Abstract
This meta-analysis examined the predictive value of admission serum and urine carboxypeptidase-B activation peptide (CAPAP) in patients with severe acute pancreatitis. PubMed, Embase, and the Cochrane Library were searched for relevant studies published up to February 21, 2022. Seven studies (439 acute pancreatitis patients) were included. The summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), the area under the receiver operating characteristic curve (AUC) of admission serum CAPAP were 0.86 (95%CI: 0.76-0.93), 0.78 (95%CI: 0.68-0.85), 3.89 (95%CI: 2.56-5.91), 0.18 (95%CI: 0.09-0.33), 18.56 (95%CI: 6.99-49.30), and 0.90 (95%CI: 0.87-0.92), respectively; these values for admission urine CAPAP were 0.82 (95%CI: 0.50-0.95), 0.78 (95%CI: 0.68-0.86), 3.75 (95%CI: 2.40-5.85), 0.23 (95%CI: 0.07-0.79), 9.87 (95%CI: 3.71-26.22), and 0.84 (95%CI: 0.80-0.87), respectively. There were no significant differences between admission serum and urine CAPAP in sensitivity (1.05, 95%CI: 0.75-1.47; P = 0.781), specificity (1.00, 95%CI: 0.85-1.18; P = 1.000), PLR (1.04, 95%CI: 0.56-1.91; P = 0.906), NLR (0.78, 95%CI: 0.20-3.10; P = 0.727), and DOR (1.88, 95%CI: 0.47-7.49; P = 0.370). The AUC of serum CAPAP (1.07, 95%: 1.02-1.13; P = 0.007) was higher than that of urine CAPAP in predicting acute pancreatitis severity. Both admission serum and urine CAPAP had moderate predictive values for assessing the severity of acute pancreatitis.
Key highlights
Both admission serum and urine carboxypeptidase-B activation peptide (CAPAP) had moderate predictive values for assessing the severity of acute pancreatitis.
The predictive value of admission serum CAPAP was higher than that of admission urine CAPAP in predicting acute pancreatitis severity.
Including the measurement of serum CAPAP at admission in patients with suspected pancreatitis could help triage the patients and determine the course of management.
Hospital policymakers could consider including it among the routine tests at admission for such patients.
Introduction
Acute pancreatitis involves inflammation and results in a local and systemic inflammatory response syndrome; it remains the leading cause of gastrointestinal-related hospital admissions. The incidence of acute pancreatitis is increasing, leading to an increased risk of mortality (Peery et al. Citation2015; Roberts et al. Citation2017). Acute pancreatitis is divided into mild (no organ failure or local complications), moderate (transient organ failure that lasts for <48 h and/or local complications), and severe (persistent organ failure for ≥48 h, with or without local complications) (Banks et al. Citation2013). Most patients have mild acute pancreatitis, whereas nearly 20% of the patients are diagnosed with moderate to severe acute pancreatitis (van Dijk et al. Citation2017). Due to sterile necrosis and infected necrosis, hospitalization can be prolonged, and there is a higher risk of mortality (Mounzer et al. Citation2012). Therefore, early detection of severe acute pancreatitis in patients is imperative for improving prognosis.
Several scoring systems (Bedside Index for Severity in Acute pancreatitis (BISAP), Ranson score, Acute Physiology, and Chronic Health Examination II (APACHE II) score, Atlanta classification, and Computed Tomography Severity Index (CTSI)) are widely used for assessing the severity of acute pancreatitis (Ranson et al. Citation1974; Larvin and McMahon Citation1989; Wu et al. Citation2008; Banks et al. Citation2013). Moreover, patients with metabolic syndrome and diabetes mellitus have an accelerated progression due to severe acute pancreatitis (Huh et al. Citation2018). Previous studies have already illustrated that apolipoprotein B/A-I ratio, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio could assist in predicting severe acute pancreatitis (Huh et al. Citation2018). The concentration of carboxypeptidase-B activation peptide (CAPAP) in serum and urine could be a promising predictor for assessing severe acute pancreatitis. Serum and urine CAPAP levels are significantly related to the extent of pancreatic injury (Appelros et al. Citation1998). Nevertheless, the predictive value of CAPAP in assessing the severity of acute pancreatitis remains unknown. In addition, two studies reported a high diagnostic odds ratio (DOR), whereas the remaining three indicated a moderate DOR. These two studies used 100 and 40 nmol/L as cutoff values of admission urine CAPAP (Appelros et al. Citation2001; Abu Hilal et al. Citation2007). Although the study conducted by Hjalmarsson et al. used 100 nmol/L as the cutoff value, the study included a smaller number of severe acute pancreatitis patients, and the false-negative rate was higher than expected, inducing lower DOR (Hjalmarsson et al. Citation2009). A previous meta-analysis of six studies reported the diagnostic value of admission serum CAPAP (Deng et al. Citation2015), but an important study was not included, and stratified analyses were not conducted.
Hence, this meta-analysis was performed to review systematically the predictive value of admission CAPAP for the severity of acute pancreatitis.
Methods
Data sources, search strategy, and selection criteria
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. Citation2009). The meta-analysis was not registered.
Electronic databases such as PubMed, Embase, and the Cochrane Library were systematically screened for relevant studies published up to February 21, 2022. The following keywords and text words were used: (‘carboxypeptidase-B activation peptide’ or ‘carboxypeptidase B’ or ‘procarboxypeptidase-B’ or ‘active carboxypeptidase-B’ or ‘activation peptide of carboxypeptidase B’) and (‘acute pancreatitis’ or ‘severe acute pancreatitis’ or ‘post endoscopic retrograde cholangiopancreatography pancreatitis’). No filters were set for the search. A manual search of the reference lists of the included studies was performed for eligibility of any new study.
Two independent reviewers performed the study selection process following a standardized form, and any inconsistencies were resolved with the help of an additional author by referring to the original article for making a final decision.
The inclusion criteria were (1) patients: all included patients were diagnosed with acute pancreatitis; (2) exposure: serum or urine CAPAP measured by radioimmunoassay (RIA) or enzyme-linked immuno-sorbent assay (ELISA); (3) study design: irrespective of prospective or retrospective design; (4) outcome: study with sufficient data to calculate the true and false positive and false and true negative rates. The exclusion criteria were 1) studies that showed no differentiation between severe and mild acute pancreatitis; 2) reported other factors than CAPAP; 3) no sufficient data for meta-analysis.
Data collection and quality assessment
The following data were extracted from the eligible studies: first authors’ surname, publication year, country, sample size, mean age, number of males and females, number of severe and mild acute pancreatitis, etiology, test method, sample, cutoff value, and true and false positive and false and true negative rates. The quality of the retrieved studies was assessed using Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), which is based on a yes/no/unclear assessment of patient selection, index test, reference standard, flow, and timing (Whiting et al. Citation2011). Data collection and quality assessment were independently conducted by two authors. Any disagreement was settled by discussion.
Statistical analysis
The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), DOR, and the area under the receiver operating characteristic curve (AUC) with corresponding 95% confidence intervals (CIs) for serum and urine CAPAP were calculated based on the true positive, false positive, false negative, and true negative rates from each study. The summary sensitivity, specificity, PLR, NLR, DOR, and AUC were calculated using bivariate generalized linear mixed, random-effects modes, and hierarchical regression, respectively (DerSimonian and Laird Citation1986; Walter Citation2002). Heterogeneity among the eligible studies was assessed using the Q statistics, and P < 0.10 was regarded as significant heterogeneity. Subgroup analyses for DOR of serum and urine CAPAP were calculated based on the test method. The ratio of DOR was conducted to indirectly compare the DOR between serum and urine CAPAP or RIA and ELISA methods (Woodward Citation2013). The publication bias was assessed using funnel plots and Deeks’ asymmetry tests (Deeks et al. Citation2005). The P-values were two-sided, and P-values <0.05 were considered statistically significant. All statistical analyses were conducted using Stata (version 10.0; Stata Corporation, TX, USA).
Results
Literature search
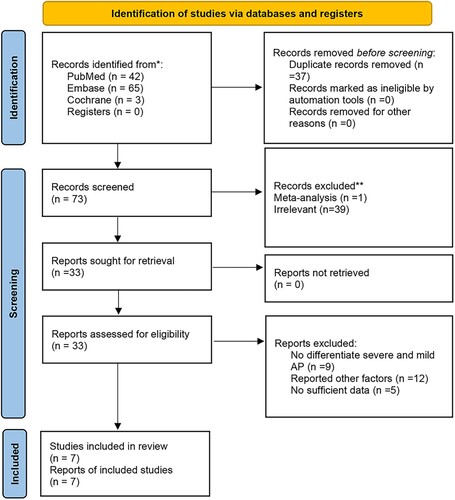
The study selection process is presented in Figure . The initial electronic search yielded a total of 110 records. Of these, 77 were excluded due to duplications and irrelevant topics. Among the remaining 33 studies, 26 were excluded for the following reasons: studies showed no differentiation between severe and mild acute pancreatitis, reported other factors than CAPAP, and had insufficient data. Finally, seven studies were eligible for this meta-analysis (Pezzilli et al. Citation2000; Appelros et al. Citation2001; Müller et al. Citation2002; Sáez et al. Citation2004; Abu Hilal et al. Citation2007; Regnér et al. Citation2008; Hjalmarsson et al. Citation2009). The manual search of the reference lists of the retrieved studies did not yield any additional studies.
Study characteristics
The characteristics of the studies included in this meta-analysis are summarized in Table . Of the seven included studies, 439 acute pancreatitis patients were recruited in the final analysis. Of these, 229 patients had acute biliary pancreatitis, 95 patients had alcoholic acute pancreatitis, and 115 had other types of acute pancreatitis. These studies were published between 2000 and 2009, and 20–140 patients were included in each study. Moreover, the number of severe and mild acute pancreatitis patients was 103 and 336, respectively. Four studies used RIA as the test method, and the remaining three studies employed ELISA. The predictive value of serum CAPAP was available in seven studies, and the predictive value of urine CAPAP was available in five studies. All the studies were of moderate to high quality (Supplementary Table 1).
Table 1. Baseline characteristics of studies included in this systematic review and meta-analysis.
Sensitivity and specificity
Data for the sensitivity and specificity of admission serum and urine CAPAP were available from seven and five studies, respectively. The pooled sensitivity and specificity of admission serum CAPAP were 0.86 (95%CI: 0.76-0.93) and 0.78 (95%CI: 0.68-0.85), respectively (Supplementary Figure 1A). Moreover, the summary sensitivity and specificity of admission urine CAPAP were 0.82 (95%CI: 0.50-0.95) and 0.78 (95%CI: 0.68-0.86), respectively (Supplementary Figure 1B). There were no significant differences between admission serum and urine CAPAP regarding the sensitivity (1.05, 95%CI: 0.75-1.47; P = 0.781) and specificity (1.00, 95%CI: 0.85-1.18; P = 1.000).
PLR and NLR
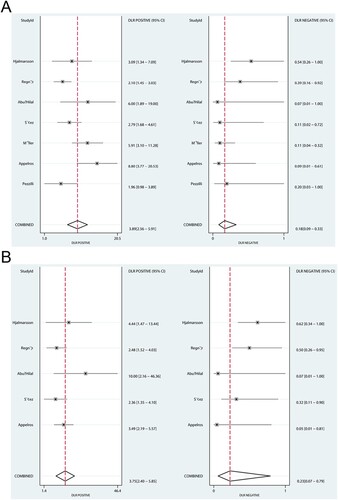
The summary values of PLR and NLR of admission serum CAPAP in the prediction of acute pancreatitis severity were 3.89 (95%CI: 2.56-5.91) and 0.18 (95%CI: 0.09-0.33), respectively (Figure A). Moreover, the pooled PLR and NLR for admission urine CAPAP were 3.75 (95%CI: 2.40-5.85) and 0.23 (95%CI: 0.07-0.79), respectively (Figure B). The PLR (ratio: 1.04; 95%CI: 0.56-1.91; P = 0.906) and NLR (ratio: 0.78; 95%CI: 0.20-3.10; P = 0.727) between admission serum and urine CAPAP showed no statistically significant association.
DOR
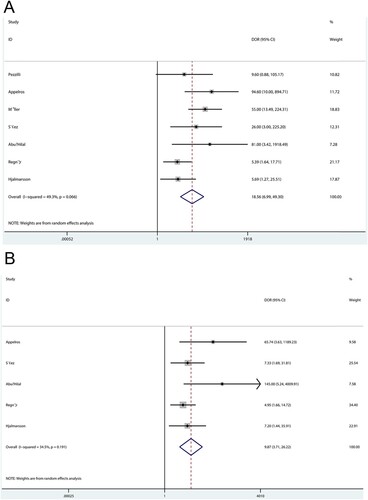
The summary DOR of admission serum CAPAP in the prediction of acute pancreatitis severity was 18.56 (95%CI: 6.99-49.30; P < 0.001), and significant heterogeneity was observed among the included studies (Figure A). Moreover, the pooled DOR of admission urine CAPAP was 9.87 (95%CI: 3.71-26.22; P < 0.001), and moderate heterogeneity was observed among the included studies (Figure B). The DOR between admission serum and urine CAPAP in the prediction of acute pancreatitis severity showed no statistically significant association (ratio: 1.88; 95%CI: 0.47-7.49; P = 0.370).
AUC
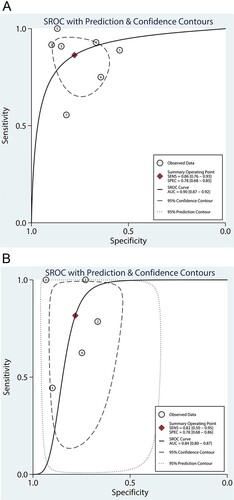
The summary ROC AUCs for admission serum and urine CAPAP were 0.90 (95%CI: 0.87-0.92; Figure A) and 0.84 (95%CI: 0.80-0.87; Figure B), respectively. We noted that the admission serum CAPAP was associated with a higher AUC for predicting the severity of acute pancreatitis than admission urine CAPAP (ratio: 1.07; 95%: 1.02-1.13; P = 0.007).
Subgroup analyses
Subgroup analyses for DOR of serum and urine CAPAP based on the test methods were conducted and listed in Supplementary Table 2. Overall, the serum CAPAP measured by RIA showed no significant DOR compared with serum CAPAP measured by ELISA (ratio: 2.94; 95%CI: 0.43-20.01; P = 0.270). Moreover, the DOR of urine CAPAP as measured by RIA was not significantly higher than the urine CAPAP as measured by ELISA (ratio: 4.79; 95%CI: 0.55-41.57; P = 0.155). Furthermore, there were no significant differences for DOR between serum and urine CAPAP whether measured by RIA (ratio: 1.29; 95%CI: 0.13-12.96; P = 0.826) or ELISA (ratio: 2.11; 95%CI: 0.37-12.02; P = 0.401).
Discussion
The present study evaluated the published articles regarding the predictive value of admission serum and urine CAPAP regarding acute pancreatitis severity. This study included 439 patients with acute pancreatitis from seven studies with a wide range of characteristics. This meta-analysis study suggested that both admission serum and urine CAPAP could be used as a marker with moderate sensitivity, specificity, PLR, NLR, DOR, and AUC for predicting acute pancreatitis severity. Moreover, the admission serum and urine CAPAP showed no significant differences regarding sensitivity, specificity, PLR, NLR, and DOR, whereas the AUC of admission serum CAPAP was significantly higher than the admission urine CAPAP in predicting acute pancreatitis severity. Subgroup analyses were performed according to assay, and the results of the RIA and ELISA subgroups were in agreement with the overall analysis, respectively; the DOR between admission serum and urine CAPAP remained statistically insignificant.
CAPAP is not a routine biomarker measured in patients with acute pancreatitis. Nevertheless, the present meta-analysis suggests that it has some value in predicting the severity of acute pancreatitis. Future studies should examine whether CAPAP could be combined with other biomarkers to build a strong predictive model for acute pancreatitis severity.
A previous meta-analysis of six studies indicated that the pooled sensitivity, specificity, DOR, and AUC for admission serum CAPAP were 0.90, 0.70, 19.08, and 0.86, respectively. Moreover, the pooled sensitivity, specificity, DOR, and AUC for urine CAPAP were 0.81, 0.77, 18.58, and 0.88, respectively (Deng et al. Citation2015). They pointed out that both serum and urine CAPAP could be used as a marker for acute pancreatitis severity during admission. However, an important study was not included, and stratified analyses were not conducted. Moreover, indirect comparisons of the predictive values between serum and urine CAPAP were not conducted. Therefore, to update the information, the present meta-analysis evaluated the predictive values of serum and urine CAPAP in predicting acute pancreatitis severity during admission.
The predictive value of admission CAPAP in serum was relatively high, and six of seven included studies reported admission serum CAPAP with significant predictive value for the severity of acute pancreatitis. Nevertheless, the study by Pezzilli et al. used 1.14-1.19 nmol/L as the cutoff value (Pezzilli et al. Citation2000), but no admission serum CAPAP with marginal DOR for predicting and evaluating acute pancreatitis severity on admission to the emergency room was found. The potential reason for this could be due to the inclusion of smaller sample size and lower cutoff values of admission serum CAPAP. Moreover, the other two included studies reported serum CAPAP with lower DOR when compared with other studies, which might be potentially due to a high false-positive rate.
There were no significant differences between admission serum and urine CAPAP for the sensitivity, specificity, PLR, NLR, and DOR for predicting acute pancreatitis severity, while the AUC of serum CAPAP was significantly higher than urine CAPAP. Nevertheless, significant heterogeneity was observed in many analyses, and the results of such analyses should be taken with caution. This heterogeneity could be due to differences in patient characteristics, populations, disease severity, causes of acute pancreatitis, test methods, and management. Nevertheless, the subgroup analyses indicated that the DOR of admission serum and urine CAPAP was unaltered when stratified by the test method. The high AUC of admission serum CAPAP could be due to the high sensitivity of serum CAPAP, although similar specificities were present between admission serum and urine CAPAP. Furthermore, the DOR between serum and urine CAPAP is not obtained from similar populations, and there are various cutoff values among the included studies, yielding potential confounders.
There are several limitations in this study that should be noted. First, both prospective and retrospective studies were included in this meta-analysis, resulting in uncontrolled biases. Prospective studies usually select the participants based on strict criteria designed to reduce confounding from other sources but at the cost of lower generalizability. On the other hand, retrospective studies are often more representative of the actual patient population, but they are at risk of documentation bias, patient identification bias, and missing data bias, but they often have higher representativeness. Still, whether including both study types overestimates or underestimates the associations is unknown. Secondly, studies with a small sample size were included, which can overestimate the associations. Thirdly, the cutoff values of CAPAP in serum and urine among the included studies varied, affecting the predictive value of serum and urine CAPAP. The studies that used a lower positivity threshold will contribute to association overestimation, while the studies that use a higher threshold will contribute to an underestimation of the associations. Fourthly, the current study was based on published articles, and the unpublished data and the gray literature were not included, inducing inevitable publication biases. Indeed, negative studies are notoriously difficult to publish, and many researchers fail to publish them. Therefore, including only published results probably contributes to an overestimation of the associations.
In conclusion, this study indicated that admission serum and urine CAPAP demonstrated moderate to high predictive value for acute pancreatitis severity. Moreover, the admission serum and urine CAPAP showed no significant differences regarding the sensitivity, specificity, PLR, NLR, and DOR, whereas the AUC of admission serum CAPAP was significantly higher than urine CAPAP. Furthermore, the DOR of admission serum and urine CAPAP was unchanged when stratified by the test method. Furthermore, comparing the predictive values of admission serum with urine CAPAP in predicting acute pancreatitis severity compared with other markers or models should be conducted in future prospective studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Liang Dai and Wei-Yan Guo provided the conceptualization of this study, drafted the manuscript, and performed data analysis. Yin-Hua Wang and Jian-Yi Pu contributed to the study’s design and collection of data. Xing-Wei Xuan, Hai-Xia Chai, and Xiao-Dong Meng worked on investigation and data collection. Jian-Li Chen and Jun-Mao Chen conducted the critical revision of the manuscript. All authors read and approved the final manuscript.
Data availability statement
The data that support the findings of this study are publicly available in the published articles which have been cited.
References
- Abu Hilal M, Ung CT, Westlake S, et al. 2007 Mar. Carboxypeptidase-B activation peptide, a marker of pancreatic acinar injury, but not L-selectin, a marker of neutrophil activation, predicts severity of acute pancreatitis. J Gastroenterol Hepatol. 22(3):349–354. doi:10.1111/j.1440-1746.2006.04550.x.
- Appelros S, Petersson U, Toh S, et al. 2001 Feb. Activation peptide of carboxypeptidase B and anionic trypsinogen as early predictors of the severity of acute pancreatitis. Br J Surg. 88(2):216–221.
- Appelros S, Thim L, Borgström A. 1998 Jan. Activation peptide of carboxypeptidase B in serum and urine in acute pancreatitis. Gut. 42(1):97–102. doi:10.1136/gut.42.1.97.
- Banks PA, Bollen TL, Dervenis C, et al. 2013 Jan. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 62(1):102–111. doi:10.1136/gutjnl-2012-302779.
- Deeks JJ, Macaskill P, Irwig L. 2005 Sep. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 58(9):882–893. doi:10.1016/j.jclinepi.2005.01.016.
- Deng L, Wang L, Yong F, et al. 2015 Jul. Prediction of the severity of acute pancreatitis on admission by carboxypeptidase-B activation peptide: a systematic review and meta-analysis. Clin Biochem. 48(10–11):740–746. doi:10.1016/j.clinbio-chem.2015.06.003.
- DerSimonian R, Laird N. 1986 Sep. Meta-analysis in clinical trials. Control Clin Trials. 7(3):177–188. doi:10.1016/0197-2456(86)90046-2.
- Hjalmarsson C, Stenflo J, Borgström A. 2009. Activated protein C-protein C inhibitor complex, activation peptide of carboxypeptidase B and C-reactive protein as predictors of severe acute pancreatitis. Pancreatology: Official Journal of the International Association of Pancreatology (IAP) [et al]. 9(5):700–707. doi:10.1159/000215577.
- Huh JH, Jeon H, Park SM, et al. 2018 Feb. Diabetes mellitus is associated with mortality in acute pancreatitis. J Clin Gastroenterol. 52(2):178–183. doi:10.1097/MCG.0000000000-000783.
- Huh JH, Jung S, Cho SK, et al. 2018 Feb. Predictive value of apolipoprotein B and A-I ratio in severe acute pancreatitis. J Gastroenterol Hepatol. 33(2):548–553. doi:10.1111/jgh.13860.
- Larvin M, McMahon MJ. 1989 Jul 22. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet (London, England). 2(8656):201–205.
- Moher D, Liberati A, Tetzlaff J, et al. 2009 Jul 21. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7):e1000097. doi:10.1371/journal.pmed.1000100.
- Mounzer R, Langmead CJ, Wu BU, et al. 2012 Jun. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 142(7):1476–1482; quiz e15–6. doi:10.1053/j.gastro.2012.04.034.
- Müller CA, Appelros S, Uhl W, et al. 2002 Aug. Serum levels of procarboxypeptidase B and its activation peptide in patients with acute pancreatitis and non-pancreatic diseases. Gut. 51(2):229–235. doi:10.1136/gut.51.2.229.
- Peery AF, Crockett SD, Barritt AS, et al. 2015 Dec. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 149(7):1731–1741.e3. doi:10.1053/j.gastro.2015.08.045.
- Pezzilli R, Morselli-Labate AM, Barbieri AR, et al. 2000 Sep. Clinical usefulness of the serum carboxypeptidase B activation peptide in acute pancreatitis. JOP: Journal of the Pancreas. 1(3):58–68.
- Ranson JH, Rifkind KM, Roses DF, et al. 1974 Jun. Objective early identification of severe acute pancreatitis. Am J Gastroenterol. 61(6):443–451.
- Regnér S, Appelros S, Hjalmarsson C, et al. 2008. Monocyte chemoattractant protein 1, active carboxypeptidase B and CAPAP at hospital admission are predictive markers for severe acute pancreatitis. Pancreatology: Official Journal of the International Association of Pancreatology (IAP) [et al]. 8(1):42–49. doi:10.1159/000114866.
- Roberts SE, Morrison-Rees S, John A, et al. 2017 Mar-Apr. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology: Official Journal of the International Association of Pancreatology (IAP) [et al]. 17(2):155–165.
- Sáez J, Martínez J, Trigo C, et al. 2004 Jul. A comparative study of the activation peptide of carboxypeptidase B and trypsinogen as early predictors of the severity of acute pancreatitis. Pancreas. 29(1):e9–14.
- van Dijk SM, Hallensleben NDL, van Santvoort HC, et al. 2017 Nov. Acute pancreatitis: recent advances through randomised trials. Gut. 66(11):2024–2032. doi:10.1136/gutjnl-2016-313595.
- Walter SD. 2002 May 15. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 21(9):1237–1256. doi:10.1002/sim.1099.
- Whiting PF, Rutjes AW, Westwood ME, et al. 2011 Oct 18. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 155(8):529–536. doi:10.7326/0003-4819-155-8-201110180-00009.
- Woodward M. 2013. Epidemiology: study design and data analysis. 3rd Edition. Chapman & Hall/CRC Texts in Statistical Science Ser. Chapman & Hall/CRC.
- Wu BU, Johannes RS, Sun X, et al. 2008 Dec. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 57(12):1698–1703. doi:10.1136/gut.2008.152702.