?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The burgeoning multi-field applications of diamond concurrently bring up a foremost consideration associated with nitrogen. Ubiquitous nitrogen in both natural and artificial diamond in most cases as disruptive impurity is undesirable for diamond material properties, eg deterioration in electrical performance. However, the feat of this most common element-nitrogen, can change diamond growth evolution, endow diamond fancy colors and even give quantum technology a solid boost. This perspective reviews the understanding and progress of nitrogen in diamond including natural occurring gemstones and their synthetic counterparts formed by high temperature high pressure (HPHT) and chemical vapor deposition (CVD) methods. The review paper covers a variety of topics ranging from the basis of physical state of nitrogen and its related defects as well as the resulting effects in diamond (including nitrogen termination on diamond surface), to precise control of nitrogen incorporation associated with selective post-treatments and finally to the practical utilization. Among the multitudinous potential nitrogen related centers, the nitrogen-vacancy (NV) defects in diamond have attracted particular interest and are still ceaselessly drawing extensive attentions for quantum frontiers advance.
1. Introduction
Various fancy colors of diamonds have been associated with values ever since diamonds were discovered as gemstones, eg brown, pink, orange, yellow, green, and blue, etc. Diamond has a 5.5 eV bandgap energy much wider than the energies of visible light, which results in the implicit colorlessness for naked eyes [Citation1]. However, the rarest and most valuable gem diamonds are those that contain abundant impurities or certain defects that produce fancy colors because each impurity and/or defect selectively absorbs different wavelengths of light to induce eye-visible colors [Citation2]. The dream of growing gem-quality diamonds had existed for many centuries before diamond was achieved by high temperature high pressure (HPHT) in the 1950s, and then by the chemical vapor deposition (CVD) method for making thick colorless single crystal diamond (SCD) in the 1990s [Citation3,Citation4]. At the same time, diamond as a versatile material, endowed with numerous astonishing physical properties, holds the key to launch practical technology advances, eg tools, heat manager, optical component, semiconductor and even quantum platform. While these applications are also fatefully determined by the different characteristic of diamond resulting from materials processing control [Citation5].
Nitrogen is the most common foreign impurity in diamond. It can be easily incorporated into the diamond lattice due to the similarity of atomic sizes and valence shells between these two elements [Citation6]. Nearly all diamonds contain more or less the nitrogen: indeed, about 98% natural diamond contains about tens to several hundred parts per million (ppm) nitrogen atoms while the rest could have nitrogen concentration at 10 ppm. Although the introduction mechanism of nitrogen in natural diamond is complex, the identification of nitrogen within diamond matrix has been used to distinguish the gem diamond types which are frequently discussed in gemology. This concept is also geologically introduced to roughly describe synthetic SCD stones [Citation7,Citation8]. For the similar situation of constantly existence of nitrogen impurities in synthetic diamonds, eliminating and controlling nitrogen is a perennial concern for the final applications of diamonds as it is detrimental for the materials properties in most cases. For instance, the change of quality grade of gemstones and deterioration of mechanical performance as well as reduction of thermal conductivity can be resulted from the nitrogen aggregation, or the carrier mobility and quantum coherence are affected by the nitrogen background. This is an unavoidable consequence of working with a nitrogen-containing environment or of impurities in the gaseous reactants. Delightedly, the nitrogen content in synthetic diamond crystals is becoming to be maturely suppressed below 5 parts per billion (ppb) not only for CVD but also for HPHT method [Citation9].
Nitrogen in diamond has many aggregation states and would be combined with defects such as other substitutional atoms, vacancies, and clusters to form a variety of optical centers which would change the absorption band and give coloration of diamond crystal [Citation10]. The color of diamond is associated with the concentration and the aggregation state of those centers. High quality diamonds with plump coloration can be cut and polished for use as gemstone; some of them can be much more valuable than colorless ones. Through artful post treatment, such as thermal annealing and radiation treatment, some diamonds with dim luster can be changed to fancy color grade, and some nitrogen-related centers can be modified or even deliberately created. At the same time, some of the nitrogen-related optical centers have great potential in laser application, such as tunable high definition (HD) blue femtosecond lasers [Citation11, Citation12]. Or the spin tunneling of some nitrogen-related optical centers promisingly provide good quantum bits and logic gates for quantum computer [Citation13]. Among these color centers, the nitrogen-vacancy (NV) is an important physical system for strongly boosting quantum technologies, including quantum metrology, information processing and communications, biological marking and imaging, or the tests of quantum entanglement [Citation10,Citation14,Citation15]. Concurrently, advanced progress such as nitrogen termination and near-surface shallow NV mutually have been promoted by the extensive boost of NV quantum utilization [Citation16]. And different to the disruptive effects mentioned earlier, nitrogen existence would contrarily improve the rate of diamond growth and surface morphology of diamond [Citation17].
As a whole, both huge advances in the understanding of relationship of diamond with nitrogen, and burgeoning availability of diamond samples grown under well-defined conditions, as well as the need to resolve the ongoing issues motivate this detailed review of diamond with nitrogen in which we discuss avenues to the gate of crucial status of nitrogen in diamond world. The review paper covers a range of topics from the basis of physical state of nitrogen and its related defects as well as resulting effects in diamond including nitrogen surface termination, to precise control of nitrogen incorporation associated with selective post-treatments and finally to the practical applications.
2. Nitrogen for diamond: effects and states
For gem-quality diamond, the “type” is a concept associated with the role of nitrogen, which is correlated with various properties. This type classification is broadly divided into two types, ie type-I with nitrogen impurity and type-II without (extremely low) nitrogen, based on the state of nitrogen impurities. Type-I diamond can be divided into Type-Ia (with aggregated nitrogen) and Type-Ib (with isolated nitrogen). Type-II diamond can be divided into Type-IIa (very pure high quality) and Type-IIb (containing boron impurities) [Citation7,Citation9,Citation10]. As presented in , natural diamonds often show colors that correlate to their diamond types resulting from the listed characteristics, and now it also has been transferred to treated natural or treated synthetics stones.
Figure 1. Four main types of diamonds including natural and treated natural as well as synthetic diamond stones exhibit many different colors depending on their impurity levels together with the listed characteristics of natural diamonds according to diamond type [Citation7].
![Figure 1. Four main types of diamonds including natural and treated natural as well as synthetic diamond stones exhibit many different colors depending on their impurity levels together with the listed characteristics of natural diamonds according to diamond type [Citation7].](/cms/asset/c97f6340-63b2-400d-9343-41e5b1d6e7be/tfdi_a_1877021_f0001_c.jpg)
2.1 Natural diamond with nitrogen from deep of the Earth
Natural diamonds originate deep in the Earth’s mantle since billions of years ago. It is believed to occur at depths of kilometers (generally > 150 km), corresponding to pressures and temperatures of 40–80 kbar and 1200–1600 °C. These conditions create a natural HPHT processing which has been mimicked, to artificially transform graphite to diamond. The equilibrium phase change occurs right on the surface of the graphite. Most lithospheric diamonds were created from carbonate-bearing high-density fluids that migrated through and reacted with rocks at the base of the subcontinental lithosphere. During the growth of the diamond crystal, nitrogen as a foundation element of the evolution of Earth both in the phase of carbon source and surrounding environment was consequentially doped into the diamond lattice [Citation13].
The vast majority of natural diamonds turned out to be type-Ia and only a rare few were found to be type-Ib. Some of those rare type-Ib diamonds showed extraordinary yellow color in the gem trade. It has been found that the formation of type-II diamonds occurred very rarely in nature and were nearly “perfect” in terms of their crystal structure comparing with the “less perfect” character of type-I diamonds resulted from carbon atoms in the diamond structure being in an “abnormal state” and from the presence of “chemical impurities” [Citation18]. Some impurities and defects are generated during diamond growth, while some defects are produced when the diamond is transported beneath earth’s surface or by interaction with radioactive fluids over millions of years under high temperatures and pressures. Each impurity and/or defect selectively absorbs different wavelengths of light to induce eye-visible colors [Citation19,Citation20]. The color of natural dominantly type-Ia diamonds that have higher contents of aggregated nitrogen than isolate nitrogen contrasts often with that of synthetic diamonds of the same type [Citation21]. Most of these are type-Ia with a minor type-Ib component, but there are very rare cases for which the A (two adjacent nitrogen atoms, 2N), B (four nitrogen atoms around a vacancy, 4NV) and isolate centers are present together naturally [Citation22].
High concentration of nitrogen aggregation over hundreds ppm (even thousands ppm) level generally exists in natural type-I diamond and it generally up to hundreds ppm (even thousands ppm) [Citation23]. While type-II diamonds have much lower nitrogen contents (<30 ppm or even 5 ppm), the IIb diamond is associated with blueish color owing to the existence of boron (even with parts per billion (ppb) level). However, the extremely rare “perfect” colorless IIa natural diamond is not only the dream as gemstone (a 118.28 ct D-Flawless stone with astronomical price of $30.06 million [Citation24]) but also the ideal platform for advanced applications owing to the extremely low nitrogen (<10 ppm nitrogen) and absence of other impurities [Citation25]. Certainly, in natural environment, the trace element such as mineral contamination at sub-ppm level is inevitably generated [Citation26].
Nitrogen in the diamond lattice also can provide information about the amount of time that diamonds spent at mantle temperatures. The following annealing during the long history of the diamond growth in the mantle forced the nitrogen atoms to form different kinds of aggregations [Citation10]. Nitrogen enters as a single atom substituting for a carbon atom (C center). Over millions of years at mantle temperatures, the nitrogen diffuses and aggregates into A centers and then, over billions of years at high geological temperature, into B centers. Most natural single crystal diamonds carry both A and B centers, while fibrous diamonds mostly carry A centers, attesting to their short residence time in the mantle [Citation27,Citation28]. The aggregation processes follow second-order kinetics, shown as 1/C − 1/C0 = Kt, where C0 and C are respectively the initial and final concentrations of A aggregates, and K is the thermally activated rate constant and t is the time over which aggregation has occurred. The estimated activation energy is extremely huge, so it was annealed at the maximum plausible geological temperature of 1200 °C and transform at the conversion rate of nearly 50% from A-nitrogen to B-nitrogen. Therefore, there is hardly pure Type-Ib natural diamond existing in this natural environment, and typical natural Type-Ia diamond contains both A and B centers.
2.2 Artificially synthetic diamonds with nitrogen
The synthesis of diamond was firstly achieved by General Electric Co. in 1955. The HPHT process was generated by simulating conditions for natural diamond growth. However, the artificial process conditions are not as powerful as the nature, only small size SCDs up to several millimeters could be obtained. Most of the synthesized diamonds present some degree of Type-Ib characteristics [Citation29]. The generally accepted equilibrium relationship between the A-nitrogen and B-nitrogen can also prove the mutability of nitrogen aggregations. It is reported that any process requiring lattice diffusion of nitrogen could not be expected to proceed rapidly when the temperature is below 2000 K [Citation28]. Therefore, optical centers in synthesized diamonds are mainly NV and H3. There are also some special centers according to particular catalysts. For example, NE4 center which contains of a nickel atom and two vacancies will be observed if Fe–Ni–Co catalysis system has been utilized [Citation29].
Alternatively, the CVD process is currently the most typical and favorite way to produce colorless diamonds under low pressure. Most CVD synthetic diamonds are near colorless type-IIa. With the recent advances in CVD growth techniques especially the microwave plasma (MP)-CVD, nitrogen content in SCD can be less than 1 ppm. In most cases, a mixture of hydrogen and methane is used for diamond growth. The excited hydrocarbon can bond on this exposed carbon and form sp2 graphite or sp3 bonded carbon. The preferential etching on graphite than diamond by active hydrogen atoms in the plasma makes the growth of CVD diamond films become true [Citation30]. In addition to the slight nitrogen defects in CVD diamond from the latent background or feeding gas, the morphology and growth strategy of CVD diamond can be regulated by the nourishing of nitrogen [Citation31]. As shown in , during the HPHT synthesis, the crystallization process exhibits several stages with the rising of nitrogen concentration, ie from single crystal to block crystal with micro-twins and then to aggregate of crystals and/or twins. When the nitrogen concentrations are higher than 0.4 atom%, graphite would begin to crystallize, instead of the nucleation and growth of diamond. Also, it is considered that nitrogen would stabilize octahedral growth form [Citation33]. For CVD progress, as displayed in , nitrogen addition would result in an all columnar crystals have a {110} orientation (even this fraction reaches 45.5% of the overall texture) and with nearly coplanar smooth surface (100) facets in polycrystalline diamond (PCD) film associated with a prevailed growth selection mechanism [Citation33,Citation34]. However, for SCD, an evolution of the growth mode from step flow to bidimensional mode was observed owing to the resulted carbon species supersaturation [Citation35]. It was beneficial to the creation of a macroscopic smooth (100) face avoiding the growth of hillocks, similar to the grain exhibition in PCD. However, the (100) surfaces looked microscopically rough by bunched steps, as illustrated in [Citation36]. This difference of course can be affected by temperature and SCD substrate holder. No matter for what forms of diamonds products, the rate of growth additionally can be remarkably boosted [Citation37]. The integration of nitrogen in diamond and its physical states turned to be the consequent concerns for the deeper understanding of entanglement relationship between nitrogen and diamond.
Figure 2. (a) Diamond crystals grown from the Fe–Ni–C system with addition of nitrogen-containing compounds [Citation32]; (b) Electron backscattering diffraction mapping images which based orientation distribution functions of the four sample with 0.5%, 1.5%, 2.5%, and 3.5% N2/CH4 addition [Citation33]; (c) Surface morphology of epitaxially grown diamonds on (100) diamond substrates with different nitrogen feeding [Citation36].
![Figure 2. (a) Diamond crystals grown from the Fe–Ni–C system with addition of nitrogen-containing compounds [Citation32]; (b) Electron backscattering diffraction mapping images which based orientation distribution functions of the four sample with 0.5%, 1.5%, 2.5%, and 3.5% N2/CH4 addition [Citation33]; (c) Surface morphology of epitaxially grown diamonds on (100) diamond substrates with different nitrogen feeding [Citation36].](/cms/asset/212addf7-3443-44d5-ae57-19f650aecb3d/tfdi_a_1877021_f0002_c.jpg)
2.3 Defects states of nitrogen in diamond
Essentially nitrogen-free diamonds show only the intrinsic diamond absorption bands from 4000 to 1500 cm−1 of typical infrared (IR) absorption spectrum, while nitrogen induces characteristic absorption bands in the one-phonon domain from 1500 to 400 cm−1 [Citation38] (refer to ). The notation used to describe defects in diamond is not universal, and the historic descriptors can be confusing [Citation10]. In addition to the substitutional defect A (at 1215 cm−1), B (at 1175 cm−1), and C (at 1135 cm−1) centers which commonly being found in natural diamonds (also in some nature-like condition of HPHT treated diamond), other nitrogen-based defect families including defects revolve around the vacancy NnVm (n ≤ 4, m ≤ 2), N–H complex and even metal-interacted defects, which were frequently contained in different types of diamond [Citation39].
Figure 3. The transmission IR spectra of diamonds of the pure poles of the different types [Citation38].
![Figure 3. The transmission IR spectra of diamonds of the pure poles of the different types [Citation38].](/cms/asset/46178176-a68e-4cd9-97fc-78021de62920/tfdi_a_1877021_f0003_c.jpg)
The single substitutional nitrogen (NS) readily incorporates into natural and synthetic diamond produced by CVD or HPHT. Its incorporation usually preferentially locates into {111} rather than other growth sectors [Citation40,Citation41]. This NS dopants in diamond behave as deep donors with an energy –1.7 eV below the conduction band minimum [Citation42]. While the NS defects can act as electron acceptor states and the charged nitrogen defects are neutralized within approximately 10 ns [Citation43]. Isolated nitrogen in diamond can exist in serval charge states, including neutral one (), positive one (
) and even negative one (
). Among them, only the
has unpaired electron, it may form from
trapping electron and/or
trapping hole [Citation44]. Electrons were observed to predominantly recombine into neutral nitrogen states rather than their original ionized nitrogen defects, thereby creating
. However, the electron mobility was observed to be limited by scattering with neutral
. Meanwhile, the
is also a product of charge-transfer and its infrared (IR) absorption bands strength decreased after irradiation and annealing, but the concentration of
and neutral vacancy (V0) increased. The defect-induced one-phonon region of irradiated and annealed synthetic diamonds arise from
[Citation45].
The conditions under which the natural or synthetic diamonds grow and experience after growth determine the degree of nitrogen aggregation or related defects, which can be differentiated by IR spectra. However, these defects involve NS; interstitial nitrogen (NI) has proved much more elusive [Citation46]. There are no definitive assignments to any spectroscopic signature of single interstitial nitrogen-related defect structures in diamond, however, the <001>-split dinitrogen interstitial, N2I, is assigned to the so-called H1a local–vibrational modes transition at 1450 cm−1 [Citation47]. Carbon interstitials could be released when B centers were formed, and these were then condensed onto {001} planes forming platelets. {001} interstitial platelets in diamond are unlike the planar interstitial aggregates seen in silicon: the rodlike defects observed there consisting of <110> chains of interstitials have a relatively high energy in diamond, probably because of the difficulty of inserting chains into small <110> channels [Citation48]. The double C interstitial (3H center) generally efficiently forms above temperatures of 300 °C-400 °C and anneals out at different temperatures depending on factors such as the N concentration or the doping level [Citation49].
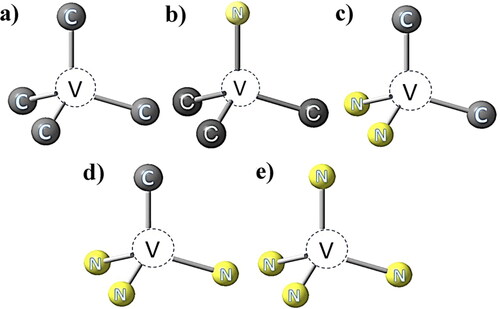
Vacancies (single vacancy GR1 center as shown in ) are easily formed by faultily crystallization or eternal damage introduction. When a vacancy is trapped by a NS atom in the lattice, it becomes a NV center, as shown in . Meanwhile, two NS atoms (A aggregation) bonded to a vacancy is a H3 center which has been mentioned earlier, three NS atoms on a {111} plane ‘bonded’ to a common carbon atom construct a N3 center, four NS atoms bonded to a vacancy construct a B aggregation mentioned earlier [Citation21,Citation50], respectively as shown in –e). Capturing of a vacancy by B aggregation and subsequent N–V exchange to place the two vacancies nearest-neighbor to each other, resulting in the H4 center atomic configuration from N4V–V to N3V2N [Citation51]. The zero-phonon lines (ZPL) energy is generally considered to be the main parameter for distinguishing and characterizing an optical center in diamond. In addition to the ZPL of GR1 center at 741 nm, examples of such centers shown in are the NV center with different charge states exhibit optical ZPLs at 637 and 575 nm respectively, H3 center with ZPL at 502.3 nm, the N3 center with ZPL at 415.2 nm, and the H4 center with ZPL at 496.2 nm [Citation39,Citation52,Citation53].
Figure 4. Schematic of typical N-related centers in diamond: (a) indicates a vacancy in diamond lattice; (b) indicates a nitrogen-vacancy center; (c) indicates a H3 center; (d) indicates a N3 center; (e) indicates a H4 center.
Among the NnVm complex family, NV, not only the most general defect but also a significant physical system in synthetic diamond rather than natural counterpart, typically is point defect with trigonal C3v symmetry consisting of a pair of NS and lattice vacancy. This center may be found in an as-grown CVD diamond or as a product of radiation damage and annealing or ion implantation and annealing in bulk and nanocrystalline diamond [Citation54]. Three charge states of NV are also known as well, ie +, 0, and −, with total ground state spin S = 0, 1/2, and 1, respectively.
shows a visualization of the NV electronic wave function. The center’s atom-like properties include a paramagnetic triplet ground state (refer to ) which interacts strongly with both microwave and optical fields [Citation55]. No optical transition has been definitively assigned to NV+, and its presence is inferred only through the lack of emission from NV0/− in active or passive charge control experiments. Theoretical calculations predict that the NV+ defect has an S = 0 (rather than S = 1) ground state and around 1 eV above the ground state, but not yet observed. Among those two counterparts NV− also shows an IR transition at 1042 nm (refer to , between a pair of spin singlet states, while the NV0 ground state can be degenerated by dynamic Jahn–Teller coupling and strain broadening [Citation56,Citation57]. Owing to the rare properties of spin-dependent fluorescence of the NV− center compared with other solid-state systems, NV− has been employed in milestone and is currently being intensively explored not just at cryogenic temperatures (below ∼10 K) but also at room temperature. Its long spin-coherence times (T2) could be extended into the millisecond range even at room temperature [Citation10,Citation58–60]. If the NS concentration higher than 100 ppb, the NV− would be the dominant charge state [Citation61]. For each individual NV, the inevitable lattice strain and applied electric field in diamond can typically result in the shifting and splitting of central resonance and it should be accompanied by a comparable shift of the overall spectrum (refer to ). During the diamond growth, different to the nitrogen-containing solid carbon source for diamond formation by natural and artificially mimicked HPHT process, the incorporation efficiency of nitrogen from gas phase to diamond is in the range of ca. 10−3 to 10−5 and the ratio of NV0/− center to NS in diamond is also variable. Teraji et al. [Citation62] reported the conversion efficiency from gas phase to diamond is ca. 10−4 and the number density ratio of NV center to the NS in diamond is reportedly 4 × 10−3–2 × 10−2. Of course, these incorporation efficiencies for nitrogen strongly depend on the growth condition and crystal orientation. Its tunable controlling and promising applications are reviewed sequentially in the following sections.
Figure 5. (a) Visualized schematic of the NV electronic wave function in diamond lattice [Citation55]; (b) A schematic illustration of the NV center’s level structure [Citation59]; Both strain and electric fields lead to (c) shifting Πz and (d) splitting 2Π⊥of the |ms = ±1> manifold; (e) When averaged over an ensemble of NV centers, random local strain fields lead to a single broad spectral feature (at large strain); (f) By contrast, random local electric fields lead to two distinct spectral regimes: at small electric fields, the center hyperfine resonance splits, leading to a total of four resolvable features; at large electric field, one obtains the characteristic split resonance seen in typical high-density NV ensembles [Citation63].
![Figure 5. (a) Visualized schematic of the NV electronic wave function in diamond lattice [Citation55]; (b) A schematic illustration of the NV center’s level structure [Citation59]; Both strain and electric fields lead to (c) shifting Πz and (d) splitting 2Π⊥of the |ms = ±1> manifold; (e) When averaged over an ensemble of NV centers, random local strain fields lead to a single broad spectral feature (at large strain); (f) By contrast, random local electric fields lead to two distinct spectral regimes: at small electric fields, the center hyperfine resonance splits, leading to a total of four resolvable features; at large electric field, one obtains the characteristic split resonance seen in typical high-density NV ensembles [Citation63].](/cms/asset/7379c0bc-796d-4061-88b8-cdba6b34e551/tfdi_a_1877021_f0005_c.jpg)
The H3 defect is one of the most common luminescence centers observed in natural diamond (usually accompanied by N3 luminescence). It is also easily produced by irradiating and annealing HPHT-grown type Ib material. The defect formation in this case is understood to be dominated by mobile NV centers moving as a unit via emission and recapture of the same vacancy. In turn, the NV center is captured at an Ns center, forming N2V. Furthermore, with increasing temperature N atoms tend to aggregate and build more complex defects. Unlike NV and N2V, only the neutral charge state of N3V0 has been identified experimentally, and it shows a distinctive optical spectrum in natural diamond, which to some extent is one of the primary markers employed in the identification of natural and synthetic diamond. Typically, N3 center has similar magnitude of the hyperfine and quadrupole interactions with each of the three equivalent nitrogen atoms.
In addition, as a large quantity of hydrogen is brought in in the hydrogen rich CVD environment, some kinds of N–H complexes can be contained in the lattice [Citation64]. Under certain conditions, some of the A-aggregations fail to be combined with vacancies. Instead, the C–N bonds have been substituted by C–H bonds; hydrogen atoms are brought in as the interstice of the A centers. Consequently, N2–H or N2–H2 centers can be formed, as shown in .
Figure 6. Calculated formation energies (Eform) of various N- and H-related defects in diamond, as a function of Δμ = μH − μN, where μH and μN are chemical potentials of H and N, respectively [Citation65]. Schematic of N–H complexes: (b) pure diamond; (c) an A-center (N pairs); (d) a N2–H complex; (e) a N2–H2 complex [Citation18].
![Figure 6. Calculated formation energies (Eform) of various N- and H-related defects in diamond, as a function of Δμ = μH − μN, where μH and μN are chemical potentials of H and N, respectively [Citation65]. Schematic of N–H complexes: (b) pure diamond; (c) an A-center (N pairs); (d) a N2–H complex; (e) a N2–H2 complex [Citation18].](/cms/asset/c09f536f-959d-4143-b232-fbb5d7160c76/tfdi_a_1877021_f0006_c.jpg)
According to , the Eform of N–H–N is lower than those of V as well as of other H-related defects. At the same time, it is higher than those of VNx as well as of Ns and the A center. Since V’s may exist in CVD-grown diamond at a high content, it is highly expected to have a substantial population of the N–H–N defects. However, the populations of the B centers and possibly of the VN3 defects may become extremely large because of their negative formation energies [Citation65]. Generally, there has been a focus on complexes of N with lattice vacancies, and assignment of a range of complexes to optical transitions, such as those from the aforementioned NV complex at 1.945 and 2.516 eV, and those labeled N2, N3, N4, H2, H3, and H4 [Citation66]. Furthermore, many fancy colors are inseparable with these nitrogen related defects. A revealed coexisting point defect featuring a deep acceptor level is considered related to nitrogen and hydrogen as well as vacancy, however, its concrete state is not clear yet and additional work will therefore be mandatory [Citation67]. This situation is similar to a so-called Y center, of which the absorption with a relatively broad apparent maximum centered at ∼1145 cm−1 [Citation68].
2.4. Nitrogen-termination on diamond
Surface termination and functionalization of diamond is also an extremely important topic for the application of diamond electronics and quantum photonics. Optically active defects are being increasingly located within nano scale of the diamond surface, where their quantum properties such as coherence time and spectral diffusion would be significantly degraded, compared to that in bulk. Most of the attempts for controlling the surface of diamond are associated with terminations, however, the unwanted electronic spins still remain on those surfaces [Citation69]. In addition to the common terminations associated with hydrogen or oxygen, nitrogen termination being considered as an effective surface bond state for affecting the shallow color center began came into sight recently.
shows the full nitrogen coverage in a 2 × 1 surface reconstruction. If it is possible to replace diamond surface carbon with nitrogen atoms, the dangling bonds would be replaced by (spin-neutral) lone pairs, thereby reducing the free spins on the surface. The N-terminated diamond is basically optically inactive; only a few occupied surface bands appear near the valence band maximum (VBM) that do not interfere with the level of NV, as shown in . The instability of shallow NV− can be caused by upward band-bending near the surface due to negative electron affinity (NEA), eg which observed for hydrogen-terminated diamond, because shallower NV− were more unstable. In addition to the intensively studied diamond hydrogen and oxygen termination associated with hydrophobicity, EA or two-dimensional hole channel, completely passivated surface with NS would be optimal to achieve enhanced photon collection efficiency of NV−. displays Rabi oscillations and histograms of the Rabi oscillation of N-terminated diamond for showing the charge stability of NV center. Meanwhile, as summarized in , the various terminations and their associated effects on shallow NV centers are illustrated. Utilizing nitrogen terminations would effectively passivate the dangling bonds on diamond surface and downshift the band bending [Citation72]. It was predicted that nitrogen termination would induce a positive electron affinity (PEA), but otherwise allow the near-surface carbon atoms of diamond to exhibit bulk-like electronic states. This gives rise to an almost ideal electronic environment for near-surface color centers, especially the NV−, for quantum sensing or information applications, and, of course, it also provides a potential nuclear spin resource at the surface [Citation69]. The nitrogen related surface bands will not perturb the single photon absorption of the NV− center in the photoexcitation process, under normal conditions. displays the nitrogen K-edge result of the nitrogen-terminated diamond measured by near edge X-ray absorption fine structure (NEXAFS) technique, which could be a powerful reference for identifying the nitrogen terminations. The first peak located near 396 eV has been attributed to full N termination, which strongly contributes to stabilized shallow NV− because of its high PEA. The second peak near 399 eV is considered to be assigned to the N/H termination [Citation71]. Furthermore, these surfaces are predicted to exhibit PEA of 3.46 and 0.32 eV for the “full N” and “N/H” surfaces, respectively, making them attractive for stabilizing the emission from NV− centers in the near-surface region.
Figure 7. (a) Band structures of N-terminated diamond in a 2 × 1 surface structure showing minimal intrusion of surface states into the bandgap [Citation69]; (b) Schematic energy diagram of H- and N-terminated (111) diamond surfaces [Citation70]; (c) Charge stability evaluation of NV center based on Rabi oscillations and Histograms of the Rabi oscillation of N-terminated diamond; (d) Schematic illustration of diamond surface terminations with positive electron affinity associated with stabilizing of NV−; (e) Typical near edge X-ray absorption fine structure surface analysis spectroscopy of nitrogen-terminated diamond [Citation71].
![Figure 7. (a) Band structures of N-terminated diamond in a 2 × 1 surface structure showing minimal intrusion of surface states into the bandgap [Citation69]; (b) Schematic energy diagram of H- and N-terminated (111) diamond surfaces [Citation70]; (c) Charge stability evaluation of NV center based on Rabi oscillations and Histograms of the Rabi oscillation of N-terminated diamond; (d) Schematic illustration of diamond surface terminations with positive electron affinity associated with stabilizing of NV−; (e) Typical near edge X-ray absorption fine structure surface analysis spectroscopy of nitrogen-terminated diamond [Citation71].](/cms/asset/d8e062aa-458a-431c-8eea-4622abbd0d01/tfdi_a_1877021_f0007_c.jpg)
3. Coloration of diamond resulting from nitrogen defects
Defects potentially generate electronic states within the diamond bandgap, allowing for absorption of light at various wavelengths and leading to an apparent coloration of the diamond. The optical centers could be evidenced by absorption or photoluminescence (PL) properties. For example, N3 center presents a broad absorption band at about 415 nm, which contributes to the famous cape system among yellow diamonds [Citation49]. The intensity of cape yellow can be shifted by controlling the N3 concentration and distribution. And two absorption bands at about 575 nm and 637 nm associated with NV0 and NV− center thus absorbing blue and purple in the visible wavelength [Citation73]. If combined with the N3 centers, the diamond will show red or aubergine and, meanwhile, H3 center can be combined with N3 centers to create orange coloration in diamond [Citation74].
While most of the synthesized diamond represent brown and light yellow, seldom pink, since the density of some strong centers such as N3 is usually low. Dislocations have long been considered to be the origin of the brown color since dislocation configurations and typical of plastically deformed crystals were observed in brown diamonds [Citation75, Citation76]. However, Jones [Citation77] supported that vacancies and especially the clusters should be the cause of the brown coloration since dislocation density seems too low to lead enough absorption and recent electron energy loss spectroscopy (EELS) studies showed that the brown centers lay between (but not on) the slip bands. What’s more, Fujita et al. [Citation78] used density function theory (DFP) to calculate on the properties of a large spherical vacancy clusters which consists of 71 vacancies, and they concluded that spherical clusters had particularly low energy. The vacancies still can be combined with hydrogen to decrease the energy further, and also make the color centers more complex. The brown color is also considered a result of the vacancies, nitrogen and hydrogen clusters in synthesized diamonds [Citation79, Citation80]. Experimentally, the intensity of the brown absorption continuum correlates well only with the concentration of nitrogen C-defects. Zaitsev et al. [Citation81] discussed that nitrogen doping contributed to the brown color not only for natural diamond but also synthetic counterpart. Furthermore, the vast majority of natural pink diamonds colored by the 550 nm absorption band are produced through deformation processes deep within the earth. However, this as-yet-unidentified “550 nm” defect (s) that are correlated with shear stress and natural plastic deformation or, very rarely, the NV0/– centers [Citation82]. Wang et al. reported that strong absorptions from N–V centers are the main causes of the observed pinkish orange bodycolor of CVD diamond. The very attractive orange color was achieved by introducing the proper concentrations of N–V centers while limiting the formation of other defects [Citation83]. While green diamonds are colored either by simple structural defects generated by radiation exposure or by more complex defects involving nitrogen, hydrogen, or nickel impurities. One of the coloring mechanisms is the green luminescence from H3 defects [Citation84]. In summary, lists a brief collection of common causes of color in diamond.
Table 1. A brief description of common causes of color in diamond [Citation2,Citation22,Citation23,Citation78,Citation85–Citation88].
Gem diamonds associated with color grading can be classified into two categories, ie the yellow-system and the rest. The yellow-system gem diamonds are strictly valuated and divided into different color grades. General standard for color grading of diamonds is shown in . The degree of coloration in the yellow-system also depends on the concentration and state of nitrogen.
Table 2. A contrast table of a general color grading of gem diamond [Citation89,Citation90].
The other category of colored diamonds, including blue, green, pink, orange ones and so on, are related to combination of the absorption and luminescence of different centers. Sometimes highly unusual ultraviolet–visible (UV–VIS) luminescence color of some optical centers may provide blue and white coloration; it makes the colored diamond look like purer. By certain blending of different kinds of color center varieties of coloration of diamond could be obtained. The formation of some particular coloration could even increase the value of some gem quality diamonds. For example, annealing at a certain high temperature could modify the coloration of a diamond and make a localized coloration to be homogeneous or even remove the coloration.
Some luminescence of the optical centers which differ under different incident light energies or densities could lead to the so called “chameleon” diamond [Citation91], which typically changes color from greyish-green to yellow when they are heated or cooled (thermochromic behavior) or kept in the dark (photochromic behavior). Post treatment for regulating colors and state of N–V centers is effective and even indispensable not only for increasing commercial value of diamond gemstone but also for functionalization.
4. Post treatments for nitrogen-related defects controlling
4.1 High temperature annealing
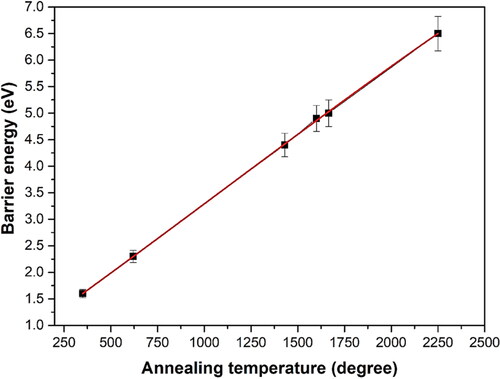
Heat treatment has been widely used (especially in jewelry industry) for increasing the color grade of the diamond associated with the state changing of nitrogen related defects and for modifying the coloration of synthesized and radiated diamond. Annealing provides thermal energy to break bonds within the material, allowing elements, vacancies, or defect complexes to diffuse through the lattice. By appropriate methods, different patterns of nitrogen-related centers can be tuned, thereby changing the properties of diamond associated with coloration, optical transmission and even quantum properties, etc [Citation92]. collects the effect of annealing temperature on the defects in diamond associated with or interacted with N–V centers.
Table 3. Effect of annealing temperature on defect in diamond [Citation23,Citation49,Citation93–101].
The effect of high temperature annealing on defects in diamond is directly related to the energy of those imperfections. shows the relationship between annealing temperature and barrier energy. For example, for the most common defect of NS, theoretical calculations (at 0 K) showed an activation energy of 6.3 eV for the direct exchange of NS with a neighbor C atom [Citation102], while the rate-limiting step for V-assisted diffusion was found to be the jump of Ns into a next-neighbor V with a calculated barrier height of 4.8 eV, as the V would become mobile when the temperature is above 600 °C (agree with the experimental value of 2.3 + 0.2 eV) [Citation103, Citation104]. In practical, its diffusion activation energy of 5.0 ± 0.3 eV was obtained between 1700 and 2100 °C at a pressure of 7 GPa. This is probably assisted by intrinsic defects under external pressure. For example, the activation energy for the transformation could be reduced to 2.6 eV under external high pressure [Citation28]. In addition, the barrier energy of 4.5 eV indicates that NV center remains immobile up to around 1700 °C, unless self-interstitials (which might assist diffusion) are released from larger aggregates [Citation105].
4.1.1 High-pressure and high-temperature method
Gemologists have paid attention to the color change of natural diamonds with heat treatment under high pressure conditions since 1999 and thereby this HPHT treatment turned to be a common method to tune diamond colors [Citation106]. In order to reduce the graphitization rate during high temperature annealing, annealing is frequently performed under high pressures to make diamond thermodynamically stable [Citation107]. The external pressure could reduce the barrier energy of defects as mentioned earlier, and also facilitate the aggregation and dissociation of nitrogen or vacancy during their migration. The brown and yellow color is mainly attributed to reduction of plastic deformation and release of vacancies clusters, and the free vacancies are trapped to form NV, H3 and H2 centers [Citation108]. For instance, annealing at the higher temperature and pressures very close to the diamond/graphite transition, seems to be effective to reduce the non-radiative re-combinations in diamonds, and, in white light, diamonds exhibit bright green luminescence, produced by absorbed energy being re-emitted in the H3 band [Citation109]. The emission of H3 centers and additional unusual platelet (resulting from pressure caused aggregation) spectroscopic feature are reliable indicators for HPHT-treated diamonds as they are not observed in untreated natural diamonds [Citation110]. However, the birefringence pattern similar to that of natural diamond could be formed with the HPHT treatment, indicating that plastic deformation was produced by the treatment [Citation111,Citation112]. The final coloration of diamond after the treatment can range from yellow, pink to nearly colorless. Blackening of diamond can be observed in nitrogen flow at temperatures as low as 850°С. The temperature of treatment must be high enough for facilitating diffusion of nitrogen associated with NV centers or aggregations as listed in , whereas the applied pressure must be sufficient to prevent excessive graphitization. The stabilizing pressure (>5 GPa) would prevent the diamond from graphitization effectively at 1700 °C. The major difference, therefore, between HPHT and atmospheric pressure high temperature (APHT) as well as low-pressure high temperature (LPHT) annealing is the quality of the process in terms of structural integrity of the annealed diamonds and their graphitization.
4.1.2 Atmospheric pressure high temperature technique
The main problem in APHT treatment is to minimize diamond graphitization [Citation113]. It has long been known that diamonds do not turn to graphite during a high temperature annealing in vacuum. To avoid or minimize diamond graphitization during APHT treatment at extreme temperatures (exceeding 1700 °С), it is necessary to make the treatment time a hundred times shorter than HPHT treatment.
A special heating like intermittent “thermal shock” (heating and cooling) is used to minimize the time. The APHT treatment is performed by a succession of 5 or 6 thermal shocks. Consequently, the total time during which each treated sample was exposed to extreme temperatures (1800–2100 °С) ranging from 8 to 11 s. The coloration of dark brown diamond changed obviously, as shown in . After APHT treatment, the concentrations of N3 and H3 centers are increased obviously evidenced by the intensity of the absorption peaks, showing that the clusters have been released and transited to sorts of nitrogen related centers, thus directly leading to the fading of the brown coloration.
Figure 9. The coloration of dark brown diamond before and after APHT treatment [Citation113].
![Figure 9. The coloration of dark brown diamond before and after APHT treatment [Citation113].](/cms/asset/259f1506-28f9-4df9-a35e-f11f5d5b865e/tfdi_a_1877021_f0009_c.jpg)
4.1.3 Low pressure high temperature treatment
An alternative way to replace HPHT or APHT treatments is the annealing in vacuum, inert gases, or hydrogen with low pressure, ie so-called LPHT treatment, which could be realized directly by different CVD technologies or tube furnace with an air pump. Meng et al. [Citation114] annealed SCD produced by MPCVD at very high growth rates (up to 150 um/h) without graphitization at temperature up to 2200 °С and pressure <300 tor.
Great changes in optical properties and defect structure have been induced by this facile LPHT processing [Citation115,Citation116]. LPHT annealing can reduce nitrogen-induced yellow color in synthetic diamonds by converting from type-Ib into type-IaA associated with considerable aggregation of dispersed nitrogen [Citation117]. However, LPHT annealing alone shows small effect on the diamond color due to a modest increase in H3 intensity although most brown diamonds show a shift towards yellow coloration but displays significant change on surface graphitization and inclusions [Citation23]. Therefore, the LPHT annealing is considered effective in aggregation of nitrogen into A-defects. However, sole LPHT treatment is insufficient in aggregation of nitrogen into B-defects [Citation118]. The HPHT method enhancing lattice distortions around point defects resulted in a considerable broadening of zero-phonon lines of “soft” (vacancy-related) optical centers. Unlikely, LPHT annealing may enhance overall intensity of luminescence of synthetic diamonds [Citation119]. Meanwhile, irradiation in combination with LPHT annealing would create a significant shift towards more commercially viable diamond colors owing to the moving of irradiated vacancies without externally produced distortion. In addition, LPHT approach can also be employed to enhance the electrical conductivity and mechanical performance of diamond with the assistance of plasma treatment [Citation120,Citation121].
4.2 Alternative effective treatment approaches
A higher density of shallow NV center in the diamond crystal is crucial to enhance magnetometer sensitivity, stronger fluorescent emission, etc. Previous studies showed an increased creation efficiency of NV centers with increasing fluence by irradiation method [Citation122], which was attributed to the formation of multi-vacancies as a result of high irradiation doses [Citation75]. In addition to the ion implantation (eg scanning focused ion beam of lightweight helium) or high energy rays such as gamma rays for creating defect, electron irradiation also has been intensively introduced not only for producing vacancy but also for generating negative charges. A conversion of up to 24% of vacancies to NV centers was carried out by using electron irradiation of samples cooled by liquid nitrogen [Citation123,Citation124]. At such extremely low temperature, the self-interstitial defect created by irradiation is not mobile. Since the self-interstitial is the main source of vacancy annihilation [Citation125], cold irradiation followed by rapid thermal annealing could produce an increased number of vacancies and promote the formation of vacancy-related defects. Simultaneous electron irradiation and annealing can significantly increase NV creation efficiency [Citation126].
Electron irradiation followed by annealing at moderate temperature (∼1100 °C) would create a high concentration of NV defects [Citation127]. Actually, this change in intensity of NV centers is the result of interaction of NV defects with other defects created by irradiation. The annealing of radiation-induced NV defects does not contribute much to the creation of H3 defects. Instead, the majority of H3 defects formed after irradiation and annealing is the result of interaction of vacancies with the A-aggregates which were created independently of the transformation of NV defects. The intrinsic optical center after irradiation has been concluded that it relates to a negatively charged interstitial–vacancy pair (I–V− defect), which could be considered as an origin of NV−. Generally, the spectra of electron treated samples only exhibit the common radiation and radiation/annealing related absorptions such as GR1, H2, H3, H4, NV−, NV0, H1a, H1b, and the H1c features [Citation128].
The NV− will most likely be neutralized to NV0/+ and several attempts have changed this neutralization. The surface termination of diamond such as nitrogen-termination could give rise to a change of NV charge state. For shallow subsurface NV centers in diamond, conversion of NV0 to NV− through selective oxidation, fluorination associated with PEA were realized [Citation129, Citation130]. The charge state conversion of NV center can also be modulated by optical illumination with lasers of different wavelengths and powers [Citation131]. Modulation of NV charge state can also be directly affected by external electric fields (refer to ). An exponential rise in the NV−/NV0 ratio with increasing negative bias was done and its relationship follows the NV−/NV0 = 1.13 × e−0.125V, where the V was the applied voltage in volts [Citation132]. Depending on this effect, a diamond p–i–n (p-type–intrinsic–n-type) junction was fabricated to control the charge state of NV centers in the i-layer by applying a forward bias below the built-in voltage [Citation133]. Engineering of the Fermi level by a n–i–n diamond junction was demonstrated for the control of the charge state of the NV centers in the intrinsic layer region. By changing the size of the i-layer region between the phosphorus-doped n-type layer regions, the gradual change in the NV− charge-state population was realized [Citation134].
Figure 10. Modulation of NV− charge state by electrons: (a) PL spectrum of NV diamond at various electric field (−7, −5, −2, 0, 5, and 7 V) at 300 K with the inset showing band bending and shifting of Fermi level with the application of external electric field, and (b) Remarkable increase in the NV− concentration at −7 V with the inset showing an exponential rise in NV−/NV0 ratio with increase in negative bias voltage [Citation132].
![Figure 10. Modulation of NV− charge state by electrons: (a) PL spectrum of NV diamond at various electric field (−7, −5, −2, 0, 5, and 7 V) at 300 K with the inset showing band bending and shifting of Fermi level with the application of external electric field, and (b) Remarkable increase in the NV− concentration at −7 V with the inset showing an exponential rise in NV−/NV0 ratio with increase in negative bias voltage [Citation132].](/cms/asset/5ae750ba-6e61-43bc-8b02-e5b708b78cd9/tfdi_a_1877021_f0010_c.jpg)
5. Applications of nitrogen-related centers
The stable color centers in diamond, together with the unique physical and chemical properties offers diamond as a suitable platform for versatile technological promise. Applications based on the nitrogen impurity in diamond with variable forms were constantly realized, including geophysical or geochemical analysis, identification of gemstones, biomarker tracing and especially the solid-state quantum electronics [Citation10,Citation135]. Among the famous NnVm complex family in over 500 electronic optical centers, NV− center is most intensively studied and applied, owing to its outstanding properties such as strong fluorescence, long-lived ground state electron spin coherence, optical spin polarization or readout, state-selective intersystem crossing probabilities, etc [Citation10, Citation54, Citation136]. It is thus could be used to detect the electric field of a single electron at a distance of ∼25 nm within 1 s of averaging, absorb and re-emit single photon for quantum computing and information processing, provide robust quantum memories, and sense magnetic field, stress, temperature or current, etc [Citation10, Citation136–143].
A new pathway associated with magnetoelastic drive of ferromagnetic resonance and NV magnon coupling combines with acoustics and magnetics that enables highly energy-efficient and local excitation of NV centers. This could lead to completely integrated, on-chip, atomic sensors [Citation139]. An imaging strain sensor under high pressure and magnetic field based on NV center was realized (refer to ) [Citation140,Citation141].
Figure 11. NV centers integrated into a diamond anvil cell (DAC): (a) Schematic of the DAC geometry; (b) The DAC sample chamber is defined by the gasket-anvil assembly, it is loaded with the sample of interest, a pressure-transmitting medium, and a single ruby microsphere. Meanwhile, a ∼50-nm layer of NV centers is embedded into the near surface of diamond anvil directly below the sample chamber; (c) Top: Stress both shifts and splits the ms = ±1 sub-levels at first order. Bottom: An axial magnetic field splits the ms = ±1 sub-levels at first order, but a transverse magnetic field leads to shifts only at second order; (d) A representative optically detected magnetic resonance ODMR spectrum from an NV center ensemble under an applied magnetic field; (e) Each pair of resonances in (d) corresponds to one of the four NV crystallographic orientations [Citation141].
![Figure 11. NV centers integrated into a diamond anvil cell (DAC): (a) Schematic of the DAC geometry; (b) The DAC sample chamber is defined by the gasket-anvil assembly, it is loaded with the sample of interest, a pressure-transmitting medium, and a single ruby microsphere. Meanwhile, a ∼50-nm layer of NV centers is embedded into the near surface of diamond anvil directly below the sample chamber; (c) Top: Stress both shifts and splits the ms = ±1 sub-levels at first order. Bottom: An axial magnetic field splits the ms = ±1 sub-levels at first order, but a transverse magnetic field leads to shifts only at second order; (d) A representative optically detected magnetic resonance ODMR spectrum from an NV center ensemble under an applied magnetic field; (e) Each pair of resonances in (d) corresponds to one of the four NV crystallographic orientations [Citation141].](/cms/asset/b5b5995b-2588-4026-873e-f9949a4570cb/tfdi_a_1877021_f0011_c.jpg)
Temperature and vibration also can be detected by NV center in diamond. The intensity and linewidth of the ZPL of NV centers are conformed to be highly depended on the environmental temperature, and the energy level shifts of NV centers in diamond follow the modified Varshni model very well. Accordingly, the NV color center shows the ability in temperature measurement with a high accuracy of up to 98% [Citation142]. Besides, the 10 nm-scale faint machinery vibration has been measured based on the spin magnetic resonant effect of NV centers, although the theoretical detection limit of mechanical vibration is as high as 5.7 nm [Citation143].
In the field of biological sciences, NV− centers can detect the magnetic field in tiny amounts of magnetic nanoparticles for future biomedical applications. The NV− quantum spin states can be optically probed to form rapidly reconstructed images of the vector components of the magnetic field created by chains of magnetic nanoparticles (magnetosomes) produced in the bacteria (refer to ) [Citation144]. Their bright luminescence, combined with their readily modifiable surface and biocompatibility, makes diamond nanoparticles containing fluorescent NV− centers, which are extremely promising for biomedical applications. Depending on the correlated magnetic plus the fluorescence, cancer biomarkers expressed by rare tumor cells in a large population of healthy cells was quantified [Citation145]. Nano-diamond biomarkers containing NV− have a number of advantages over competing luminescent probes, such as quantum dots, fluorescent proteins or organic dyes. It has extremely small size which could be implanted into tissues, no toxicity to most cell types and the wavelength range 625–800 nm which is easy for penetrating through tissues [Citation146,Citation147]. Understanding the functioning of the brain is one of the most significant challenge, it was shown that sensitivity of upcoming generation of NV magnetic sensors may not be enough for the measurement of single neuron action potential, while monitoring electromagnetic signals in brain slices or cardiac tissues seems very promising [Citation148].
Figure 12. Wide-field magnetic imaging microscope: (a) Widefield fluorescence microscope used for combined optical and magnetic imaging of live magnetotactic bacteria on the surface of a diamond chip with NV− centers; (b) Energy-level diagram of the NV centers; (c) Typical transmission electron microscope (TEM) image of an M. magneticum AMB-1 bacterium. Magnetite nanoparticles appear as spots of high electron density [Citation142].
![Figure 12. Wide-field magnetic imaging microscope: (a) Widefield fluorescence microscope used for combined optical and magnetic imaging of live magnetotactic bacteria on the surface of a diamond chip with NV− centers; (b) Energy-level diagram of the NV centers; (c) Typical transmission electron microscope (TEM) image of an M. magneticum AMB-1 bacterium. Magnetite nanoparticles appear as spots of high electron density [Citation142].](/cms/asset/8059896c-cc76-4926-a958-c63a11a8f940/tfdi_a_1877021_f0012_c.jpg)
‘Quantum diamond spectrometer’ was also obtained for performing nuclear magnetic resonance (NMR), electron spin resonance (ESR), and Electron paramagnetic resonance (EPR) microscopy [Citation149]. Macroscale and even nanoscale NMR have been a longstanding challenge, while using a combination of electron spin echoes and proton spin manipulation, the NV− center senses the nanotesla (nT) field fluctuations from the protons, enabling both time-domain and spectroscopic NMR measurements on the extremely small scale [Citation150]. A diamond magnetometer with a handheld sensing head, with a sensitivity of 7 nT/ and an ultimate noise floor of 3 nT/
has newly developed [Citation151]. NV− center in diamond also provides enhanced ability with diffraction limited spatial resolution of the target spin species over a field of view of 50 × 50 µm2 with a spin sensitivity of 104 spins per voxel or ∼100 zmol of EPR [Citation152].
Furthermore, scaling a quantum computer to the large number of qubits required to outperform classical algorithms is a grand challenge, which requires the ability to correct the inevitable errors due to the delicate, analogue nature of quantum states. The utilization of the Quantum Experience quantum computing system to simulate different scenarios involving common hybrid quantum system components, the NV center, and the flux qubit was proposed [Citation153]. By readout the NV signal using multicolor optical microscopy, one can read, write, and reset arbitrary data sets with two-dimensional (2D) binary bit density comparable to present digital-video-disk (DVD) technology for long-term data storage () [Citation154].
Figure 13. (a) Information can be stored and accessed in three dimensions, as demonstrated for the case of a three-level stack; (b) Charge-conditional initialization of the 14N nuclear spin host into mI = 0 is attained via spin transfer from the optically polarized NV− electronic spin; (c) Measured NV− ODMR spectra after the application of the pulse sequence in (b). The upper images show the NV− fluorescence in a vicinity of the probed sample spot (coincident with the image center) after charge initialization; (d) With the 14N spin in the mI = 0 state, NV− undergo a cycle of ionization and recharge [Citation154].
![Figure 13. (a) Information can be stored and accessed in three dimensions, as demonstrated for the case of a three-level stack; (b) Charge-conditional initialization of the 14N nuclear spin host into mI = 0 is attained via spin transfer from the optically polarized NV− electronic spin; (c) Measured NV− ODMR spectra after the application of the pulse sequence in (b). The upper images show the NV− fluorescence in a vicinity of the probed sample spot (coincident with the image center) after charge initialization; (d) With the 14N spin in the mI = 0 state, NV− undergo a cycle of ionization and recharge [Citation154].](/cms/asset/88338ca1-5fe0-4ec5-aeef-c0591cc4af8c/tfdi_a_1877021_f0013_c.jpg)
Applications for masers — the microwave equivalent of lasers — have been hindered by their extreme operating conditions and the inability to produce continuous emissions. A diamond maser overcomes these limitations. Using a laser to ‘pump’ the NV centers into the 0 state. The defects can relax to the −1 state, and in doing so produce the microwave radiation associated with a maser [Citation155]. Most previous maser technologies have required cryogenic refrigeration and high-vacuum systems and been restricted to niche applications. While a room-temperature maser based on NV centers in diamond, which features the longest known solid-state spin lifetime (∼5 ms) at room temperature, high optical pumping efficiency (∼106s−1) and material stability has been realized [Citation156].
Continuingly, several breakthroughs in other field also have been achieved with diamond NV center, eg the first successful “loophole-free Bell’s inequality test” [Citation157], detection of a single spin-labeled protein under ambient conditions [Citation158], the longest spin lifetime without the use of any cryogens and a 10-qubit register storing quantum information for up to 75 s [Citation159] as well as nanoscale electrometry based on magnetic-field-resistant spin sensor enabling a quantitative investigation of electric noise [Citation160]. Interestingly, different to the most utilized NV− mentioned above, the analysis of NV0 shows that the spin state can be selectively occupied optically may be read out optically, the electron spin state can be manipulated by time-varying magnetic field. Based on this NV0 is a hope for realizing qubit in diamond without the need of nitrogen donors. Therefore, it has been suggested that NV0 may be more sensitive magnetometer than the ultrasensitive NV− [Citation161].
Nitrogen-related defects in diamond also can be the clue of formation of diamond crystal [Citation162]. The very high sensitivity of nitrogen aggregations in diamond to temperature and relatively weak dependence on residence time makes an aggregation transition (nitrogen A- to B-center) a useful geo-thermometer [Citation10]. Radiogenic dating of inclusions trapped during growth can give a reliable measure of the age of natural diamond stones, therefore, the aggregation state can be employed to detect the mantle temperature experienced by the diamond [Citation163]. Meanwhile, isotopic compositions, ie δ13C and δ15N, values in diamond have been introduced to build a reappearance of natural diamond formation [Citation164]. Aside from the dislocation types and Si–V which gemologist generally concerned, the nitrogen related state is also a representative symbol for distinguishing artificial stones from their natural counterparts.
In addition, stable H3, NV, and NE4 color centers in natural and synthetic diamonds exhibit broad vibrionic emission bands, so the use of these centers in solid state tunable and femtosecond lasers in the visible and NIR spectral range seems to have great potential. The first successful generation of stimulated emission was achieved on H3 color centers (at 530 nm) in natural diamonds [Citation12], however, that research has not continued due to low laser efficiency (lower than 0.1%). The laser efficiency can be greatly increased by deliberately creating those optical centers [Citation12,Citation165]. Ion bombardment and N2 beam are two methods to bring in H3 centers to diamond, and these deliberately created optical centers have high values of stimulated emission gain factors in broad spectral bands, which is necessary for generating femtosecond pulses and makes them promising for various laser systems, including femtosecond and tunable VIS-NIR lasers.
6. Conclusion and outlook
Diamond, a symbol of luxury, was generally considered as a gemstone with various fancy colors endowed by impurities. But more than that, diamond with nitrogen has offered great potential for applications in advanced electronics, photonics, and biomedical fields.
Nitrogen impurity is ubiquitously contained in natural and synthetic diamond. The concentration and form state of nitrogen differ greatly depending on situations during the growth of diamond, and the growing conditions of natural diamond make it more stable in aggregation. In turn nitrogen presence would make a change of diamond growth. It also can be combined easily with other impurities such as hydrogen and vacancies to form various kinds of color centers. These nitrogen-related centers not only give rise to the coloration of diamond by affecting the absorption spectrum, but also represent many special physical properties.
With the advent of upgraded synthetic methods (HPHT and, particularly, CVD), diamond grown with controllable nitrogen impurity content under well-defined and well-controlled conditions is no longer difficult. By thorough studies on the relationship between the structure of nitrogen-related defects and absorption features, the coloration of diamond and many related properties can be controlled or even modified by many selectable approaches with multi-purpose, eg post annealing or external electric field applying. Therefore, such versatile modification of diamond properties offers diamond great potential for many advanced technological applications. In addition to the nitrogen defects with specific state which act as clue of synthesis conditions for tracing the origin of gemstone diamonds and their classification, the NV centers endows amazing properties such as tunable stimulated emission, continuous and stable spin can greatly expand quantum applications of diamond and make it a potential platform for the micro or nano-electric, photoelectric and photonic devices. However, NV center still need to be further improved as it is not mature enough and the performance sometimes is not competent as expected. With the further understanding of nitrogen in diamond, practical application of diamond-based quantum devices is believed not far away.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Yuting Zheng
Dr. Yuting Zheng, Postdoctoral Researcher in Materials Science and Engineering (MSE) at University of Science and Technology Beijing (USTB); Joint-training PhD in MSE at USTB and University of Leicester, UK.
Chengming Li
Prof. Chengming Li, Chair of Laboratory of Carbon-based Materials and Functional Films at USTB; Standing director of China Heat Treatment Society; Director of Pan-Pacific International R & D and Industry Alliance of Single Crystal Diamond and its Electronic Devices; Member of National Technical Committee for Standardization of abrasive and abrasive tools, etc.
Jinlong Liu
Prof. Jinlong Liu, Associate Professor in MSE at University of Science and Technology Beijing (USTB).
Junjun Wei
Prof. Junjun Wei, Associate Professor in MSE at University of Science and Technology Beijing (USTB).
Haitao Ye
Prof. Haitao Ye, Chair in Materials Engineering at School of Engineering, University of Leicester, UK. PhD in Electronic Engineering at University College London, UK; MEng in Materials Science ar Nanyang Tech. Uni., Singapore.
References
- Guyer RL, Koshland DE Jr. Diamond: glittering prize for materials science. Science. 1990; 250(4988): 1640–1643.
- Breeding CM. Colored diamonds: the rarity and beauty of imperfection. Gems Gemology. 2018; 54(3): 274–277.
- Renfro N, Koivula JI, Wang WY, et al. Synthetic gem materials in the 2000s: a decade in review. Gems Gemology. 2010; 46(4): 260–274.
- Butler JE, Woodin RL, Brown LM, et al. Thin film diamond growth mechanisms. Philos Trans R Soc Lond. 1993; 342: 15–30.
- Wort CJH, Balmer RS. Diamond as an electronic material. Mater Today. 2008; 11(1-2): 22–28.
- Baker JM. Deducing atomic models for point defects in diamond: the relevance of their mechanism of formation. Diam Relat Mater. 2007; 16(2): 216–219.
- Breeding CM, Shigley JE. The “TYPE” classification system of diamonds and its importance in gemology. Gems Gemology. 2009; 45(2): 96–111.
- Field JE. The mechanical and strength properties of diamond. Rep Prog Phys. 2012; 75(12): 126505.
- Nebel CE. Nitrogen-vacancy doped CVD diamond for quantum applications: a review. Semicond Semimet. 2020; 103: 14–18.
- Ashfold MNR, Goss JP, Green BL, et al. Nitrogen in diamond. Chem Rev. 2020; 120(12): 5745–5794.
- Khomich AV, Ralchenko VG, Vlasov AV, et al. Effect of high temperature annealing on optical and thermal properties of CVD diamond. Diam Relat Mater. 2001; 10(3-7): 546–551.
- Vins VG, Pestryakov EV. Color centers in diamond crystals: their potential use in tunable and femtosecond lasers. Diam Relat Mater. 2006; 15(4-8): 569–571.
- Aharonovich I, Greentree AD, Prawer S. Diamond photonics. Nature Photon. 2011; 5(7): 397–405.
- Markham M, Twitchen D. The diamond quantum revolution. Phys World. 2020; 33(4): 39–43.
- Atatüre M, Englund D, Vamivakas N, et al. Material platforms for spin-based photonic quantum technologies. Nat Rev Mater. 2018; 3(5): 38–51.
- Rosskopf T, Dussaux A, Ohashi K, et al. Investigation of surface magnetic noise by shallow spins in diamond. Phys Rev Lett. 2014; 112(14): 147602.
- Dunst S, Sternschulte H, Schreck M. Growth rate enhancement by nitrogen in diamond chemical vapor deposition—a catalytic effect. Appl Phys Lett. 2009; 94(22): 224101.
- Fedortchouk Y. A new approach to understanding diamond surface features based on a review of experimental and natural diamond studies. Earth Sci Rev. 2019; 193: 45–65.
- Cohen H, Ruthstein S. Evaluating the color and nature of diamonds via EPR spectroscopy. Gems Gemology. 2018; 54(3): 276.
- Willems B, Tallaire A, Achard J. Optical study of defects in thick undoped CVD synthetic diamond layers. Diam Relat Mater. 2014; 41: 25–33.
- Weerdt FD, Royen JV. Defects in coloured natural diamonds. Diam Relat Mater. 2001; 10: 474–479.
- Magaña SE, McElhenny G, Breeding CM, et al. Comparison of gemological and spectroscopic features in type IIa and Ia natural pink diamonds. Diam Relat Mater. 2020; 105: 107784.
- Magaña SE, Ardon T, Zaitsev AM. LPHT annealing of brown-to-yellow type Ia diamonds. Diam Relat Mater. 2017; 77: 159–170.
- https: //www.gia.edu/gia-news-research-sothebys-diamond-auction-2013-shor.
- Simakov SK. On the origin of large type IIa gem diamonds. Ore Geol Rev. 2018; 102: 195–203.
- Melton GL, McNeill J, Stachel T, et al. Trace elements in gem diamond from Akwatia, Ghana and DeBeers Pool, South Africa. Chem Geol. 2012; 314-317: 1–8.
- Collins AT. Things we still don’t know about optical centres in diamond. Diam Relat Mater. 1999; 8(8-9): 1455–1462.
- Chrenko RM, Tuft RE, Strong HM. Transformation of the state of nitrogen in diamond. Nature. 1977; 270(5633): 141–144.
- Yin LW, Li MS, Cui JJ, et al. Planar defects and dislocations in HPHT as-grown diamond crystals. Diam Relat Mater. 2002; 11(2): 268–272.
- Schwander M, Partes K. A review of diamond synthesis by CVD processes. Diam Relat Mater. 2011; 20(9): 1287–1301.
- Lin LTS, Popovici G, Mori Y, et al. Study of color centers in hot-filament CVD diamond films by cathodoluminescence and photoluminescence and their correlations with film quality. Diam Relat Mater. 1996; 5(11): 1236–1245.
- Palyanov YN, Borzdov YM, Khokhryakov AF, et al. Effect of nitrogen impurity on diamond crystal growth processes. Cryst Growth Des. 2010; 10(7): 3169–3175.
- Liu T, Raabe D. Influence of nitrogen doping on growth rate and texture evolution of chemical vapor deposition diamond films. Appl Phys Lett. 2009; 94(2): 021119.
- Locher R, Wild C, Herres N, et al. Nitrogen stabilized 100 texture in chemical vapor deposited diamond films. Appl Phys Lett. 1994; 65(1): 34–36.
- Achard J, Silva F, Brinza O, et al. Coupled effect of nitrogen addition and surface temperature on the morphology and the kinetics of thick CVD diamond single crystals. Diam Relat Mater. 2007; 16(4-7): 685–689.
- Chayahara A, Mokuno Y, Horino Y, et al. The effect of nitrogen addition during high rate homoepitaxial growth of diamond by microwave plasma CVD. Diam Relat Mater. 2004; 13(11-12): 1954–1958.
- Müller‐Sebert W, Wörner E, Fuchs F, et al. Nitrogen induced increase of growth rate in chemical vapor deposition of diamond. Appl Phys Lett. 1996; 68(6): 759–760.
- Hainschwang T. Gemstone analysis by spectroscopy. In: Encyclopedia of spectroscopy and spectrometry . 3rd ed. Netherlands: Elsevier; 2017. p. 18–24.
- Zaitsev AM. Optical properties of diamond: a data handbook. SpringerBerlin Heidelberg; 2010.
- Burns RC, Cvetkovic V, Dodge CN, et al. Growth-sector dependence of optical features in large synthetic diamonds. J Cryst Growth. 1990; 104(2): 257–279.
- Samlenski R, Haug C, Brenn R, et al. Incorporation of nitrogen in chemical vapor deposition diamond. Appl Phys Lett. 1995; 67(19): 2798–2800.
- Liu X, Chen X, Singh DJ, et al. Boron–oxygen complex yields n-type surface layer in semiconducting diamond. Proc Natl Acad Sci USA. 2019; 116(16): 7703–7711.
- Ulbricht R, van der Post ST, Goss JP, et al. Single substitutional nitrogen defects revealed as electron acceptor states in diamond using ultrafast spectroscopy. Phys Rev B. 2011; 84(16): 165202.
- Shao T, Lyu F, Guo X, et al. The role of isolated nitrogen in phosphorescence of high-temperaturehigh-pressure synthetic type IIb diamonds. Carbon. 2020; 167: 888–895.
- Lawson SC, Fisher D, Hunt DC, et al. On the existence of positively charged single-substitutional nitrogen in diamond. J Phys Condens Matter. 1998; 10(27): 6171–6180.
- Kiflawi I, Mainwood A, Kanda H, et al. Nitrogen interstitials in diamond. Phys Rev B. 1996; 54(23): 16719–16726.
- Liggins S, Newton ME, Goss JP, et al. Identification of the dinitrogen 〈001〉 split interstitial H1a in diamond. Phys Rev B. 2010; 81(8): 085214.
- Goss JP, Coomer BJ, Jones R, et al. Extended defects in diamond: the interstitial platelet. Phys Rev B. 2003; 67(16): 165208.
- Lühmann T, Raatz N, John R, et al. Screening and engineering of colour centres in diamond. J Phys D Appl Phys. 2018; 51(48): 483002.
- Budker D. The sense of colour centres. Nature Phys. 2011; 7(6): 453–454.
- Wyk van JA, Woods GS. Electron spin resonance of excited states of the H3 and H4 centres in irradiated type Ia diamonds. J Phys Condens Matter. 1995; 7(29): 5901–5911.
- Davies G, Lawson SC, Collins AT, et al. Vacancy-related centers in diamond. Phys Rev B. 1992; 46(20): 13157–13170.
- Zaitsev AM. Vibronic spectra of impurity-related optical centers in diamond. Phys Rev B. 2000; 61(19): 12909–12922.
- Doherty MW, Manson NB, Delaney P, et al. The nitrogen-vacancy colour centre in diamond. Phys Rep. 2013; 528(1): 1–45.
- Acosta V, Hemmer P. Nitrogen-vacancy centers: physics and applications. MRS Bull. 2013; 38(2): 127–130.
- Felton S, Edmonds AM, Newton ME, et al. Electron paramagnetic resonance studies of the neutral nitrogen vacancy in diamond. Phys Rev B. 2008; 77(8): 081201.
- Barson MSJ, Krausz E, Manson NB, et al. The fine structure of the neutral nitrogen-vacancy center in diamond. Nanophotonics. 2019; 8(11): 1985–1991.
- Goldman ML, Sipahigil A, Doherty MW, et al. Phonon-induced population dynamics and intersystem crossing in nitrogen-vacancy centers. Phys Rev Lett. 2015; 114(14): 145502.
- Fu KMC, Santori C, Barclay PE, et al. Observation of the dynamic Jahn-Teller effect in the excited states of nitrogen-vacancy centers in diamond. Phys Rev Lett. 2009; 103(25): 256404.
- Balasubramanian G, Neumann P, Twitchen D, et al. Ultralong spin coherence time in isotopically engineered diamond. Nature Mater. 2009; 8(5): 383–387.
- Goss JP, Briddon PR, Jones R, et al. Donor and acceptor states in diamond. Diam Relat Mater. 2004; 13(4-8): 684–690.
- Teraji T, Yamamoto T, Watanabe K, et al. Homoepitaxial diamond film growth: high purity, high crystalline quality, isotopic enrichment, and single color center formation. Phys Status Solidi A. 2015; 212(11): 2365–2384.
- Mittiga T, Hsieh S, Zu C, et al. Imaging the local charge environment of nitrogen-vacancy centers in diamond. Phys Rev Lett. 2018; 121(24): 246402.
- Goss JP, Ewels CP, Briddon PR, et al. Bistable N2–H complexes: the first proposed structure of a H-related colour-causing defect in diamond. Diam Relat Mater. 2011; 20(7): 896–901.
- Miyazaki T, Okushi H, Uda T. Shallow donor state due to nitrogen-hydrogen complex in diamond. Phys Rev Lett. 2002; 88(6): 066402.
- Goss JP, Briddon PR, Papagiannidis S, et al. Interstitial nitrogen and its complexes in diamond. Phys Rev B. 2004; 70(23): 235208.
- Lozovoi A, Daw D, Jayakumar H, et al. Dark defect charge dynamics in bulk chemical-vapor-deposition-grown diamonds probed via nitrogen vacancy centers. Phys Rev Mater. 2020; 4(5): 053602.
- Hainschwang T, Fritsch E, Notari F, et al. The origin of color in natural C center bearing diamonds. Diam Relat Mater. 2013; 39: 27–40.
- Stacey A, O’Donnell KM, Chou J-P, et al. Nitrogen terminated diamond. Adv Mater Interfaces. 2015; 2(10): 1500079.
- Chou JP, Retzker A, Gali A. Nitrogen-terminated diamond (111) surface for room-temperature quantum sensing and simulation. Nano Lett. 2017; 17(4): 2294–2298.
- Kawai S, Yamano H, Sonoda T, et al. Nitrogen-terminated diamond surface for nanoscale NMR by shallow nitrogen-vacancy centers. J Phys Chem C. 2019; 123(6): 3594–3604.
- Chandran M, Shasha M, Michaelson S, et al. Nitrogen termination of single crystal (100) diamond surface by radio frequency N2 plasma process: An in-situ x-ray photoemission spectroscopy and secondary electron emission studies. Appl Phys Lett. 2015; 107(11): 111602.
- Botsoa J, Sauvage T, Adam MP, et al. Optimal conditions for NV− center formation in type-Ib diamond studied using photoluminescence and positron annihilation spectroscopies. Phys Rev B. 2011; 84(12): 125209.
- Fritsch E, Rondeau B, Hainschwang T, et al. A contribution to the understanding of pink color in diamond: the unique, historical «Grand Condé. Diam Relat Mater. 2007; 16(8): 1471–1474.
- Humble P, Hannink RHJ. Plastic deformation of diamond at room temperature. Nature. 1978; 273(5657): 37–40.
- Pantea C, Gubicza J, UngáR T, et al. High-pressure effect on dislocation density in nanosize diamond crystals. Diam Relat Mater. 2004; 13(10): 1753–1756.
- Jones R. Dislocations, vacancies and the brown colour of CVD and natural diamond. Diam Relat Mater. 2009; 18(5-8): 820–826.
- Fujita N, Jones R, Öberg S, et al. Large spherical vacancy clusters in diamond – origin of the brown colouration? Diam Relat Mater. 2009; 18(5-8): 843–845.
- Fujita N, Jones R, Öberg S, et al. Theoretical investigation on the interaction of nitrogen with dislocations in single crystal CVD diamond. Diam Relat Mater. 2008; 17(2): 123–126.
- Hounsome LS, Jones R, Martineau PM, et al. Origin of brown coloration in diamond. Phys Rev B. 2006; 73(12): 125203.
- Zaitsev AM, Kazuchits NM, Kazuchits VN, et al. Nitrogen-doped CVD diamond: nitrogen concentration, color and internal stress. Diam Relat Mater. 2020; 105: 107794.
- Magaña SE, Ardon T, Smit KV, et al. Natural-color pink, purple, red, and brown dimonds: band of many colors. Gems Gemology. 2018; 54: 352–377.
- Wang WY, Moe KS. CVD Synthetic diamond with fancy vivid orange color. Gems Gemology. 2014; 50(4): 299.
- Breeding CM, Eaton-Magaña S, Shigley JE. Natural-color green diamonds: a beautiful conundrum. Gems Gemology. 2018; 54(1): 2–27.
- Gu TT, Wang WY. Optical defects in milky type IaB diamonds. Diam Relat Mater. 2018; 89: 322–329.
- Collins AT. The detection of colour-enhanced and synthetic gem diamonds by optical spectroscopy. Diam Relat Mater. 2003; 12(10-11): 1976–1983.
- Fritsch E, Shigley JE, Moses T, et al. A Green diamond a study of chameleonism. Leeds, England: Maney and Sons; 1995.
- Hainschwang T, Notari F, Fritsch E, et al. Natural, untreated diamonds showing the A, B and C infrared absorptions (“ABC diamonds”), and the H2 absorption. Diam Relat Mater. 2006; 15(10): 1555–1564.
- https: //www.gia.edu/diamond-quality-factor.
- https: //shopidc.com/pages/education.
- Byrne KS, Butler JE, Wang WY, et al. Chameleon diamonds: thermal processes governing luminescence and a model for the color change. Diam Relat Mater. 2018; 81: 45–53.
- Yamamoto T, Umeda T, Watanabe K, et al. Extending spin coherence times of diamond qubits by high-temperature annealing. Phys Rev B. 2013; 88(7): 075206.
- Kalish R. Ion implantation in diamond for quantum information processing (QIP): doping and damaging. Netherlands: Elsevier; 2014.
- Chakravarthi S, Moore C, Opsvig A, et al. Window into NV center kinetics via repeated annealing and spatial tracking of thousands of individual NV centers. Phys Rev Mater. 2020; 4(2): 023402.
- Zaitsev AM, Moe KS, Wang W. Defect transformations in nitrogen-doped CVD diamond during irradiation and annealing. Diam Relat Mater. 2018; 88: 237–255.
- Hood RQ, Kent PRC, Needs RJ, et al. Quantum Monte Carlo study of the optical and diffusive properties of the vacancy defect in diamond. Phys Rev Lett. 2003; 91(7): 076403.
- Kalish R, Reznik A, Prawer S, et al. Ion‐implantation‐induced defects in diamond and their annealing: experiment and simulation. Phys Stat Sol (a). 1999; 174(1): 83–99.
- Preciado‐Flores S, Meléndrez R, Chernov V, et al. Thermal annealing effects on the TL response of beta‐irradiated HPHT Ib type synthetic diamond. Phys Stat Sol (a). 2007; 204(9): 3041–3046.
- Huang GF, Jia XP, Yin JW, et al. Preparation of IaA-type diamond crystals containing a high concentration of nitrogen by annealing {111}-oriented N-doped diamond crystals. Int J Refract Met Hard Mater. 2013; 41: 517–521.
- Rueda FA, Gordillo N, Ynsa MD, et al. Lattice damage in 9-MeV-carbon irradiated diamond and its recovery after annealing. Carbon. 2017; 123: 334–343.
- Charles SJ, Butler JE, Feygelson BN, et al. Characterization of nitrogen doped chemical vapor deposited single crystal diamond before and after high pressure, high temperature annealing. Phys Stat Sol (a). 2004; 201(11): 2473–2485.
- Mainwood A. Nitrogen and nitrogen-vacancy complexes and their formation in diamond. Phys Rev B. 1994; 49(12): 7934–7940.
- Lombardi EB, Mainwood A, Osuch K, et al. Computational models of the single substitutional nitrogen atom in diamond. J Phys Condens Matter. 2003; 15(19): 3135–3149.
- Avalos V, Dannefaer S. Vacancy-type defects in brown diamonds investigated by positron annihilation. Phys B Condens Matter. 2003; 340-342: 76–79.
- Deák P, Aradi B, Kaviani M, et al. Formation of NV centers in diamond: a theoretical study based on calculated transitions and migration of nitrogen and vacancy related defects. Phys Rev B. 2014; 89(7): 075203.
- Kanda H, Watanabe K. Change of cathodoluminescence spectra of natural diamond with HPHT treatment. Diam Relat Mater. 2004; 13(4-8): 904–908.
- Dobrinets IA, Vins VG, Zaitsev AM. HPHT-treated diamonds: diamonds forever. Heidelberg, New York, Dordrecht, London: Springer; 2013.
- Fisher D. Brown diamonds and high-pressure high temperature treatment. Lithos. 2009; 112: 619–624.
- Collins AT, Kanda H, Kitawaki H. Colour changes produced in natural brown diamonds by high-pressure, high-temperature treatment. Diam Relat Mater. 2000; 9(2): 113–122.
- Lai MY, Breeding CM, Stachel T, et al. Spectroscopic features of natural and HPHT-treated yellow diamonds. Diam Relat Mater. 2020; 101: 107642.
- Kanda H, Ahmadjan A, Kitawaki H. Change in cathodoluminescence spectra and images of type II high-pressure synthetic diamond produced with high pressure and temperature treatment. Diam Relat Mater. 2005; 14(11-12): 1928–1931.
- Kupriyanov IN, Palyanov YN, Kalinin AA, et al. The effect of HPHT treatment on the spectroscopic features of type IIb synthetic diamonds. Diam Relat Mater. 2008; 17(7-10): 1203–1206.
- Vins VG, Yelisseyev AP, Lobanov SS, et al. APHT treatment of brown type Ia natural diamonds: dislocation movement or vacancy cluster destruction?Diam Relat Mater. 2010; 19(7-9): 829–832.
- Meng Y-f, Yan C-s, Lai J, et al. Enhanced optical properties of chemical vapor deposited single crystal diamond by low-pressure/high-temperature annealing. PNAS. 2008; 105(46): 17620–17625.,
- Tallaire A, Barjon J, Brinza O, et al. Dislocations and impurities introduced from etch-pits at the epitaxial growth resumption of diamond. Diam Relat Mater. 2011; 20(7): 875–881.
- López-Santos C, Yubero F, Cotrino J, et al. Lateral and in-depth distribution of functional groups on diamond-like carbon after oxygen plasma treatments. Diam Relat Mater. 2011; 20(2): 49–56.
- Kazuchits NM, Rusetsky MS, Kazuchits VN, et al. Aggregation of nitrogen in synthetic diamonds annealed at high temperature without stabilizing pressure. Diam Relat Mater. 2016; 64: 202–207.
- Kazuchits NM, Rusetsky MS, Kazuchits VN, et al. Cathodoluminescence of synthetic diamonds annealed at high temperature without stabilizing pressure. Diam Relat Mater. 2017; 74: 41–44.
- Kazuchits NM, Rusetsky MS, Kazuchits VN, et al. Comparison of HPHT and LPHT annealing of Ib synthetic diamond. Diam Relat Mater. 2019; 91: 156–164.
- Liu S, Liu JL, Li CM, et al. The mechanical enhancement of chemical vapor deposited diamond film by plasma low-pressure/high-temperature treatment. Carbon. 2013; 65: 365–370.
- Riedel M, Ristein J, Ley L. Recovery of surface conductivity of H-terminated diamond after thermal annealing in vacuum. Phys Rev B. 2004; 69(12): 125338.
- Acosta VM, Bauch E, Ledbetter MP, et al. Diamonds with a high density of nitrogen-vacancy centers for magnetometry applications. Phys Rev B. 2009; 80(11): 115202.
- Tang Z, Chiba T, Nagai Y, et al. Positron annihilation study for enhanced nitrogen-vacancy center formation in diamond by electron irradiation at 77 K. Appl Phys Lett. 2014; 104(17): 172101.
- Huang Z, Li WD, Santori C, et al. Diamond nitrogen-vacancy centers created by scanning focused helium ion beam and annealing. Appl Phys Lett. 2013; 103(8): 081906.
- Newton M, Campbell B, Twitchen D, et al. Recombination enhanced diffusion of self-interstitial atoms and vacancy interstitial recombination in diamond. Diamond Related Materials. 2002; 11(3-6): 618–622.
- Capelli M, Heffernan AH, Ohshima T, et al. Increased nitrogen-vacancy centre creation yield in diamond through electron beam irradiation at high temperature. Carbon. 2019; 143: 714–719.
- Zaitsev AM, Moe KS, Wang W. Optical centers and their depth distribution in electron irradiated CVD diamond. Diamond Related Materials. 2017; 71: 38–52.
- Hainschwang T, Respinger A, Notari F, et al. A comparison of diamonds irradiated by high fluence neutrons or electrons, before and after annealing. Diamond Related Materials. 2009; 18(10): 1223–1234.
- Osterkamp C, Scharpf J, Pezzagna S, et al. Increasing the creation yield of shallow single defects in diamond by surface plasma treatment. Appl Phys Lett. 2013; 103(19): 193118.
- Shanley TW, Martin AA, Aharonovich I, et al. Localized chemical switching of the charge state of nitrogen-vacancy luminescence centers in diamond. Appl Phys Lett. 2014; 105(6): 063103.
- Chen XD, Zou CL, Sun FW, et al. Optical manipulation of the charge state of nitrogen-vacancy center in diamond. Appl Phys Lett. 2013; 103(1): 013112.
- Bhaumik A, Sachan R, Narayan J. Tunable charge states of nitrogen-vacancy centers in diamond for ultrafast quantum devices. Carbon. 2019; 142: 662–672.
- Shimizu M, Makino T, Iwasaki T, et al. Charge state modulation of nitrogen vacancy centers in diamond by applying a forward voltage across a p–i–n junction. Diamond Related Materials. 2016; 63: 192–196.
- Murai T, Makino T, Kato H, et al. Engineering of Fermi level by nin diamond junction for control of charge states of NV centers featured. Appl Phys Lett. 2018; 112(11): 111903.
- Prawer S, Greentree AD. Diamond for quantum computing. Science. 2008; 320(5883): 1601–1603.
- Dolde F, Doherty MW, Michl J, et al. Nanoscale detection of a single fundamental charge in ambient conditions using the NV− center in diamond. Phys Rev Lett. 2014; 112(9): 097603.
- Robledo L, Childress L, Bernien H, et al. High-fidelity projective read-out of a solid-state spin quantum register. Nature. 2011; 477(7366): 574–578.
- Bucher DB, Craik D, Backlund MP, et al. Quantum diamond spectrometer for nanoscale NMR and ESR spectroscopy. Nat Protoc. 2019; 14(9): 2707–2747.
- Labanowski D, Bhallamudi VP, Guo Q, et al. Voltage-driven, local, and efficient excitation of nitrogen-vacancy centers in diamond. Sci Adv. 2018; 4(9): eaat6574.
- Lesik M, Plisson T, Toraille L, et al. Magnetic measurements on micrometer-sized samples under high pressure using designed NV centers. Science. 2019; 366(6471): 1359–1362.
- Hsieh S, Bhattacharyya P, Zu C, et al. Imaging stress and magnetism at high pressures using a nanoscale quantum sensor. Science. 2019; 366(6471): 1349–1354.
- Yang M, Yuan Q, Gao J, et al. A Diamond Temperature Sensor Based on the Energy Level Shift of Nitrogen-Vacancy Color Centers. Nanomaterials. 2019; 9(11): 1576.
- Liu Y, Guo H, Zhang W, et al. Nanoscale detection of faint machinery vibration using the NV center in diamond. Phys Lett A. 2020; 384(32): 126832.
- Sage DL, Arai K, Glenn DR, et al. Optical magnetic imaging of living cells. Nature. 2013; 496(7446): 486–489.
- Glenn DR, Lee K, Park H, et al. Single-cell magnetic imaging using a quantum diamond microscope. Nat Methods. 2015; 12(8): 736–738.
- Hui YY, Hsiao WW, Haziza S, et al. Single Particle Tracking of Fluorescent Nanodiamonds in Cells and Organisms. Current Opinion in Solid State Materials Science. 2017; 21(1): 35–42.
- Ho D, Wang CHK, Chow EKH. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci Adv. 2015; 1(7): e1500439.
- Moreva E. The biosensing with NV centers in diamond: Related challenges. Int J Quantum Inform. 2020; 18(01): 1941023.
- Meijer J, Burchard B, Domhan M, et al. Generation of single-color centers by focused nitrogen implantation. Appl Phys Lett. 2005; 87(26): 261909.
- Mamin HJ, Kim M, Sherwood MH, et al. Nanoscale Nuclear Magnetic Resonance with a Nitrogen-Vacancy Spin Sensor. Science. 2013; 339(6119): 557–560.
- Webb JL, Clemen JD, Troise L, et al. Nanotesla sensitivity magnetic field sensing using a compact diamond nitrogen-vacancy magnetometer. Appl Phys Lett. 2019; 114(23): 231103.
- Simpson DA, Ryan RG, Hall LT, et al. Electron paramagnetic resonance microscopy using spins in diamond under ambient conditions. Nat Commun. 2017; 8(1): 458.
- Mazhandu F, Mathieson K, Coleman C, et al. Experimental simulation of hybrid quantum systems and entanglement on a quantum computer. Appl Phys Lett. 2019; 115(23): 233501.
- Dhomkar S, Henshaw J, Jayakumar H, et al. Long-term data storage in diamond. Sci Adv. 2016; 2(10): e1600911.
- Liu RB. A diamond age of masers. Nature. 2018; 555(7697): 447–449.
- Jin L, Pfender M, Aslam N, et al. Proposal for a room-temperature diamond maser. Nat Commun. 2015; 6(1): 8251.
- Hensen B, Bernien H, DréAu AE, et al. Loophole-free Bell inequality violation using electron spins separated by 1.3 kilometres. Nature. 2015; 526(7575): 682–686.
- Shi FZ, Zhang Q, Wang PF, et al. Single-protein spin resonance spectroscopy under ambient conditions. Science. 2015; 347(6226): 1135–1138.
- Bradley CE, Randall J, Abobeih MH, et al. A Ten-Qubit Solid-State Spin Register with Quantum Memory up to One Minute. Phys Rev X. 2019; 9(3): 031045.
- Li R, Kong F, Zhao PJ, et al. Nanoscale electrometry based on a magnetic-field-resistant spin sensor. Phys Rev Lett. 2020; 124(24): 247701.
- Gali A. Theory of the neutral nitrogen-vacancy center in diamond and its application to the realization of a qubit. Phys Rev B. 2009; 79(23): 235210.
- Stachel T, Harris JW. The origin of cratonic diamonds - constraints from mineral inclusions. Ore Geol Rev. 2008; 34(1-2): 5–32. −
- Taylor WR, Jaques AL, Ridd M. Nitrogen-defect aggregation characteristics of some australasian diamonds time-temperature constraints on the source regions of pipe and alluvial diamonds. Am Mineral. 1990; 75: 1290−1310.
- Cartigny P, Palot M, Thomassot E, et al. Diamond formation: a stable isotope perspective. Annu Rev Earth Planet Sci. 2014; 42(1): 699–732.
- Waldermann FC, Olivero P, Nunn J, et al. Creating diamond color centers for quantum optical applications. Diamond Related Materials. 2007; 16(11): 1887–1895.