Abstract
The World Health Organization has acknowledged the gap in schizophrenia diagnoses and recommended further research to identify tools and biomarkers for the disease’s early detection. Because the skin and brain have a common ectodermal origin, dermatoglyphics are hypothesized to serve as a potential mirror for the identification of risks and characteristics of neuropsychiatric diseases. This study aimed to determine the digito-palmar dermatoglyphic patterns of schizophrenia patients in Ghana. Digito-palmar dermatoglyphics were obtained using a digital scanner, and the details studied. Individuals living with schizophrenia in Ghana were significantly characterized by low odds of vestige pattern in the hypothenar region, low odds of palmar creases 300, and high and low odds of radial loop (RL) and plain arch (PA) respectively at the finger patterns relative to the control group [OR (95% CI): vestige, 0.2 (0.06–0.69), P = 0.01; palmar creases 300, 0.1 (0.01–0.99), P = 0.049; PA, 0.4 (0.3–0.7), P < 0.001; RL, 1.9 (1.0–3.7), P = 0.044]. Individuals with schizophrenia were also characterized by low mean left-hand a-b ridge count and mean right hand atd angle compared to controls. These differences could be explored as a potential biomarker in diagnosing and early detection of schizophrenia in Ghanaians.
Background
Schizophrenia is a neuropsychiatric condition with a global prevalence of 0.3–0.7%, and a global annual incidence from 7.7 to 43/100,000 per year (Farah Citation2018; Lin et al. Citation2022). Although the cause is not fully understood, it involves the brain and is associated with congenital malformations or distortion of the corpus callosum and septum cavum pellucidum in the brain (Innocenti et al. Citation2003; Flashman et al. Citation2007; Achalia et al. Citation2014). The burden of mental health in Ghana and Africa has sharply risen in the last decade. In Ghana alone, it was estimated in the year 2021 that 3.1 million of 31 million people living in Ghana had a mental illness (CITI NEWSROOM Citation2021). Schizophrenia, which is the leading cause of admissions to psychiatric hospitals in Ghana, constitutes 32% of the total number of hospital admissions (Roberts et al. Citation2014). The World Health Organization (WHO) about 2 decades ago, acknowledged the gap in schizophrenia diagnoses and sub-classification and recommended further research to identify tools and biomarkers that could help in the early detection and sub-classification of schizophrenia (Saraceno Citation1998). Several studies have unsuccessfully explored identifying biomarkers for diagnosing schizophrenia (Wang et al. Citation2008; Ahmed-Popova et al. Citation2014; Norovsambuu et al. Citation2021), limiting diagnoses of schizophrenia to heavy dependence on clinical interviews, observation of patient behavior, and clusters of symptoms over a period of time. Globally, two primary documents are used to diagnose schizophrenia: The World Health Organization’s International Statistical Classification of Diseases and Related Health Problems (ICD) and the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM). Studies have acknowledged the gaps in the diagnostic accuracy of these contemporary tools in schizophrenia and recommend further research for improvement (Valle Citation2020). This gap includes the absence of a defined boundary between schizophrenia and other psychotic disorders and the lack of sufficient information for subcategorization of schizophrenia and treatment planning (Ahmed-Popova et al. Citation2014; Valle Citation2020). Although the 11th revision of ICD i.e. ICD11 has addressed some of these limitations, the need for continuing research for the identification of biomarkers to improve the diagnoses and early detection of schizophrenia and mental health at large is still recommended. The brain is a crucial organ commonly affected in psychotic disorders. However, the difficulty in obtaining brain tissues from living donors and the lack of accurate experimental animal models are significant barriers in neuropsychiatric research. Attempts have therefore been made to explore alternative options as proxies for predicting brain health, one of which is the skin. Embryonically, the skin and brain originate from the same ectoderm, hence, the patterns of epidermal ridges on the feet, fingers, palms, toes, and soles termed dermatoglyphics, are hypothesized to serve as a potential mirror for the identification of risk and characteristics of neuropsychiatric disorders and being explored as potential biomarkers for schizophrenia (Haroun Citation2019). Dermatoglyphics development occurs between the 10th and 13th weeks of gestation and is fully formed by 21 weeks of fetal life (Kumbnani Citation2007; Singh et al. Citation2016). These epidermal ridge imprints are known to have polygenic inheritance and remain stable throughout life in the absence of any mechanical injury (Kumbnani Citation2007). Therefore we hypothesize that digito-palmar dermatoglyphic patterns of individuals living with schizophrenia will not be the same when compared to those without schizophrenia in the Ghanaian population.
Although there are several studies on the association between dermatoglyphics and schizophrenia, the inconsistency of the results is influenced by methodological differences, and genetic characteristics of the studied populations. These reports suggest the importance of exploring dermatoglyphic patterns of individuals living with schizophrenia among the Ghanaian population. Therefore, this study aimed at assessing the digito-palmar dermatoglyphic patterns of patients living with schizophrenia in Ghana.
Methodology
Study design
The study was a cross-sectional study involving purposive recruitment of patients diagnosed with schizophrenia from Accra Psychiatry Hospital (APH) and simple random sampling of individuals with no family history of psychiatric illness from a community as control.
Study sites
The study was conducted at the Accra Psychiatric Hospital (APH). The Accra Psychiatric Hospital is a specialized health facility located in Accra, the capital of Ghana. The primary healthcare in this facility is the rehabilitation and management of persons with mental illnesses. It has about six hundred (600) beds with an average in-patient population of about 1200 (Fournier Citation2011). The hospital specializes in the treatment of severe mental disorders, such as schizophrenia, bipolar disorder, and major depressive disorder.
Ethical issues
Approval for the study was obtained from the Ethical and Protocol Review Committee of the College of Health Sciences, the University of Ghana, with Protocol Identification Number: CHS-Et/M.4-P4.7/2020-2021. The Accra Psychiatric Hospital gave full administrative permission for the conduct of the study.
Sample size determination
The sample size for the control group was determined using Cochran’s formula (n = z2pq/e2) (Israel Citation1992); where n is the sample size, z is the selected critical value of desired confidence level, p is the estimated proportion of the attribute of interest in the population, q = 1 – p, and e is the desired allowable error. If the estimated proportion of the attribute of interest in the population is unknown, a prevalence of 50% is assumed. Therefore, an estimated proportion of not having any psychiatric condition of 50% was assumed. At a 95% confidence level, the z value for the area under the normal curve is 1.96. At ± 9.6% precision, a minimum sample size of 104 was obtained for the control group: n ≥ [(1.96)2*0.5*0.5 /(0.096)2]. Therefore 106 apparently healthy individuals from the community were randomly recruited as controls. A total of 69 schizophrenia patients consented and were recruited purposively for the study.
Study population
All the study participants were recruited from a multiethnic Ghanaian population. The Ghanaian population is predominantly of black Africans and is made up of diverse ethnic groups. Akan, Dagomba, Ewe, Ga-Adangbe, Gurma, Guan, Gurunsi, and Bissa are the major ethnic groups in Ghana, among many.
Participant selection
Individuals living with schizophrenia were recruited purposively from the Accra Psychiatry Hospital. They were inpatients who were already diagnosed by a specialist psychiatrist and were being managed for schizophrenia. Schizophrenia patients who were assessed by the consultant psychiatrist as being fit to participate and being able to provide consent willingly were recruited into the study. Schizophrenic patients with current mania and those who declined to participate in the study after explanation were excluded. Also, patients were able to withdraw their consent at any point of the assessment. The apparently healthy controls were randomly recruited from communities in Ghana. Individuals who had no history of psychiatric illness and no family history of psychiatric illness up to second-generation were included in the study as controls. The Brief Psychiatric Rating Scale (BPRS) was used to assess the controls for any psychiatric symptoms. Individuals with physical damage or deformation of the palmar skin of the hand were excluded from the study.
Sample collection
Following written informed consent, the patient’s demographic data and clinical history, such as past psychiatry history, family history, past medical and surgical history, drug history, and forensic history, were obtained from the patient’s hospital folder. A structured questionnaire and BPRS were used to document the biodata, family history of psychiatric illness and measure psychotic symptoms among the control study participants. The digito-palmar dermatoglyphics of each study participant was obtained using a digital scanner as reported by Igbigbi et al. (Citation2018) with slight modification. A CanoScan LiDE220 scanner was used to obtain the palmar and fingerprint patterns of the study participants. The palms and digits were placed flat on the scanner and scanned, after which the thumb was also placed in the scanner and scanned. The images were then recorded in PDF format on an HP laptop. To ensure uniformity, all the images were read using Adobe Reader 9.0 at 100 to 150% magnification. The scanned images were analyzed for the following dermatoglyphic parameters; fingertip patterns (whorls, loops, and arches), total finger ridge count (TFRC), palmar patterns (thenar and hypothenar), palmar creases, a-b ridge count, atd angle and total triradii count with the aid of OnScreen Protractor version 0.4. The parameters were read twice each by two independent scorers, and the concordance was assessed using the Kappa test in IBM SPSS version 20. For the continuous variables, the average of the readings from the two individuals was recorded as the final value for the parameter.
Parameter description
Finger ridge count and fingerprint patterns
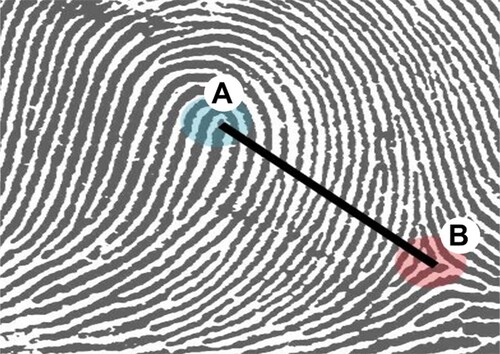
Finger ridge count (FRC) was obtained by counting the number of ridges when a straight line is drawn between a core and a triradius (delta), as shown in Figure , without counting the point of the triradius and the point of the core. To obtain a TFRC, the individual finger ridge counts of the ten fingers were summed together as reported by others (Sharma et al. Citation2018; Modiano Citation2019). The patterns at the distal ends of the palmar surface of the fingers were determined as an arch, a whorl, or a loop and their various subclassifications according to Galton and the FBI classification as reported by Singh et al. (Citation2016).
Figure 1. Diagram showing a straight line drawn between a core (A) and a triradius (B) for finger ridge count. Adopted from Gnanasivam and Vijayarajan (Citation2019).
Palmar creases
The pattern of the palmar creases was described in a three-digit format according to the PIC model as reported by Ali et al. (Citation2021). The first digit represented the number of the primary creases, which ranges from 1 to 3; the middle digit represented the number of intersections between the primary creases, which ranges from 0 to 2; and the last digit represented the number of complete transverse creases which ranges from 0 to 2.
Palmprints
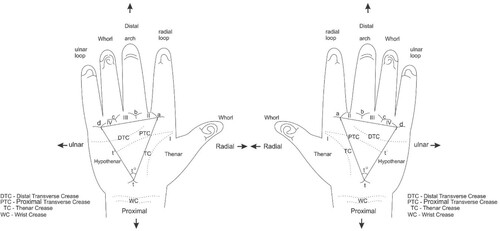
The palmar surface was divided into six anatomically defined dermatoglyphic areas: hypothenar, thenar, and the four inter-digital areas as reported by Singh et al. (Citation2016). Triradius is the basic landmark of digito-palmar dermatoglyphics. The palm has four digital triradii (a, b, c, d) located in proximal relation to the bases of the digits I, II, III, and IV (index finger to the little finger, respectively), as shown in Figure . The axial triradius (t) is located at the base of the palm, distal to the wrist, and in the depression between the thenar and hypothenar eminences. The a-b ridge count was obtained by counting the number of ridges between triradius ‘a’ and triradius ‘b’. The angle between straight lines drawn from the distal ‘t’ triradius to the medial ‘a’ triradius and the lateral ‘d’ triradius was determined as the atd angle as demonstrated in Figure . The hypothenar and thenar areas were examined for patterns. Straight ridges that did not form any pattern were recorded as open fields (O) and poorly arranged ridges such that they did not form a true pattern were recorded as vestige (V) according to Sharma et al. (Citation2018).
Figure 2. A diagram showing the palmar surface and method of determining the atd angle of the right and left hands. Demonstrated are the four digital triradii (a, b, c, d) located in proximal relation to the bases of the digits I, II, III, and IV (index finger to the little finger, respectively). The lines drawn from the distal ‘t’ triradius to the medial ‘a’ triradius and the lateral ‘d’ triradius form the atd angle (t°). Adapted from Singh et al. (Citation2016).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows version 20. The normality of the continuous data was assessed using Kolmogorov–Smirnov. Means of total and absolute finger ridge count, a-b ridge count, and atd angles between individuals with schizophrenia and the control were compared using the T-test / Mann–Whitney U test for statistical significance. The proportion of digito-palmar dermatoglyphic patterns between schizophrenia and control was compared using Chi-square test. Multinomial logistic regression was used to calculate odds ratio to determine the association between the digito-palmar dermatoglyphic patterns and schizophrenia. P-value < 0.05 was considered statistically significant.
Results
Demographic characteristics of the study participants
A total of one hundred and seventy-five (175) study participants of Ghanaian descent were recruited for this study. This included sixty-nine (69) schizophrenic patients with a mean age of 45 ± 1.8 years and a hundred and six (106) controls with a mean age of 23.7 ± 0.7 years (mean ± standard error of the mean). Of the 69 schizophrenic participants, 11.6% (8) were females, and 88.4% (61) were males. The control group consisted of 63.2% (67) females and 36.8% (39) males.
Clinical characteristics of the schizophrenic study participants
About 79.8% of the patients had lived with schizophrenia for between 1 and 20 years, while the remaining 20.2% had lived with schizophrenia for more than 20 years. The age of onset of schizophrenia ranged between 10 and 70 years. The majority (71%) reported onset within the ages 20–50 years while for 17.4% and 11.6% of them, the age of onset was below 20 years and above 50 years, respectively. The modal age of onset of schizophrenia was within the age range 31 to 40 years and the modal number of years living with schizophrenia was within the range 1 to 10 years (age of onset; 31–40 = 26.1%, years living with schizophrenia; 1–10 years = 44.9%).
Comparison of finger and palmar patterns between patients with schizophrenia and control
The prevalence of finger patterns assessed was not significantly different when compared between the schizophrenic group and the control except for radial loop (RL), ulnar loop (UL), and plain arch (PA). The prevalence of RL and the UL were found to be significantly higher while PA was significantly lower in the schizophrenic group compared to the control group. Moreover, schizophrenia was found to be associated with high and low odds of RL and PA respectively relative to the control group (Table ). Also, no significant difference was observed when the proportion of the palmar patterns assessed was compared between the schizophrenic group and the control group except the proportion of vestige in the hypothenar region and palmar crease 300 that were found to be significantly lower in patients with schizophrenia compared to the controls (Table ).
Table 1. Comparison of digito-palmar dermatoglyphic patterns between schizophrenia and control groups and its association with schizophrenia.
The sample size for the palmar pattern was obtained by adding the two palms per individual and multiplying by the number of individuals per study category while the sample size for the finger pattern was obtained by adding the five fingers per individual and multiplying by the number of individuals per study category. Palmar creases: the pattern of the palmar creases was described in three-digit according to the PIC model; the first number represents the number of the primary creases, which ranges from 1 to 3, and the middle number represents the number of intersections between the primary creases, which ranges from 0 to 2, and the last number represents the number of complete transverse creases which ranges from 0 to 2. DLW: double loop whorl, TA: tented arch, CPLW: central pocket loop whorl, RL: radial loop, PA: plain arch, PW: plain whorl, UL: ulnar loop. Regression analysis, open field in the hypothenar and thenar region, palmar creases 320, and ulnar loop in the finger patterns were the categories of comparison with control as the reference category. OR: odds ratio, CI: confidence interval; the odds of schizophrenia relative to the control group (reference category).
Comparison of finger ridge counts, triradii, a–b ridge count, and atd angle between patients with schizophrenia and controls
No significant difference was observed when the finger ridge counts and triradii were compared between the controls and the participants with schizophrenia (Mann–Whitney U-test: P > 0.05) (Table ). However, the mean left-hand a-b ridge count was significantly higher in the control group than in the schizophrenia group (Mann–Whitney U-test: P = 0.01). Additionally, the mean right atd angle was significantly higher in controls than in the schizophrenia group (Mann–Whitney U-test: right, P = 0.02) (Table ).
Table 2. Comparison of finger ridge count (FRC), triradius, a–b ridge count, and atd angle between patients with schizophrenia and control group.
Discussion
Dermatoglyphics is one of the areas being explored by many scientists as a tool for understanding the genetic association with psychiatric illness (Markow and Wandler Citation1986; Golembo-Smith et al. Citation2012; Akbarova Citation2018). This is because of the close association between intrauterine development of dermatoglyphic patterns and the brain, both of which develop at the same time and have a common ectodermal origin (Ahmed-Popova et al. Citation2014). A hypothesis, therefore, exists that any congenital disturbances in the development of the brain that may lead to any psychiatric illness could potentially reflect in dermatoglyphics. An example is the single palmar crease associated with Down’s syndrome (Rignell Citation1987; Afework Citation2019). Moreover, the stability of dermatoglyphic patterns after development makes them a good marker to track any abnormality that could have happened during development (Babler Citation1991) as they remain unchanged throughout life. Although several studies have reported associations between dermatoglyphic patterns and schizophrenia (Wang et al. Citation2008; Özyurt et al. Citation2010; Igbigbi et al. Citation2018; Norovsambuu et al. Citation2021), the inconsistency of the results in different populations necessitated the exploration of the association of dermatoglyphic patterns among people living with schizophrenia in the Ghanaian population.
Characteristic of digito-palmar dermatoglyphic patterns of patients with schizophrenia
In this study, the finger dermatoglyphic patterns of patients with schizophrenia were characterized by significantly high prevalence of RL, UL and low prevalence of PA with a significant association between RL and schizophrenia [RL, control = 17 (1.6) versus schizophrenia = 23 (3.3), X2 = 5, P = 0.02; UL, control = 661(62.4) versus schizophrenia = 464 (67.2), X2 = 4.2, P = 0.04; PA, control = 84 (7.9) versus schizophrenia = 24 (3.5), X2 = 14, P = 0.0002. RL, OR (95% CI) = 1.9 (1.0–3.6), P = 0.04]. This observation agrees with Igbigbi et al. (Citation2018) who reported a significant increase in loops and a decrease in arches in the schizophrenic group compared to the control group in the Nigerian population. In contrast, no significant difference was observed in the frequency of fingerprint patterns when compared between schizophrenia and the controls in Chilean and Indian populations (Rothhammer et al. Citation1971; Ponnudurai et al. Citation1997) suggesting that the digito-palmar dermatoglyphic patterns may be influenced by the genetic makeup of a specified population. Several studies have reported the influence of genetic makeup of a specified population on dermatoglyphics patterns and the significance of dermatoglyphics patterns as a tool to identify a person’s specific population (Sunderland and Coope Citation1973; Awuah et al. Citation2017; de Jongh et al. Citation2018; Jaiyeoba-Ojigho et al. Citation2019; Baryah and Krishan Citation2020). This variability could be because of environmental influence during the differentiation of the ridges in utero. Environmental factors such as exposure to infectious pathogens and lifestyles such as alcohol use and stress have been associated with disturbances in the formation of dermatoglyphics (Wang et al. Citation2008). Some studies have specifically documented the association between embryonic stress and low ridge count formation, the association between schizophrenia and stress (Babler Citation1978), and the association between schizophrenia and developmental stress (Özyurt et al. Citation2010). These pieces of evidence could explain why stress and some infections can trigger schizophrenia in high-risk individuals. Thus, it is likely that the damage to the brain in-utero leads to future risk of schizophrenia caused by the infection or stress that happens at the same time the brain and the dermatoglyphics are developing. This may also cause disturbances in the formation of the dermatoglyphics reflecting the damage in the brain since both the brain and the dermatoglyphics develop from the same ectoderm. Some of these disturbances in the dermatoglyphics may include low a-b ridge counts and atd angles in patients with schizophrenia as observed in this study and reported by several studies (Fananas et al. Citation1996; Özyurt et al. Citation2010; Norovsambuu et al. Citation2021). Moreover, this may also account for low prevalence of vestige pattern in the hypothenar region and palmar crease 300, with schizophrenic patients significantly less likely to have vestige pattern in the hypothenar region and palmar crease 300 [OR (95% CI); vestige pattern = 0.2 (0.06–0.69), P = 0.01; palmar crease 300 = 0.1 (0.01–0.99), P = 0.049] as observed in this study. Since Africa is characterized by a high burden of infectious pathogens, patients with schizophrenia may likely have been exposed to some of these environmental factors in-utero leading to low levels of these parameters among patients with schizophrenia. This observation also suggests that these disturbances in the dermatoglyphics may be explored for their diagnostic significance in schizophrenia.
Limitations
The composition of the two study categories, the control and schizophrenia groups, were not closely matched in terms of sample size, gender and age. Also schizophrenia was considered a single disease entity rather than the subcategories of schizophrenia, which may have affected some of the findings. Moreover, the representative of the heterogeneous population of Ghana was not considered. These limitations could have affected some of the results obtained since the subclassification of schizophrenia, genetic makeup of a specified population and sample size have been reported to be a source of variations in digito-palmar dermatoglyphics patterns (Igbigbi et al. Citation2018).
Recommendation
Further studies should be conducted to evaluate the finger and the palmar patterns associated with schizophrenia among Ghanaians using a matched case control study with consideration to the sample size and the heterogeneous population of Ghana.
Conclusion
In this study, the finger dermatoglyphic patterns of patients with schizophrenia were characterized by a significantly high prevalence of RL, UL, and low prevalence of PA, with a significant association between RL and schizophrenia, while the palmar patterns were characterized by a significantly low prevalence of vestige pattern in the hypothenar region, and palmar crease 300. Also, the mean left-hand a-b ridge count and the atd angle on the right hand was found to be significantly low in patients with schizophrenia compared to controls. These observations could be explored further as a potential biomarker in diagnosing and early detection of schizophrenia similar to the association between single palmar creases and Down’s syndrome.
Acknowledgements
We thank the staff of the Accra Psychiatry Hospital who provided guidance and support for participant recruitment. Special mention is made of Dr. Susan Seffah, Dr. Pinaman Appau and Dr. Emmanuel Azusong. We also say a big ‘thank you’ to all the study participants. A special thanks to the technical support staff of the Department of Anatomy, UGMS, for their relevant support. Conceptualization: SNM, BAB, DF. Design & data generation: SNM, BAB, NKKK, KAO, RMB, BAH, JA, MAR, DF. Data analysis & interpretation: SNM, BAB, NKKK, KAO, RMB, BAH, JA, MAR, DF. Manuscript development: SNM, BAB, NKKK, JA. All authors critically reviewed and approved the final manuscript. SNM & BAB are co-first Authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Supporting source files and raw data are deposited in Mendeley Data repository as Arko-Boham, Benjamin (2022), ‘Schizophrenia and Digital-Palmar Dermatoglyphics’, Mendeley Data, V6. https://doi.org/10.17632/p2hds3wj2h.6.
Additional information
Funding
References
- Achalia R, Bhople KS, Ahire P, Andrade C. 2014. Late-onset schizophrenia with isolated cavum vergae: case report and literature review. Indian J Psychiatry. 56(4):399–401.
- Afework M. 2019. Prevalence of the different types of palmar creases among medical and dental students in Addis Ababa, Ethiopia. Ethiop J Health Sci. 29(3):391–400.
- Ahmed-Popova FM, Mantarkov MYJ, Sivkov ST, Akabaliev VH. 2014. Dermatoglyphics – a possible biomarker in the neurodevelopmental model for the origin of mental disorders. Folia Med. 56(1):5–10.
- Akbarova SN. 2018. Dermatogliphics can be as method of behavior genetics. GESJ Educ Sci Psychol. 4(50):26–37.
- Ali MB, Alrashed AW, Alateeq AE, Alkhawfi AM. 2021. Incidence of primary palmar creases variants and their correlation to academic performance in KFU College of Medicine-2020: a cross-sectional descriptive study. Med Sci. 25(114):1803–1811.
- Awuah D, Dzogbefia VP, Chattopadhyay PK. 2017. Finger dermatoglyphics of the Asante population of Ghana. IJIRAS. 4(4):333–336.
- Babler WJ. 1978. Prenatal selection and dermatoglyphic patterns. Am J Phys Anthropol. 48(1):21–27.
- Babler WJ. 1991. Embryologic development of epidermal ridges and their configurations. Birth Defects Orig Artic Ser. 27(2):95–112.
- Baryah N, Krishan K. 2020. Exploration of digital dermatoglyphics of two ethnicities of North India-forensic and anthropological aspects. Forensic Sci Int Reports. 2:100055.
- CITI NEWSROOM. 2021. Ghana: CITI; [cited 2021 July 8]. Available from: https://citinewsroom.com/2021/07/3-1-million-ghanaians-suffering-from-mental-health-issues-health-minister/.
- de Jongh A, Lubach AR, Kwie SLL, Alberink I. 2018. Measuring the rarity of fingerprints patterns in the Dutch population using an extended classification set. J Forensic Sci. 64(1):108–119.
- Fananas L, Van Os J, Hoyos C, McGrath J, Mellor CS, Murray R. 1996. Dermatoglyphic a-b ridge count as a possible marker for developmental disturbance in schizophrenia: replication in two samples. Schizophr Res. 20(3):307–314.
- Farah FH. 2018. Schizophrenia: an overview. Asian J Pharm. 12(2):77–87.
- Flashman LA, Roth RM, Pixley HS, Cleavinger HB, McAllister TW, Vidaver R, Saykin AJ. 2007. Cavum septum pellucidum in schizophrenia: clinical and neuropsychological correlates. Psychiatry Res. 154(2):147–155.
- Fournier O. 2011. The status of mental health care in Ghana, West Africa and signs of progress in the Greater Accra Region. Berkeley Undergrad J. 24(3):9–34.
- Gnanasivam P, Vijayarajan R. 2019. Gender classification from fingerprint ridge count and fingertip size using optimal score assignment. Complex Intell Syst. 5:343–352.
- Golembo-Smith S, Walder DJ, Daly MP, Mittal VA, Kline E, Reeves G, Schiffman J. 2012. The presentation of dermatoglyphic abnormalities in schizophrenia: a meta-analytic review. Schizophr Res. 142:1–11.
- Haroun HSW. 2019. Digito-palmar dermatoglyphics: variations and prediction of brain disorders. MOJ Anat Physiol. 6(3):103–106.
- Igbigbi PS, Ominde BS, Oyibojoba OA. 2018. Dermatoglyphics patterns of schizophrenic patients in a Nigerian population. Int J Anat Res. 6(2.1):5114–5121.
- Innocenti GM, Ansermet F, Parnas J. 2003. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 8(3):261–274.
- Israel GD. 1992. Determining sampe size. Program and Evaluation and Organizational Development, IFAS, University of Florida. POED-6:1–5.
- Jaiyeoba-Ojigho EJ, Odokuma IE, Igbigbi PS. 2019. Comparative study of fingerprint patterns of two ethnic groups: A Nigerian study. J Coll Med Sci. 15(4):270–275.
- Kumbnani HK. 2007. Dermatoglyphics: a review. Anthropol Today Trends Scope Appl. 3:285–295.
- Lin P, Sun J, Lou X, Li D, Shi Y, Li Z, Ma P, Li P, Chen S, Jin W, et al. 2022. Consensus on potential biomarkers developed for use in clinical tests for schizophrenia. Gen Psychiatry. 35(1):1–11.
- Markow TA, Wandler K. 1986. Fluctuating dermatoglyphic asymmetry and the genetics of liability to schizophrenia. Psychiatry Res. 19(4):323–328.
- Modiano YA. 2019. Dermatoglyphic measures in relation to depressive symptoms among non-clinical adolescents and young adults [PhD thesis]. The City University of New York; 2019.
- Norovsambuu O, Tsend-Ayush A, Lkhagvasuren N, Jav S. 2021. Main characteristics of dermatoglypics associated with schizophrenia and its clinical subtypes. PLoS One. 16(6):1–17.
- Özyurt B, Songur A, Sarsilmaz M, Akyol Ö, Namli M, Demirel R. 2010. Dermatoglyphics as markers of prenatal disturbances in schizophrenia: a case-control study. Turkish J Med Sci. 40(6):917–924.
- Ponnudurai R, Menon MS, Muthu M. 1997. Dermatoglyphic fluctuating asymmetry and symmetry in familial and non familial schizophrenia. Indian J Psychiatry. 39(3):205–211.
- Rignell A. 1987. Simian crease incidence and the correlation with thenar and hypothenar pattern types in Swedish patients with trisomy 21 (Down’s syndrome). Am J Phys Anthropol. 72(3):277–286.
- Roberts M, Mogan C, Asare JB. 2014. An overview of Ghana’s mental health system: results from an assessment using the World Health Organization’s assessment instrument for mental health systems (WHO-AIMS). Int J Ment Health Syst. 8(1):1–13.
- Rothhammer F, Pereira G, Camousseight A, Benado M. 1971. Dermatoglyphics in schizophrenic patients. Hum Hered. 21:198–202.
- Saraceno B. 1998. Nations for mental health: A new who action programme on mental health for underserved populations. Eur Psychiatry. 13(S4):164s.
- Sharma A, Sood V, Singh P, Sharma A. 2018. Dermatoglyphics: a review on fingerprints and their changing trends of use. CHRISMED J Heal Res. 5(3):167–172.
- Singh A, Gupta R, Zaidi S, Singh A. 2016. Dermatoglyphics: a brief review. Int J Adv Integr Med Sci. 1(3):111–115.
- Sunderland E, Coope E. 1973. The tribes of south and central Ghana: a dermatoglyphic investigation. Man. 8(2):228–265.
- Valle R. 2020. Schizophrenia in ICD-11: comparison of ICD-10 and DSM-5. Rev Psiquiatr Salud Ment. 13(2):95–104.
- Wang JF, Lin CL, Yen CW, Chang YH, Chen TY, Su KP, Nagurka ML. 2008. Determining the association between dermatoglyphics and schizophrenia by using fingerprint asymmetry measures. Int J Pattern Recognit Artif Intell. 22(3):601–616.
