?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
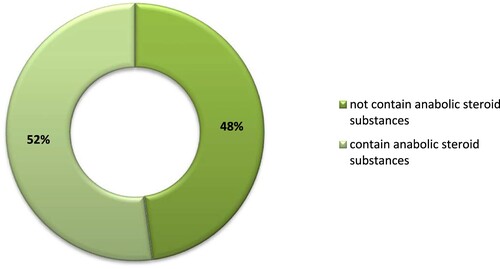
The goal of this study is to establish the presence or absence of anabolic androgenic steroids in food supplements implemented in sports practice, to assess the health risk from intake of food supplements with quality and content discrepancies in conflict with the recommendations. The authors analysed 23 samples of food supplements by using liquid chromatographic methods. The presence/absence of substances with steroid structure was determined by considering the resulting chromatograms and using reference substances. The analysis of the resulting data showed that 11 out of 23 samples of food supplements did not contain anabolic steroid substances, while different amounts of androgenic steroid substances were identified in the other 12 samples. The results from the analyses confirmed the hypothesis formulated by the authors for possible presence of undeclared steroid ingredients in food supplements, and enabled the following conclusion: more than half of the tested food supplements contained undeclared anabolic steroids, all banned by the World Anti-Doping Agency, and their number in one of the samples could be more than one. The authors also revealed that the information on the product label and package concealed the undeclared ingredients of the food supplement and claimed for high efficacy and safety.
Highlights
The findings of the analyses with chromatographic methods confirmed the hypothesis set by the authors for the possible presence of undeclared steroid ingredients in food supplements (FS) and point to the following highlights:
More than half of the tested FS (52.2%) contained undeclared anabolic steroids and their number in one of the samples could be more than one (sample 16 contained 9 steroid substances).
The label and product package information conceal the undeclared ingredients of the food supplement and claim high efficacy and safety.
All anabolic steroids detected in the composition of the tested FS have been banned by the World Anti-Doping Agency (WADA).
Introduction
Increased interest in food supplements (FS) has been observed during recent years. The global dietary supplements market was valued at $155.2 billion in 2022 and is projected to reach $220.8 billion by 2027, growing at a compound annual growth rate of 7.3% from 2022 to 2027 (Dietary Supplements Market Citation2023).
Numerous factors contribute to the sustainable FS market growth: large-scale advertising focused on both users and health specialists; and enhanced access to FS due to the annual increase of suppliers (pharmacies, drug stores, specialized shops for sport supplementation, food stores), as well as Internet sales. The global legislation allows the relatively quick launching of novel FS on the market. Some countries have established a notification regime (USA, Mexico, Australia, and Bulgaria) while others (Brazil, Canada, and Russia) have enforced a registration regime for novel FS on the market. Often the users consider FS safe, although the analytical and quality control of these products before launching them on the market is poor and not obligatory. In most cases, no quality and quantity control is implemented on input materials or ready-to-use products, which could lead to compromised quality and hazardous risks for the consumer. Because of the global liberal legislative framework, it is a frequent practice to detect undeclared ingredients in FS or discrepancies between the real quality and quantity content and what is declared by the manufacturer. It has been revealed that some supplements placed on the market contain hormones which are not shown on the product label. Androgenic anabolic steroids (AAS) as ingredients in sports supplements can be assumed as a contamination that affects the health of consumers, the majority of which are athletes (Alaedini S. et al. Citation2021).
Professional athletes are exposed every day to intensive physical fatigue/exhaustion (Ivanov and Ivanova Citation2016). Because of that, the use of various stimulants to improve muscle power and stamina, to strengthen the mental and physical conditions, has gone along human civilization since its origin and up to now. Athletes’ intake of FS aims to reduce fats and increase muscle mass as well as improving stamina and stimulating post-exercise recovery (Deijen et al. Citation1999). The substances used in sports are divided into two large groups: chemicals banned by the World Anti-Doping Agency (WADA) (doping substances), and substances permitted for use.
According to the joint work of the American College of Sports Medicine, American Dietetic Association, and dietologists from Canada, an athlete’s nutritional regime has a major role in achieving good athletic results (Williams Citation2005; American Dietetic Association, Dietitians of Canada, American College of Sports Medicine, Nutrition and athletic performance Citation2000), and their demands for protein supply are much higher than those of physically inactive individuals. This means that the necessary protein amount cannot always be supplied only by food. The use of amino acids, protein products, creatine, and multivitamins can be observed in almost all sports disciplines.
The post-production FS quality and quantity control, unlike that of medicinal products, is not mandatory. This is the main factor leading to disturbing reports provided by many researchers about undeclared ingredients detected in FS (Da Justa Neves and Caldas Citation2015; Fouillot Citation2004; Ros et al. Citation1999; Dietary Supplement Recall Issued Due to Anabolic Steroids Citation2017). The lack of mandatory analytical control, combined with the liberal regulation policy for FS, can cause the following: the presence of deliberately undeclared ingredients; accidental contamination during the production process; falsified food supplements.
According to the Italian Ministry of Health, 28% of FS on the Italian market contain undeclared components. An investigation conducted in Italy over the period 2011–2013 covered 105 FS and established that 72% of the samples had infringements in the labels. In 37% of the samples, the manufacturer was not listed and 4% of the samples had no label at all (Gaudiano et al. Citation2016). The analytical results of the investigation established that for 7% of the samples there was no active ingredient listed; 35% of the samples did not correspond to the specifications stated on the label. Undeclared ingredients were found in 12% of the samples. The undeclared ingredients were substances that could be included in drugs administered only by medical prescription. Undoubtedly, such FS present a serious risk for consumers’ health.
The most frequently detected ingredients in FS, consumed by athletes, are testosterone and other AAS. Testosterone is a major human hormone responsible for male secondary genital features (androgenic effect) and for maintaining the nitrogen balance necessary for strengthening the tissues and supporting the muscle mass (anabolic effect); that is, testosterone has both androgenic and anabolic effects.
Anabolic steroids are obtained through modification of testosterone structure. The aim is to increase the desired nitrogen-preserving effects through modification of the testosterone molecule and to decrease the androgenic ones to achieve minimization of the undesired virilising effect. Practically though, the full elimination of androgenic effects of the anabolic steroids is impossible (Karaivanova et al. Citation2016).
AAS are prescribed for medical purposes: in the treatment of certain forms of anaemia; for faster tissue regeneration in acute or chronic wounds or severe burns; as substituting therapy of primary and secondary hypogonadism or short height; in osteoporosis; for protein-calcium malnutrition associated with weight loss.
The large-scale administration of AAS is associated with the improvement of professional athletes’ sports achievements, as well as with body-building for accelerated body shaping. The long-term administration of AAS though can cause many undesired reactions, affecting physically and mentally the whole human organism. They modify neuro-mediation with their effect on the neurotransmitters noradrenaline, dopamine, serotonin, and the production of endogenous opioids. That is why steroids have a substantial effect on mood and behaviour. AAS misuse can cause extreme changes in the mood, including the so-called ‘steroid rage’ – an episode of strong, usually groundless, aggression, resembling a psychotic episode. They can also lead to addiction and, in some cases, abstinence syndrome when their intake is discontinued – a condition resulting from physical and mental dependence, created as a result of the discontinuation of long-term intake of AAS. Other possible undesired effects are dermatological ones: acne (because of elevated estradiol and dihydrotestosterone (DHT) levels), hair loss, greasy skin, and changed skin colour (jaundice); decreased production of sex hormones by the feedback mechanism; premature closing of the epiphyses of growing young organisms; liver damage, etc. Those data testify to the unsafe long-term administration of AAS. Long-term side effects affect the cardiovascular system, mental health, and the endocrine system. Infertility, growth defects, feminization, and masculinization are very often irreversible side effects. The use of AAS is permitted only for medical reasons. The mode of administration of AAS is strictly regulated and is only by doctor’s prescription. Androgenic steroids are substances that cannot be included in the composition of FS and off the shelf products. They can only appear in the composition of medicinal products. The safety profile of the use of dietary supplements with natural ingredients is much better compared to that of steroids. Their use is legal, the effects are often satisfactory, which in turn leads to a growing interest in them on the part of users. The effects are identical, but with the use of these FS they occur much more slowly and to a lesser degree compared to the use of AAS.
Food supplements containing AAS, implemented on a large scale in professional athletes’ training-competition practice can doubtlessly create real risk for the athlete’s health depending on the administration mode and dose, intake period, and the quality of the product. This hazard is even greater if the FS contains an undeclared ingredient. Besides that, the violation of anti-doping rules, with a positive test result of an athlete who has used banned substances, creates psychological risk and possible professional or health consequences.
Analytical studies have established that declared non-hormonal supplements (containing vitamins, minerals, amino acids, or plant ingredients) could contain AAS that have not been listed on the labels or in the product leaflet (Parr et al. Citation2004). It is known that 44% of the positive results of doping testing performed by an anti-doping organization in Great Britain, came from FS containing banned substances. During the Winter Olympic Games in Sochi, 2631 doping tests were made. Seven positive doping test results were identified and the athletes supported the thesis that those results were due to the use of food supplements (Ivanov and Ivanova Citation2016).
Geyer et al. (Citation2004) conducted a large-scale screening of 634 FS for undeclared ingredients. The investigations were conducted from October 2000 to November 2001. The food supplements were purchased from 13 countries and 215 suppliers. Most of them were bought from the Netherlands. It has been established that 14.8% (94 samples) contained undeclared anabolic steroids in concentrations from 0.01 to 190 µg/g. The presence of plasma levels over 1 µg/g of steroid is sufficient to enable the detection of a positive doping result (Parr et al. Citation2004). The research team of Parr et al. (Citation2007) detected high steroid concentrations in FS and identified methandienone (16.8 mg/pill) and stanozolol (14.5 mg/pill). Another study by the same researchers established the presence of steroids in supplements produced in China containing multivitamins and magnesium (Geyer et al. Citation2008). Other surveys reported ingredients included in a FS such as hypophyseal growth hormone-releasing peptide (GHRP-2) – a peptide stimulating the production of growth hormone (Kohler et al. Citation2010).
Green et al. (Citation2011) investigated 12 FS and determined that 11 of them did not comply with the labelling requirements: the data did not correspond to the quantitative content of the ingredients as declared by the manufacturer. Besides that, one of the tested FS contained 10 mg of undeclared testosterone. This enabled the authors to consider almost 100% discrepancy with the declared data.
Baume et al. (Citation2006) from the Medical University in Lausanne, Switzerland, investigated 103 FS, purchased mainly on the Internet. These were supplements mainly of the following categories: creatine, prohormones, amino acids, and nootropics. The researchers performed a screening for the content of AAS. They established that almost 20% of the tested samples contained undeclared anabolic steroids.
Professional athletes particularly are at high risk from the lack of regulation. Aiming at higher performance levels, they consume supplements far more than other people (Green et al. Citation2011). With the growth of the nutritional supplements market and the risk of taking supplements that contain harmful, undeclared substances, the world’s anti-doping rules and doping control procedures force athletes to be strictly responsible for their health and body cleanliness under competition conditions. Undeclared composition of nutritional supplements and their free movement on the market can cause health, sports and technical problems for athletes. Because of this, and also because the cited studies in this area are not very recent, we conducted our study on the contamination of dietary supplements with anabolic steroids, without being declared on the label.
This study is relevant because it is necessary to trace and monitor the described composition of dietary supplements, perform a risk assessment related to the health, well-being and safety of FS users, as well as develop approaches to improve signalling systems and knowledge of unwanted side effects.
The purpose of this survey is to establish the presence or absence of AAS in FS implemented in sports practice, to assess the health risk from intake of FS with quality and content discrepancies with the recommendations.
Materials and methods
To determine the presence/absence of anabolic steroids in FS, we purchased 23 food supplements randomly on the Internet and from shops for sports supplementation. All the supplements selected for study had a pharmacological effect to stimulate improvement of muscle strength and endurance, and enhance physical status in athletes. In the content and characteristics of the products, only natural ingredients regulated as a FS without a medical prescription were declared. The commercial names of the nutritional supplements are deliberately not disclosed and are named as ‘sample no … ’. Daily intake and dosage were not the subject of research in this study, but undeclared ingredients in the label which may harm those taking them, in a health and sports-legal aspect, were sought. The analysis was made at a Globaltest testing centre – a laboratory in Sofia, Bulgaria, certified for testing foods, FS and drugs https://globaltest-bg.com/services-en/food/. The tested FS were blinded, pre-coded, packed in individual sterile bags, and delivered to the laboratory with a takeover record. When the laboratory delivered the test results each sample was completed with an individual record containing sample description, description of the applied methods, absence/presence of the relevant anabolic steroids, amount, test conditions, and chromatograms of the samples where an undeclared ingredient was detected.
The determination of the presence/absence of anabolic steroids was made by Ultra Performance Liquid Chromatography (UPLC) method with Photodiode Array (PDA)-detector. The active substances were detected by the generated Ultraviolet (UV)- and Mass Spectrometry (MS)-spectra as well as by the chromatograms produced by the PDA-detector and Ion Trap MS-detector. Specifically, the analyses of the tested samples were made by UPLC Shimadzu LC 20- ADc with PDA detector, combined with chromatography column Zorbax Eclipse C18 150 × 4.6 mm, 5 µm of Agilent Technologies Company.
The retention times and the areas of the obtained peaks of each sample were compared to those of the reference substances. The presence/absence of substances with steroid structure was determined based on chromatographic data and the used reference materials. The resulting chromatographic data of the samples and the pure reference material with AAS structure were processed by Xcalibur version 2.0.7 software. The chromatograms of the standards – medicinal products with steroid structure, namely: methandienone, oxandrolone, methyltestosterone, stanozolol, metenolone, boldenone, androsterone, and the chromatograms of the studied FS samples were used for comparison and identification of the undeclared steroids. The concentrations of the detected undeclared and identified FS ingredients were calculated based on the chromatographic evidence for the areas of the tested solution and the standard solution test.
Results
The results of the chromatographic analysis are presented in Table reflecting the peaks with retention time (RT) in minutes and the estimation of the identified undeclared steroid ingredient.
Table 1. Results from the chromatographic analysis of the samples.
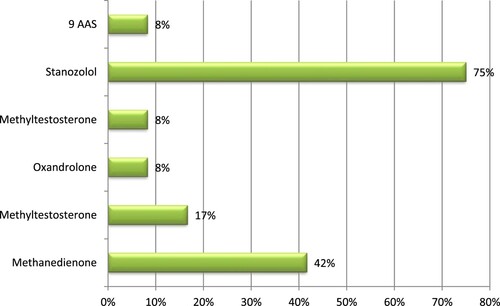
The FS samples No. 1, 2, 5, 7, 9 contained methandienone (optimal daily intake is 30–50 mg). Their chromatograms showed a peak within the relevant area and retention time in minutes (RT): 20,277–21,037.
FS samples No. 3 and No. 6 contained methyltestosterone (optimal daily intake is 25–50 mg). The chromatograms of both samples in RT: 24,541–25,116 contained a peak with the corresponding areas identical by RT and area to those of methyltestosterone.
FS samples No. 4 and 8 evidenced the undeclared ingredient oxandrolone (optimal daily intake is 5–10 mg for women, and 30–80 mg for men). On the chromatograms of both samples in RT: 43.285–43.419 min appeared similar peaks with the respective areas identical to that of oxandrolone.
FS sample No. 14 revealed the content of the undeclared ingredient metenolone (optimal daily intake is 60–100 mg). A peak with the adequate area appeared on the chromatogram in RT: 34,843.
The compiled results of the conducted chromatographic study of the steroid drugs methandienone, oxandrolone, methyltestosterone, stanozolol, metenolone, boldenone, androsterone, and the determined amounts of the detected undeclared ingredients in 23 FS are presented in Table (Figures and ).
Table 2. Compiled results for presence or absence of anabolic steroids in samples of food supplements intended for intake as testosterone stimulants.
The results showed that 11 out of 23 samples of FS did not contain anabolic steroid substances. The other samples contained various amounts of AAS. The laboratory analyses of FS for methandienone content found that samples with numbers 1, 2, 5, 7, and 9 contained methandienone in amounts 9.75, 10.03, 1.67, 9.67, and 9.80 mg/pill, respectively. This is a potential hazard as the athlete consuming the studied 5 FS could have a positive doping test. The effects could be serious for the athlete’s health as well as for their sports career. It is well known that the use of methandienone is banned by WADA. The listed 5 FS containing methandienone were purchased from Internet sites. All FS were completed with a label in Bulgarian but with an incorrect list of FS composition, with undeclared steroid content. The supplements had a similar formulation and included 150 mg soy protein isolate and 150 mg of whey protein concentrate, as well as 75 mg of amino acids.
The samples with numbers 3 and 6 showed the content of another undeclared compound, methyltestosterone, in concentrations 2.25 and 1.12 mg/pill, respectively. The label of FS sample No. 3 stated that it contained branched-chain amino acids (BCAA) – leucine, isoleucine, and valine and the permitted dose was set to 5 pills 2–3 times daily. The label of FS No. 6 stated that it contained L-carnitine in a concentration of 500 mg. These samples contained a testosterone analogue in respectively small amounts.
The chromatographic analytical results of samples No.4 and 8 revealed the presence of the undeclared compound oxandrolone in amounts respectively 4.93 and 1.79 mg/capsule. The names of these two food supplements were not found on the Food and Drug Agency (FDA) online list of FS blocked and withdrawn from the market. The label on the packages of both products listed a similar composition: soy protein isolate, amino acids, and yohimbine.
According to the results of conducted analysis for absence/presence of stanozolol, metenolone, and other substances with steroid structure, undeclared substances were found in the majority of tested samples. In one of the samples (No. 12), stanozolol (optimal daily intake is 5–15 mg for women and 40–100 mg for men) was in the amount of 4.56 mg/capsule and the presence of a steroid in the product was not declared on the manufacturer’s label. According to package information, the formulation contained BCAA, acetyl L-carnitine, and wild yam (Dioscorea villosa), and the product was claimed to contain 100% natural substances.
Sample No. 14 revealed the presence of another undeclared steroid ingredient, metenolone, in the amount of 2.80 mg/pill. The label of the product stated the composition of the FS included wheat protein concentrate (150 mg), isoleucine (100 mg), valine (100 mg), leucine (25 mg), and puncture vine (Tribulus terestris) (25 mg). Metenolone is comparatively expensive and is less frequently detected as an undeclared FS ingredient.
One of the tested samples (No. 16) contained the steroids boldenone, androsterone, and 7 more unidentified substances. The label of the product presented information that the supplement contained grape seed extract (75 mg), coneflower (Echinacea) (200 mg), coenzyme Q10 (25 mg), ginger (100 mg), and claimed that it was a 100% natural product without side effects. The presence of anabolic steroids could lead to a positive doping test and temporarily suspend the guilty athlete’s sports career. This is also associated with adverse health risks for the athlete, elevated by the presence of other unidentified substances as well. This food supplement was considered as having a false composition and being hazardous for human health and an athlete’s sports career.
The results of the pharmaceutical analyses with chromatographic methods confirmed the hypothesis set by the authors for the possible presence of undeclared steroid ingredients in FS and enabled the following conclusion: more than half of the tested FS (52.2%) contained undeclared anabolic steroids and their number in one of the samples could be more than one (sample 16 contained 9 steroid substances). All anabolic steroids detected in the composition of the tested FS have been banned by WADA. The label and product package information hide the undeclared ingredients of the food supplement and claim high efficacy and safety. At first view, all ‘samples’ are nutritional supplements for athletes without prohibited doping ingredients described in the ingredients section.
Discussion
Many nutrition supplements intended for athletes that are sold on the Internet continue to have undeclared doping substances in their composition. Therefore the possibility of unintentional doping infringements is higher due to the prescribed use of such products, and they may even lead to general health risks, according to the study of (Duivenet et al. Citation2021).
The conducted laboratory tests according to the analytical standards confirm the hypothesis for the presence of undeclared ingredients in FS, which compromises their safety. Unannounced ingredients are present in tested samples, and some of the FS do not meet the labelling requirements and should not be distributed and sold. Health risk assessment from the conducted analytical studies for the presence of undeclared ingredients shows that 52.2% of the studied 23 FS used in sports contain AAS. The samples contain methandienone, methyltestosterone, oxandrolone, stanozolol, metenolone, boldenone, and androsterone. In one of the samples, the presence of 9 undeclared substances with steroid structure was found. All the revealed anabolic steroids in this study of FS are banned by WADA. There is evidence that professional athletes are not sufficiently aware of the health risks of using some FS, in particular testosterone stimulants, despite their awareness of anti-doping programs and prevention. Key points in the study of Diuvenet et al. are that 38% of 66 of the studied potentially high-hazard sports supplements (stating to intensify workouts, help muscle growth and fat loss) were found to contain doping substances; and 4.5% of the products tested were found to have doping substances in concentrations which can have strong negative health effects, and may lead to a positive doping result (Diuvenet et al. Citation2021).
According to study of Kozhuharov et al., more than 28% of the analysed dietary supplements pose a potential risk of unintentional doping. They conclude that athletes and their teams need to be aware of the issues associated with the use of FS and should take great care before inclusion of FS in the supplementation regime (Kozhuharov et al. Citation2022).
Millman and Ross’s research states that steroids can be related to a wide range of unwanted effects that can cause physical changes, psychological disturbances, morbidity, and mortality as well. There are not enough studies on the side effects of nutritional supplements, however they can be considered as similarly dangerous. Striving for higher and better achievements, many athletes at all levels seek the potential benefits of these substances while neglecting the associated risks. Therefore focused testing at all levels is recommended according to Millman and Ross (Millman and Ross Citation2003).
In perspective, recommendations were formulated to the responsible institutions and professional health communities to increase the requirements for qualitative and quantitative control of FS and to develop a strategy for their safe and rational use.
Future scenario
The safety profile of the use of food supplements with natural ingredients is much better compared to that of steroids. Their use is legal, the effects are often satisfactory, which in turn leads to a growing interest in them on the part of users. The effects are identical, but with the use of these FS they occur much more slowly and to a lesser degree compared to the use of AAS.
There is a need to provide adequate information to patients and ensure their safety through training and exchange of experience with best practice between individual countries in order to improve legislation to be in line with Council Framework Directive 89/391 of the European Union regarding consumer safety.
Strengths and limitations of the study
Strengths: The research algorithm allowed for a systematic study to clarify the safety profile of the products offered on the market through the use of methods that provide a precise assessment of risk and risk factors. Recommendations have been formulated to the responsible institutions in Bulgaria and the professional health communities to increase the requirements for the qualitative and quantitative control of nutritional supplements and to develop a strategy for their safe and rational use.
Limitations: The limitation of the study is that not all sources of supply of the studied food supplements were covered, but only the Internet sites and health/sports shops; it would be good to include pharmacies and drugstores for a larger scope in a future study. However, this cannot limit the distribution and production of nutritional supplements containing unregulated ingredients. The multi-million army of nutritional supplement users is constantly growing, but what is more frightening is that the number of supplements containing medicinal products is constantly increasing.
Conclusion
In conclusion, the results of the study confirmed the hypothesis set by the authors on the possible presence of undeclared steroid ingredients in food supplements. Such supplements present a high risk of side effects, unanticipated by their user, as well as risks for an athlete’s career. There is undeclared content of AAS in FS implemented in sports practice, and there is a health risk from the intake of FS with quality and content discrepancies with the recommendations. This highlights the need for additional government regulations on FS, as well as penalties for breaking those regulations.
Author contributions
Each author contributed to the production of this manuscript: Conceptualization, E.P.G., S.G.; methodology, E.P.G., S.G. H.L, A.M., and V.M.; software, S.B.; validation, E.P.G., S.G. H.L, A.M., and V.M.; formal analysis, E.P.G., S.G. H.L, A.M., and V.M.; investigation, E.P.G., S.G. H.L, A.M., and V.M.; resources, E.P.G., S.G. H.L, A.M., and V.M.; data curation, E.P.G., S.G. H.L, A.M., and V.M.; writing – original draft preparation, E.P.G and S.G; writing – review and editing, H.L.; supervision, S.G.; project administration, E.P.G, S.G. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in Mendeley Data at https://doi.org/10.17632/kts9ydvc62.3.
References
- Alaedini S, Amirahmadi M, Kobarfard F, Rastegar H, Nasirahmadi S, Shoeibi S. 2021. Survey of protein-based sport supplements for illegally added anabolic steroids methyltestosterone and 4-androstenedione by UPLC-MS/MS. Steroids. 165:108758. doi:10.1016/j.steroids.2020.108758.
- American Dietetic Association. 2000. Dietitians of Canada, American College of Sports Medicine, Nutrition and athletic performance. J Am Diet Assoc. 100:1543–46. doi:10.1016/S0002-8223(00)00428-4.
- Baume N, Mahler N, Kamber M, Mangin P, Saugy M. 2006, February. Research of stimulants and anabolic steroids in dietary supplements. Scand J Med Sci Sports. 16(1):41–48. doi:10.1111/j.1600-0838.2005.00442.x.
- Da Justa Neves DB, Caldas ED. 2015 Oct. Dietary supplements: International legal framework and adulteration profiles, and characteristics of products on the Brazilian clandestine market. Regul Toxicol Pharmacol. 73(1):93–108104.
- Deijen J, Wientjes C, Vullinghs H, Cloin P, Langefeld J. 1999. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res Bull. 48(2):203–209. doi:10.1016/S0361-9230(98)00163-4.
- Dietary Supplement Recall Issued Due to Anabolic Steroids. 2017. http://www.pharmacytimes.com/news/dietary-supplement-recall-issued-due-to-anabolic-steroids.
- Dietary Supplements Market, Industry Size, Forecast - 2030 (marketsandmarkets.com) visited 25 April 2023.
- Duiven E, van Loon LJC, Spruijt L, Koert W, de Hon OM. 2021, March 22. Undeclared doping substances are highly prevalent in commercial sports nutrition supplements. J Sports Sci Med. 20(2):328–338. doi:10.52082/jssm.2021.328.
- Fouillot JP. 2004. Doping and dietary supplements. Bull Acad Natl Med. 188(6):933–942. discussion 942–43.
- Gaudiano M, Manna L, Bartolomei M, Rodomonte A, et al. 2016. Health risks related to illegal and on-line sale of drugs and food supplements results of a survey on marketed products in Italy from 2011 to 2013. Ann Ist Super Sanita. 52(1):128–132.
- Geyer H, Parr MK, Köhler K, Mareck U, Schänzer W, Thevis M. 2008. Nutritional supplements cross-contaminated and faked with doping substances. J Mass Spectrom. 43(7):892–902. doi:10.1002/jms.1452.
- Geyer H, Parr MK, Mareck U, Reinhart U, et al. 2004. Analysis of non-hormonal nutritional supplements for anabolic-androgenic steroids-results of an international study. Int J Sports Med. 25:124–129. doi:10.1055/s-2004-819955.
- Green G, Catlin D, Starcevic B. October 2011. Analysis of over-the- counter dietary supplements. Clin J Sport Med. 11(4):254–259.
- Ivanov K, Ivanova S. August 2016. Substances in sports. , Plovdiv, Zenitza Publishing House, 2-5. ISBN 978-954-9674-47-7.
- Karaivanova M, Peychev L. 2016. Georgiev St. Anabolic androgenic steroids. Drug Reference Book. Tea Dizain OOD, p. 308. ISSN978-619-90647-3-3.
- Kohler M, Thomas A, Geyer H, et al. 2010. Confiscated black market products and nutritional supplements with non-approved ingredients analysed in the Cologne Doping Control Laboratory. Drug Test Anal. 1(11-12):533–537.
- Kozhuharov VR, Ivanov K, Ivanova S. 2022 April 22. Dietary supplements as source of unintentional doping. Biomed Res Int. 2022:1–18. doi:10.1155/2022/8387271.
- Millman RB, Ross EJ. 2003. Steroid and nutritional supplement use in professional athletes. Am J Addict. 12(s2):S48–S54.
- Parr MK, Geyer H, Hoffmann B, Köhler K, Mareck U, Schänzer W. 2007. High amounts of 17-methylated anabolic-androgenic steroids in effervescent tablets on the dietary supplement market. Biomed Chromatogr. 21(2):164–168. doi:10.1002/bmc.728.
- Parr MK, Geyer H, Reinhart U, Schänzer W. 2004. Analytical strategies for the detection of non-labelled anabolic androgenic steroids in nutritional supplements. Food Addit Contam. 21(7):632–640. doi:10.1080/02652030410001701602.
- Ros JJ, Pelders MG, De Smet PA. 1999, February. A case of positive doping associated with a botanical food supplement. Pharm World Sci. 21(1):44–46. doi:10.1023/A:1008681612399.
- Williams M. 2005. Dietary supplements and sports performance: amino acids. J Int Soc Sports Nutr. 2(2):63–67.