Abstract
The present study investigated the effect of Gastrodia elata extracts (GE) on chronic electric foot-shock stress (cEFS)-induce anxiety-like behaviors in male C57BL/6 mice. Mice were administered GE (200 or 400 mg/kg/day, oral), and then exposed to cEFS (0.6 mA intensity, 1 s every 5 s interval, 3 min duration) for seven days. GE treatment ameliorates the anxiety-like behavior induced by cEFS, which were determined with elevated plus maze test and open field test. cEFS decreased the dopamine and serotonin levels in brain and both were reversed by GE treatment. cEFS increased corticosterone levels in serum and number of c-fos positive cells in brain, which were downregulated by GE treatment. These results suggested that GE ameliorates the anxiety-like behavior by regulating the levels of dopamine and serotonin, the number of c-fos positive cells in the brain, and corticosterone levels in the serum.
Introduction
External stress can cause physical and mental changes and even illnesses, including anxiety and depressive disorders (Herbison et al. Citation2017). Stress activates the hypothalamic–pituitary–adrenal (HPA) axis, and causes a sudden rise in adrenal cortical hormones and release of glucocorticoids such as corticosterone (CORT) in rodents and cortisol in humans (Joëls et al. Citation2018; Juruena et al. Citation2020). In addition, chronic stress results in appearance of anxiety-related symptoms, reduced excitability, and decrease in levels of neurotransmitters as dopamine and serotonin in the brain (Sheikh et al. Citation2007). Furthermore, exposure to chronic stimulation causes increased expression of the early-stage gene c-fos, particularly in the paraventricular nucleus (PVN) of the hypothalamus (Chen and Herbert Citation1995; Imaki et al. Citation2003). Benzodiazepines are the main class of drugs used for treating anxiety disorders, but they have many side effects including sedation, muscle relaxation, ataxia, amnesia, and drug dependence (Kang et al. Citation2023). Therefore, it is important to find more effective and safer alternatives.
Gastrodia elata Blume (G. elata), the dried tuber of orchids, is a valuable traditional medicinal plant that was first recorded in Shen Nong’s Herbal Classic thousands of years ago (Liu and Huang Citation2017). In traditional Chinese medicine, G. elata is used to treat with symptoms such as febrile convulsions in children, tetanus, limb paralysis, numbness, rheumatic arthralgia, among others. Additionally, it is employed to alleviate epileptic-like convulsions, headaches, and dizziness (Heese Citation2020; Gong et al. Citation2024). It is rich in various active components, including phenols and their glycosides, organic acids, polysaccharides, sterols, as well as a variety of amino acids and trace elements. Among these, phenolic compounds and their glycosides are the primary active constituents of G. elata (Zhou et al. Citation2023). Many studies have demonstrated that it can be used to treat Tourette syndrome in children (Wang et al. Citation2021b), vascular dementia (Shi et al. Citation2020; Wu et al. Citation2023), depression and improve cognitive function (Li et al. Citation2020). It can ameliorate migraine (Mi et al. Citation2020) and cerebral ischemia-reperfusion injury (Zhang et al. Citation2024), used to treat stroke (Zhou et al. Citation2020), lower blood pressure, used as anti-thrombotic, and for regulating blood sugar (Cheng et al. Citation2019). Recent studies have revealed the effects of G. elata including antioxidant, antidepressant, immunomodulatory, neuroplasticity, and neuroprotective activities (Huang et al. Citation2021). In terms of neuroprotection, the water extract of G. elata has been demonstrated to alter the composition and functionality of the gut microbiota by regulating monoamine metabolism pathways, thereby exhibiting antidepressant-like effects and alleviating cognitive impairment in mild social defeat stress-induced depressive mice and ApoE-/ – mice (Huang et al. Citation2021; Huang et al. Citation2023). Furthermore, fresh G. elata has been found to improve simulated weightlessness-induced cognitive impairment by regulating the inflammation apoptosis pathway (Zhang et al. Citation2023b). Although it has been shown that G. elata has neuroprotective effects, its anti-anxiety effects and the underlying mechanism are still unclear.
Materials and methods
Materials
Gastrodia elata extract (GE) was obtained from Shaanxi Healthy Biotechnology Co. LTD (Xi’an, China). Dopamine and serotonin were purchased from Sigma-Aldrich (Darmstadt, Germany). The CORT assay kit was purchased from Wuhan USCN (CEA540Ge, China). The c-fos primary antibody was provided by Santa Cruz Biotechnology (Dallas, Texas, U.S.A.), and the secondary antibody was provided by Cell Signaling Technology (Danvers, MA, U.S.A.). All other chemicals were of analytical grade.
Experimental animals
We purchased 48 adult male C57BL/6 mice, weighing (24 ± 3) g from Hangzhou Medical College, and housed them in temperature and humidity-controlled environment (12 h light/dark cycle) °C temperature, and (50 ± 5) % humidity with water and food freely available. All experiments and procedures were performed in accordance with the rules of the Experimental Animal Center of the School of Medicine, Hangzhou City University.
The establishment of anxiety-like models
The male C57BL/6 mice were placed in a foot shocker (Shanghai XinRuan Information Technology Co., Ltd, Shanghai, China) for 3 min at an intensity of 0.6 mA; 1 sec foot-shock was administered every 5 s, once a day for 7 consecutive days, as previously described (Zhao et al. Citation2015).
Experimental design
The male C57BL/6 mice were randomly divided into six groups (eight mice per group) and randomly assigned to the control, GE (200 mg/kg), GE (400 mg/kg), cEFS, cEFS + GE (200 mg/kg) and cEFS + GE (400 mg/kg) groups. The allocation was conducted using a computer-generated randomization table, with the sample size for this study determined based on preliminary experiments. The control and cEFS groups were orally administered 0.9% saline, while the drug-treated groups were orally administered GE (200 mg/kg) or GE (400 mg/kg). The drug was dissolved in saline and administered once daily, 2 h before the cEFS stimuli, for seven consecutive days. On the 7th day, behavioral tests were performed 1 h after the foot shock. On day 7, after the behavioral tests were completed, the mice were anesthetized with 3% pentobarbital sodium, and blood was collected from the hearts of anesthetized mice, and brain tissue was obtained for the assays. All experiments were performed by experimenters who were blind to the treatment of the animals.
Open field test
The open field test (OFT) apparatus (ENV-520; Med Associates Inc., USA) consisted of one floor and four transparent walls (30 cm × 30 cm × 15 cm). The male C57BL/6 mice were placed in the center of the apparatus and the distance traveled within 5 min was recorded (Zhao et al. Citation2015).
Elevated plus maze test
The elevated plus maze (EPM) apparatus and smart analytic system (Panlab S.I., Barcelona, Spain) consisted of two closed arms (30 × 5 × 16 cm) and two open arms (30 × 5 cm), connected by a central area (5 × 5 cm). The male C57BL/6 mice were placed in the center facing the open arms. After 1-min adaptation, the time spent in the open arms and entries made in the open arm within 5 min were recorded (Lister Citation1987).
Biochemical assay
Serum was obtained after whole blood centrifugation (4°C, 13,000 g, 15 min), and CORT level was determined using an ELISA kit (CEA540Ge, Cloud-Clone Corp, Wuhan, China).
The midbrain was dissected and dopamine and serotonin levels in the midbrain were detected using high-performance liquid chromatography, as described previously (Choi et al. Citation2013; Zhao et al. Citation2015).
c-fos immunohistochemical staining
The male C57BL/6 mice were anesthetized and then transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. The brains were removed and fixed with 4% PFA overnight at 4 °C. Subsequently, they were moved to a 15% sucrose solution overnight and finally to a 30% sucrose solution until they sank. The brains were then cut into 25 μm thick coronal frozen sections. Subsequently, these sections were washed with PBS, incubated with 3% hydrogen peroxide for 10 min, and blocked with 5% BSA for 1 h. The sections were then incubated with c-fos primary antibody (1:250) overnight at 4°C, and then with secondary antibody (1:500) for 2 h at room temperature. The sections were then incubated with the ABC kit and immunoreactivity was measured using a DAB kit. Cells which were positive for c-fos expression were counted and averaged. Photomicrographs of c-fos-positive cells (3-4 sections for each animal) in the PVN regions were counted and analyze by ImageJ software.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics version 26.0 for Windows (Armonk, NY, IBM Corp.). Data are presented as mean ± standard deviation (S.D.). The one-way analysis of variance ANOVA followed by the Tukey’s post-hoc test was used for comparison between different groups, and the t-test was used for comparison between control and cEFS groups or cEFS and GE treatment groups. p < 0.05 was considered as statistically significant.
Results
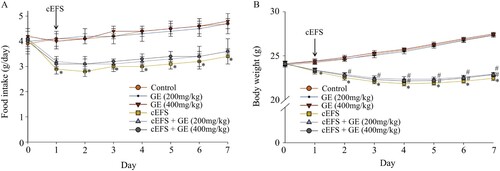
Effects of GE on food intake and body weight
The male C57BL/6 mice’s food intake and body weight were recorded daily. GE (200 and 400 mg/kg) were administered to normal mice, and neither GE (200 mg/kg) nor GE (400 mg/kg) treatments had any effect on food intake and body weight in normal mice (p > 0.05) (A and B). Both decreased during the experiment in all the mice which were given cEFS compared to the control group (p < 0.05) (A and B). The GE treatment increased food intake compared to the cEFS group, but the difference was not significant (A), and GE treatment increased body weight and was significant from day 4 to day 7 in both the GE (200 mg/kg) and GE (400 mg/kg) treatment group (p < 0.05) (B). In addition, there was no significant differences between the GE (200 mg/kg) and GE (400 mg/kg) treatment groups (p > 0.05) (A and B).
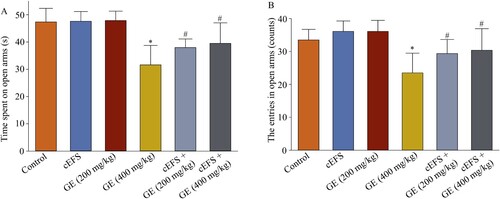
Effects of GE on EPM test
We recorded the time spent on the open arms and entries into the open arms within 5 min. Compared with the control group (47 s, 100%), the GE (200 mg/kg) group (48 s, 102.1%, p > 0.05) and the GE (400 mg/kg) group (48 s, 102.1%, p > 0.05) had no effect on the time to open arms in normal mice (A). The male C57BL/6 mice in the cEFS group spent significantly less time in the open arms 32 s (68.1%, p < 0.05) (A) compared with that of the control group (47 s), whereas it was increased by cEFS + GE (200 and 400 mg/kg) treatments to 38 s (80.9%) and 40 s (85.1%), respectively (p < 0.05). In addition, mice in control group made 34 entries (100%) in the open arms, and both of GE (200 mg/kg) and GE (400 mg/kg) groups made 36 entries in the open arms (105.9%, p > 0.05) (B); however, the number of entries decreased to 24 in the cEFS group, which was 29.4% less than that of the control group (p < 0.05) (B). cEFS + GE (200 and 400 mg/kg) treatments increased the number of entries into the open arms to 29 and 30, respectively (85.3% and 88.2%, p < 0.05). In addition, there was no significant difference between the GE (200 mg/kg) and GE (400 mg/kg) treatment groups (p > 0.05) (A and B).
Figure 2. Effects of GE on the EPM test. Effects of GE on time spent in open arms (A). Effects of GE on the number of entries in the open arms (B). Data are presented as mean ± S.D. (n = 8 per group). *p < 0.05 shows comparison with the control group, and #p < 0.05 shows comparison with the cEFS group.
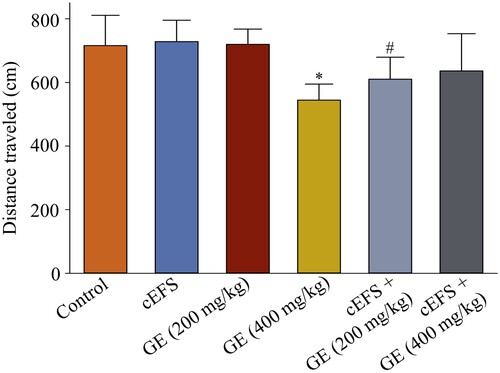
Effect of GE on distance traveled in OFT
We measured the distances traveled by the male C57BL/6 mice in OFT within 5 min. There was no significant change in the distance traveled by GE (200 mg/kg) (728.6 cm, 101.8%, p > 0.05) and GE (400 mg/kg) (719.7 cm, 100.6%, p > 0.05) treatments compared to the control group (715.7 cm, 100%). The distance traveled in the OFT was significantly reduced (544.2 cm, 76.0%, p < 0.05) (Figure ) in the cEFS group compared to the control group (100%). The distance traveled in the cEFS + GE (200 mg/kg) (610.0 cm, 85.2%) was significantly increased compared to the cEFS group (p < 0.05). Although the distance traveled in the cEFS + GE (400 mg/kg) group (635.9 cm, 88.9%) was higher than that in the cEFS group, it was not statistically significant because of its within-group differences. Whereas, there was not significance between cEFS + GE (400 mg/kg) group and cEFS + GE (200 mg/kg) group, due to the differences within group. In addition, there was no significant difference between the GE (200 mg/kg) and GE (400 mg/kg) treatment groups (p > 0.05).
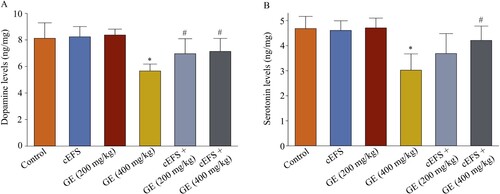
Effects of GE on the dopamine and serotonin levels in the brain
The dopamine and serotonin levels in normal mice treated with GE (200 mg/kg) (8.2 ng/mg, 101.2% and 4.6 ng/mg, 97.9%, p > 0.05) and GE (400 mg/kg) (8.4 ng/mg, 103.7% and 4.7 ng/mg, 100.0%, p > 0.05) did not significantly change compared to the control group (8.1 ng/mg, 100% and 4.7 ng/mg, 100%). However, after 7 days of exposure to foot shock, the dopamine (5.7 ng/mg, 69.5%, p < 0.05, A) and serotonin levels in the cEFS group (3.0 ng/mg, 63.8%, p < 0.05, B) were significantly lower than those in the control group. However, dopamine levels were significantly increased by treatment with cEFS + GE (200 mg/kg) (7.0 ng/mg, 85.7%, p < 0.05) and cEFS + GE (400 mg/kg) (7.1 ng/mg, 87.8%, p < 0.05). While serotonin levels were higher in both the cEFS + GE (200 mg/kg) (3.7 ng/mg, 78.7%, p > 0.05) and cEFS + GE (400 mg/kg) (4.2 ng/mg, 89.9%, p < 0.05) groups compared to the cEFS group, only the GE (400 mg/kg) group showed a statistically significant increase. In addition, there was no significant difference between the GE (200 mg/kg) and GE (400 mg/kg) treatment groups (p > 0.05) (A and B).
Figure 4. Effects of GE on the dopamine and serotonin levels in the brain. Effects of GE on dopamine (A) and serotonin (B) levels in the brain. Data are presented as mean ± S.D. (n = 8 mice per group). *p < 0.05 shows comparison with the control group, and #p < 0.05 shows comparison with the cEFS group.
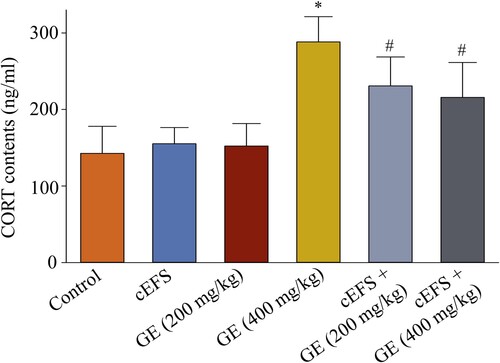
Effects of GE on the serum CORT level
In normal mice, the treatment with GE (200 mg/kg) (155.3 ng/ml, 105.9%, p > 0.05) and GE (400 mg/kg) (152.3 ng/ml, 106.8%, p > 0.05) did not affect CORT levels compared to the control group (142.6 ng/ml, 100%). The CORT level increased in the cEFS group to 202.1% (288.2 ng/ml, p < 0.05, ) compared to the control level; however, this increase was reduced upon GE (200 mg/kg) and GE (400 mg/kg) treatments (230.8 ng/ml, 161.9% and 215.9 ng/ml, 151.4%, respectively, p < 0.05). In addition, there was no significant difference between the GE (200 mg/kg) and GE (400 mg/kg) treatment groups (p > 0.05).
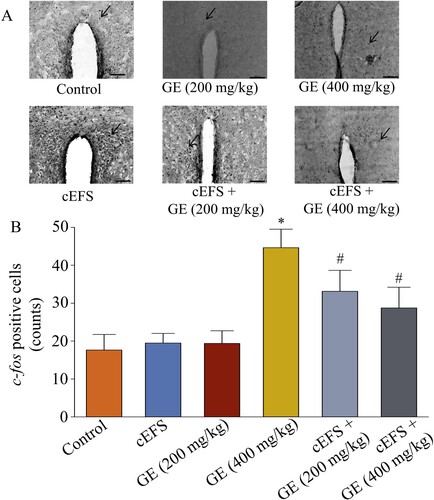
Effects of GE on c-fos expression in PVN
GE (200 mg/kg) (20 counts, 111.1%, p > 0.05) and GE (400 mg/kg) (19 counts, 105.6%, p > 0.05) had no effect on c-fos expression in normal mice compared to the control group (18 counts, 100%, p > 0.05). However, the number of c-fos positive cells in the cEFS group was significantly increased by foot shock (45 counts, 250.0%, p < 0.05, ), whereas both GE (200 mg/kg) and GE (400 mg/kg) treatments significantly decreased the number of c-fos positive cells (33 counts,183.3% and 29 counts,161.1%, respectively, p < 0.05). In addition, there was no significant difference between the GE (200 mg/kg) and GE (400 mg/kg) treatment groups (p > 0.05).
Figure 6. Effects of GE on c-fos expression in the PVN region of brain. Immunohistochemical image of c-fos positive cells in the PVN region (20x). The arrow shows the c-fos positive cells (A). Statistical comparison of the number of c-fos positive cells in the PVN brain region in different groups (B). Data are presented as mean ± S.D. (n = 8 mice per group). *p < 0.05 shows comparison with the control group, and #p < 0.05 shows comparison with the cEFS group.
Discussion
The EPM test is commonly used to assess the anxiety-like behavior by detecting the time spent and the number of entries in the open arms which helps in determining animals passive avoidance of a potential threat (Lister Citation1987). Distance traveled in the OFT is used to detect the spontaneous activity and exploratory behavior of rodents (Lister Citation1987; Verma et al. Citation2010; Zhao et al. Citation2016). The time spent and the number of entries in the open arms in EPM test, and the spontaneous activity in OFT are closely related to dopamine and serotonin levels in the brain (Broderick and Phelix Citation1997; Espejo Citation1997; Vallone et al. Citation2000). It has been reported that GE and its phenolic constituents might improve the anxiety-like behavior in the EPM test (Jung et al. Citation2006). The present study aimed to investigate the mechanisms of GE on anxiety-like behaviors induced by cEFS in male C57BL/6 mice. We performed EPM and OFT tests to determine whether the mice were anxious. We observed that cEFS stimulation reduced the time spent and number of entries made into the open arms in the EPM test, as well as the distance traveled in the OFT, which was in accordance with a previous study (Zhao et al. Citation2015). However, GE alleviated these reductions (Figures and ). In addition, the food intake and body weight in all foot-shock-stressed mice were lower than those in the control group (A and B) (Jeong et al. Citation2013). This might be due to anxiety. In addition, cEFS stimulation decreased the dopamine and serotonin levels which was reversed by GE administration (A and B). These results suggest that GE ameliorates anxiety-like behaviors induced by cEFS in mice.
Changes in the dopamine and serotonin levels under stressful conditions are closely connected to anxious and depressive behaviors, as well as learning and memory impairments (Zhao et al. Citation2015). cEFS stimulation could lead to affective disorders such as anxiety, which are closely related to decrease in dopamine and serotonin levels in the brain (Sheikh et al. Citation2007). In the present study, GE significantly improved the decrease in dopamine and serotonin levels induced by cEFS stimulation (A and B). Adrenal glands secrete corticosteroids during stress-induced anxiety. For example, CORT levels were elevated in case of cEFS groups compared to control groups (). This process was mediated by the HPA axis (Sheline Citation2000). Persistently high CORT levels could affect dopamine and serotonin levels (Sheikh et al. Citation2007). Moreover, CORT levels are known to be highly correlated with the EPM test, and its administration to rats aggravated their anxiety-like behaviors (Rodgers et al. Citation1999; Skórzewska et al. Citation2014). In the present study, cEFS stimulation increased the serum CORT levels. However, serum CORT levels were significantly reduced in GE administered mice, and their dopamine and serotonin levels were restored (A and B). Additionally, GE increased the time spent and number of entries in the open arms in the EPM test and increased spontaneous activity in the OFT ( and ). These results suggest that GE exerts anxiolytic effects by modulating the levels of CORT, dopamine, and serotonin in the serum and brain. Moreover, expression of c-fos, an immediate early gene, in PVN is associated with glucocorticoid regulation, whose expression is increased after exposure to acute and chronic stimuli in rodents. Meanwhile, PVN plays a crucial role in regulating the hormone secretion of the HPA axis by affecting cerebral CORT levels (Chen and Herbert Citation1995; Imaki et al. Citation2003). We observed that cEFS stimulation increased c-fos expression in the PVN, while treatment with both 200 and 400 mg/kg GE significantly decreased the expression of c-fos in mice (). Gastrodin from GE is reported to ameliorate depressive symptoms in mice by regulating the serotonergic and GABAergic nervous system (Jung et al. Citation2006), and JAK2-STAT3 signaling pathway in the frontal cortex (Wang et al. Citation2021a). It might also inhibit IL-1β and p38 MAPK pathway in posttraumatic stress disorder model of rat (Peng et al. Citation2013). Gastrodin promotes an Arg-1 + microglial phenotype via Nrf2, mitigating the detrimental effects of lipopolysaccharide-induced neuroinflammation and ameliorates depressive and anxiety-like behaviors (Zhang et al. Citation2023a). It attenuates CFA-induced anxiety-like behavior in mice by inhibiting ferroptosis (Chen et al. Citation2022). In chronic restraint stress mice, the anxiolytic effects of GE may be associated with the tryptophan metabolism pathway (Ma et al. Citation2021; Cheng et al. Citation2023). In the preliminary experiments, we tested four doses: 100 mg/kg, 200 mg/kg, 400 mg/kg, and 800 mg/kg. The results showed that 100 mg/kg had almost no effect, while the efficacy of 800 mg/kg was comparable to that of 400 mg/kg. Therefore, we showed 200 and 400 mg/kg as the two effective doses. Furthermore, while improvements in anxiety-like behaviors and neurotransmitter levels were observed, the active components and their precise mechanisms underlying these effects remain unclear and warrant further exploration. In future studies, there will be an analysis of the extract's components, isolation of active ingredients, and investigation into the dosage and treatment duration of these compounds. Additionally, research will delve into the potential mechanisms of action of GE and its active constituents. Our results demonstrated that both 200 and 400 mg/kg Gastrodia elata extract could alleviate cEFS-induced anxiety disorder in male C57BL/6 mice by regulating the dopamine, serotonin and CORT levels, and c-fos expression.
Author’s contribution statement
Ting-Ting Zhao designed the experiments and critically revised the manuscript. Lan-Qiao He and Jia-Yu Lin wrote the manuscript and carried out the experiments. Ming-Zhi Yu and Peng-Yu Fan analyzed the data. Cen-Jie He checked the data and contributed to the interpretation of data. All authors contributed to the article and approved the final manuscript.
Conclusions
In conclusion, GE is a phytonutrient that can relieve chronic irritable anxiety disorders in mice by regulating dopamine and serotonin levels and the expression of c-fos positive cells in the brain, and corticosterone levels in serum. However the optimal therapeutic dose determination and clinical evaluations are needed.
Ethical approval
The study was approved by Animal Ethics Committee of HANGZHOU CITY UNIVERSITY, China (approval code 22026).
Acknowledgements
The authors thank Prof. Myung-Koo LEE from Chungbuk National University from South Korea for providing the GE drug purchased from company to the research.
Data availability statement
The data that support the findings of this study are openly available in figshare https://doi.org/10.6084/m9.figshare.24219004.v5
.Disclosure statement
No potential conflict of interest was reported by the author(s).
Statement of human and animal rights
All the experimental procedures involving animals were conducted in accordance with the rules of experimental animal center of School of Medicine, Hangzhou City University, and approved by the Animal Ethics Committee of HANGZHOU CITY UNIVERSITY, China.
Additional information
Funding
References
- Broderick PA, Phelix CFI. 1997. Serotonin (5-HT) within dopamine reward circuits signals open-field behavior. II. Basis for 5-HT-DA interaction in cocaine dysfunctional behavior. Neurosci Biobehav Rev. 21(3):227–260.
- Chen X, Herbert J. 1995. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 64(3):675–685.
- Chen X, Wang J, He Z, Liu X, Liu H, Wang X. 2022. Analgesic and anxiolytic effects of Gastrodin and its influences on ferroptosis and jejunal microbiota in complete Freund’s adjuvant-injected mice. Front Microbiol. 13:841662.
- Cheng L, Wang H, Ma K, Deng Y, Li M, Ma J. 2023. A novel alcohol steamed prepara-tion from Gastrodia elata Blume: pharmacological assessment of a functional food. Front Pharmacol. 14:1092693.
- Cheng QQ, Yang WM, Liu X. 2019. Research progress on pharmacological mechanism of Gastrodiae Rhizoma in cardiovascular and metabolic diseases. Acta Univ Tradit Med Sin Pharmacol Shanghai. 33:96–100.
- Choi HS, Zhao TT, Shin KS, Kim SH, Hwang BY, Lee CK, Lee MK. 2013. Anxiolytic effects of herbal ethanol extract from Gynostemma pentaphyllum after exposure to chronic stress in mice. Molecules. 18(4):4342–4356.
- Espejo EF. 1997. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res. 762(1-2):281–284.
- Gong MQ, Lai FF, Chen JZ, Li XH, Chen YJ, He Y. 2024. Traditional uses, phyto-chemistry, pharmacology, applications, and quality control of Gastrodia elata Blume: a comprehensive review. J Ethnopharmacol. 319(Pt 1):117128.
- Heese K. 2020. Gastrodia elata Blume (Tianma): hope for brain aging and dementia. Evid Based Complement Alternat Med. 8870148.
- Herbison CE, Allen K, Robinson M, Newnham J, Pennell C. 2017. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev Psychopathol. 29(4):1443–1454.
- Huang HS, Lin YE, Panyod S, Chen RA, Lin YC, Chai LMX, Hsu CC, Wu WK, Lu KH, Huang YJ, et al. 2023. Anti-depressive-like and cognitive impairment alleviation ef-fects of Gastrodia elata Blume water extract is related to gut microbiome remodeling in ApoE-/- mice exposed to unpredictable chronic mild stress. J Ethnopharmacol. 302(Pt B):115872.
- Huang YJ, Choong LXC, Panyod S, Lin YE, Huang HS, Lu KH, Wu WK, Sheen LY. 2021. Gastrodia elata Blume water extract modulates neurotransmitters and al-ters the gut microbiota in a mild social defeat stress-induced depression mouse model. Phytother Res. 35(9):5133–5142.
- Imaki T, Katsumata H, Konishi SI, Kasagi Y, Minami S. 2003. Corticotropin-releasing factor type-1 receptor mRNA is not induced in mouse hypothalamus by either stress or osmotic stimulation. J Neuroendocrinol. 15(10):916–924.
- Jeong JY, Lee DH, Kang SS. 2013. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol Metab (Seoul). 28(4):288–296.
- Joëls M, Karst H, Sarabdjitsingh RA. 2018. The stressed brain of humans and rodents. Acta Physiol (Oxf). 223(2):e13066.
- Jung JW, Yoon BH, Oh HR, An JH, Kim SY, Park SY, Ryu JH. 2006. Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol Pharm Bull. 29(2):261–265.
- Juruena MF, Eror F, Cleare AJ, Young AH. 2020. The role of early life stress in HPA axis and anxiety. Adv Exp Med Biol. 1191:141–153.
- Kang M, Galuska MA, Ghassemzadeh S. 2023. Benzodiazepine toxicity. In: [Updated 2023 Jun 26]. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024 Jan.
- Li JM, Zhao Y, Sun Y, Kong LD. 2020. Potential effect of herbal antidepressants on cognitive deficit: pharmacological activity and possible molecular mechanism. J Ethnopharmacol. 257:112830.
- Lister RG. 1987. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl). 92(2):180–185.
- Liu Y, Huang GL. 2017. The chemical composition, pharmacological effects, clinical applications and market analysis of Gastrodia elata. Pharm Chem J. 51:211–215.
- Ma J, Deng Y, Wang Y, Liu QY, An J, Li M, Song NL, Zhang J, Cheng LJ, Ma KJ. 2021. A. comparative study on ingredient and efficiency difference between fresh and steamed Gastrodia elata Blume: an herbal material to a novel functional food. J Funct Foods. 82:104512.
- Mi YH, Wang MJ, Liu MP, Cheng H, Li SQ. 2020. Pharmacokinetic comparative study of GAS with different concentration of tetramethylpyrazine and ferulic acid on liver-yang hyperactivity migraine model by blood-brain microdianlysis method. J Pharm Biomed Anal. 191:113643.
- Peng Z, Wang H, Zhang R, Chen Y, Xue F, Nie H, Chen Y, Wu D, Wang Y, Wang H, et al. 2013. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol Res. 62(5):537–545.
- Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. 1999. Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiol Behav. 68(1-2):47–53.
- Sheikh N, Ahmad A, Siripurapu KB, Kuchibhotla VK, Singh S, Palit G. 2007. Effect of Ba-copa monniera on stress induced changes in plasma corticosterone and brain monoamines in rats. J Ethnopharmacol. 111(3):671–676.
- Sheline YI. 2000. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 48(8):791–800.
- Shi R, Zheng CB, Wang HY, Rao Q, Du TY, Bai CY, Xiao C, Dai ZL, Zhang CH, Chen C, et al. 2020. Gastrodin alleviates vascular dementia in a 2-VO-vascular dementia rat model by altering amyloid and Tau levels. Pharmacology. 105(7-8):386–396.
- Skórzewska A, Lehner M, Wisłowska-Stanek A, Krząścik P, Ziemba A, Płaźnik A. 2014. The effect of chronic administration of corticosterone on anxiety- and depression-like behavior and the expression of GABA-A receptor alpha-2 subunits in brain structures of low- and high-anxiety rats. Horm Behav. 65(1):6–13.
- Vallone D, Picetti R, Borrelli E. 2000. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 24(1):125–132.
- Verma P, Hellemans KG, Choi FY, Yu W, Weinberg J. 2010. Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiol Behav. 99(3):276–285.
- Wang S, Dong WJ, Liu PL, Yang GM, Shen BY, Yu H, Zhang DX, Li LH, Hong SJ. 2021a. Gastrodin ameliorates depression-like behavior of mice through JAK2-STAT3 signaling pathway. Prog Mod Biomed. 21:3622–3627.
- Wang Y, Zhao L, Li AY. 2021b. Gastrodin-A potential drug used for the treatment of Tourette Syndrome. J Pharmacol Sci. 145(3):289–295.
- Wu S, Huang R, Zhang R, Xiao C, Wang LL, Luo M, Song N, Zhang J, Yang F, Liu X, Yang WM. 2023. Gastrodin and Gastrodigenin improve energy metabolism disorders and mitochondrial dysfunction to antagonize vascular dementia. Molecules. 28(6):2598.
- Zhang J, Li L, Liu Q, Zhao ZH, Su DP, Xiao CH, Jin T, Chen L, Xu CY, You ZL, Zhou T. 2023a. Gastrodin programs an Arg-1 + microglial phenotype in hippocampus to ameliorate depression- and anxiety-like behaviors via the Nrf2 pathway in mice. Phytomedicine. 113:154725.
- Zhang Y, Huang H, Yao C, Sun XR, He QH, Choudharyc ML, Chen SG, Liu XM, Jiang N. 2023b. Fresh Gastrodia elata Blume alleviates simulated weightlessness-induced cognitive impairment by regulating inflammatory and apoptosis-related pathways. Front Pharmacol. 14:1173920.
- Zhang Y, Ye P, Zhu H, Gu LJ, Li YT, Feng S, Zeng Z, Chen QX, Zhou BH, Xiong XX. 2024. Neutral polysaccharide from Gastrodia elata alleviates cerebral ischemia-reperfusion injury by inhibiting ferroptosis-mediated neuroinflammation via the NRF2/HO-1 signaling pathway. CNS Neurosci Ther. 30(3):e14456.
- Zhao TT, Shin KS, Choi HS, Lee MK. 2015. Ameliorating effects of gypenosides on chronic stress-induced anxiety disorders in mice. BMC Complement Altern Med. 15:323.
- Zhao TT, Shin KS, Kim KS, Park HJ, Kim HJ, Lee KE, Lee MY. 2016. Effects of (−)-sesamin on motor and memory deficits in an MPTP-lesioned mouse model of Parkinson’s disease treated with L-DOPA. Neuroscience. 339:644–654.
- Zhou HB, Lu SZ, Yu ZS, Zhang JL, Mei ZN. 2023. Mechanisms for the biological ac-tivity of Gastrodia elata Blume and its constituents: a comprehensive review on sedative-hypnotic, and antidepressant properties. Phytomedicine. 123:155251.
- Zhou RR, Zhu Y, Yang W, Zhang FR, Wang JW, Yan RH, Tang SH, Li ZY. 2020. Discovery of herbal pairs containing Gastrodia elata based on data mining and the delphi expert questionnaire and their potential effects on stroke through network pharmacology. Evid Based Complement Alternat Med. 2020:4263591.