucoma rabbits
Table shows the effects of FPMSE on blood glucose and some serum electrolytes (Na+, K+, Cl-, HCO3-) of prednisolone-induced glaucoma rabbits. There was no significant (p > 0.05) increase in the glucose levels of the treated group compared to the untreated. However, there was an increase in the treated animals though not significant. The concentration of serum Na+ of the treated group was found to be significantly (p < 0.05) lower than the untreated at the highest concentration and 100 mg/kg b.w. though not significant (p > 0.05) in the aqueous humor. On the other hand, there was a significant (p < 0.05) increase in the concentration of serum K+ of the treated group (group 4) compared to the untreated (group 2) though not significant (p > 0.05) in the aqueous humor. There was no significant (p > 0.05) difference in the concentrations of serum Cl- and HCO3- across the animals however there was a relative reduction in bicarbonate of the standard group and treated animal (group 4). On the other hand, there was no significant (p > 0.05) difference in aqueous humor Cl- concentration, while there was a significant (p < 0.05) reduction in bicarbonate concentration at 200 mg/kg b.w. compared to the untreated.
Table 8. Effect of FPMSE on blood glucose and electrolyte levels of both aqueous humor and serum of prednisolone-induced glaucoma rabbits.
Histological findings
The histological findings of the longitudinal section of the eyes were represented on Plates 1–4. In Plate 1 (normal control), the ciliary processes with pigmented ciliary epithelia (PCE) (white arrow), ciliary muscle (CM), and sclera (S) (black arrow) are present. However, in Plate 2 (untreated), the ciliary processes (black arrow) with reduced pigmented ciliary epithelia (PCE) and ciliary muscle (CM) (star) are observed. In Plate 3 (standard control), there is a reduction in pigmentation in the simple columnar epithelium of the inner layer (white arrow). The folds are not reduced in number but reduced in size. The bundles of smooth muscle (SM) (black arrow) are arranged longitudinally. A few vacuolated (V) (red arrow) spaces within the ciliary muscle are observed. In Plate 4 (100 mg/kg b.w. of FPSME), ciliary processes (blue arrow) are increased in size and infoldings (blue arrow). The simple columnar epithelium in inner pigmented layer is adequate with melanocytes (white arrow). Smooth muscle (SM) (black arrow) of the ciliary body is also present.
Plate 1. (Normal): Ciliary processes with pigmented ciliary epithelia (PCE) (white arrow). Ciliary muscle (CM). Sclera (S) (black arrow) present. H&E. mag. 100X
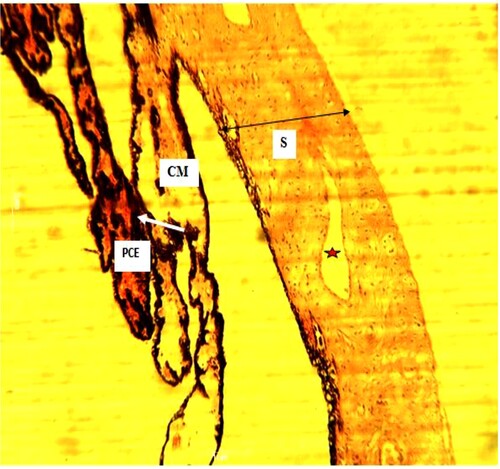
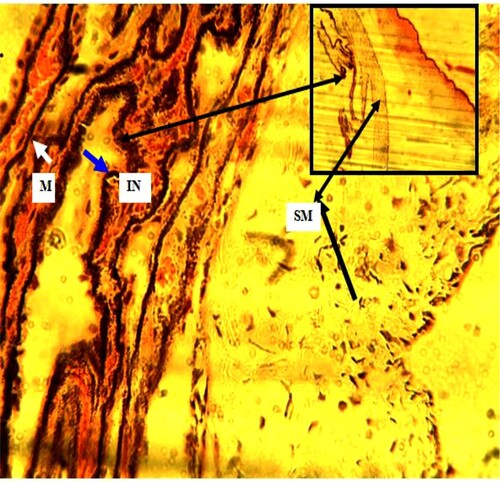
Plate 2. (Untreated): Ciliary processes (black arrow) with reduced pigmented ciliary epithelia (PCE). Ciliary muscle (CM) (star) present. H&E. mag. 100X
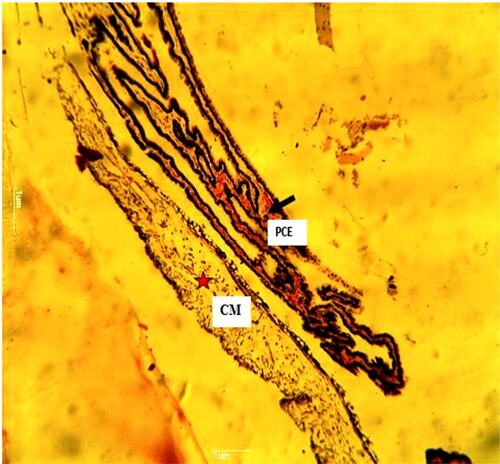
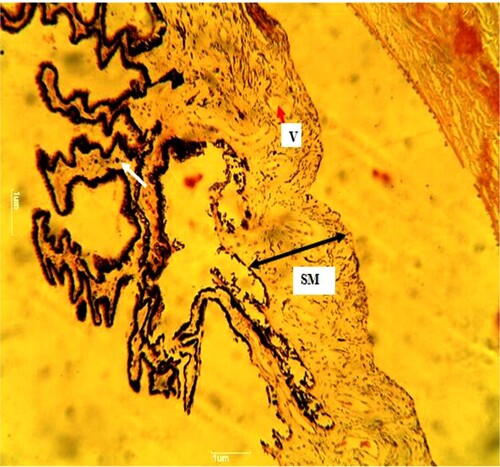
Plate 3. (Standard control): Reduced pigmentation in the simple columnar epithelium of the inner layer (white arrow). The folds are not reduced in number but reduced in size. The bundles of smooth muscle (SM) (black arrow) are arranged longitudinally. A few vacuolated (V) (red arrow) spaces within the ciliary muscle. H&E. mag. 100X.
Phospholipase A2 inhibitory activity of FPMSE
The FPMSE significantly (p < 0.05) inhibited the activity of PLA2 in a concentration-related manner from 1.25–40 mg/ml compared to the control, as shown by a reduction in absorbance of the supernatant solution. There were no significant (p > 0.05) differences in the inhibitory effect with higher concentrations of the extract (20 and 40 mg/ml) compared to prednisolone. However, there existed a slightly higher inhibition with 40 mg/ml of the extract compared to prednisolone (Table ).
Table 9. Phospholipase A2 inhibitory activity of FPMSE.
Discussion
Preliminary qualitative and quantitative analysis showed that FPMSE was high in terpenoids and alkaloids, while flavonoids, total phenols, and volatile oils were detected in moderate amounts. Carotenoids, saponins, tannins, and cardiac glycosides were all detected in small amounts, but glycosides were not. The presence of bioactive compounds in the fermented seed and its extract agrees with the report of Osabor et al. (Citation2017) and Cedric et al. (Citation2018) that recorded the presence of saponins, flavonoids, alkaloids, cardiac glycosides, polyphenols, and reducing sugar in the plant extracts. Similarly, Akinlabu et al. (Citation2019) reported some of these phytochemicals; however, their study did not detect steroids, flavonoids, and tannins, which could be attributed to the extraction method, among other factors. Phytochemicals have been shown to have a strong therapeutic role against some diseases, including asthma, arthritis, cancer, and other diseases, when consumed at safe doses with little or no side effects (Banu and Cathrine Citation2015). Moreover, phytochemicals are beneficial in the prevention and treatment of diabetes, high blood pressure, and macular degeneration (American Cancer Society Citation2000). Terpenoids have been reported to have antibacterial, antineoplastic, and other pharmaceutical functions (Nita et al., Citation2014). Therapeutic potentials of terpenoids, such as antiplasmodial, anticancer, antioxidant, and anti-inflammatory, among others, have been documented (Cox-Georgian et al. Citation2019). Alkaloids have also been shown to have antihypertensive, antiarrhythmic, antimalarial, anticancer, and hypotensive properties (Wynter-Adams et al. Citation1999; Mamta et al. Citation2013). The hypotensive effects of alkaloids could be linked to the ocular hypotensive potentials of the n-hexane extract (Woolley Citation2001). Flavonoids possess antioxidant activity which is crucial in boosting the eye's endogenous antioxidant system to prevent an oxidative assault on the eye (Kyei et al. Citation2015). The presence of flavonoids in the n-hexane extract suggests that the extract may be beneficial against ocular disorders. Macular pigment carotenoids such as lutein, zeaxanthin, and meso-zeaxanthin are broadly suggested as nutritional supplements for the prevention of vision loss caused by age-related macular degeneration and other eye defects (Paul et al. Citation2016). Hence, the presence of carotenoids in the n-hexane extract of FPMSE could contribute to the eyes’ overall health, including aiding vision and abating glaucoma-associated complications. Saponins have been demonstrated to have some hypotensive activity (Hiwatashi et al. Citation2010), and the extract containing saponins may help lower intraocular pressure.
The acute toxicity test of the FPMSE showed no death or adverse reaction up to 5000 mg/kg b.w. of the extract. The finding is consistent with Anioke (Citation2019) and Ugbogu et al. (Citation2017), who independently showed that the LD50 of the seed extracts is higher than 5000 mg/kg b.w. Hence, our findings and others show that the seed is relatively safe and supports its use as part of some local delicacies in many parts of Nigeria.
The presence of the minerals with health benefits as enzyme co-factors observed in this study agrees with Ikhuoria et al. (Citation2008) and Osabor et al. (Citation2017), who reported high calcium, iron, sodium, and potassium levels, and Igwenyi et al. (Citation2015) who reported high nitrate level. Mbah et al. (Citation2018) reported the presence of calcium, sodium, zinc, and iron, which were also found in this study. Minerals are micro-elements that are necessary for good health. Their presence in FPMSE could serve as a source of minerals in the body. Dietary nitrate has been shown to offer several vascular benefits, including lowering blood pressure, blocking platelet aggregation, and improving endothelial dysfunction and exercise performance in healthy and peripheral artery disease patients (Satnam and Andrew Citation2012). The high nitrate content identified in FPMSE may contribute to lowering IOP. According to Tribble et al. (Citation2021), metabolic deficits and abnormalities may play a key role in glaucoma pathogenesis; hence, the presence of minerals with some metabolic benefits as enzyme co-factors in the extract may contribute to ameliorating the complications associated with glaucoma.
Elevation in IOP is a crucial factor in RGC mortality; therefore, reduced IOP slows the advancement of glaucoma degenerative processes (Renu et al. Citation2009). The results of this study demonstrated a substantial (p < 0.05) drop in IOP in the FPMSE-treated group compared to the control group (group 2). Significant reduction in IOP was found in groups treated with 300 mg/kg b.w. of the extract compared to groups who received lower doses, indicating that the extract has a dose-dependent effect. The result compares well with George et al. (Citation2018), who assessed the ocular hypotensive potential of intraocular and blood pressure effects of Moringa oleifera leaf aqueous extract in normotensive individuals in Edo State, Nigeria. The decrease in intraocular and blood pressure observed in the treatment groups in this study showed that the n-hexane extract has a hypotensive property which could be attributable to its rich bioactive components and nutrients with ocular hypotensive capacities, such as flavonoids, terpenoids, and nitrates. Our findings also agree with Kyei et al. (Citation2015), who found that aqueous extract of whole plant extract of H. indicum significantly reduced the IOP of prednisolone acetate-induced experimental glaucoma rabbits.
The effects of FPMSE on both aqueous humor and plasma MDA concentrations revealed a significant (p < 0.05) reduction in the treated groups compared to group 2 (untreated animals). The MDA is a major marker of lipid peroxidation levels and oxidative stress (Kayar et al. Citation2015). The plasma MDA levels found in this study accord with Yildirim et al. (Citation2004), who suggested that changes in plasma MDA levels might be linked to the etiology of primary open angle glaucoma (POAG). MDA, a lipid peroxidation biomarker, was found to be considerably higher in the glaucoma group's serum and aqueous humor (Mumcu et al. Citation2016; Wojciech et al. Citation2017). The preceding finding is consistent with the findings of this investigation. As a result, the reduced MDA concentration in the treated groups compared to the untreated groups demonstrated FPMSE's ability to resist oxidative stress, a risk factor for glaucoma.
Serum GSH, SOD, and catalase were found to be higher though not statistically significant (p > 0.05) compared to the untreated animals. The aqueous humor concentration of GSH was significantly (p < 0.05) higher at 200 mg/kg b.w (group 5) compared to the untreated group (Group 2). This supports the findings that antioxidant enzymes such as SOD, catalase, and glutathione enzyme system (GSHPx), as well as total antioxidant activity, were reduced in the aqueous humor of POAG patients. The aqueous humor comprises substantial amounts of glutathione, which plays a significant role in defending the body from oxidative stress-induced illnesses (Richer and Rose Citation1998). The serum glutathione result corresponds with the findings of Kyei et al. (Citation2015), who found that the extract sustained GSH levels, implying that it is effective not only in lowering IOP but also in protecting against glaucomatous neurodegeneration, the product of oxidative damage (Kyei et al. Citation2015).
The effect of FPMSE on the serum antioxidant vitamins - A, C, and E revealed that there was a significant (p < 0.05) increase in the concentration of vitamins A, C, and E compared to the untreated group (group 2). This could be attributable to the rich vitamin contents in FPMSE. In normal cells, a natural defensive system including enzymatic antioxidants (glutathione peroxidase, SOD, catalase) and non-enzymatic antioxidants (vitamins C and E, glutathione, selenium) neutralizes or removes oxygen derivatives (Małgorzata et al. Citation2005). Vitamins are organic substances that are required in small amounts to keep the body's basic functions running (Bennasir et al., Citation2010). The synthesis of retinal pigments in the eye is a well-known basic function of vitamin A. Vitamin A (retinol) and its biologically active derivatives, retinoids, control important processes like cell growth inhibition, differentiation, apoptosis, embryo shape, and organogenesis (Hassen et al., 2010). Vitamin A is a key antioxidant that does a functional duty in protecting the body from oxidative damage caused by free radicals (Ramadhan and Ian Citation2012). The osmotic impact of vitamin C has been proven to reduce IOP in high doses (Kathleen Citation2001). Hence, the capacity of FPMSE to increase the concentration of these antioxidant vitamins will contribute to maintaining the oxidative state of the eye and mopping up excess free radicals associated with the pathogenesis of glaucoma.
Findings from this study revealed that the NO concentrations of the treatment groups in both aqueous humor and serum were found to be significantly (p < 0.05) higher than the untreated group (Group 2). There was no significant (p > 0.05) difference between the glutamate and CRP concentrations in the aqueous humor and serum. In the cardiovascular, neurological, and immunological systems, NO plays a key role as an intercellular messenger. The NO synthase (NOS) family generates gaseous molecules from the amino acid L-arginine; its functional roles range from vasodilation and neurotransmission to inflammatory reactions (Forstermann and Sessa Citation2012). NO signaling in the eye plays roles in various homeostatic purposes, including IOP modulation and blood supply control. Because of its physiological role in IOP regulation, NOS could be a target for IOP-lowering treatment techniques (Stamer Citation2017). As observed in the treatment groups, an increase in the concentration of NO suggests that NO plays a role in lowering IOP after treatment. The findings give credence to the opinion that NO is a potential strategy to slow glaucoma development by reducing IOP and increasing optic nerve head perfusion (Aliancy et al. Citation2017). The findings of this study corroborate those of Matos et al. (Citation2019), that individuals with glaucoma have lower NO and cGMP concentrations in plasma and aqueous humor. In individuals with POAG, reduced plasma levels of NO markers may represent an imbalance of endothelium-derived mediators (Galassi et al. Citation2004).
A significant change was not observed in the glutamate concentrations in the treated animals. Thus, the glutamate levels were maintained in the ocular tissue (Kyei et al. Citation2015). There was no significant (p > 0.05) difference within concentrations of CRP in both serum and aqueous humor. However, there was a reduction in the concentration of CRP at a high dose of the extract, although not statistically significant. CRP is an acute-phase protein and blood CRP level rises from trace levels to high microgram/ml levels during inflammatory conditions/diseases (Ansari et al. Citation2014). Our result agrees with the observation that there was no correlation between IOP and CRP levels (Ansari et al. Citation2014). In a similar manner, De Voogd et al. (Citation2006) found no significant (p > 0.05) difference between the CRP levels of patients with glaucoma and healthy persons.
Diabetic patients have been reported to have higher IOP (Baisakhiya et al. Citation2017). It has been documented that tight control of blood glucose levels reduces the risk of retinopathy (Mohamed et al., Citation2007). The foregoing compares well with this research and as such revealed that the extract does not pose risk to glaucoma pathogenesis. The ciliary epithelium actively pumps solutes or ions to the aqueous side and forms concentration gradients in the aqueous humor. The gradients then act as a force to propel the bulk flow of water into the eye, which macroscopically is known as the aqueous inflow. From the results of this work, the FPMSE was able to preserve these electrolytes, posing no risk to glaucoma pathogenesis.
The histological findings of the longitudinal section of the eyes were represented on Plates 1–4. No damage or alteration was observed in the normal control (Plate 1). However, there was reduced number of photo receptors in the outer nuclear layer of the tissue with darkened nuclei observed in Plate 2 (non-treated). The pigment around the epithelium was reduced due to reduced number of melanocytes within the tissue which may affect absorption of photons of light. The ciliary processes had reduced pigmented ciliary epithelia. Plate 3 (standard) showed a ring-like, longitudinal arranged smooth muscle with small, folded projections called ciliary processes. The inner layer had a reduced pigment with a non-pigmented region in the outer layer. Meanwhile, vacuolated spaces were observed within the cytoplasm of the epithelial cells in the ciliary muscles which is an indication of defect leading to accumulation of molecules within the epithelial cells of the ciliary processes. The presence of endogenous pigment (melanin) possibly derived from exogenous sources was observed in Plate 1. The group treatment with 100 mg/kg extract (Plate 4) showed adequate pigmentations in the inner pigmented layers of the retina with corresponding vascular infoldings on the inner surface of the ciliary body which produces aqueous humor. The smooth muscle of the ciliary body is present as seen in Plate 2. The increased pigmentation and normal folding of the ciliary processes as seen in this study on the treatment group shows the capacity of FPMSE in maintaining the integrity of the ciliary body which is responsible for the secretion of aqueous humor.
PLA2 mobilization of arachidonic acid and subsequent prostaglandin production is thought to be a critical step in inflammation. Some terpenoids derived from marine sources have been widely studied as PLA2 inhibitors and have shown to be a promising source of natural anti-inflammatory drugs (Garcia-Pastor et al. Citation1999b). The n-hexane extract significantly (p < 0.05) inhibited the activity of PLA2 in a concentration-related manner from 1.25–40 mg/ml compared to the control, as seen by the reduced absorbance of the supernatant solution. High concentrations of terpenoids in the extract, as reported in this study, could be responsible for the PLA2-inhibitory activities of the plant extract (Garcia-Pastor et al. Citation1999b). Hence, the high concentration of terpenoids found in this study could contribute to lowering the IOP by regulating inflammatory processes associated with glaucoma.
Conclusion
Pentaclethra macrophylla seed extract demonstrated a significant hypotensive effect on IOP when administered orally to experimental rabbits. The lowering effect on IOP may have resulted from the high content of some minerals, such as nitrate, that led to an increase in NO, which in turn helps in the dilation of the blood vessels. More so, the high terpenoid composition, which could serve as an anti-inflammatory agent, may have contributed to the lowering of IOP. Therefore, fermented P. macrophylla seeds may be consumed as a form of adjunct therapy in ocular hypertensive humans but further investigation may be required to determine the therapeutic dose for managing ocular hypertension.
Authors’ contributions
The authors made significant contributions in the conceptualization and design of the study, reading, and approval of the final version of the manuscript. Ikechukwu Jacob Okoro: Conceptualized and designed the work, acquired, analyzed and interpreted the data, drafted the paper, approved the final version paper to be published and agreed to be accountable for all aspects of the work. Victor Nwadiogbu Ogugua: Conceptualized and designed the work, interpreted the data, revised the paper critically for intellectual content, approved the final version paper to be published, and agreed to be accountable for all aspects of the work. Parker Elijah Joshua: Conceptualized and designed the work, interpreted the data, revised the paper critically for intellectual content, approved the final version paper to be published and agreed to be accountable for all aspects of the work. Samuel Kyei: Interpreted the data, revised the paper critically for intellectual content, approved the final version paper to be published and agreed to be accountable for all aspects of the work. Innocent Uzochukwu Okagu: Designed the work, interpreted the data, revised the paper critically for intellectual content, approved the final version paper to be published and agreed to be accountable for all aspects of the work. Christian Chijioke Amah: Acquired and interpreted the data, revised the paper critically for intellectual content, approved the final version paper to be published and agreed to be accountable for all aspects of the work. Nnamani, Vitus Ikenna: Acquired and interpreted the data, revised the paper critically for intellectual content, approved the final version paper to be published and agreed to be accountable for all aspects of the work.
Data availability statement
The data that support the findings of this study are openly available in figshare.com at: https://doi.org/10.6084/m9.figshare.19616007.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aebi HE. 1983. Catalase. In: Bergmeyer H. U., editor. Methods of enzymatic analysis (3rd Edn.). Weinheim: Verlag Chemie; p. 273–286.
- Akinlabu DK, Owoeye TF, Owolabi FE, Audu OY, Ajanaku CO, Falope F, Ajani OO. 2019. Phytochemical and Proximate Analysis of African Oil Bean (Pentaclethra macrophylla Benth). Seed. J Phys Conf Ser. 1–6. doi:10.1088/1742-6596/1378/3/032057.
- Aliancy J, Stamer WD, Wirostkom B. 2017. A review of nitric oxide for the treatment of glaucomatous disease. Ophthalmol Ther. 6:1–12.
- Alinnor IJ, Oze R. 2011. Chemical evaluation of the nutritive value of Pentaclethra macrophylla Benth (African oil bean) seeds. Pakistan J Nutr. 10(4):355–359.
- American Cancer Society. 2000. Phytochemicals. Available at http://www.cancer.org/eprise/main/docroot/ETO/content/ETO_5_3X_Phytochemicals, June 2000.
- Andersen HC, McCarthy M. 1950. Determination of C-reactive protein in the blood as a measure of the activity of the disease process in acute rheumatic fever. Am J Med. 8:445–455.
- Anioke IC. 2019. Effect of fermented pentaclethra macrophylla benth (African Oil bean) seed extract on plasma lipid profile in healthy Rat model-A preliminary study. South Asian Research J Nat Prod. 2(1):1–9.
- Ansari TJ, Kamble PH, Parate V. 2014. Study of C - reactive protein and intraocular pressure in Type 2 Diabetics. Indian J Basic Appl Med Res. 3(3):197–203.
- Association of official analytical chemists (AOAC). 1990. Official methods of analysis (15th Edn). Oxford University Press, Virginia, USA. pp 12–98.
- Baisakhiya S, Garg P, Singh S. 2017. Association between glycemic control and intraocular pressure in patients with Type II diabetes mellitus. National J Physiol, Pharm Pharmacol. 7(1):43–46.
- Bancroft JD, Stevens A. 1977. Theory and practice of histological techniques. Edinburgh: Churchill Livingstone. pp. 16-64.
- Banu KS, Cathrine L. 2015. General techniques involved in phytochemical analysis. Int J Adv Res Chem Sci. 2(4):25–32.
- Bennasir H, Sridhar S, Abdel-Razek TT. 2010. Vitamin A from physiology to disease prevention. Int. J Pharm Sci Rev Res. 1(1):68–73.
- Bernt E, Bergmeyer HU. 1965. L- Glutamate UV-assay with glutamate dehydrogenase and NAD. In: Bergmeyer H. U, editor. Methods of enzymatic analysis vol. 6. 2nd ed. New York: Academic Press; p. 384–388.
- Bryan NS, Grisham MB. 2007. Methods to detect nitric oxide and its metabolites in biological samples. Free Radical Biol Med. 43(5):645–657.
- Cangao C. 2011. African indigenous fruit with potential health benefits. International Tropical Fruits Network. Available on: http://www.itfnet.org/v1/2011/12/african-indigenous-fruits-with-potentialhealth-benefits/.
- Cedric Y, Payne VK, Nadia NAC, Kodjio N, Kollins E, Megwi L, Kuiate JR. 2018. In vitro antioxidant and anticoccidial studies of pentaclethra macrophylla extracts against eimeria magna, eimeria flavescens, eimeria intestinalis eimeria stiedae oocysts and sporozoites of rabbits. J Adv Parasitol. 5(3):38–48. doi:10.17582/journal.jap/2018/5.3.38.48.
- Cox-Georgian D, Ramadoss N, Dona C, Basu C. 2019. Therapeutic and medicinal uses of terpenes. In: Joshee N., Dhekney S., Parajuli P., editor. Medicinal plants. Cham: Springer. doi:10.1007/978-3-030-31269-5_15.
- Debjit B, Sampath KP, Lokesh K, Shravan D, Dutta P, S A. 2012. Glaucoma-A Eye disorder Its causes, risk factor, prevention and medication. The Pharma Innovation. 1(1):66.
- De Voogd S, Wolfs RC, Jansonius NM, Witteman JC, Hofman A, de Jong PT. 2006. Atherosclerosis, C-reactive protein, and risk for open-angle glaucoma: the Rotterdam study. Invest Opthalmol Visual Sci. 47(9):3772–3776.
- El-Shemy HA, Aboul-Enein AM, Aboul-Enein KM, Fujita K. 2007. Willow leaves’ extracts contain antitumor agents effective against three cell types. PLoS ONE. 2:e178.
- Enujiugha VN, Akanbi CT. 2005. Compositional changes in African oil bean (Pentaclethra macrophylla Benth) seeds during processing. Pakistan J Nutr. 4(1):27–31.
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. 2004. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 137:62–69.
- Forstermann U, Sessa WC. 2012. Nitric oxide synthases: regulation and function. Eur Heart J. 33(7):829–837.
- Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L, Masini E. 2004. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 88(6):757–760.
- Garcia-Pastor P, Randazzo A, Gomez-Paloma L, Alcaraz MJ, Paya M. 1999b. Effects of petrosaspongiolide M, a novel phospholipase A2 inhibitor, on acute and chronic inflammation. J Pharmacol Exp Ther. 289:166–172.
- George GO, Ajayi OB, Oyemike AA. 2018. Effect of Moringa Oleifera leaf aqueous extract on intraocular and blood pressure of normotensive adults in Edo state, Nigeria. J Nigerian Optometric Assoc. 20(2):75–81.
- Harborne JB. 1973. Phytochemical methods: a guide to modern technique of plant analysis, 1st Edn. Chapman and Hall Ltd, London. pp. 107–150.
- Hiwatashi K, Shirakawa H, Hori K, Yoshiki Y, Suzuki N, Hokari M. 2010. Reduction of blood pressure by soybean saponins, renin inhibitors from soybean, in spontaneously hypertensive rats. Biosci, Biotechnol, Biochem. 74:2310–2312.
- Igwenyi IO, Isiguzo OE, Aja PM, Okechukwu PCU, Ezeani NN, Uraku A. J.(2015). Proximate composition, mineral content and phytochemical analysis of the African oil bean (Pentaclethra macrophylla) Seed. American-Eurasian J Agric Environ Sci. 15(9):1873–1875.
- Ikhuoria EU, Aiwonegbe AE, Okoli P, Idu M. 2008. Characteristics and composition of African oil bean seed (Pentaclethra macrophylla benth). J Appl Sci. 8(7):1337–1339.
- Iwueke AV, Nwodo OFC, Okoli CO. 2006. Evaluation of the Anti-inflammatory and Analgesic activities of Vitex doniana leaves. Afr J Biotechnol. 5(20):1929–1935.
- Kathleen H. 2001. Natural therapies for ocular disorders part Two: cataracts and glaucoma. Altern Med Rev. 6(2):141–166.
- Kaushik S, Pandav SS, Ram J. 2003. Neuroprotection in glaucoma. J Postgrad Med. 49:90–95.
- Kayar A, Dokuzeylul B, Kandemir F, Kirbas A, Bayrakal A, Or M. 2015. Total oxidant and antioxidant capacities, nitric oxide and malondialdehyde levels in cats seropositive for the feline coronavirus. Veterinární Medicína. 60:274–281.
- King EJ, Wootton IDP. 1959. Microanalysis in medical biochemistry, 3rd Edn. London: Churchill. p. 14.
- Kyei S, George AK, Paul R, Osei O. 2015. Anti-glaucoma potential of Heliotropium indicum Linn in experimentally-induced glaucoma. Eye Vision. 2:16.
- Lorke D. 1983. Determination of acute toxicity. Archives toxicity. 53:275–279.
- Małgorzata S, Jolanta G, Wojciech W. 2005. Roles of reactive oxygen species and selected antioxidants in regulation of cellular metabolism. Int J Occup Med Environ Health. 18(1):15–26.
- Mamta S, Jyoti S, Rajeev N, Dhatmendra S, Abhishek G. 2013. Phytochemistry of medicinal plants. J Pharmacogn Phytochem. 1(6):168–182.
- Maruna RFL. 1958. Colorimetric determination of sodium. Clin Chem. 2:581.
- Matos AG, Gurgel VP, Callou AL. 2019. The influence of nitric oxide on the pathophysiology of glaucomatousneuropathy. Rev Bras Oftalmol. 78:70–73.
- Mbah GO, Onyeabo UA, Udeh BC. 2018. Effect of fermentation on nutritional composition of African Oil bean seed. Pac J Sci Technol. 19(1):244–250.
- Melena J, Santafe J, Segarra J. 1998. The effects of topical Diltiazem on the IOP in Betamethasone induced ocular hypertensive rabbits. J Pharmacol Exp Ther. 284:278–282.
- Mohamed Q, Gillies MC, Wong TY. 2007. Management of diabetic retinopathy: a systematic review. Jama. 298(8):902–916.
- Mumcu UY, Kocer I, Ates O, Alp HH. 2016. Decreased paraoxonase1 activity and increased malondialdehyde and oxidative DNA damage levels in primary open angle glaucoma. Int J Ophthalmol. 9(10):1518–1520.
- Natalie S, Gustav S, Selente B. 2015. Glaucoma: a brief review. South African Pharm J. 82(5):18–22.
- Nita Y, Rajesh Y, Anju GI. 2014. Chemistry of terpenoids. Int JPharm Sci Rev Res. 27(2):272–278.
- Nwanjo H, Iroagba I, Nnatuanya I, Eze N. 2006. Is fermented Pentactethra macrophylla nutritional or antinutritional? Response from hamatological studies in protein malnourished Guinea pigs. Int J Nutr Wellness. 4(2):1–5.
- Nwosu UC, Essien EB, Ohiri RC. 2017. Phytochemical, mineral composition and anti-hyperlipidemic effects of processed pentaclethra macrophylla seeds on high Fat diet and streptozocin-induced diabetic wistar rats. Int J Agric Earth Sci. 3(6):31–41.
- Obeta JAN. 1983. A note on the micro-organisms associated with the fermentation of seeds of the African oil bean tree (Pentaclethra macrophylla). J Appl Bacteriol. 54(3):433–435.
- Osabor VN, Okonkwo PC, Ikeuba AI. 2017. Chemical profile of plant and seeds of Pentaclethra macrophylla Benth. J Med Herb Ther Res. 5:11–17.
- Paul SB, Binxing L, Preejith PV, Aruna G, Rajalekshmy S, Bradley SH, John MN. 2016. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retinal Eye Res. 50:34–66.
- Pearson D. 1976. The Chemical analysis of foods, 7th ed. Livingstone.: Churchill.
- Ramadhan O, Ian FP. 2012. The biological significance of vitamin a in humans: a review of nutritional aspects and clinical considerations. Science Jet. 1:19.
- Renu A, Suresh KG, Puneet A, Rohit S, Shyam SA. 2009. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 57:257–266.
- Richer SP, Rose RC. 1998. Water soluble antioxidants in mammalian aqueous humor: interaction with UV B and hydrogen peroxide. Vision Res. 38:2881–2888.
- Russell W, Duthie G. 2011. Plant secondary metabolites and gut health: The case for phenolic acids. Proc Nutr Soc. 70:389–396.
- Satnam L, Andrew JW. 2012. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br J Clin Pharmacol. 75(3):677–696.
- Stamer DW. 2017. Nitric oxide in ocular physiology. In: Tsai J. C., Gray M. J., Cavallerano T., editor. Nitric oxide in glaucoma: what clinicians need to know. New York: Candeo Clinical/Science Communications; p. 31.
- Terri AE, Sesin PG. 1985. Colorimetric determination of potassium. Am J Clin Pathol. 29:86.
- Tietz NW. 1976. Fundamentals of clinical chemistry, W B Saunders, Philadelphia, P A pp 897.
- Trease GE, Evans WC. 1989. Pharmacognosy. 13th ed. Bailliere Tindall Books Publishers. By Cas Sell and Collines Macmillan Publishers., Ltd. London. pp. 1-105.
- Tribble JR, Hui F, Jöe M, Bell K, Chrysostomou V, Crowston JG, Williams PA. 2021. Targeting diet and exercise for neuroprotection and neurorecovery in glaucoma. Cells. 10:295. doi:10.3390/cells10020295.
- Trinder P. 1951. A rapid method for the determination of sodium in serum. Analyst. 76:596–599.
- Ufelle SA, Emeka EN, Peter UA, Samuel G. 2015. Potential haematopoietic properties of crude methanolic seed extract of Pentaclethra macrophylla in Wistar rats. Int J Nigerian Soc Exp Biol. 27(1):22–25.
- Ugbogu AE, Arunsi UO, Uche-Ikonne OO, Ude VC, Okezie E. 2017. Antiulcerogenic Potentials of Fermented Aqueous Extract of Pentaclethra macrophylla (Benth) Seeds. American J Biomed Res. 5(3):57–64.
- Wallin B, Rosengren B, Shertzer HG, Camejo G. 1993. Lipoprotein oxidation and measurement of thiobarbituric acid reacting substances formation in a single microtiter plate: Its Use for evaluation of antioxidants. Anal Biochem. 208:10–15.
- Wojciech R, Jolanta Z, Dorota P, Alicja HWM, Serap O, Ewa M. 2017. Differences in serum oxidative status between glaucomatous and nonglaucomatous cataract patients. BMC Ophthalmol. 17:13.
- Woolley JG. 2001. Plant alkaloids. Encyclopedia Of life sciences; Nature Publishing Group / www.els.net.
- Wynter-Adams DM, Simon OR, Gossell-Williams MD, West ME. 1999. Isolation of a muscarinic alkaloid with ocular hypotensive action from Trophis racemosa. Phytother Res. 13:670–674.
- Xin Z, Waterman DF, Henken RM, Harmon RJ. 1991. Effects of copper status on neutrophil function, superoxide dismutase and copper distribution in steers. J Diary Sci. 74:3078–3082.
- Yildirim O, Ates TL, Muslu N, Ercan B, Atik U. 2004. Changes in antioxidant enzyme activity and malondialdehyde level in patients with age-related macular degeneration. Ophthalmologica. 218:202–206.