Abstract
Aim: To quantitatively analyze the vascular and structure abnormalities in the papillary areas in eyes with noninfectious uveitis during different periods using optical coherence tomography angiography (OCTA). Methods: Forty eyes of 40 uveitis patients in the acute phase, 12 eyes of 12 patients with inactive uveitis and 21 eyes of 21 healthy controls were included in this study. RPCvd% and pRNFL thickness in different regions were automatically measured and calculated by OCTA. Correlation between RPCvd% and pRNFL was assessed. Results: RPCvd% was significantly higher in uveitis-inactive eyes than in active eyes in the inferior quadrant (p = 0.036), whole image (p = 0.041) and inferior nasal (p = 0.001) sector. The RPCvd% in active uveitis eyes was lower compared to healthy eyes in the inside disc sector (p = 0.026). pRNFL was notably greater in the active group compared to the healthy group. Positive correlations were identified between pRNFL and RPCvd% both in active and inactive uveitis eyes. Conclusions: Compared with active uveitis, papillary microcirculation showed a significant improvement in inactive uveitis eyes (anterior or pan-uveitis). RPCvd% may serve as a sensitive indicator to supervising uveitis progression and treatment response using OCTA.
Background
Uveitis refers to any process involving an inflammation of the uveal tissue which includes the iris, ciliary body and the choroid (Touhami et al. Citation2021). It may lead to macular edema and neovascularization. Early identification and monitoring of these changes is key to the management of patients with uveitis (Tranos et al. Citation2019). Fundus autofluorescence (FAF), fluorescein angiography (FA), indocyanine green angiography (ICGA), optical coherence tomography (OCT) and enhanced depth imaging-OCT (EDI-OCT) have become the standard imaging methods.
Chorioretinal vasculature can now be visualized with optical coherence tomography angiography (OCTA) in a non-invasive way. Quantification of chorioretinal perfusion including the optic nerve head (ONH) perfusion is possible (Chen et al. Citation2016; Lauermann et al. Citation2018). Previous studies have shown that OCTA, in combination with other imaging methods, can reveal the characteristics of patients with ocular inflammation and provide help in our understanding of the disease (Waizel et al. Citation2018; Khan et al. Citation2019; Kleerekooper et al. Citation2020; Liang et al. Citation2021).
Quite a few OCTA studies have revealed macular vascular and structural changes in patients with uveitis (Waizel et al. Citation2018; Ye et al. Citation2018; Tian et al. Citation2019; Accorinti et al. Citation2020; Aksoy et al. Citation2020; Karaca et al. Citation2020; Koca et al. Citation2020; Yalcinkaya et al. Citation2022). However, no reports have been made on performing papillary analyses of patients with uveitis during different disease status. We focused on the papillary area in uveitis patients because of the following reasons: First, previous studies have confirmed that any inflammatory condition of the eye can demonstrate a ‘hot disc’ on FA (Lee et al. Citation2020), indicating that the optic disc alternation may be a characteristic in uveitis. Second, papillary capillary network is more vulnerable to vessel flow changes due to the correspondingly dense unmyelinated nerve fibers with high metabolic demands (Abri Aghdam et al. Citation2021). In our study, vascular and structural changes in the papillary regions in active uveitis and inactive uveitis patients using OCTA were comprehensively analyzed, and correlation between these findings was also evaluated.
Methods
Study design and ethical approval
This comparative study adhered to the Tenets of the Declaration of Helsinki. Approval for data collection and analysis was obtained from the Ethics Committee of Soochow University Affiliated No 1 People’s Hospital (NO.:2020(040)). After collecting the enrolled cases, we followed up the patients by telephone, in accordance with the work practices of our department. Patients were informed about requests for participation and publication, and electronic informed consent was obtained. At the same time, some patients who came to the hospital for follow-up signed informed consent in the consulting room.
Study subjects
All patients above 18 years older who have or have had non-infectious anterior or pan-uveitis were recruited, mediate and posterior uveitis were not included because of inadequate number of patients. Upon entry in the study, data including gender, age, and uveitis diagnosis were collected. Diagnosis of anatomic location of uveitis and determination of location of disease activity were made according to the criteria established by the standardization of uveitis nomenclature (SUN) criteria. ‘Inactive’ stage was defined in those patients, being on the same therapy or without therapy for at least 6 months prior to the evaluation, with no sign of inflammation in the anterior chamber, vitreous or retina, and no vascular leakage or macular edema according to FA, which has been believed as the best tool to detect retinal vascular alterations in uveitis. In all the other conditions, uveitis was defined as active. Results of laboratory testing, chest X-ray, MRI imaging, or biopsy, if necessary, were used to further classify uveitis diagnosis by underlying etiology. For patients with both eyes affected, only the right eye was included. Age and sex-matched healthy individuals who received a general physical examination in our hospital were recruited as a control group, and right eyes were selected for evaluation and analysis.
Exclusion criteria were: patients with age <18 years old and age >70 years old; spherical refractive error ≥ ± 6 diopters; astigmatism ≥ ± 3 diopters; intraocular pressure above 21㎜Hg in any eye (with or without anti-glaucoma drugs); presence of significant media opacities (e.g. cataract or corneal opacity) that could have influenced retinal examination; patients with masquerades, retinal and optic nerve diseases other than uveitis, any anterior segment surgery performed less than 6 months prior to the examination, and previous vitreoretinal surgery or ocular trauma.
OCTA images acquisition
The OCTA images were obtained using the RTVue XR Avanti device (Optovue, Fremont, California, USA), which performs 70,000 A-mode scans per second using an 840nm light source. The split-spectrum amplitude-decorrelation angiography (SSADA) algorithm improves the image quality and its scanning time. The algorithm distinguishes the movement of red blood cells (RBCs) within the lumen of both retinal and choroidal vessels between cross-sectional scans. The motion artifacts were removed by the motion correction technology. Vascular density was defined as the percent of the proportion of the examined area occupied by vessels. RPCvd% images were acquired between the inner limiting membrane (ILM) and retinal nerve fiber layer (RNFL) within a 4.5㎜×4.5㎜ (400 × 400 pixels) cube scan centered on the optic disc. Scan images with scan quality lower than 8/10 were excluded due to media opacities or poor cooperation.
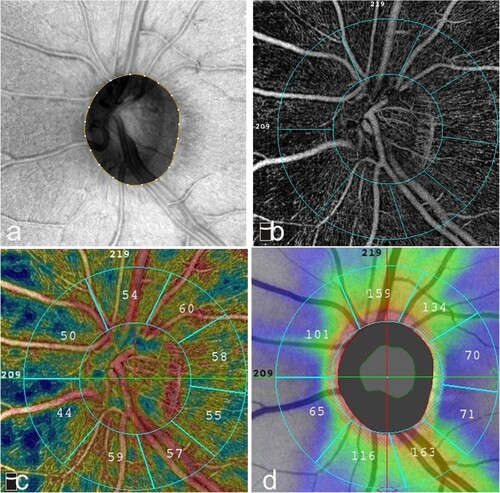
To quantify the RPCvd% and pRNFL thickness, all images were first segmented using built-in software (Software Version 2018,1,1,63). The measurements of RPCvd% and pRNFL thickness were obtained in 4 equal quadrants: superior (S), temporal (T), inferior (I), and nasal (N); two hemispheres: superior-hemi (SH) and inferior-hemi (IH) defined by a horizontal line drawn through the disc; and 8 peripapillary sectors: nasal superior (NS), nasal inferior (NI), inferior nasal (IN), inferior temporal (IT), temporal inferior (TI), temporal superior (TS), superior temporal (ST), and superior nasal (SN) by modified Garway-Heath sector grid with 2 rings of 2㎜ and 4㎜ (Figure ). Within the 2㎜ area was defined as inside disc area, and the ring between 2㎜ and 4㎜ was the peripapillary region. Any notable segmentation error was manually corrected.
Figure 1. RPCvd% and pRNFL thickness in modified Garway-Heath sectors in a normal subject. The Angio Disc radial peripapillary capillary (RPC) enface image was separated by modified Garway-Heath sector grid into 8 sectors by 2 circles with a diameter of 2㎜ and 4㎜. (a) En-face OCT image of the left eye, which is the scan of interest for capillary density measurement. (b) En-face angioflow RPC image of 8 sectors by 2 circles diameter around the ONH. c RPCvd% map. (d) pRNFL thickness map, arcuate regions look like butterfly wings because of warm color code with higher pRNFL thickness.
Statistical analysis
IBM SPSS Statistics (Armonk, NY, IBM Corp.) for windows version 26.0 was used for statistical analysis. Quantitative data are presented as mean and standard deviation, while data are presented as median with 25th and 75th percentile for categorical variables. Differences among groups were evaluated by analysis of variance (ANOVA) or Kruskal–Wallis variance analysis with post-hoc test (Bonferroni adjustments), Pearson’s or Spearman correlation analyses were performed between RPCvd% and pRNFL as appropriate. Statistical significance was set at p < 0.05.
Results
Demographic summary
Forty patients with active uveitis, 12 patients with inactive uveitis and 21 healthy participants were recruited at the Department of Ophthalmology, Soochow University Affiliated No 1 People’s Hospital, China, between September 2020 and September 2021. Table shows the demographic characteristics of the subjects. There was no significant difference in terms of age and sex distribution.
Table 1. Characteristics of the patients included in the study.
RPC vessel parameters
The RPC vessel parameters measured from active uveitis, inactive uveitis and healthy eyes are summarized in Table . Overall, patients with active uveitis showed a decreased RPCvd% than those with inactive uveitis and healthy controls. While significant difference was seen in the inferior quadrant (p = 0.036), whole image (p = 0.041) and IN (p = 0.001) sector between the active eyes and the inactive eyes, and in the inside disc sector (p = 0.026) between the active eyes and the healthy eyes.
Table 2. RPCvd% measurement in patients with active and inactive uveitis and healthy controls: comparison between groups and test post hoc results pairwise.
pRNFL thickness parameters
The OCTA findings of pRNFL thickness parameters in active uveitis, inactive uveitis and healthy eyes are summarized in Table . Patients with active uveitis exhibited increased pRNFL thickness compared to inactive uveitis patients and healthy controls, while the statistically significant difference between active and healthy controls was in the temporal quadrant (p = 0.009), inferior-hemi (p = 0.010), NI (p = 0.011), IT (p = 0.019) and TI (p = 0.001) sector.
Table 3. pRNFL thickness measurement in patients with active and inactive uveitis and healthy controls: comparison between groups and test post hoc results pairwise.
RPC and pRNFL parameters during active phase in anterior and pan uveitis
The more subtly separated analysis of RPC vessels and pRNFL parameters in the anterior and pan-uveitis patients during active phase listed in Table . No difference was noted in RPC vessels between the anterior and pan-uveitis eyes during active status. pRNFL were greater in pan-uveitis eyes than in anterior uveitis eyes in the IH (p = 0.042), NI (p = 0.027) and TS (p = 0.004) sector.
Table 4. Differences in patients with anterior and pan uveitis in active phase.
Correlation between RPCvd% and pRNFL
The results of correlation analysis between RPCvd% and pRNFL in active uveitis patients are summarized in Table , which reveals a positive association in the superior quadrant (r = 0.427, p = 0.006), nasal quadrant (r = 0.453, p = 0.003), peripapillary (r = 0.394, p = 0.012), SH (r = 0.379, p = 0.016), IH (r = 0.396, p = 0.011), NI (r = 0.557, p = 0.000), TI (r = 0.391, p = 0.013), ST (r = 0.324, p = 0.042) and SN (r = 0.481, p = 0.002) sector. The results of correlation analysis between RPCvd% and pRNFL in inactive uveitis patients are summarized in Table . In terms of each region, a high positive correlation was obtained (p < 0.05 for all) except the inferior quadrant and IT sector.
Table 5. Correlation analysis between RPCvd% and pRNFL in active uveitis.
Table 6. Correlation analysis between RPCvd% and pRNFL in inactive uveitis.
Discussion
Uveitis is a generic term that encompasses various types of intraocular inflammation. Uveitis constitutes a major cause of ocular morbidity. It leads to 5–10% of visual impairment worldwide (Tsirouki et al. Citation2018). OCTA overcomes some of the drawbacks of FA and ICGA, such as invasiveness, risk of allergy-associated complications and cost. Alexander demonstrated that the peripapillary capillary density measurement obtained using OCTA is highly repeatable in eyes with optic nerve and retinal vascular pathology (Vu et al. Citation2021). Prior works have identified decreased peripapillary vascular density in glaucoma, nonarteritic anterior ischemic optic neuropathy and Behcet’s uveitis using OCTA (Akil et al. Citation2017; Liu et al. Citation2020; Yan et al. Citation2021). Our study tried to identify OCTA changes in patients with noninfectious uveitis and compare the different findings among different disease status of the patients.
We did not expect a significant increment in RPCvd% in patients with a strictly defined inactive uveitis compared to active patients in the inferior quadrant, whole image and IN sector. Improved RPCvd% in inactive eyes appears to recover towards the direction of healthy controls. At the same time, pRNFL thickness was notably greater in active-uveitis patients than healthy controls in the temporal quadrant, IH, NI, IT and TI sector; the pathogenesis of the result appears to be associated with an increase in vascular permeability due to break down in the blood-aqueous and blood-retina barriers and leukocyte migration caused by prostaglandins, inflammatory cytokines and vascular endothelial growth factor (Lee et al. Citation2020).
It should be noted that the ‘inferior’ regions (inferior quadrant, IN, IH, NI, IT and TI sector mentioned before) were the most involved areas both in RPCvd% and in pRNFL thickness alteration. In line with Poon’s study, the order of the pRNFL thickness follows the pattern of I > S, known as the ‘IS’ rule (Poon et al. Citation2017). We propose that regions with thicker RNFL need greater RPC vessel density due to a higher energy demand, making these regions more susceptible to ocular impairment. The most plausible explanation for an apparent decrease in RPCvd% during the acute phase could be the mechanical compression and impedance of RPC vessels from the edema of the pRNFL, rather than vascular occlusion or non-perfusion (Pichi et al. Citation2017). Additionally, it is possible that the reduced RPCvd% results from the progressive hypoxia and ischemia with the early axon loss which would be masked by the edema in some patients (Liu et al. Citation2020). As for the decreased RPCvd% in active uveitis eyes compared to the healthy controls in the inside disc sector, we believe that the swelling pRNFL exacerbated the crowd of the optic disc, making it more vulnerable to ischemic damage than other peripapillary sectors.
pRNFL thickened during the active phase either in anterior or pan uveitis, and it is not surprising that pRNFL was thicker in pan-uveitis eyes than that in anterior uveitis eyes because of the wider and more severe inflammation involved in pan-uveitis.
Correlation analysis was conducted to further clarify the relationship between pRNFL thickness and vascular density in the peripapillary region. A positive correlation of RPC vessel density and pRNFL was observed in uveitis eyes, both in active and inactive status, which has not been reported up to now. Generally speaking, a reduced RPCvd% trend and an increased pRNFL tendency were seen in active uveitis patients. Considering the positive correlation between the two, we assumed that subjects with greater RPCvd% and thicker pRNFL still showed a greater RPCvd% and thicker pRNFL during active stage than others, even with a relatively decreased RPCvd% and thickened pRNFL, while greater RPCvd% along with thicker pRNFL in inactive eyes suggests a better prognosis, getting back to normal status. However, a similar result was not found in the previous studies. Multicenter prospective studies with a larger number of patients with a heterogeneous uveitis type, along with different disease duration, as well as the number of episodes, should be undertaken in the future.
Conclusions
Our study may reveal the evolution of vascular abnormalities and structural disorders in the papillary region of patients with anterior uveitis or pan uveitis during different disease phases using OCTA. Particularly, the ‘inferior’ regions (inferior quadrant, IN, IH, NI, IT and TI sector) are the most involved areas, indicating that OCTA may be a valuable modality for observing the microvascular changes associated with uveitis, and that RPCvd% could serve as a sensitive indicator for disease status, therapeutic response and prognosis among uveitis patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Both authors have participated directly in planning and execution of the work and have approved the final version of the manuscript. YX: acquisition and analysis of data, drafting and writing the article; XZ: participated in the design of the study, analysis of data, and revised the article critically for important intellectual content.
Availability of data and materials
The data that support the findings of this study are available at https://doi.org/10.6084/m9.figshare.22503784.v1.
Additional information
Funding
References
- Abri Aghdam K, Aghajani A, Razi-Khosroshahi M, Soltan Sanjari M, Chaibakhsh S, Falavarjani KG. 2021. Optical coherence tomography angiography and structural analyses of the pale optic discs: is it possible to differentiate the cause. Curr Eye Res. 46(12):1876–1885.
- Accorinti M, Gilardi M, De Geronimo D, Iannetti L, Giannini D, Parravano M. 2020. Optical coherence tomography angiography findings in active and inactive ocular Behçet disease. Ocul Immunol Inflamm. 28(4):589–600.
- Akil H, Huang AS, Francis BA, Sadda SR, Chopra V. 2017. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS One. 12:e0170476.
- Aksoy FE, Basarir B, Altan C, et al. 2020. Retinal microvasculature in the remission period of Behcet's uveitis. Photodiagnosis Photodyn Ther. 29:101646.
- Chen CL, Bojikian KD, Xin C, Wen JC, Gupta D, Zhang Q, Mudumbai RC, Johnstone MA, Chen PP, Wang RK. 2016. Repeatability and reproducibility of optic nerve head perfusion measurements using optical coherence tomography angiography. J Biomed Opt. 21:65002.
- Karaca I, Yılmaz SG, Afrashi F, Nalçacı S. 2020. Assessment of macular capillary perfusion in patients with inactive Vogt-Koyanagi-Harada disease: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 258(6):1181–1190.
- Khan HA, Iqbal F, Shahzad MA, Khan QA, Rashid F, Sharjeel M, Khan N, Pizzimenti J. 2019. Textural properties of choriocapillaris on OCTA in healed inflammatory choriocapillaropathies. Ophthalmic Surg Lasers Imaging Retina. 50:566–572.
- Kleerekooper I, Houston S, Dubis AM, Trip SA, Petzold A. 2020. Optical coherence tomography angiography (OCTA) in multiple sclerosis and neuromyelitis optica spectrum disorder. Front Neurol. 11:604049.
- Koca S, Onan D, Kalaycı D, Allı N. 2020. Comparison of optical coherence tomography angiography findings in patients with Behçet's disease and healthy controls. Ocul Immunol Inflamm. 28(5):806–813.
- Lauermann JL, Eter N, Alten F. 2018. Optical coherence tomography angiography offers new insights into choriocapillaris perfusion. Ophthalmologica. 239:74–84.
- Lee MW, Lee TH, Won YK, Shin YI, Kim JY. 2020. Characteristics of retinal layer thickness in acute anterior uveitis: an optical coherence tomography study. Acta Ophthalmol. 98(1):e50–e55.
- Liang A, Zhao C, Jia S, Gao F, Han X, Pei M, Qu Y, Xiao J, Zhang M. 2021. Retinal microcirculation defects on OCTA correlate with active inflammation and vision in Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm. 29:1417–1423.
- Liu J, Chen C, Li L, Yi Z, Zheng H. 2020. Peripapillary and macular flow changes in nonarteritic anterior ischemic optic neuropathy (NAION) by optical coherence tomography angiography (OCT-A). J Ophthalmol. 2020:3010631.
- Pichi F, Sarraf D, Morara M, Mazumdar S, Neri P, Gupta V. 2017. Pearls and pitfalls of optical coherence tomography angiography in the multimodal evaluation of uveitis. J Ophthalmic Inflamm Infect. 7(1):20–32.
- Poon LY, Solá-Del Valle D, Turalba AV, et al. 2017. The ISNT rule: how often does it apply to disc photographs and retinal nerve fiber layer measurements in the normal population. Am J Ophthalmol. 184:19–27.
- Tian M, Tappeiner C, Zinkernagel MS, Huf W, Wolf S, Munk MR. 2019. Evaluation of vascular changes in intermediate uveitis and retinal vasculitis using swept-source wide-field optical coherence tomography angiography. Br J Ophthalmol. 103(9):1289–1295.
- Touhami S, Gueudry J, Leclercq M, Touitou V, Ghembaza A, Errera MH, Saadoun D, Bodaghi B. 2021. Perspectives for immunotherapy in noninfectious immune mediated uveitis. Expert Rev Clin Immunol. 17(9):977–989.
- Tranos P, Karasavvidou EM, Gkorou O, Pavesio C. 2019. Optical coherence tomography angiography in uveitis. J Ophthalmic Inflamm Infect. 9(1):21.
- Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, Androudi S. 2018. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 26:2–16.
- Vu AF, Alber SA, Chang MY, Park SS. 2021. Prospective cross-sectional study of repeatability of peripapillary capillary density measurement using optical coherence tomography angiography in eyes with optic nerve and retinal vascular pathology. J Neuroophthalmol. 42(1), 73–78.
- Waizel M, Todorova MG, Terrada C, LeHoang P, Massamba N, Bodaghi B. 2018. Superficial and deep retinal foveal avascular zone OCTA findings of non-infectious anterior and posterior uveitis. Graefes Arch Clin Exp Ophthalmol. 256(10):1977–1984.
- Yalcinkaya G, Altan C, Basarir B, Cakir I. 2022. Retinal optical coherence tomography angiography findings of acute anterior uveitis. Int Ophthalmol. 42(5):1409–1418.
- Yan C, Li F, Hou M, Ye X, Su L, Hu Y, Luo J, Chi W. 2021. Vascular abnormalities in peripapillary and macular regions of behcet's uveitis patients evaluated by optical coherence tomography angiography. Front Med (Lausanne). 8:727151.
- Ye L, Zhou SS, Yang WL, et al. 2018. Retinal microvasculature alteration in active thyroid-associated ophthalmopathy. Endocr Pract. 24(7):658–667.
