Abstract
Schistosomiasis is a parasitic disease that is endemic in tropical and subtropical areas. Its diagnosis is crucial for effective treatment and control, particularly in resource-limited settings where the disease burden is high. Various diagnostic methods are available. However, these methods are associated with low accuracy, efficiency or accessibility. This review summarizes the published literature on the diagnostics of schistosomiasis based on the techniques of microscopy, serology, molecular, antigen-based and aptamer-based assays. The limitations of each technique were summarized to encourage future research. Furthermore, we highlight the need for point-of-care diagnostics that are sensitive, specific, and easy to use in resource-limited settings may address the challenges associated with commonly used diagnostic techniques. The review concludes that further research and development are needed to improve the diagnosis of schistosomiasis and enable effective treatment and control of this debilitating disease.
Introduction
Schistosomiasis is a parasitic disease caused by blood flukes of the genus Schistosoma, which affects an estimated 240 million people worldwide (Exum et al. Citation2019). The disease is endemic in many tropical and subtropical regions, particularly in sub-Saharan Africa (Sacolo et al. Citation2018). Schistosomiasis is associated with significant morbidity and mortality, particularly in children and young adults (Vester et al. Citation1997; Mduluza-Jokonya et al. Citation2020). The impact of schistosomiasis is multifaceted, affecting both physical and economic aspects of the lives of those affected. The disease can cause chronic illness, leading to complications such as anemia, cognitive impairment, and hepatosplenomegaly (Cimini et al. Citation2021). In addition to its direct health impact, schistosomiasis is also linked to poverty and poor socioeconomic conditions. It is most prevalent in areas with poor sanitation and inadequate access to clean water (Mwanga et al. Citation2013; Nascimento et al. Citation2019). Early diagnosis and treatment are critical in preventing the progression of the disease and thus, the development of severe complications (Shiff et al. Citation2010; Wu et al. Citation2018). Several diagnostic methods are available for detecting schistosomiasis, each with its advantages and limitations. The gold standard is the microscopic examination of stool or urine samples which is the most widely used method (Katz and Chaves Citation1972). Microscopy involves identifying the presence of Schistosoma eggs in the patient’s urine or excreta. The eggs can be visualized using various staining techniques.
Accurate and efficient diagnostics are essential for disease control and management (Amoah et al. Citation2020), as they play a critical role in identifying and treating diseases and in early diagnosis. Early and accurate diagnosis is key to improving patient outcomes, reducing transmission rates, and minimizing healthcare costs associated with delayed or misdiagnosed conditions (Shiff et al. Citation2010; Wu et al. Citation2018). Early diagnosis allows for prompt treatment, which can lead to better outcomes and a reduced risk of complications. Also, in the case of infectious diseases, early diagnosis can help prevent the spread of the disease to others. Accurate and efficient diagnostics are critical for disease surveillance and control (Weerakoon et al. Citation2018). In many cases, diseases can be asymptomatic or have non-specific symptoms, making diagnosis difficult. Accurate diagnostics can help identify cases that might otherwise go undetected, allowing for effective control measures to be implemented (Amoah et al. Citation2020). Efficient diagnostics help reduce healthcare costs associated with disease management (Krishnan Citation2016). Delayed or misdiagnosed conditions can result in unnecessary tests, treatments, and hospitalizations, leading to increased healthcare costs (Krishnan Citation2016; de Wilton et al. Citation2021). Accurate and efficient diagnostics ensure that patients receive appropriate care and treatment in a timely manner. Moreover, accurate and efficient diagnostics guide the development of new treatments and therapies (Fasogbon et al. Citation2023; Manciulli et al. Citation2023).
This review is aimed at discussing the diagnostic methods for schistosomiasis and identifying their limitations in terms of accuracy, efficiency and their field usability as point-of-care diagnostics especially in resource-limited centers. The data presented in the study were curated from Scopus, PubMed and Google Scholar databases. They were not systematically screened but studies were included based on relevance to our objective.
Challenges in accuracy and efficiency for schistosomiasis diagnostics
Microscopy methods
Microscopic examination of stool or urine samples is a common method for diagnosing schistosomiasis caused by many of the Schistosoma species. Kato-Katz technique is commonly associated with the diagnosis of S. mansoni, (Katz and Chaves Citation1972; Menezes et al. Citation2023), S. japonicum (Xu et al. Citation2023) and S. mekongi (Rahman et al. Citation2021). The technique is simple being that it provides quantitative data by counting the number of parasite eggs in a known volume of fecal sample. The technique involves filtering a portion of the stool through a mesh screen, placing the filtered material on a microscope slide, and examining it under low-power magnification for the presence of Schistosoma eggs. Similar to the Kato-Katz technique for stool samples urine filtration method is being used more specifically for the detection of S. haematobium in urine samples collected from individuals suspected of having S. haematobium infection (Deribew et al. Citation2022). Typically, the first-morning urine sample is preferred as it may contain higher concentrations of parasite eggs. The sensitivity of Kato-Katz and urine filtration varies depending on factors such as the number of stool/urine samples examined, the distribution of eggs in the sample, and the skill of the personnel (Assaré et al. Citation2021). The eggs of Schistosoma species can be identified based on their characteristic shape and the presence of a lateral spine (Boon et al. Citation2017; Gryseels Citation2020; Reguera-Gomez et al. Citation2021). While this method is widely used, its sensitivity is limited by factors such as low parasite burden and the presence of other helminth infections (Oliveira et al. Citation2018).
In a study that compared diagnostic methods in low burden infection, the prevalence rates of 8.5%, 43.0% and 56.2% were reported by Da Silva et al. (Citation1998) for Kato-Katz, ELISA (detecting immunoglobulin G antibodies against adult worm antigens- IgG-ELISA), and immunofluorescence test (detecting immunoglobulin M antibodies to gut associated antigens- IgM-IFT), respectively. The results suggest that parasitological methods by Kato-Katz had very poor sensitivity for the detection of S. mansoni eggs in individuals with low worm burden, which is the situation commonly observed in low-endemic areas. The Kato-katz thick smear is the most recommended method for the epidemiological study of S. mansoni, yet it has lower sensitivity when compared with the Formol-Ether concentration methods used for the diagnosis of other intestinal helminthic infections according to a report among school children of Wonji Shoa town, Eastern Ethiopia (Taye Citation2014). The Kato-Katz technique was even reported to be less sensitive in evaluating S. mansoni infection, even in a high-prevalence setting in central Sudan (Ibrahim and Elbasheir Citation2016). A sensitivity of 41% and 66% was reported respectively for sedimentation and filtration methods to prepare fecal samples for microscopic examination of Schistosoma eggs (Giovanoli Evack et al. Citation2020). Also, Ferreira et al. (Citation2017) reported a sensitivity and specificity of the Kato-Katz technique for diagnosing S. mansoni infection in Brazil at 25.6% and 94.6%, respectively.
The poor sensitivity of microscopic methods results in false positive or negative results, although Benjamin-Chung et al. (Benjamin-Chung et al. Citation2020) demonstrated that a double-slide Kato-Katz had relatively few false positives compared to the conventional approach. While comparing the diagnostic performance of Kato-Katz with Real-Time PCR in urine samples from Kenyan children infected with S. haematobium, Vinkeles Melchers et al. (Citation2014), suggested the day-to-day variation often associated with microscopic methods of schistosomiasis diagnosis as a major setback in this method. Given the very poor sensitivity of egg detection in non-schistosomiasis-endemic settings, most tropical and travel medicine clinics in Europe use conventional microscopy systematically combined with other diagnostic methods for schistosomiasis diagnosis (Gautret et al. Citation2016).
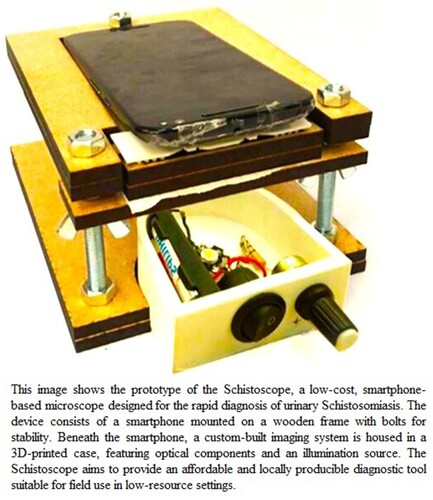
Recently, there has been interest in the use of digital microscopy for the diagnosis of schistosomiasis. Digital microscopy involves the capture of images of fecal or urine samples using a microscope and the analysis of these images using computer software, as shown in Figure (reproduced after obtaining appropriate permission). A digital microscope, called Schistoscope has been shown to have higher sensitivity than traditional microscopy methods and can be used for the simultaneous detection of multiple parasites (Agbana et al. Citation2019; Diehl and Oyibo Citation2020; Meulah et al. Citation2022). Holmström et al. (Citation2017) also provided evidence for the applicability of the digital microscope at the point-of-care settings.
Figure 1. Components of a smartphone-based Schistoscope, a digital microscopic device for schistosomiasis diagnosis (Agbana et al. Citation2019).
Serology methods
Serological methods play an important role in the diagnosis of schistosomiasis by detecting specific antibodies or antigens produced in response to Schistosoma infection. ELISA is widely used to detect specific antibodies (IgG, IgA, IgM, or IgE) against Schistosoma antigens in serum or other body fluids (Beck et al. Citation2008). Many reports suggest that ELISA is the only reliable test for the measurement of IgG and IgM antibodies for both clinical diagnosis and epidemiological studies (Parker and Weber Citation1993; Hoermann et al. Citation2022). However, ELISA may produce false-negative results during the early stages of infection when antibody levels are low (Doenhoff et al. Citation2004; Xu et al. Citation2014). Cross-reactivity with other parasitic infections or exposure to unrelated antigens can also lead to false-positive results (Alarcón De Noya et al. Citation1996; Hamilton et al. Citation1999; Ishida et al. Citation2003; Lv et al. Citation2016), necessitating confirmatory tests. Other researchers have reported diverse approaches to reduce or eliminate cross-reactivity in ELISA assays. Examples include the use of sodium metaperiodate to successfully reduce the false reactivity of soluble egg antigen (SEA)-ELISA, with no decrease in sensitivity (Alarcón de Noya et al. Citation2000). The application of magnetic beads in ELISA assay in the form of immunomagnetic beads (IMB)-ELISA for diagnosing S. mansoni infection was also reported to reduce false positive results (Méabed and Hassan Citation2019). ELISA, like other serological assays, is often found challenging to distinguish between current or past infection (Hinz et al. Citation2017). However, the sandwich ELISA assays developed against S. japanicum protein (Sj29) were reported to have potential diagnostic capability that could distinguish between current or past infection and assess responses to drug treatment (Ren et al. Citation2017).
Indirect Hemagglutination Assay (IHA) is another serological assay that detects antibodies against Schistosoma antigens by causing red blood cell agglutination (Abdalla Citation2018; Suleiman et al. Citation2022). It is relatively simple and inexpensive, but its sensitivity and specificity may vary depending on the antigen used and geographical variations in Schistosoma species. False-positive results due to cross-reactivity with other helminthic infections also occur in IHA like ELISA and other serological assays (Malone et al. Citation2017). In general, serological methods primarily indicate exposure to Schistosoma parasites, rather than the current infection status or the worm burden so serological test may present positive results for many years (‘serological scar’) post infection, therefore the method is inappropriate to monitor the treatment efficacy.
Molecular methods
Molecular methods have become increasingly popular for the diagnosis of schistosomiasis, as they offer improved sensitivity and specificity compared to traditional microscopy-based methods. PCR, the most commonly used molecular method for the diagnosis of schistosomiasis, is a sensitive and specific technique that can detect small amounts of Schistosoma DNA in a variety of sample types, including stool, urine, blood, and tissue (Kato-Hayashi et al. Citation2010; Cai et al. Citation2019; Guegan et al. Citation2019), even when the sample has low parasite load (Magalhães et al. Citation2020). There have been various types of PCR assays developed to detect either the parasite nuclei DNA, ribosomal DNA or cell free DNA from the parasite in host samples. One of such novel technology is the development of a 18S rRNA gene (ribosomal DNA [rDNA])-specific PCR-based assay, to detect avian schistosomes in water samples, coupled with a follow-up 28S rDNA-specific PCR and sequencing to identify the schistosomes to the species or genus level (Jothikumar et al. Citation2015). Blin et al. (Citation2023) also developed another rapid, reliable and cost-effective duplex tetra-primer amplification refractory mutation system (T-ARMS)-PCR assay to distinguish between three S. haematobium (human parasite), S. bovis and S. curassoni (animal parasites), and their hybrids. The technique has the advantage of amplifying multiple alleles in a single reaction with the inclusion of an internal molecular control. Hamburger et al. (Hamburger et al. Citation1991) reported a set of primers are used to amplify a 121-basepair highly repeated sequence specific to S. mansoni. These primers were designed to bind specifically to this repetitive sequence, allowing for the amplification of DNA fragments that can be further analyzed through techniques such as PCR to detect the presence of S. mansoni DNA in biological samples. Similarly, repetitive nuclear DNA sequences have been reported, that specifically identifies of S. haematobium (Abbasi et al. Citation2017).
Quantitative or real-time PCR provides an advantage in terms of its capability in multiplexed detection and ability to distinguish several intestinal parasites from Schistosome (Siqueira et al. Citation2021). However, PCR assays are not without limitations. Despite their high accuracy and sensitivity, a major challenge is the higher probability of the test generating false-positive or false-negative results (Kato-Hayashi et al. Citation2010; Keller et al. Citation2020), which may be due to contamination or poor sample quality. Additionally, PCR requires specialized equipment and expertise, which may limit its availability in resource-limited settings (Jothikumar et al. Citation2015; Magalhães et al. Citation2020; Blin et al. Citation2023).
PCR assays based on the amplification of the Internal Transcribed Spacer (ITS) region of the ribosomal DNA (rDNA) of Schistosoma parasites have been reported to present improved specificity (Hoffmann et al. Citation2021), as PCR that utilize DraI, a restriction endonuclease enzyme, also increase sensitivity in the detection and amplification of Schistosoma DNA (Frickmann et al. Citation2021b). DraI digestion was reported to also allow for the detection of low parasite burdens and can be adapted for use with various sample types (Frickmann et al. Citation2021a, Citation2021b).
Both ITS1 and ITS2 regions are utilized in PCR for Schistosoma diagnosis, though they differ in their nucleotide sequences and primers used for amplification. However, ITS2 region is generally preferred for its higher variability among Schistosoma species, which aids in species identification and differentiation. (Meurs et al. Citation2015; Menezes et al. Citation2023). ITS1 region may also be used in conjunction with ITS2 for comprehensive analysis, particularly in epidemiological studies and genetic characterization of Schistosoma populations. The ITS region is highly conserved within Schistosoma species but differs significantly from other organisms, making it a suitable target for specific detection. ITS-based PCR diagnosis is useful for early diagnosis and monitoring treatment efficacy (Meurs et al. Citation2015).
Another molecular method that has been used for the diagnosis of schistosomiasis is loop-mediated isothermal amplification (LAMP). LAMP is a sensitive and specific nucleic acid amplification technique that can also detect Schistosoma DNA in stool, urine, and blood samples (Kumagai et al. Citation2022). LAMP was developed as an alternative to PCR to overcome the bottleneck of application in resource-limited settings and for field studies (García-Bernalt Diego et al. Citation2019; Diego et al. Citation2021). Mesquita et al. (Citation2021) reported that the LAMP assay was effective as the parasitological examination for the detection of S. mansoni infection when performed in triplicate, and is more directly applicable in the field than other molecular techniques. Several LAMP-based assays have been developed for the diagnosis of different Schistosoma species, with high sensitivity and specificity; LAMP for detection of S. mekongi detection in human stools and even in the snails intermediate host (Kumagai et al. Citation2022). A report on the use of LAMP in the snail screening to characterize schistosomiasis transmission zones makes LAMP quite promising. The technique also gives useful information for surveillance services as it can detect Schistosoma species, even when snails are not shedding cercariae (Hamburger Citation2020; Mesquita et al. Citation2021); LAMP was also developed for rapid detection of S. japanicum (Xu et al. Citation2010), where the percentage sensitivity of LAMP was 96.7%, whereas that of PCR was only 60%, indicating that LAMP was more sensitive than conventional PCR for clinical diagnosis of schistosomiasis cases in endemic areas. Moreso, Crego-Vicente et al. (Citation2021) reported a genus-Specific LAMP Assay that was used to detect S. haematobium and S. bovis hybrids that could be adapted for diagnosis in the field and also for surveillance in settings of hybrids endemicity. LAMP assays, however, often have a challenge of inconclusive results when LAMP products are run in gels for analysis. As an alternative to this, visual inspection of reaction tubes by the naked eye became prioritized, which also aided the field application of the LAMP assay (Mesquita et al. Citation2021). Also, there have been reports of non-specific amplification in LAMP assay, hence results presented are unreliable (Gandasegui et al. Citation2018; Mesquita et al. Citation2021).
Overall, molecular methods offer significant advantages over traditional microscopy-based methods for the diagnosis of schistosomiasis, including improved sensitivity and specificity. However, challenges exist with the accuracy and efficiency of these methods, which may limit their effectiveness in certain contexts. Further research is needed to address these challenges and improve the utility of molecular methods for the diagnosis of schistosomiasis.
Antigen detection methods
In recent years, antigen tests have emerged as a promising alternative for schistosomiasis diagnosis. The antigen test for schistosomiasis diagnosis is primarily based on the detection of schistosome antigens in infected individuals (Casacuberta-Partal et al. Citation2020). These antigens are glycoproteins released by adult schistosomes into the bloodstream of infected individuals. The most widely used antigen test is the point-of-care circulating cathodic antigen (POC-CCA) assay, which utilizes immunochromatographic techniques to detect CCA in urine or stool samples (Neumayr et al. Citation2019). The test provides rapid results in about 20 min, enabling immediate diagnosis and initiation of treatment (de Sousa et al. Citation2020). This quick turnaround time is crucial for disease management, especially in endemic regions where laboratory facilities may be limited. The POC-CCA assay has demonstrated good sensitivity for detecting Schistosoma infections. Its simplicity makes it ideal for field settings and resource-limited areas (Ferreira et al. Citation2017; de Sousa et al. Citation2020; Hoekstra et al. Citation2020). However, its sensitivity has been reported to be lower in detecting early or low-intensity infections. False-negative results can occur, particularly in areas with low infection rates, potentially leading to missed diagnoses (Cai et al. Citation2021; Graeff-Teixeira et al. Citation2021). The results of POC-CCA assay are determined by visual reading, and the interpretation of a ‘Trace’ reading as ‘positive’ or ‘negative’ is problematical (Colley et al. Citation2017). Also, the assay primarily detects S. mansoni infections, while its sensitivity for other Schistosoma species such as S. haematobium or S. japonicum may vary (Neumayr et al. Citation2019; de Sousa et al. Citation2020; Cai et al. Citation2021). In regions with mixed infections, complementary tests may be required for accurate species identification.
The varied sensitivity, specificity and inaccuracy of the currently available diagnostic methods of schistosomiasis led to the development of the up-converting phosphor lateral flow assay (UCP-LF) for circulating anodic antigen (CAA) quantification. The UCP-LF CAA assay presents high specificity, sensitivity and affinity for schistosomiasis diagnosis although, it is a laboratory-based assay requiring specialized equipment, protocol and skills that limit its application at the POC and in resource-limited settings (Corstjens et al. Citation2008, Citation2017; van Dam et al. Citation2012, Citation2015; Knopp et al. Citation2015; Fasogbon et al. Citation2023).
Aptamer-based diagnosis for schistosomiasis
Aptamers are synthetic nucleic acid molecules with precise target binding, facilitating the selective identification of the target (Molefe et al. Citation2018; Fasogbon et al. Citation2022). Aptamers are often generated from a synthetic random library with 1013–1016 single-stranded DNA or RNA molecules by an in vitro iterative process known as systematic evolution of ligands by exponential enrichment (SELEX) (Long et al. Citation2016). Aptamer-based diagnostic presents an exciting approach in the schistosomiasis diagnosis, presenting high specificity, sensitivity, and potential for rapid detection of biomarkers associated with Schistosoma parasites. However, the translation of aptamer-based diagnostics for schistosomiasis encounters challenges and limitations like; the laborious task of identifying and validating aptamers with high specificity and affinity for Schistosoma-specific biomarkers (Long et al. Citation2016; Molefe et al. Citation2018) and the intricate nature of Schistosoma infections, encompassing diverse parasite species and distinct life cycle stages, poses a great challenge in selecting aptamers targeting diagnostic biomarkers (Colley et al. Citation2017). Hence, there are paucity of information on the development and application aptamers aptamers in schistosomiasis diagnosis.
However, Long et al. (Citation2016) have reported two aptamers, namely, LC6 and LC15 exhibiting high specificity and affinity S. japonicum eggs. Although, this discovery advances knowledge regarding the potential of aptamer in the diagnosis of S. japonicum infection, however, the aptamer would not be applicable for early phase diagnosis (before shedding of egg), since it would only detect the parasite’s egg. It would also be subject to variability in egg shedding. Fasogbon et al. (Citation2023) recently proposed the development of aptamers that could specifically bind the genus-specific Schistosoma CAA as an alternative to monoclonal antibodies in the UCP-LF assay for early diagnosis of schistosomiasis.
Summary of the advantages and limitations of diagnostic methods
Each diagnostic method has its own strengths and weaknesses, and the choice of technique depends on factors such as the stage of infection, available resources, and specific requirements of the healthcare setting. Often, a combination of methods may be employed for accurate diagnosis and monitoring of schistosomiasis. The major advantages and limitations of the reviewed methods are summarized in Table .
Table 1. Major advantages and limitations of reviewed methods.
Conclusion
The diagnosis of schistosomiasis, like other diseases, is crucial for effective treatment and control, particularly in resource-limited settings where the disease burden is high. Various methods are available for schistosomiasis diagnosis, and their selection depends on the prevalence of the disease in the population being tested, the availability of resources and personnel, and the sensitivity and specificity of the tests. However, they all have limitations in terms of accuracy, efficiency, and feasibility in resource-limited settings. Hence we recommend further research on point-of-care testing that would be applicable in resource-limited settings for schistosomiasis diagnosis.
Acknowledgements
Conceptualization and Design: Ilemobayo Victor Fasogbon and Patrick Maduabuchi Aja. Article Curation: Ilemobayo Victor Fasogbon, Erick Nyakundi Ondari, Deusdedit Tusubira and Patrick Maduabuchi Aja. Resources: Ilemobayo Victor Fasogbon, Erick Nyakundi Ondari, Deusdedit Tusubira, Egwu Chinedu Ogbonnia, Jon Ashley, Swamiappan Sasikumar, Patrick Maduabuchi Aja. Writing – Original Draft: Ilemobayo Victor Fasogbon. Writing – Review & Editing: Ilemobayo Victor Fasogbon, Erick Nyakundi Ondari, Deusdedit Tusubira, Egwu Chinedu Ogbonnia, Jon Ashley, Swamiappan Sasikumar, Patrick Maduabuchi Aja. Supervision: Erick Nyakundi Ondari, Deusdedit Tusubira and Patrick Maduabuchi Aja. Final Approval of the Version to be Published: Ilemobayo Victor Fasogbon, Erick Nyakundi Ondari, Deusdedit Tusubira, Egwu Chinedu Ogbonnia, Jon Ashley, Swamiappan Sasikumar, Patrick Maduabuchi Aja.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Abbasi I, Webster BL, King CH, Rollinson D, Hamburger J. 2017. The substructure of three repetitive DNA regions of Schistosoma haematobium group species as a potential marker for species recognition and interbreeding detection. Parasites Vectors. 10(1):1–9. doi:10.1186/s13071-017-2281-7.
- Abdalla MA. 2018. Comparative study between direct microscopy and indirect haemagglutination methods used in diagnosis of urinary schistosomiasis. MOJ Immunol. 6(1). doi:10.15406/moji.2018.06.00186.
- Agbana T, Van G, Oladepo O, Vdovin G, Oyibo W, Diehl JC. 2019. Schistoscope: towards a locally producible smart diagnostic device for Schistosomiasis in Nigeria. IEEE Globa, p. 1–8, 2019. [cited: 23 May 2023]. [Online]. Available: https://ieeexplore.ieee.org/abstract/document/9033049/.
- Alarcón de Noya B, Colmenares C, Lanz H, Caracciolo MA, Losada S, Noya O. 2000. Schistosoma mansoni: immunodiagnosis is improved by sodium metaperiodate which reduces cross-reactivity due to glycosylated epitopes of soluble egg antigen. Exp Parasitol. 95(2):106–112. doi:10.1006/expr.2000.4515.
- Alarcón De Noya B, Colmenares C, Losada S, Fermin Z, Masroua G, Ruiz L, Soto L, Noya O. 1996. Do intestinal parasites interfere with the seroepidemiologic surveillance of schistosoma mansoni infection? Epidemiol Infect. 116(3):323–329. doi:10.1017/S095026880005264X.
- Amoah AS, Hoekstra PT, Casacuberta-Partal M, Coffeng LE, Corstjens PLAM, Greco B, van Lieshout L, Lim MD, Markwalter CF, Odiere MR, et al. 2020. Sensitive diagnostic tools and targeted drug administration strategies are needed to eliminate schistosomiasis. Lancet Infect Dis. 20(7):e165–e172. doi:10.1016/S1473-3099(20)30254-1.
- Assaré RK, Tra-Bi MI, Coulibaly JT, Corstjens PLAM, Ouattara M, Hürlimann E, van Dam GJ, Utzinger J, N’Goran EK. 2021. Accuracy of two circulating antigen tests for the diagnosis and surveillance of schistosoma mansoni infection in low-endemicity settings of côte d’Ivoire. Am J Trop Med Hyg. 105(3):677–683. doi:10.4269/ajtmh.21-0031.
- Beck L, Van-Lüme DSM, Souza JR, Domingues ALC, Favre T, Abath FGC, Montenegro SML. 2008. Discriminating acute from chronic human Schistosomiasis mansoni. Acta Trop. 108(2–3):229–233. doi:10.1016/j.actatropica.2008.08.012.
- Benjamin-Chung J, Pilotte N, Ercumen A, Grant JR, Maasch JRMA, Gonzalez AM, Ester AC, Arnold BF, Rahman M, Haque R, et al. 2020. Comparison of multi-parallel qPCR and double-slide kato-katz for detection of soil-transmitted helminth infection among children in rural Bangladesh. PLoS Negl Trop Dis. 14(4):e0008087. doi:10.1371/journal.pntd.0008087.
- Blin M, Dametto S, Agniwo P, Webster BL, Angora E, Dabo A, Boissier J. 2023. A duplex tetra-primer ARMS-PCR assay to discriminate three species of the Schistosoma haematobium group: Schistosoma curassoni, S. bovis, S. haematobium and their hybrids. Parasites Vectors. 16(1):1–11. doi:10.1186/S13071-023-05754-9.
- Boon NAM, Fannes W, Rombouts S, Polman K, Volckaert FAM, Huyse T. 2017. Detecting hybridization in African schistosome species: does egg morphology complement molecular species identification? Parasitology. 144(7):954–964. doi:10.1017/S0031182017000087.
- Cai P, Mu Y, Weerakoon KG, Olveda RM, Ross AG, McManus DP. 2021. Performance of the point-of-care circulating cathodic antigen test in the diagnosis of Schistosomiasis japonica in a human cohort from Northern Samar, the Philippines. Infect Dis Poverty. 10(1):40–51. doi:10.1186/s40249-021-00905-5.
- Cai P, Weerakoon KG, Mu Y, Olveda RM, Ross AG, Olveda DU, McManus DP. 2019. Comparison of Kato Katz, antibody-based ELISA and droplet digital PCR diagnosis of Schistosomiasis japonica: lessons learnt from a setting of low infection intensity. PLoS Negl Trop Dis. 13(3):e0007228. doi:10.1371/journal.pntd.0007228.
- Casacuberta-Partal M, Janse JJ, van Schuijlenburg R, de Vries JJC, Erkens MAA, Suijk K, van Aalst M, Maas JJ, Grobusch MP, van Genderen PJJ, et al. 2020. Antigen-based diagnosis of schistosoma infection in travellers: a prospective study. J Travel Med. 27(4):1–9. doi:10.1093/JTM/TAAA055.
- Cimini A, Ricci M, Gigliotti PE, Pugliese L, Chiaravalloti A, Danieli R, Schillaci O. 2021. Medical imaging in the diagnosis of schistosomiasis: a review. Pathogens. 10(8):1058. doi:10.3390/pathogens10081058.
- Colley DG, Andros TS, Campbell CH. 2017. Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect Dis Poverty. 6(1):1–8. doi:10.1186/s40249-017-0275-5.
- Corstjens PLAM, Hoekstra PT, de Dood CJ, van Dam GJ. 2017. Utilizing the ultrasensitive schistosoma up-converting phosphor lateral flow circulating anodic antigen (UCP-LF CAA) assay for sample pooling-strategies. Infect Dis Poverty. 6(1):155. doi:10.1186/s40249-017-0368-1.
- Corstjens PLAM, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, van Dam GJ. 2008. Up-converting phosphor technology-based lateral flow assay for detection of schistosoma circulating anodic antigen in serum. J Clin Microbiol. 46(1):171–176. doi:10.1128/JCM.00877-07.
- Crego-Vicente B, Fernández-Soto P, Febrer-Sendra B, García-Bernalt Diego J, Boissier J, Angora EK, Oleaga A, Muro A. 2021. Application of a genus-specific LAMP assay for schistosome species to detect Schistosoma haematobium x schistosoma bovis hybrids. J Clin Med. 10(6):1308. doi:10.3390/jcm10061308.
- Da Silva RM, Kanamura HY, Camargo ED, Chiodelli SG, Nakamura PM, Gargioni C, Vellosa SA, Antunes JL. 1998. A comparative study on IgG-ELISA, IgM-IFT and Kato-Katz methods for epidemiological purposes in a low endemic area for schistosomiasis. Mem Inst Oswaldo Cruz. 93(Suppl. 1):279–282. doi:10.1590/S0074-02761998000700054.
- Deribew K, Yewhalaw D, Erko B, Mekonnen Z. 2022. Urogenital schistosomiasis prevalence and diagnostic performance of urine filtration and urinalysis reagent strip in schoolchildren, Ethiopia. PLoS One. 17(7):e0271569. doi:10.1371/journal.pone.0271569.
- de Sousa SRM, Nogueira JFC, Dias IHL, Fonseca ÁLS, Favero V, Geiger SM, Enk MJ. 2020. The use of the circulating cathodic antigen (CCA) urine cassette assay for the diagnosis and assessment of cure of Schistosoma mansoni infections in an endemic area of the Amazon region. Rev Soc Bras Med Trop. 53:1–7. doi:10.1590/0037-8682-0562-2019.
- de Wilton A, Aggarwal D, Jäger HR, Manji H, Chiodini PL. 2021. Delayed diagnosis of spinal cord schistosomiasis in a non-endemic country: a tertiary referral centre experience. PLoS Negl Trop Dis. 15(2):e0009161. doi:10.1371/journal.pntd.0009161.
- Diego JGB, Fernández-Soto P, Febrer-Sendra B, Crego-Vicente B, Muro A. 2021. Loop-mediated isothermal amplification in schistosomiasis. J Clin Med. 10(3):511. doi:10.3390/jcm10030511.
- Diehl J, Oyibo P. 2020. Schistoscope: smartphone versus Raspberry Pi based low-cost diagnostic device for urinary Schistosomiasis. IEEE Globa, p. 1–8, 2020. [cited 23 May 2023]. [Online]. Available: https://ieeexplore.ieee.org/abstract/document/9342871/.
- Doenhoff MJ, Chiodini PL, Hamilton JV. 2004. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol. 20(1):35–39. doi:10.1016/j.pt.2003.10.019.
- Exum NG, Kibira SPS, Ssenyonga R, Nobili J, Shannon AK, Ssempebwa JC, Tukahebwa EM, Radloff S, Schwab KJ, Makumbi FE. 2019. The prevalence of schistosomiasis in Uganda: a nationally representative population estimate to inform control programs and water and sanitation interventions. PLoS Negl Trop Dis. 13(8):e0007617. doi:10.1371/journal.pntd.0007617.
- Fasogbon IV, Aja PM, Ondari E N, Adebayo I, Ibitoye OA, Egesa M, Tusubira D, Sasikumar S, Onohuean H. 2023. UCP-LF and other assay methods for schistosome circulating anodic antigen between 1978 and 2022. Biol Methods Protocols. 8(1):1–9. doi:10.1093/biomethods/bpad006.
- Fasogbon IV, Yakubu MN, Adam M, Obi C, Nuhu T, Alegbe SD, Zenoh DA, Mshelia MB, Aja PM. 2022. Non-selex-based in-silico modeled aptamers against sars-cov-2 proteins: a systematic review. KIU J Heal Scii. 2(1):69–79.
- Ferreira FT, Fidelis TA, Pereira TA, Otoni A, Queiroz LC, Amâncio FF, Antunes CM, Lambertucci JR. 2017. Sensitivity and specificity of the circulating cathodic antigen rapid urine test in the diagnosis of Schistosomiasis mansoni infection and evaluation of morbidity in a low- endemic area in Brazil. Rev Soc Bras Med Trop. 50(3):358–364. doi:10.1590/0037-8682-0423-2016.
- Frickmann H, Loderstädt U, Nickel B, Poppert S, Odermatt P, Sayasone S, Van Esbroeck M, Micalessi I, Cnops L, Adisakwattana P, et al. 2021a. Low sensitivity of real time PCRs targeting retrotransposon sequences for the detection of Schistosoma japonicum complex DNA in human serum. Pathogens. 10(8):1067. doi:10.3390/pathogens10081067.
- Frickmann H, Lunardon L-M, Hahn A, Loderstädt U, Lindner AK, Becker SL, Mockenhaupt FP, Weber C, Tannich E. 2021b. Evaluation of a duplex real-time PCR in human serum for simultaneous detection and differentiation of Schistosoma mansoni and Schistosoma haematobium infections – cross-sectional study. Travel Med Infect Dis 41:102035. doi:10.1016/j.tmaid.2021.102035.
- Gandasegui J, Fernández-Soto P, Muro A, Simões Barbosa C, Lopes de Melo F, Loyo R, de Souza Gomes EC. 2018. A field survey using LAMP assay for detection of schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: assessment in human and snail samples. PLoS Negl Trop Dis. 12(3):e0006314. doi:10.1371/journal.pntd.0006314.
- García-Bernalt Diego J, Fernández-Soto P, Crego-Vicente B, Alonso-Castrillejo S, Febrer-Sendra B, Gómez-Sánchez A, Vicente B, López-Abán J, Muro A. 2019. Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: towards a ready-to-use test. Sci Rep. 9(1):1–11. doi:10.1038/s41598-019-51342-2.
- Gautret P, Mockenhaupt FP, von Sonnenburg F, Rothe C, Libman M, Van De Winkel K, Bottieau E, Grobusch MP, Hamer DH, Esposito DH, et al. 2016. Schistosomiasis screening of travelers to Corsica, France. Emerg Infect Dis. 22(1):160–161. doi:10.3201/eid2201.151606.
- Giovanoli Evack J, Kouadio JN, Achi L, Balmer O, Hattendorf J, Bonfoh B, Zinsstag J, N’Goran EK, Utzinger J. 2020. Accuracy of the sedimentation and filtration methods for the diagnosis of schistosomiasis in cattle. Parasitol Res. 119(5):1707–1712. doi:10.1007/s00436-020-06660-0.
- Graeff-Teixeira C, Favero V, Pascoal VF, de Souza RP, Rigo FdV, Agnese LHD, Bezerra FSM, Coelho PMZ, Enk MJ, Favre TC, et al. 2021. Low specificity of point-of-care circulating cathodic antigen (POC CCA) diagnostic test in a non-endemic area for schistosomiasis mansoni in Brazil. Acta Trop. 217:105863. doi:10.1016/j.actatropica.2021.105863.
- Gryseels B. 2020. Schistosomiasis. In: Bennett JE, Dolin R, Blaser MJ, editors. Hunter's tropical medicine and emerging infectious diseases. 10th ed. Philadelphia, PA: Elsevier; p. 905–917. doi:10.1016/B978-0-323-55512-8.00126-5.
- Guegan H, Fillaux J, Charpentier E, Robert-Gangneux F, Chauvin P, Guemas E, Boissier J, Valentin A, Cassaing S, Gangneux J-P, et al. 2019. Real-time PCR for diagnosis of imported schistosomiasis. PLoS Negl Trop Dis. 13(9):e0007711. doi:10.1371/journal.pntd.0007711.
- Hamburger J. 2020. Molecular tools and schistosomiasis transmission elimination. Am J Trop Med Hyg. 103(4):1376–1379. doi:10.4269/ajtmh.20-0111.
- Hamburger J, Turetski T, Kapeller I, Deresiewicz R. 1991. Highly repeated short DNA sequences in the genome of Schistosoma mansoni recognized by a species-specific probe. Mol Biochem Parasitol. 44(1):73–80. doi:10.1016/0166-6851(91)90222-R.
- Hamilton JV, Chiodini PL, Fallon PG, Doenhoff MJ. 1999. Periodate-sensitive immunological cross-reactivity between keyhole limpet haemocyanin (KLH) and serodiagnostic Schistosoma mansoni egg antigens. Parasitology. 118(1):83–89. doi:10.1017/S0031182098003461.
- Hinz R, Schwarz NG, Hahn A, Frickmann H. 2017. Serological approaches for the diagnosis of schistosomiasis – a review. Mol Cell Probes. 31:2–21. doi:10.1016/j.mcp.2016.12.003.
- Hoekstra PT, Schwarz NG, Adegnika AA, Andrianarivelo MR, Corstjens PLAM, Rakotoarivelo RA, Rakotozandrindrainy R, Sicuri E, Kreidenweiss A, van Dam GJ. 2020. Fast and reliable easy-to-use diagnostics for eliminating bilharzia in young children and mothers: an introduction to the freeBILy project. Acta Trop. 211:105631. doi:10.1016/j.actatropica.2020.105631.
- Hoermann J, Kuenzli E, Schaefer C, Paris DH, Bühler S, Odermatt P, Sayasone S, Neumayr A, Nickel B. 2022. Performance of a rapid immuno-chromatographic test (Schistosoma ICT IgG-IgM) for detecting Schistosoma-specific antibodies in sera of endemic and non-endemic populations. PLoS Negl Trop Dis. 16(5):e0010463. doi:10.1371/journal.pntd.0010463.
- Hoffmann T, Carsjens I, Rakotozandrindrainy R, Girmann M, Randriamampionona N, Maïga-Ascofaré O, Podbielski A, Hahn A, Frickmann H, Schwarz NG. 2021. Serology- and blood-PCR-based screening for schistosomiasis in pregnant women in Madagascar—a cross-sectional study and test comparison approach. Pathogens. 10(6):722. doi:10.3390/pathogens10060722.
- Holmström O, Linder N, Ngasala B, Mårtensson A, Linder E, Lundin M, Moilanen H, Suutala A, Diwan V, Lundin J. 2017. Point-of-care mobile digital microscopy and deep learning for the detection of soil-transmitted helminths and Schistosoma haematobium. Glob Health Action. 10(sup3):1337325. doi:10.1080/16549716.2017.1337325.
- Ibrahim AM, Elbasheir M. 2016. The unreliability of Kato-Katz technique limits its usefulness for evaluating Schistosoma mansoni infections in high prevalence area in central Sudan. Int J Trop Med Public Health. 6(1):10–13. doi:10.5455/220123/ijtmph.
- Ishida MMI, Rubinsky-Elefant G, Ferreira AW, Hoshino-Shimizu S, Vaz AJ. 2003. Helminth antigens (Taenia solium, Taenia crassiceps, Toxocara canis, Schistosoma mansoni and Echinococcus granulosus) and cross-reactivities in human infections and immunized animals. Acta Trop. 89(1):73–84. doi:10.1016/j.actatropica.2003.09.005.
- Jothikumar N, Mull BJ, Brant SV, Loker ES, Collinson J, Secor WE, Hill VR. 2015. Real-time PCR and sequencing assays for rapid detection and identification of avian schistosomes in environmental samples. Appl Environ Microbiol. 81(12):4207–4215. doi:10.1128/AEM.00750-15.
- Kato-Hayashi N, Kirinoki M, Iwamura Y, Kanazawa T, Kitikoon V, Matsuda H, Chigusa Y. 2010. Identification and differentiation of human schistosomes by polymerase chain reaction. Exp Parasitol. 124(3):325–329. doi:10.1016/j.exppara.2009.11.008.
- Katz PJ, Chaves A. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 14(6):397–400. Accessed: May 22, 2023. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/4675644/.
- Keller D, Rothen J, Dangy J-P, Saner C, Daubenberger C, Allan F, Ame SM, Ali SM, Kabole F, Hattendorf J, et al. 2020. Performance of a real-time PCR approach for diagnosing Schistosoma haematobium infections of different intensity in urine samples from Zanzibar. Infec Dis Poverty. 9(5):22–34. doi:10.1186/s40249-020-00726-y.
- Knopp S, Corstjens PLAM, Koukounari A, Cercamondi CI, Ame SM, Ali SM, de Dood CJ, Mohammed KA, Utzinger J, Rollinson D, van Dam GJ. 2015. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis. 9(5):e0003752. doi:10.1371/journal.pntd.0003752.
- Krishnan SM. 2016. Application of Analytics to Big Data in Healthcare. 2016 32nd South. Biomed. Eng. Conf., p. 156–157. doi:10.1109/SBEC.2016.88.
- Kumagai T, Matsumoto-Takahashi ELA, Ishikawa H, Keomalaphet S, Khattignavong P, Soundala P, Hongvanthong B, Oyoshi K, Sasaki Y, Mizukami Y, et al. 2022. Detection of Schistosoma mekongi DNA in human stool and intermediate host snail neotricula aperta via loop-mediated isothermal amplification assay in Lao PDR. Pathogens. 11(12):1413. doi:10.3390/pathogens11121413.
- Long Y, Qin Z, Duan M, Li S, Wu X, Lin W, Li J, Zhao Z, Liu J, Xiong D, et al. 2016. Screening and identification of DNA aptamers toward Schistosoma japonicum eggs via SELEX. Sci Rep. 6:1–9. doi:10.1038/srep24986.
- Lv C, Hong Y, Fu Z, Lu K, Cao X, Wang T, Zhu C, Li H, Xu R, Jia B, et al. 2016. Evaluation of recombinant multi-epitope proteins for diagnosis of goat schistosomiasis by enzyme-linked immunosorbent assay. Parasites Vectors. 9(1):1–11. doi:10.1186/s13071-016-1418-4.
- Magalhães FDC, Resende SD, Senra C, Graeff-Teixeira C, Enk MJ, Coelho PMZ, Oliveira E, Negrão-Corrêa DA, Geiger SM, Carneiro M. 2020. Accuracy of real-time polymerase chain reaction to detect Schistosoma mansoni – infected individuals from an endemic area with low parasite loads. Parasitology. 147(10):1140–1148. doi:10.1017/S003118202000089X.
- Malone J, Bergquist R, Rinaldi L. 2017. Geospatial Surveillance and Response Systems for Schistosomiasis. In: Rinaldi M, Bergquist R, Malone J, editors. Surveillance systems for vector-borne diseases. p. 487–505. doi:10.1201/9781315368900-30
- Manciulli T, Marangoni D, Salas-Coronas J, Bocanegra C, Richter J, Gobbi F, Motta L, Minervini A, Bartoloni A, Zammarchi L. 2023. Diagnosis and management of complicated urogenital schistosomiasis: a systematic review of the literature. Infection. 51(5):1185–1221. doi:10.1007/s15010-023-02060-5.
- Mduluza-Jokonya T, Vengesai A, Jokonya L, Thakataka A, Midzi H, Mduluza T, Sibanda E, Naicker T. 2020. Impact of indolent schistosomiasis on morbidity and mortality from respiratory tract infections in preschool age children from a schistosomiasis endemic area. medRxiv. doi:10.1101/2020.11.06.20227173.
- Méabed EMH, Hassan EA. 2019. Immuno-magnetic beads elisa for diagnosis of schistosoma mansoni infection. J Egypt Soc Parasitol. 49(1):51–59. doi:10.21608/jesp.2019.68286.
- Menezes DL, Santos CTJ, Oliveira YLDC, Campos VTC, Negrão-Corrêa DA, Geiger SM, Silva JRS, Jain S, Oliveira LM, Fujiwara RT, et al. 2023. Accuracy study of Kato-Katz and helmintex methods for diagnosis of Schistosomiasis mansoni in a moderate endemicity area in Sergipe, Northeastern Brazil. Diagnostics. 13(3):527. doi:10.3390/diagnostics13030527.
- Mesquita SG, Neves FGdS, Scholte RGC, Carvalho OdS, Fonseca CT, Caldeira RL. 2021. A loop-mediated isothermal amplification assay for Schistosoma mansoni detection in biomphalaria spp. from schistosomiasis-endemic areas in Minas Gerais, Brazil. Parasites Vectors. 14(1):1–12. doi:10.1186/s13071-021-04888-y.
- Meulah B, Oyibo P, Bengtson M. 2022. Performance evaluation of the schistoscope 5.0 for (semi-) automated digital detection and quantification of schistosoma haematobium eggs in urine: a field. Am J Trop Med Hygiene. 107(5):1047–1054. doi:10.4269/ajtmh.22-0276.
- Meurs L, Brienen E, Mbow M, Ochola EA, Mboup S, Karanja DMS, Secor WE, Polman K, van Lieshout L. 2015. Is PCR the next reference standard for the diagnosis of schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Negl Trop Dis. 9(7):e0003959. doi:10.1371/journal.pntd.0003959.
- Molefe PF, Masamba P, Oyinloye BE, Mbatha LS, Meyer M, Kappo AP. 2018. Molecular application of aptamers in the diagnosis and treatment of cancer and communicable diseases. Pharmaceuticals. 11(4):93–24. doi:10.3390/ph11040093.
- Mwanga JR, Lwambo NJS, Rumisha SF, Vounatsou P, Utzinger J. 2013. Dynamics of people’s socio-economic status in the face of schistosomiasis control interventions in Ukerewe district, Tanzania. Acta Trop. 128(2):399–406. doi:10.1016/j.actatropica.2013.01.004.
- Nascimento GL, Pegado HM, Domingues ALC, Ximenes RAA, Itria A, Cruz LN, Oliveira MRF. 2019. The cost of a disease targeted for elimination in Brazil: the case of schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 114(1). doi:10.1590/0074-02760180347.
- Neumayr A, Chernet A, Sydow V, Kling K, Kuenzli E, Marti H, Paris DH, Nickel B, Labhardt ND. 2019. Performance of the point-of-care circulating cathodic antigen (POC-CCA) urine cassette test for follow-up after treatment of S. mansoni infection in Eritrean refugees. Travel Med Infect Dis. 28:59–63. doi:10.1016/j.tmaid.2018.09.004.
- Oliveira WJ, Magalhães FdC, Elias AMS, de Castro VN, Favero V, Lindholz CG, Oliveira ÁA, Barbosa FS, Gil F, Gomes MA, et al. 2018. Evaluation of diagnostic methods for the detection of intestinal schistosomiasis in endemic areas with low parasite loads: saline gradient, Helmintex, Kato-Katz and rapid urine test. PLoS Negl Trop Dis. 12(2):e0006232. doi:10.1371/journal.pntd.0006232.
- Parker CA, Weber JM. 1993. An enzyme-linked immunosorbent assay for the detection of IgG and IgM antibodies to human herpesvirus type 6. J Virol Methods. 41(3):265–275. doi:10.1016/0166-0934(93)90017-L.
- Rahman MO, Sassa M, Parvin N, Islam MR, Yajima A, Ota E. 2021. Diagnostic test accuracy for detecting Schistosoma japonicum and S. mekongi in humans: a systematic review and meta-analysis. PLoS Negl Trop Dis. 15(3):e0009244. doi:10.1371/journal.pntd.0009244.
- Reguera-Gomez M, Valero MA, Oliver-Chiva MC, de Elias-Escribano A, Artigas P, Cabeza-Barrera MI, Salas-Coronas J, Boissier J, Mas-Coma S, Bargues MD. 2021. First morphogenetic analysis of parasite eggs from schistosomiasis haematobium infected sub-Saharan migrants in Spain and proposal for a new standardised study methodology. Acta Trop. 223:106075. doi:10.1016/j.actatropica.2021.106075.
- Ren CP, Liu Q, Liu F-C, Zhu F-Y, Cui S-X, Liu Z, Gao W-D, Liu M, Ji Y-S, Shen J-J. 2017. Development of monoclonal antibodies against Sj29 and its possible application for schistosomiasis diagnosis. Int J Infect Dis. 61:74–78. doi:10.1016/j.ijid.2017.04.009.
- Sacolo H, Chimbari M, Kalinda C. 2018. Knowledge, attitudes and practices on schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infect Dis. 18:46. doi:10.1186/s12879-017-2923-6.
- Shiff C, Naples JM, Isharwal S, Bosompem KM, Veltri RW. 2010. Non-invasive methods to detect schistosome-based bladder cancer: is the association sufficient for epidemiological use? Trans R Soc Trop Med Hyg. 104(1):3–5. doi:10.1016/j.trstmh.2009.05.013.
- Siqueira LMV, Senra C, de Oliveira ÁA, Carneiro NFdF, Gomes LI, Rabello A, Coelho PMZ, Oliveira E. 2021. A real-time PCR assay for the diagnosis of intestinal schistosomiasis and cure assessment after the treatment of individuals with low parasite burden. Front Immunol. 11:3857. doi:10.3389/FIMMU.2020.620417.
- Suleiman J, Isyaku NT, Ukatu VE, Yusuf IA, Hafiz A. 2022. A review on the immunological techniques use for detection of Schistosoma spp infection. UMYU Sci. 1(1):303–314. doi:10.56919/USCI.1122.039.
- Taye S. 2014. Comparison of Kato-Katz and formol-ether concentration methods for the diagnosis of intestinal helminthic infections among school children of Wonji Shoa town, Eastern Ethiopia: a school based cross-sectional study. Am J Health Res. 2(5):271. doi:10.11648/j.ajhr.20140205.18.
- van Dam GJ, de Dood CJ, Lewis M, Deelder AM, van Lieshout L, Tanke HJ, van Rooyen LH, Corstjens PLAM. 2012. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol. 23(1):274–282. doi:10.1016/j.exppara.2013.06.017.
- van Dam GJ, Xu J, Bergquist R, de Dood CJ, Utzinger J, Qin Z-Q, Guan W, Feng T, Yu X-L, Zhou J, et al. 2015. An ultra-sensitive assay targeting the circulating anodic antigen for the diagnosis of Schistosoma japonicum in a low-endemic area, People’s Republic of China. Acta Trop. 141(Part B):190–197. doi:10.1016/j.actatropica.2014.08.004.
- Vester U, Kardorff R, Traoré M, Traoré HA, Fongoro S, Juchem C, Franke D, Korte R, Gryseels B, Ehrich JHH, Doehring E. 1997. Urinary tract morbidity due to Schistosoma haematobium infection in Mali. Kidney Int. 52(2):478–481. doi:10.1038/ki.1997.356.
- Vinkeles Melchers NVS, van Dam GJ, Shaproski D, Kahama AI, Brienen EAT, Vennervald BJ, van Lieshout L. 2014. Diagnostic performance of schistosoma real-time PCR in urine samples from Kenyan children infected with Schistosoma haematobium: day-to-day variation and follow-up after praziquantel treatment. PLoS Negl Trop Dis. 8(4):e2807. doi:10.1371/journal.pntd.0002807.
- Weerakoon KG, Gordon CA, McManus DP. 2018. DNA diagnostics for schistosomiasis control. Trop Med Infect Dis. 3(3):81. doi:10.3390/tropicalmed3030081.
- Wu Y, Liu J, Lin Y, Weng R, Chen R, Li J, Lv Z. 2018. Diagnosis, monitoring, and control of schistosomiasis—an update. J Biomed Nanotechnol. 14(3):430–455. doi:10.1166/jbn.2018.2517.
- Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM. 2010. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int J Parasitol. 40(3):327–331. doi:10.1016/j.ijpara.2009.08.010.
- Xu X, Chen X, Wu F, Wu C, Liu T, Dai B, Wang T, Zhang S. 2023. Comparison of the efficiency of different etiological assays for detection of Schistosoma japonicum infections in wild mice. Chinese J Schistosomiasis Control. 35(6):573. Accessed: Mar. 22, 2024. [Online]. Available: https://www.zgxfzz.com/EN/.
- Xu X, Zhang Y, Lin D, Zhang J, Xu J, Liu Y-m, Hu F, Qing X, Xia C, Pan W. 2014. Serodiagnosis of Schistosoma japonicum infection: genome-wide identification of a protein marker, and assessment of its diagnostic validity in a field study in China. Lancet Infect Dis. 14(6):489–497. doi:10.1016/S1473-3099(14)70067-2.
