ABSTRACT
Biochar as a biomass derived, low cost, carbon conductive material is considered as an important supplement in the anaerobic digestion (AD) of organic matter. It functions as an electrical grid to allow direct electron transfer from fatty acid oxidizers to methanogenic archaea, thereby promoting syntophy between various microbial groups and leading to efficient methanogenesis. Specific properties of biochar play an important role in promoting syntrophic interactions in AD. As a physical indicator, surface area, porosity, particle size and surface texture of biochar play an important role in governing microbial attachment and enrichment on biochar. This influences the microbial degradation of fatty acids and their subsequent conversion to methane by methanogens. Chemical properties such as the presence of hydrophobic functional groups, molecular nature and redox active groups on biochar surface promote interaction between biochar and microorganisms and provide an increased degree of electron transfer between the attached microorganisms. The above characteristics depend on feedstock and pyrolysis conditions used for biochar production. Unlike previous reviews, herein the desired physical and chemical properties of biochar that promote syntrophy in AD and the factors that influence them have been discussed in detail. Furthermore, engineering of biochar properties by various activation methods to harness favourable characteristics of biochar as an effective AD additive is described. Such a comprehensive account would be useful for engineering efficient biochar-mediated digestions with enhanced syntrophy and overall AD performance.
PUBLIC INTEREST STATEMENT
Anaerobic digestion (AD) involves the treatment of organic waste and its conversion to biomethane. Diverse microbial communities degrade the organic matter into intermediate compounds including hydrogen, formate, and volatile fatty acids that become the substrates for methanogens in AD. This assimilation of intermediates to methane depends on syntrophic interactions between hydrolytic bacteria and acetogenic bacteria as well as between fatty acid oxidizers and methanogenic archaea that occurs by interspecies electron transfer. Biochar, a carbonaceous material obtained from biomass, can promote such syntrophic metabolism by enabling long-range electron exchange between electron donating and electron accepting microbial groups. Specific biochar properties such as alkalinity, microstructure, molecular nature and surface chemistry play an important role to facilitate syntrophy in AD. Such desired physical and chemical properties of biochar that promote syntrophy in AD and the factors that influence them have been discussed in detail in this review. Such a comprehensive account would be useful for engineering efficient biochar-mediated digesters with enhanced syntrophy and overall AD performance.
1. Introduction
Anaerobic digestion (AD) is an important biotechnological process which allows the treatment of organic wastes and production of biomethane. Mechanistically, during AD, the organic matter undergoes fermentation degradation using protons and HCO3- as electron acceptors due to the absence of oxygen. Primary fermentation bacteria convert the complex organic matter into simple fermentation products such as volatile fatty acids (VFA), ethanol, etc. which are then used as substrates by proton-reducing acetogenic bacteria during the conversion to methanogenic substrates such as acetate, CO2 and H2, at low hydrogen partial pressures. Finally, the methanogenic archaea convert acetate, CO2 and H2 to methane (de BOK et al., Citation2004). The degradation of VFAs produced as AD intermediates is an endothermic process and cannot be carried out spontaneously. On the other hand, as the precursors of methanogenesis, these can thermodynamically stimulate methane production more efficiently. This type of symbiosis between the hydrolytic bacteria and acetogenic bacteria as well as acetogenic bacteria and methanogenic archaea is called as syntrophy and these microbial groups are regarded as syntrophic microbes, whose interactions make material conversions in AD proceed efficiently. For instance, acetate can be produced from butyrate oxidation intermediate via syntrophic bacteria under-elevated H2 partial pressure in order to avoid inhibition of accumulated butyrate (JUNICKE et al., Citation2016). In addition, homoacetogens, as syntrophic partners of acetogens and fermenting bacteria also play a vital role in the production of acetate through reduction of CO2 by H2 (LIU et al., Citation2021; SIRIWONGRUNGSON et al., Citation2007). Therefore, a syntrophic metabolism between microbial communities is a critical need in AD for organic substrate degradation to methane.
Microbial syntrophic interaction in AD is associated with interspecies electron transfer (IET) which is an important means by which syntrophic microbes can break thermodynamic constraints to maintain growth and perform organic matter degradation and methanogenesis efficiently. It includes three modes depending on the mechanism used for electron transfer and energy exchange between syntrophic microbes, i.e. (i) mediated by H2 (called as interspecies hydrogen transfer (IHT)), (ii) or formate (called as interspecies formate transfer (IFT)), and (iii) direct interspecies electron transfer (DIET) (ZHAO et al., Citation2020). H2 is an important substrate for hydrogenotrophic methanogenesis and in IHT, H2 is used as a carrier for the transfer of electrons from VFA, ethanol to CO2. H2 is produced upon the degradation of propionate and butyrate; however, their degradation is thermodynamically feasible only when the H2 partial pressure is low. This critical requirement of low H2 partial pressure can only be met by effective consumption of H2 by the hydrogenotrophic methanogens. This implies that once the utilization of H2 is slow due to the environmental conditions, the consumption and degradation of propionate and butyrate by acetogens would also be slow, thereby leading to the accumulation of these acids which eventually causes digestion failure. Similar constraint exists for formate and therefore any impediment in the consumption of these mediators (H2 and formate) causes an accumulation of VFA which in turn obstructs the syntrophic process due to the inhibitory effect of VFA on microbial cells (NOZHEVNIKOVA et al., Citation2020).
DIET involving an electrical contact between microbial groups has been identified as an alternative mechanism to solve this issue with IHT and IFT. It involves the direct transfer of electrons from VFA oxidizing bacteria to methanogens, thereby reducing CO2 to CH4 with lower energy. Unlike conventional IET which depends on the diffusion of mediator molecules to transfer electrons, DIET is independent of this requirement and instead is mediated by non-biological conductive materials (Chen et al., Citation2014). As such, these materials operate in a manner similar to an electrical grid and connect the electron-donating partners with electron-accepting ones, thereby improving syntrophic metabolism, and provide interesting opportunities to engineer and optimize AD (Lin et al., Citation2018). To this, the conductive carbon materials such as granular/powdered activated carbon (GAC/PAC), carbon cloth, carbon nanotubes, graphene, biochar, and conductive magnetite have been investigated as DIET materials (ZHAO et al., Citation2020). Carbon conductive materials provide the simplicity to microorganisms to connect with each other by attaching anywhere on the surface of these materials rather than locating each other and establishing direct contact. On the other hand, the mechanism by which magnetite stimulates DIET is different and this difference is mainly based on the size of magnetite particles. Studies from defined co-culture studies have indicated that carbon conductive materials are much larger than cells whereas the magnetite particles are much smaller, i.e., 20–50 nm. This implies that two cells need to be within nanometres of each other to be simultaneously in contact with the same magnetite crystal since the crystal only contacts a small proportion of the cell surface. On the other hand, carbon conductive materials can facilitate long range (up to 100 µm or more) electron exchange between microbial partners.
Compared to other carbon conductive materials, biochar, as a low cost, carbonaceous solid material obtained from waste biomass has emerged as an effective supplement for the AD digester. Thermochemical conversion methods, such as pyrolysis, hydrothermal carbonization (HTC), gasification and torrefaction are employed for biochar production. To obtain the maximum yield of biochar, the selection of technique should be appropriate based on both feedstock type and the specific process conditions used which include heating rate, temperature, residence time, etc. shows the different operating conditions for various thermal processes and the yields of biochar expected. Among the various methods, pyrolysis is the most commonly used and extensively investigated technique for biochar production (YAASHIKAA et al., Citation2020). It involves the thermal decomposition of organic matter at temperatures ranging from 250°C to 900°C in an oxygen-free environment. Biochar yields in pyrolysis depend on the type and nature of the feedstock as well as the temperature used. In general, the yield decreases with the increase in temperature. Pyrolysis is further characterized as fast and slow based on the heating rate, temperature, and residence time used during the process (). Generally, slow pyrolysis uses as slow temperature heating rate (0.01–2°C/s) and a longer residence time of more than 1 h as compared to fast pyrolysis. Slow pyrolysis results in higher biochar yields, while fast pyrolysis produces greater bio-oil and lower biochar quantities. Furthermore, fast pyrolysis yields biochar with greater surface area (183 ± 17.3 versus 98.6 ± 3.53 m2/g) and lower average particle size (52.3 ± 40.2 versus 1190 ± 565 nm) than slow pyrolysis. On the other hand, slow pyrolysis leads to biochar with greater ash content and reduced abundance of acidic functional groups (IPPOLITO et al., Citation2020). Another dry thermochemical process is torefaction, which is performed at a temperature range of 200–300°C under an inert atmosphere. On the other hand, HTC is a wet thermal process which is performed in the presence of water at low temperatures around 180–250°C and pressure above 1 MPa. The product formed is called hydrochar to differentiate it from the one produced in dry thermal processes. HTC produces a char with lower ash content but similar mass yield at much lower temperatures than torrefaction. It is favoured if the feedstock has a higher moisture content as no drying process is required (Y. Wang et al., Citation2019b; ZHOU et al., Citation2020). Compared to the above methods, gasification produces syngas as the main product, while biochar is the by-product with less yield.
Table 1. Comparison of thermochemical conversion methods for production of biochar
Biochar addition to AD has been shown to promote syntrophic VFA oxidation, methane production and process stability (HU et al., Citation2020; KAUR et al., Citation2020). Suggested mechanisms for the increased methane production by addition of biochar include (a) reduction of acid and ammonia stress by virtue of the buffering capacity of biochar and consequent increase in methanogenic activity in the AD reactor (LÜ et al., Citation2016; LUO et al., Citation2015; SUNYOTO et al., Citation2016), (b) acting as a conductor for electron transfer between different microbial groups colonizing the biochar surface, thereby increasing the rate of methanogenesis (BAEK et al., Citation2018), (c) facilitating growth of bacteria and methanogens via biochar’s immobilization effect and microbial enrichment ability (LÜ et al., Citation2019; ZHANG et al., Citation2020), and (d) enabling the capacity of fermentative bacteria to metabolize long-chain VFA during AD (KAUR et al., Citation2020; LÜ et al., Citation2019). provides a summary of reports on biochar addition in AD and its impact on enhancing methane production.
Table 2. Summary of reports on biochar addition in AD and its impact on enhancement of methane production
The properties of biochar are influenced by the feedstock and pyrolysis conditions used for their production (INDREN et al., Citation2020a). Consequently, this influences their functioning upon addition to the AD digester. Therefore, it is important to gain a comprehensive knowledge about the physical and chemical properties of biochar to allow an optimal biochar selection for promoting syntrophic metabolism and AD performance. Furthermore, the biochar properties can also be engineered using physical and/or chemical activation methods in order to harness desirable features that enhance its efficiency when added to AD (KAZEMI SHARIAT PANAHI et al., Citation2020). The present review discusses the properties of biochar desirable for improving syntrophy in AD. Previously published reviews have generally focussed on the means by which biochar can promote AD including the alleviation of inhibition by VFA and ammonia; adsorption of heavy metals; increasing methane production or improving biogas and digestate quality (MASEBINU et al., Citation2019, Codignole LUZ et al., Citation2018; Qiu et al., Citation2019; CHIAPPERO et al., Citation2020). Additionally, there are other reviews on mechanisms of IET, mediation of DIET by various modes, i.e., membrane-bound electron transport proteins, conductive pili and abiotic conductive materials, and microbiology of IET (NOZHEVNIKOVA et al., Citation2020; ZHANG & ZANG, Citation2019; ZHAO et al., Citation2020). However, none of these reviews provides a comprehensive description and analysis of biochar properties in relation to syntrophic metabolism in AD and the biochar engineering strategies to obtain desired properties for enhanced syntrophy and AD efficiency. This is the focus of the present review. Such information will be useful to propose research strategies for engineering biochar mediated DIET-based digesters to achieve improved AD performance.
2. Properties of biochar and their specific roles to promote syntrophy in AD
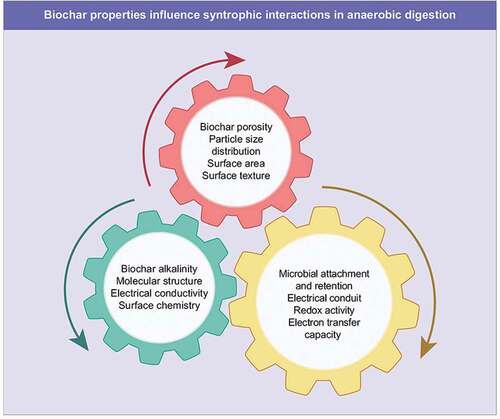
The stability and efficiency of AD depends on the crucial balance between organic substrate degradation, rapid utilization and conversion of intermediate metabolites to methanogenic substrates and their assimilation to methane. However, this balance is upset by the accumulation of intermediate products, unfavourable pH, acid/ammonia stress, and hindrance of methanogenic activity. This leads to reactor instability and failure, limited decomposition rate and low methane yields, particularly at high organic loading rates (KAUR et al., Citation2020). Specific properties of biochar namely pH, particle size, surface area, porous structure, conductivity, and surface chemistry can govern the reactions during AD to alleviate VFA accumulation, ammonia stress and promote syntrophic interactions among microbial consortia leading to enhanced process efficiency. Such desired physical and chemical characteristics of biochar along with factors that influence them are discussed in the sections below to allow optimal biochar selection for enhanced syntrophy in AD ().
2.1. Physical properties of biochar to facilitate syntrophy
2.1.1. Modification of physical properties of biochar and their influence on microbial interactions during AD
As a physical indicator, surface area, pore size, porosity and particle size distribution of biochar are important factors to evaluate its structure for microbial attachment and possible formation of connections between them to enhance DIET. Upon pyrolysis of biomass, the resultant biochars exhibit a high similarity to the original biomass in terms of the basic structure and porosity of the latter (KAZEMI SHARIAT PANAHI et al., Citation2020). For example, wood-derived biochars have been reported to possess high macroporosity which is linked to their derivatization reactions from inherently porous feedstock (plant cellular structures). The macropores having an internal diameter >50 nm can convert into mesopores (diameter up to 5 nm) and micropores (diameter <2 nm). Presence of micropores on biochar surface is considered to be favourable for CO2 sequestration which in turn can lead to enhanced buffering capacity during AD (INDREN et al., Citation2020a). Additionally, the surface area of biochar is predominantly influenced by the presence of micropores. It has been reported that the processing of biochars at high pyrolysis temperatures produces biochars possessing a higher abundance of micropores that represent 50–78% of the total pores on the biochar surface. In general, an increase in the specific surface area of biochars is achieved with an increase in pyrolysis temperature to up to 400°C (S. Li et al., Citation2019b). This can be attributed to a greater vaporization of gaseous compounds from biomass, releasing more volatile content from it and forming micropores. This eventually leads to a rapid increase in the specific surface of biochars at high pyrolysis temperatures. An increase in biochar surface area is also reported upon particle size reduction of biochar. This can be explained through the interrelation between particle size, pore size and surface area. With a decrease in particle size, the pore size distribution moves towards micropores (i.e., less than 2 nm diameter) and this in turn results in higher surface area of the biochar due to the maximum contribution of micropores among all types of pores to the surface area. Thus, fine biochar with particle sizes of <5 µm has been shown to aggregate more microorganisms, thereby resulting in a higher degree of microbial colonization. The large surface area of biochar benefits microbial attachment, growth and enrichment in AD, thereby promoting methane production (FAGBOHUNGBE et al., Citation2016). Supporting this theory, increased rates of methanogenesis between 23% and 47% have been observed when biochar particle size was reduced, mainly resulting from the increased surface area of biochar (LÜ et al., Citation2019; ZHANG et al., Citation2020). To this, the surface areas of biochar varying from 37.2 to 248.6 m2/g have been shown in literature to promote microbial growth and enhance methane production (Wang et al., Citation2019aa, Johnravindar et al., Citation2021).
Furthermore, the surface texture also affects microbial attachment. LUO et al. (Citation2015) reported a selective colonization of microorganisms and a preferential enrichment of Methanosarcina on a coarse biochar surface as opposed to a smooth surface. This selective enrichment of functional microbes facilitated by biochar can be possibly explained by the specific metabolism of different microorganisms. To illustrate this further, LUO et al. (Citation2015) found that the increase in substrate loading conditions led to the abundance of Methanosarcina in the digester even though the inoculum was initially dominated by Methanobacterium and Methanosaeta. Methanosarcina is known to utilize multiple nutrients for its growth. The pore channels in coarse biochar promoted the colonization of Methanosarcina deep within the biochar wherein it was protected from the high organic loading (i.e., high acid concentration) while also being able to access multiple nutrients and establish methanogenic zones. Therefore, specific surface properties of biochar could lead to a diversification and/or shift in microbial communities to allow enhanced methanogenesis under various AD conditions.
Microbial colonization is conducive to microbial retention in the digester and promotes syntrophic interactions among different microbial groups (He et al., Citation2020; Pan et al., Citation2019). Furthermore, the microstructure of biochar is a significant factor governing the retention of microbes on its surface as well as in the pores with a potential formation of biofilm that supports the colonization of acetogens and methanogens. In addition to serving as a microbial support, the biochar also promotes their acclimatisation to various inhibitory substances while being retained in close proximity on biochar, thereby increasing the chances of a more rapid exchange and assimilation of intermediates from acetogens to partnering methanogens. Thus, structural differences between biochars play an important role in governing microbial interactions and their catalysed reactions on biochar surface.
2.1.2. Effect of modifications in biochar properties on microbial degradation of VFA during AD
With respect to VFA assimilation into methane during AD, synergistic impacts due to the physical structure of biochar on increased degradation of inhibitory short-chain VFA (e.g. propionic acid) and long-chain VFA including valeric acid, iso-valeric acid, caproic acid, isobutyric acid have been reported (KAUR et al., Citation2020). While acetate can be directly consumed for methane production, all other VFA need to undergo syntrophic oxidation to acetate. The feasibility of the latter is governed by the thermodynamics of the individual reactions e.g. ΔG0´ for butyrate and propionate conversion to acetate is 48.1 kJ/mol and 76.1 kJ/mol, respectively (de BOK et al., Citation2004). Furthermore, the strong inhibitory effect of propionic acid on methanogenesis makes its accumulation more problematic. Their degradation is feasible only if the products of their oxidation, i.e., acetate, formate, and H2 are maintained at non-inhibitory concentrations via consumption by the partnering methanogens.
Biochar addition has been reported to speed up the degradation and oxidation of propionate and isobutyrate to acetate as the digestion proceeds (Johnravindar et al., Citation2021). This has been usually attributed to the presence of colonizing areas provided by biochar surface to accommodate syntrophic VFA utilizing bacteria and methanogens and promote the utilization of acetate and other VFA for methane production. This relief from acid stress upon biochar addition has been studied in conjunction with investigation of microflora and their interactions responsible for VFA oxidation and subsequent methanogenesis.
For the bacterial population, Firmicutes, Proteobacteria and Bacteroidetes have been reported to increase in abundance upon biochar addition. Among the syntrophic VFA-oxidizing bacteria, Syntrophomonas was found to be more abundant with biochar amendment in the digester, reaching as high as 50% abundance (LUO et al., Citation2015). Syntrophomonas can establish a syntrophic metabolism with methanogens. Furthermore, Syntrophorhadbus and Syntrophobacter have also been reported to be increased in abundance and these could degrade propionate to acetate, thereby promoting VFA oxidation upon biochar addition. In fact, the ratio of propionate to VFA was found to be between 0 and 0.07 in digesters enriched with these bacteria (D. Li et al., Citation2019a). Additionally, the relative abundances of Pseudomonas and Pseudoxanthomonas which are known exoelectrogens and play important roles in organic matter degradation and electron transfer was also reported to increase with biochar amendment. It was also found that acetic acid/VFA was increased with biochar addition which indicated that butyric acid could be smoothly degraded to acetic acid without any accumulation. Furthermore, the efficiencies of acidogenesis and acetogenesis were similar which indicated that the higher diversity of acetogens obtained upon biochar supplementation facilitated VFA microbial degradation to acetate during AD which can then be easily converted to methane by methanogens. In another study using sawdust biochar, Tepidimicrobium was enriched in the digester (Wang et al., Citation2019aa). A metabolic type of Tepidimicrobium can reduce ferric oxide, with low molecular organics as the electron donors. This characteristic is similar to Geobacter metallireducens, a typical ferric oxide reducing microorganism which has been demonstrated to be involved in DIET with methanogens under mesophilic conditions. This exoelectrogenic capacity of Tepidimicrobium makes it an important syntrophic microorganism in AD.
In some thermophilic AD, a selective enrichment of bacterial community Thermotogae due to biochar addition has also been reported (Wang et al., Citation2019aa, ZHANG et al., Citation2020). These have been particularly described to lead to a stable AD operation at elevated organic loading conditions which indicates their vital role in degradation of VFA and avoiding digester failure from VFA accumulation. Within this phylum, the bacterial genus Defluviitoga, a typical fermentative bacterium responsible for degradation of complex groups including C3-C5 VFA to acetate, H2, and CO2 has been found to play a vital role in enhanced thermophilic AD.
For the syntrophy to occur, the methanogenesis archaeal community is also transformed with the presence of biochar in AD. Methanobacterium, a strictly hydrogenotrophic methanogen has been reported to be the most abundant one followed by acetate/H2- consuming Methanosaeta and strictly acetate-consuming Methanosarcina. In a study by LUO et al. (Citation2015), biochar amendment led to the replacement of Methanobacterium by Methanosarcina which could utilize multiple nutrients (VFA) after their conversion to acetate. Differences in terms of location of specific methanogens on biochar have also been observed (GIWA et al., Citation2019). For instance, Methanosarcina resided deep in the pore channels of coarse biochar wherein it established methanogenic zones, whereas Methanosaeta remained attached to the outer surface of biochar. This preferential attachment on biochar surface might be related to the possibility of DIET between Methanosaeta and other bacteria. For example, in co-culture studies, methane production has been shown to be facilitated by biochar addition whose surface was inhabited by Methanosaeta and electron donating bacteria Geobacter sp. (INDREN et al., Citation2020b). This implied that the metabolism of Methanosaeta did not only rely on acetate rather it was supported by other nutrients through interaction with other microorganisms via DIET.
Similar results were reported for cardboard digestion using biochar addition in which the abundance of Methanobacterium was found to decrease upon biochar amendment (D. Li et al., Citation2019a). The digester was instead conspicuously enriched with acetoclastic Methanosaeta. In some thermophilic AD, the hydrogenotrophic Methanothermobacter has been found to be enriched along with Methanosarcina (Wang et al., Citation2019aa, ZHANG et al., Citation2020). This points to a more balance state of the two methanogenic pathways, i.e., hydrogenotrophic and acetoclastic, allowing a more efficient consumption of diverse intermediates during methanogenesis. Furthermore, Methanothermobacter also has the ability to accept electrons from electron donors and achieve DIET for syntrophic metabolism with other bacteria such as Tepidimicrobium. Relating the findings for methanogenic population with bacterial groups enriched upon biochar treatment, e.g., Syntrophomonas and Bacteroidetes, it suggests that the bacterial groups had specific synergistic effects with methanogens in AD. It further illustrates that the enhanced methane production observed with biochar addition is achieved through synergistic efficiencies among acidogenesis, acetogenesis and methanogenesis. By virtue of its properties as a porous conductive material, biochar facilitates enrichment of synergistic microorganisms Syntrophomonas, Bacteroidetes and Methanosaeta and/or Methanosarcina in a microhabitat with high metabolic activity. To this, the specific properties of biochar such as high surface area and surface roughness of biochars increase the likelihood of presence of fatty acid oxidizing bacteria and methanogens in close proximity on biochar surface thereby increasing the above syntrophic reactions for VFA conversions in AD. Moreover, the floating of biochar having a relatively large particle size, e.g., diameter > 1 cm, and a density of <1000 kg/m3 (e.g. 847 kg/m3) was not found to be conducive to microbial growth (ZHANG et al., Citation2020). This indicated that the biochars derived from pyrolysis or gasification processes need simple pretreatment (e.g. smash) to reduce the particle size, which can guarantee the desired intensification performance during AD.
Considering the above properties of biochar, it is also necessary to discuss their impact in low-solid (liquid) and high-solid digestions. The attachment of microorganisms to biochar surfaces requires sufficient contact time between biochar and microorganisms. In the case of high-solid AD, this level of contact is expected to be less due to the high solid content in the digester which decreases the mass transfer within the bulk contents (sludge) of the digester. Fine biochar particles of a few micrometers in size would be more suitable in this scenario to provide more opportunities for microbial attachment and growth on the biochar surface and enable syntrophic interactions.
2.2. Chemical properties of biochar to facilitate syntrophy
2.2.1. Modifications of chemical structure of biochar and their impact on AD
Biochar with diverse chemical properties can be formed under different pyrolysis conditions using the same biomass feedstock. In general, the biochars produced from the same feedstock have been shown to have a high pH when high pyrolysis temperatures are used and a linear relationship between pyrolysis temperature and alkalinity (pH) of biochar is seen at the rate of 0.5–1.4/100°C (S. Li et al., Citation2019b). As studied in detail by S. Li et al. (Citation2019b), the mechanism behind the increase in the biochar pH lies in the cleavage of weak bonds such as hydroxyl bonds in the biochar structure when high pyrolysis temperatures are used. Such weak bonds are present in the original feedstock used for biochar production and easily undergo thermal decomposition upon being subjected to high temperatures. The subsequent release of oxygenated carbon yields a deprotonated biochar surface at high temperatures which causes the elevated biochar pH. Thus, the biochar pH can be altered i.e., increased or decreased based on the pyrolysis temperature, thereby allowing the use of biochar both as a corrector of acidic and/or alkaline environment in the digester.
pH fluctuation is a commonly encountered problem during AD and it results from feedstock composition and its degradation products. For example, food waste degradation results in a highly acidic environment due to VFA accumulation from carbohydrate-rich feedstock whereas higher pH values over the methanogenic range are seen for high nitrogen containing feedstocks e.g., poultry litter and manure, etc., that result in ammonia accumulation in the digester (INDREN et al., Citation2020a; Wong et al., Citation2018). The inability to self-buffer the system indicates a poor interaction between different microbial groups to assimilate the intermediate compounds produced during AD. Oftentimes, chemical additives are needed for pH correction in the digestor (e.g., lime to increase the pH), however such additives have been reported to inhibit methanogenesis when added at high concentrations. Biochar can be a useful additive to exert a buffering action and restore the system pH balance (MUMME et al., Citation2014; SUNYOTO et al., Citation2016). Furthermore, the ability to modify the biochar pH based on pyrolysis temperature offers a great opportunity to tailor it according to the specific requirement in AD, i.e., to increase or decrease the digester pH.
Additional alkalinity is contributed by the presence of high concentration of nutrient elements, such as N, P, K, and S in biochar (Fidel et al., Citation2017; INDREN et al., Citation2020a). The concentration of these alkaline metals is determined by the ash elemental composition of the biochar. Due to the role played by these metals in the second stage cracking reaction of volatiles in biochar, their presence is also potentially significant to the increase in biochar porosity. These alkaline metals influence AD performance by their ability to prevent acidification due to the accumulation of VFA along with enhanced buffering capacity via CO2 sequestration. The concentration of these alkaline metals is usually higher in biochars derived from agricultural residues than those from forestry residues (SHEN et al., Citation2017).
Biochar surface shows the presence of various chemical groups with different properties such as basic, acidic, hydrophobic, and hydrophilic, and so on which is dictated by the feedstock as well as pyrolysis conditions used for biochar production. The presence of hydrophobic functional groups promotes attraction between microorganisms and biochar (SCHIMMELPFENNIG & Glaser, Citation2012). Furthermore, various functional groups are formed on biochar surface by the association of heteroatoms including S, P, N, O and H with aromatic rings, thereby resulting in varied surface chemistry of biochars. Consequently, electron accepting (NO2, (C = O)H, (C = O)OH) or electron donating (O(C = O)R, OR, NH2,OH) functional groups can be formed (Amonette & Joseph, Citation2009). To this, a high abundance of oxygen containing functional groups is considered to be favourable for electron transfer between microorganisms (Xu et al., Citation2020). This can probably be explained by the fact that oxygen functional groups promote interaction between biochar and microbial groups, thereby increasing the likelihood of electron transfer between the attached microorganisms on biochar surface (LÜ et al., Citation2019).
2.2.2. Modifications of molecular structure of biochar and their impact on microbial interactions for improved AD performance
Further characterization of biochars to support biochar’s electron transfer capacity and promote syntrophic interactions is based on their molecular structure. This property is reflected by their molar H/C and O/C ratios. These ratios correspond to the amounts of O2 and H2 remaining after pyrolysis; thus, their loss during pyrolysis produces a biochar with higher amount of fixed carbon and lower H/C and O/C molar ratios. H/C is related to the degree of carbonisation due to the fact that hydrogen is related to the organic matter in feedstock (MASEBINU et al., Citation2019). According to the International Biochar Initiative, an H/C molar ratio of 0.7 is used as a threshold value to differentiate between biochar produced and the biomass that has been modified as a result of thermal and/or chemical treatment.
In recent studies, H/C and O/C molar ratios of biochar have been related to electron transfer properties of biochar and used to explain the mechanism of biochar-induced syntrophic interactions during AD. Sun et al. (Citation2017) reported that the molar H/C and O/C ratios of lower than 0.35 and 0.09, respectively, lead to the emergence of graphite carbon structures which allow a high conductance and easy transfer of electrons (Sun et al., Citation2017). Such structures arise in biochars produced at high pyrolysis temperatures exceeding 700°C while those produced at lower temperatures have amorphous structures. This was supported by a recent study on biochar-amended AD conducted by LIM et al. (Citation2020), who found that the addition of 5–10 g/L of biochar produced from gasification of wood chips at 800 °C promoted the growth of electroactive Clostridia class and other electroactive bacteria. Clostridia, belonging to the phylum Firmicutes, are key microorganisms in anaerobic fermentation and the enriched homoacetogenic Clostridia are critical for elevated efficiency of syntrophic acetic acid oxidation to methane (Shin et al., Citation2019; WESTERHOLM et al., Citation2016). Thus, biochar exhibiting H/C and O/C within the above limits are favourable to promote microbial interactions between acetogenic/methanogenic communities and promote methanogenesis. Furthermore, a low H/C indicates a high abundance of fused ring aromatic structures and aromatic groups in biochar. This property has been related to the high biochar conductance which allows biochar to function as an electron mediator between various microbial groups (Johnravindar et al., Citation2021). Additionally, the low O/C along with the high alkalinity of biochar can indicate biochar’s hydrophobicity which is a desirable property to attract microbes to the biochar surface (SCHIMMELPFENNIG & Glaser, Citation2012). The O/C value of biochar is also an important property to determine the functionality of biochar to mitigate the problem of ammonia inhibition during AD (Johnravindar et al., Citation2021). GAI et al. (Citation2014) developed a correlation between biochar properties influencing their ammonium adsorption capacity and reported that low O/C i.e., low polarity of biochar was the predominant factor causing its low ability to adsorb ammonium. Accumulation of high ammonium concentration has been reported to affect the microbial growth, metabolism and result in lower methane yields. Thus, the use of biochar with a high O/C and thus low hydrophobicity would be beneficial to adsorb ammonium and relieve this inhibition during AD. The H/C and O/C of biochar can be modified based on the pyrolysis conditions to suit-specific needs for AD. Typically, the H/C and O/C values are reported to decrease when high temperatures and/or residence times are used during pyrolysis. For example, biochars with low H/C ratios and aromatic structures are obtained at pyrolysis temperatures >350°C. The dominance of these aromatic structures is further increased at conditions of temperatures exceeding 500°C and extended residence time. This occurs at the expense of removal of O-aryl carbon functional groups which cause a higher mass loss and low H/C (KAZEMI SHARIAT PANAHI et al., Citation2020).
A feature of biochar that has been extensively discussed to explain its beneficial role in influencing microbial interactions in AD is its electrical conductivity. In fact, this property of biochar has been used to draw parallels with other conductive minerals/materials, e.g., magnetite and GAC, that can facilitate DIET in anaerobic cultures. It has been suggested that biochar facilitates interspecies electron transfer via a conduction-based mechanism, i.e., the electron flow from electron donating cells through biochar to electron accepting cells (Chen et al., Citation2014). This bulk conductivity of biochar has also been cited as one of the phenomena causing a direct and quicker transfer of electrons to methanogens, thereby leading to increased efficiency of methanogenesis (KAUR et al., Citation2020; D. Li et al., Citation2019a). Although it has been shown in various studies that DIET mediated syntrophic methanogenesis is stimulated by biochar, a complete understanding of its exact mechanism remains limited. Furthermore, the role of bulk conductivity of biochar to promote this phenomenon is also inexplicit (Wang et al., Citation2019aa, SHEN et al., Citation2021). For instance, mitigation of VFA accumulation and higher methane yields have been reported for biochars with lower conductivity as compared to those with higher electrical conductivity values.
Opposed to bulk conductivity, surface functional groups, e.g., quinone moieties identified on the surface of biochar have been suggested to mediate microbial redox reactions with their ability to reversibly donate or accept electrons (SHEN et al., Citation2021). For instance, Yuan et al. (Citation2018) reported that rice straw-derived biochar and manure-derived biochar had higher charging and discharging capacities (i.e., redox-active properties) than wood chip-derived biochar, and the functional groups (e.g., quinones) on the biochar surface played an essential role in facilitating methanogenesis. Typically, biochars derived at low pyrolysis temperature are greatly enriched with redox active functional groups and their presence is significantly related to H/C (0.35–0.62) and O/C (0.09–0.24) values of biochar (Sun et al., Citation2017). As discussed above, low H/C and O/C ratios of biochar of less than 0.35 and 0.09, respectively, result in more ordered graphite-like structures that allow a direct, shortcut pathway for electron transfer through the biochar matrix. Such biochars are usually derived at pyrolysis temperatures exceeding 700°C and function like an electric conduit owing to their high electrical conductivity and reduced presence of functional moieties. Greater abundance of functional groups on biochar surface improves the electron transfer to methane producers, thereby promoting syntrophic interactions between microbial consortia and supporting methanogenesis via CO2 reduction. For instance, the syntrophic and electro-active partners of Petrimonas and Methanosarcina, responsible for high-efficient VFA degradation, were found to be synergistically enriched in sawdust-based biochar-amended anaerobic digesters of food waste and sewage sludge under high organic loading rate (e.g. 6.8–16.2 gVS/L/d), which demonstrated that the biochar likely acted as a redox-active mediator to facilitate DIET between the syntrophic partners for promoted methanogenesis process (Wang et al., Citation2020a). However, a detailed kinetic and quantitative information on redox activity of biochar to support interspecies electron transfer in AD is needed to gain a deeper understanding of this mechanism.
2.3. Ecotoxicological effects of biochar and the role of biochar properties
Biochar has been increasingly considered as an environment-friendly material which can be used in various applications, such as soil remediation, water treatment, energy production, catalysis, etc. This is mainly attributed to its properties including high external surface area, presence of inorganic nutrients, modifiable structure, and environmental stability (YANG et al., Citation2019). However, biochar itself can be a source of contaminants which can arise from the feedstock used for its production (e.g. heavy metals) or as by-products, such as polycyclic aromatic hydrocarbons (PAH), volatile organic compounds, dioxins, etc., which are generated during the pyrolysis process (OLESZCZUK et al., Citation2013; Xing et al., Citation2019). Such pollutants from biochar can result in toxicity to organisms during environmental applications of biochar. As assessed by various methods, such as cell viability, Ames test etc., PAH are classified as human pathogens owing to their carcinogenic and mutagenic properties. These arise from incomplete combustion of organic matter in the feedstock and condensation and aromatization of solid materials during the formation of biochar. Similarly, the volatile organic compounds which arise during pyrolysis can re-condense to form compounds in biochar which are bioavailable and phytotoxic.
Toxicity of biochar can limit their applications. To this, the impact of feedstock selection and pyrolysis temperature has been studied in relation to biochar toxicity. It has been reported that the levels of heavy metals are mainly dependent on the feedstock i.e., if the raw material itself is naturally free of heavy metals, the resultant biochar has no heavy metal content (YANG et al., Citation2019). For PAHs, biochars derived from fast pyrolysis and gasification have been found to contain higher total PAH concentrations than those obtained from slow pyrolysis (Hale et al., Citation2012). In a study by Lyu et al. (Citation2016), the total concentration of PAHs in biochar was found to be inversely proportional to pyrolysis temperature. In general, a low-temperature biochar has a higher PAH content and would therefore cause more toxicity (TOMCZYK et al., Citation2021). Furthermore, the molar ratios of H/C and O/C have been proposed as an index of toxicity. Along with the increment of carbonization, thermal decomposition of organic matter in biomass leads to a decrease in H/C and O/C values. As discussed in Section 2.2.2., the use of high pyrolysis temperatures above 350°C and high residence times during pyrolysis is beneficial to yield biochars with low H/C and O/C and hence low toxicity.
A relation between porosity and surface area of biochar and the resultant biochar toxicity has also been obtained. YANG et al. (Citation2019) reported that deepening the pyrolysis reaction could strengthen the biochar porosity and lead to a higher biochar surface area. The increase in surface area favours to reduce its toxicity because all intrinsic toxicants are captured by the porous structure of biochar in this case. Additionally, the fine powdered and low-density forms of biochar can also increase their likelihood of causing toxicity via their resuspension in atmosphere, accumulation in river sediments, and eventually leading to surface waters. Therefore, the correct selection of biochar, i.e., coarse and high-density particles, can minimize their toxicological effects.
3. Engineering of biochar with tailored properties to promote syntrophy in AD
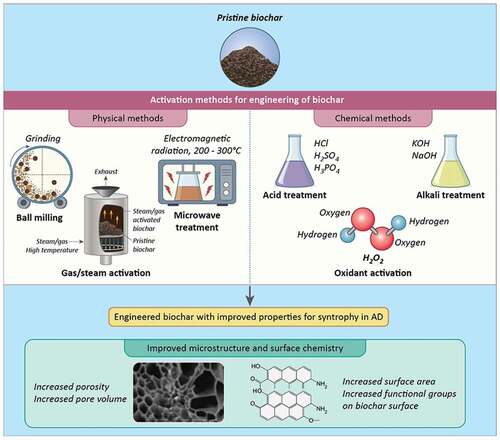
The microstructure and surface chemistry of biochar can be engineered by various physical and chemical methods. Owing to the significant role played by these biochar characteristics as detailed in Section 2, the engineered biochars with improved properties can be useful to obtain enhanced syntrophy upon addition to AD.
The biochar can be physically modified without the addition of any chemicals and/or additives or engineered using certain chemicals and/or inert gases (). Pertinent to facilitating syntrophy in AD, an improved pore structure and increased presence of oxygen containing functional groups is obtained as a result of physical methods of biochar modification. For this, three types of physical methods are particularly useful, including ball milling, gas/steam activation, and microwave treatment. Grinding or physical ball milling of biochar enhances the particle size and specific surface area of biochar. PETERSON et al. (Citation2012) reported a 3.2-fold increase in the surface area of biochar derived from corn stover using this method (PETERSON et al., Citation2012). In another type of ball milling which involves the use of specific chemicals (chemical ball milling), improvements in micropores, surface area and modification of functional groups could be achieved (B. Wang et al., Citation2017). Similar enhancements of surface area, surface reactivity and porosity can be obtained by activation of biochar with gas/steam using air, CO2, and water vapour, etc. This type of activation method causes the development of activated carbon at the applied elevated temperature of 700°C-1100°C that results in the above microstructural changes in biochar. Application of steam activation also increases the presence of functional groups on biochar and provides improved aromaticity that are desirable characteristics to facilitate syntrophy in AD, as discussed in Section 2. An additional advantage of this method is the removal of impurities and products of incomplete combustion from the resultant biochar (L. Wang et al., Citation2020b). This adds further merit to its safe use in a biological process, such as AD.
The third physical method for engineering biochar properties is microwave modification. It involves the use of electromagnetic-based irradiation during pyrolysis, which is conducted at temperatures between 200°C and 300°C. The irradiation frequencies can range between 0.03 and 300 GHz with varying wavelengths, thereby allowing greater process control in this method. A higher level of carbonization, increased surface area as well as deeper and narrower pores have been reported as a result of microwave irradiation during pyrolysis. On the other hand, it resulted in biochar containing a lesser number of oxygenic functional groups (Nair & VINU, Citation2016; Paunovic et al., Citation2019).
As opposed to physical modification methods, chemical methods employ certain chemicals or gases for modifying biochar characteristics. In this regard, the treatments of biochar are usually performed with acids or bases. Specifically, the main objective of acid activation is to clean the pores and increase the pore volume and surface area and introduce hydrophobic groups such as carbonyl, phenolic, etc., on biochar. The use of sulphuric acid, hydrochloric acid, citric acid, phosphoric acid, oxalic acid has been explored for acid activation of biochars (PANWAR & PAWAR, Citation2020). In contrast, alkaline activation of biochar using KOH or NaOH is usually performed to increase the number of oxygen-containing groups (e.g., hydroxyl). Generally, alkali treated biochars exhibit a greater increase in surface area than those treated with acid (L. Wang et al., Citation2020b). Overall, the increase with acid/alkali treatment results from demineralization of biochar. A similar effect of increased abundance of oxygenic functional groups can be obtained by the activation of biochar with hydrogen peroxide. Wang and LIU (Citation2018) reported an increase in oxygen and carbon content of manure-derived biochar by 63% and 101%, respectively, by using this method (Wang & LIU, Citation2018).
While improved biochar properties can be obtained using the above-described methods, it is important to ensure their sustainability both in regard to the cost of the activation method and its impact on the environment. In other words, a sustainable engineering method would be the one which minimizes the environmental footprint and cost while maximizing the benefit in terms of improved properties of the resultant biochar. To this, factors such as cost effectiveness, energy and water consumption, use of mild processing conditions, and toxicity of reagents and materials being used, and the effluents generated at the end of the process are all important considerations to ensure the sustainability of the approach. In light of this, the use of steam and microwave irradiation appears promising due to the mild conditions used in these processes. Steam activation is an easy to apply and a relatively more energy-efficient process as compared to other methods used to achieve increased surface area of biochar, such as increasing the pyrolysis temperature which consumes greater energy. Similarly, the methods which employ non-toxic agents would be given a higher priority. For example, the acid/alkali activation method which involves simple mixing and washing steps using natural and/or non-toxic agents under mild conditions would be encouraged for engineering of biochar. However, the water footprint for these methods is high since large volumes of water are required to reduce the introduced high acidity/alkalinity before biochar can be applied to the AD digester. Therefore, more research is required in this area to develop modification methods with simplified procedures, reduced cost and minimal environmental impact to enable the development of sustainably engineered biochar-mediated digesters.
4. Conclusion
Syntrophy between various microbial groups within the AD community is an important requirement for an efficient AD performance. Syntrophic interactions between hydrolytic bacteria and acetogenic bacteria as well as between synergistic interactions between the fatty acid oxidizers and methanogens are required to keep the intermediates’ concentrations at the accepted levels for the reactions to proceed smoothly. This can be achieved by efficient electron transfer between the various microbial groups. Biochar with its favourable physical and chemical characteristics including surface area, alkalinity, porosity and surface chemistry is a suitable additive in AD. It provides the conditions allowing requisite microbial groups to be present in close proximity thereby increasing the chances of electron exchange between them and enhancing the reaction efficiency. Engineering of biochar properties by various activation methods further provides an opportunity to modify them to engineer more biochar-mediated AD digesters with improved performance. However, care needs to be taken to ensure sustainable engineering with respect to the design of simplified procedures with reduced cost and minimal environmental impact. This would broaden the application of biochars for desired performance intensification of AD and high bioenergy recovery while also supporting its positioning as an environment-friendly material.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article.
Additional information
Funding
Notes on contributors
Guneet Kaur
Davidraj Johnravindar is a Postdoctoral Research Associate at Hong Kong Baptist University (HKBU), Hong Kong, and an expert in anaerobic digestion, organic waste treatment and bioenergy production. Yen Wah Tong is a Professor at Department of Chemical and Biomolecular Engineering, NUS. He is the Programme Co-Director Director for the National Research Foundation CREATE programme with Shanghai Jiaotong University “Energy and Environmental Sustainability Solutions for Megacities”. Yong Sik Ok is a Professor at and global research director, APRU Sustainable Waste Management Program and Korea Biochar Research Center, Korea University, Seoul. Guneet Kaur is an Adjunct Professor at Department of Civil Engineering, York University (YorkU), Canada. Her research focusses on biorefinery, microbial biotechnology, sustainable resource management and resource recovery from waste streams. Before joining YorkU, she was an Assistant Professor at HKBU. Her recent government funded research projects involve the development of closed-loop advanced biorefinery platforms based on urban wastes such as food waste, textile waste, and wastewater sludge.
References
- Amonette, J. E., & Joseph, S. (2009). Characteristics of biochar: Microchemical properties. In J. Lehmann & S. Joseph (Ed.) Pacific Northwest National Laboratory (PNNL), Richland, WA (US), Environmental Molecular Sciences Laboratory (EMSL).
- BAEK, G., Kim, J., Kim, J., & Lee, C. (2018). Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies, 11(1), 107. https://doi.org/https://doi.org/10.3390/en11010107
- BOK, D. E., PLUGGE, F. A., C., M., & STAMS, A. J. (2004). Interspecies electron transfer in methanogenic propionate degrading consortia. Water Research, 38(6), 1368–15. https://doi.org/https://doi.org/10.1016/j.watres.2003.11.028
- Chen, S., ROTARU, A.-E., Shrestha, P. M., MALVANKAR, N. S., LIU, F., Fan, W., Nevin, K. P., & Lovley, D. R. (2014). Promoting interspecies electron transfer with biochar. Scientific Reports, 4(1), 5019. https://doi.org/https://doi.org/10.1038/srep05019
- CHIAPPERO, M., NOROUZI, O., HU, M., Demichelis, F., BERRUTI, F., Di Maria, F., MAŠEK, O., & FIORE, S. (2020). Review of biochar role as additive in anaerobic digestion processes. Renewable and Sustainable Energy Reviews, 131, 110037. https://doi.org/https://doi.org/10.1016/j.rser.2020.110037
- FAGBOHUNGBE, M. O., Herbert, B. M., Hurst, L., Li, H., USMANI, S. Q., & SEMPLE, K. T. (2016). Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresource Technology, 216, 142–149. https://doi.org/https://doi.org/10.1016/j.biortech.2016.04.106
- Fidel, R. B., Laird, D. A., Thompson, M. L., & Lawrinenko, M. (2017). Characterization and quantification of biochar alkalinity. Chemosphere, 167, 367–373. https://doi.org/https://doi.org/10.1016/j.chemosphere.2016.09.151
- GAI, X., Wang, H., LIU, J., ZHAI, L., LIU, S., REN, T., & LIU, H. (2014). Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PloS One, 9(12), e113888. https://doi.org/https://doi.org/10.1371/journal.pone.0113888
- GIWA, A. S., Xu, H., Chang, F. M., Wu, J. J., Li, Y. H., Ali, N., Ding, S. M., & Wang, K. J. (2019). Effect of biochar on reactor performance and methane generation during the anaerobic digestion of food waste treatment at long-run operations. Journal of Environmental and Chemical Engineering, 7(4), 103067. https://doi.org/https://doi.org/10.1016/j.jece.2019.103067
- Hale, S. E., Lehmann, J., Rutherford, D., Zimmerman, A. R., BACHMANN, R. T., SHITUMBANUMA, V., O’TOOLE, A., SUNDQVIST, K. L., Arp, H. P. H., & CORNELISSEN, G. (2012). Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environmental Science & Technology, 46(5), 2830–2838. https://doi.org/https://doi.org/10.1021/es203984k
- He, P., ZHANG, H., DUAN, H., SHAO, L., & LÜ, F. (2020). Continuity of biochar-associated biofilm in anaerobic digestion. Chemical Engineering Journal, 390, 124605. https://doi.org/https://doi.org/10.1016/j.cej.2020.124605
- HU, Q., Jung, J., Chen, D., Leong, K., Song, S., Li, F., MOHAN, B. C., YAO, Z., PRABHAKAR, A. K., Lin, X. H., LIM, E. Y., ZHANG, L., Souradeep, G., Ok, Y. S., KUA, H. W., Li, S. F. Y., Tan, H. T. W., DAI, Y., Tong, Y. W., Peng, Y., … Wang, C. H. (2020). Biochar industry to circular economy. Science of the Total Environment, 143820. https://doi.org/https://doi.org/10.1016/j.scitotenv.2020.143820
- INDREN, M., BIRZER, C. H., Kidd, S. P., Hall, T., & MEDWELL, P. R. (2020a). Effects of biochar parent material and microbial pre-loading in biochar-amended high-solids anaerobic digestion. Bioresource Technology, 298, 122457. https://doi.org/https://doi.org/10.1016/j.biortech.2019.122457
- INDREN, M., BIRZER, C. H., Kidd, S. P., & MEDWELL, P. R. (2020b). Effect of total solids content on anaerobic digestion of poultry litter with biochar. Journal of Environmental Management, 255, 109744. https://doi.org/https://doi.org/10.1016/j.jenvman.2019.109744
- IPPOLITO, J. A., CUI, L., Kammann, C., WRAGE-MÖNNIG, N., Estavillo, J. M., FUERTES-MENDIZABAL, T., CAYUELA, M. L., SIGUA, G., NOVAK, J., Spokas, K., & BORCHARD, N. (2020). Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar, 2(4), 421–438. https://doi.org/https://doi.org/10.1007/s42773-020-00067-x
- JANG, H. M., CHOI, Y. K., & Kana, E. (2018). Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresource Technology, 250, 927–931. https://doi.org/https://doi.org/10.1016/j.biortech.2017.11.074
- Johnravindar, D., Wong, J. W. C., CHAKRABORTY, D., BODEDLA, G., & KAUR, G. (2021). Food waste and sewage sludge co-digestion amended with different biochars: VFA kinetics, methane yield and digestate quality assessment. Journal of Environmental Management, 290, 112457. https://doi.org/https://doi.org/10.1016/j.jenvman.2021.112457
- JUNICKE, H., LOOSDRECHT, V. A. N., M. C., M., & KLEEREBEZEM, R. (2016). Kinetic and thermodynamic control of butyrate conversion in non-defined methanogenic communities. Applied Microbiology and Biotechnology, 100(2), 915–925. https://doi.org/https://doi.org/10.1007/s00253-015-6971-9
- KAUR, G., Johnravindar, D., & Wong, J. W. C. (2020). Enhanced volatile fatty acid degradation and methane production efficiency by biochar addition in food waste-sludge co-digestion: A step towards increased organic loading efficiency in co-digestion. Bioresource Technology, 308, 123250. https://doi.org/https://doi.org/10.1016/j.biortech.2020.123250
- KAZEMI SHARIAT PANAHI, H., DEHHAGHI, M., Ok, Y. S., NIZAMI, A.-S., KHOSHNEVISAN, B., Mussatto, S. I., AGHBASHLO, M., TABATABAEI, M., & Lam, S. S. (2020). A comprehensive review of engineered biochar: production, characteristics, and environmental applications. Journal of Cleaner Production, 270, 122462. https://doi.org/https://doi.org/10.1016/j.jclepro.2020.122462
- LÜ, F., LIU, Y., SHAO, L., & He, P. (2019). Powdered biochar doubled microbial growth in anaerobic digestion of oil. Applied Energy, 247, 605–614. https://doi.org/https://doi.org/10.1016/j.apenergy.2019.04.052
- LÜ, F., LUO, C., SHAO, L., & He, P. (2016). Biochar alleviates combined stress of ammonium and acids by firstly enriching methanosaeta and then methanosarcina. Water Research, 90, 34–43. https://doi.org/https://doi.org/10.1016/j.watres.2015.12.029
- Li, D., song, L., Fang, H., Li, P., TENG, Y., Li, -Y.-Y., LIU, R., & NIU, Q. (2019a). Accelerated bio-methane production rate in thermophilic digestion of cardboard with appropriate biochar: dose-response kinetic assays, hybrid synergistic mechanism, and microbial networks analysis. Bioresource Technology, 290, 121782. https://doi.org/https://doi.org/10.1016/j.biortech.2019.121782
- Li, S., Harris, S., ANANDHI, A., & Chen, G. (2019b). Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. Journal of Cleaner Production, 215, 890–902. https://doi.org/https://doi.org/10.1016/j.jclepro.2019.01.106
- LIM, E. Y., TIAN, H., Chen, Y., Ni, K., ZHANG, J., & Tong, Y. W. (2020). Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresource Technology, 314, 123751. https://doi.org/https://doi.org/10.1016/j.biortech.2020.123751
- Lin, R., CHENG, J., Ding, L., & Murphy, J. D. (2018). Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chemical Engineering Journal, 350, 681–691. https://doi.org/https://doi.org/10.1016/j.cej.2018.05.173
- LIU, C., REN, L., YAN, B., LUO, L., ZHANG, J., & AWASTHI, M. K. (2021). Electron transfer and mechanism of energy production among syntrophic bacteria during acidogenic fermentation: A review. Bioresource Technology, 323, 124637. https://doi.org/https://doi.org/10.1016/j.biortech.2020.124637
- LUO, C., LÜ, F., SHAO, L., & He, P. (2015). Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Research, 68, 710–718. https://doi.org/https://doi.org/10.1016/j.watres.2014.10.052
- LUZ, C. O. D. I. G. N. O. L. E., CORDINER, F., MANNI, S., MULONE, A., & Rocco, V. (2018). Biochar characteristics and early applications in anaerobic digestion-a review. Journal of Environmental Chemical Engineering, 6(2), 2892–2909. https://doi.org/https://doi.org/10.1016/j.jece.2018.04.015
- Lyu, H., He, Y., Tang, J., HECKER, M., LIU, Q., Jones, P. D., Codling, G., & GIESY, J. P. (2016). Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environmental Pollution, 218, 1–7. https://doi.org/https://doi.org/10.1016/j.envpol.2016.08.014
- MASEBINU, S. O., AKINLABI, E. T., MUZENDA, E., & ABOYADE, A. O. (2019). A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renewable and Sustainable Energy Reviews, 103, 291–307. https://doi.org/https://doi.org/10.1016/j.rser.2018.12.048
- MUMME, J., Srocke, F., HEEG, K., & Werner, M. (2014). Use of biochars in anaerobic digestion. Bioresource Technolology, 164, 189–197. https://doi.org/https://doi.org/10.1016/j.biortech.2014.05.008
- Nair, V., & VINU, R. (2016). Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Bioresource Technology, 216, 511–519. https://doi.org/https://doi.org/10.1016/j.biortech.2016.05.070
- NOZHEVNIKOVA, A. N., RUSSKOVA, Y. I., LITTI, Y. V., PARSHINA, S. N., ZHURAVLEVA, E. A., & Nikitina, A. A. (2020). Syntrophy and interspecies Electron transfer in methanogenic microbial communities. Microbiology, 89(2), 129–147. https://doi.org/https://doi.org/10.1134/S0026261720020101
- OLESZCZUK, P., JOSKO, I., & KUSMIERZ, M. (2013). Biochar properties regarding to contaminants content and ecotoxicological assessment. Journal of Hazardous Materials, 260, 375–382. https://doi.org/https://doi.org/10.1016/j.jhazmat.2013.05.044
- Pan, J., Ma, J., ZHAI, L., & LIU, H. (2019). Enhanced methane production and syntrophic connection between microorganisms during semi-continuous anaerobic digestion of chicken manure by adding biochar. Journal of Cleaner Production, 240, 118178. https://doi.org/https://doi.org/10.1016/j.jclepro.2019.118178
- PANWAR, N. L., & PAWAR, A. (2020). Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Conversion and Biorefinery, 1–23. https://doi.org/https://doi.org/10.1007/s13399-020-00870-3
- Paunovic, O., Pap, S., MALETIC, S., TAGGART, M. A., BOSKOVIC, N., & Turk Sekulic, M. (2019). Ionisable emerging pharmaceutical adsorption onto microwave functionalised biochar derived from novel lignocellulosic waste biomass. Journal of Colloid and Interface Science, 547, 350–360. https://doi.org/https://doi.org/10.1016/j.jcis.2019.04.011
- PETERSON, S. C., Jackson, M. A., Kim, S., & Palmquist, D. E. (2012). Increasing biochar surface area: optimization of ball milling parameters. Powder Technology, 228, 115–120. https://doi.org/https://doi.org/10.1016/j.powtec.2012.05.005
- Qiu, L., Deng, Y. F., Wang, F., DAVARITOUCHAEE, M., & YAO, Y. Q. (2019). A review on biochar-mediated anaerobic digestion with enhanced methane recovery. Renewable and Sustainable Energy Reviews, 115, 109373. https://doi.org/https://doi.org/10.1016/j.rser.2019.109373
- SCHIMMELPFENNIG, S., & Glaser, B. (2012). One step forward toward characterization: Some important material properties to distinguish biochars. Journal of Environmental Quality, 41(4), 1001–1013. https://doi.org/https://doi.org/10.2134/jeq2011.0146
- SHEN, Y., FORRESTER, S., KOVAL, J., & URGUN-DEMIRTAS, M. (2017). Yearlong semi-continuous operation of thermophilic two-stage anaerobic digesters amended with biochar for enhanced biomethane production. Journal of Cleaner Production, 167, 863–874. https://doi.org/https://doi.org/10.1016/j.jclepro.2017.05.135
- SHEN, Y., YU, Y., ZHANG, Y., URGUN-DEMIRTAS, M., Yuan, H., ZHU, N., & DAI, X. (2021). Role of redox-active biochar with distinctive electrochemical properties to promote methane production in anaerobic digestion of waste activated sludge. Journal of Cleaner Production, 278, 123212. https://doi.org/https://doi.org/10.1016/j.jclepro.2020.123212
- Shin, J., JANG, H. M., Shin, S. G., & Kim, Y. M. (2019). Thermophilic anaerobic digestion: effect of start-up strategies on performance and microbial community. Science of the Total Environment, 687, 87–95. https://doi.org/https://doi.org/10.1016/j.scitotenv.2019.05.428
- SIRIWONGRUNGSON, V., ZENG, R. J., & ANGELIDAKI, I. (2007). Homoacetogenesis as the alternative pathway for H2 sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis. Water Research, 41(18), 4204–4210. https://doi.org/https://doi.org/10.1016/j.watres.2007.05.037
- Sun, T., Levin, B. D. A., Guzman, J. J. L., ENDERS, A., Muller, D. A., ANGENENT, L. T., & Lehmann, J. (2017). Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nature Communications, 8(1), 14873. https://doi.org/https://doi.org/10.1038/ncomms14873
- SUNYOTO, N. M. S., ZHU, M., ZHANG, Z., & ZHANG, D. (2016). Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresource Technology, 219, 29–36. https://doi.org/https://doi.org/10.1016/j.biortech.2016.07.089
- TOMCZYK, B., SIATECKA, A., BOGUSZ, A., & OLESZCZUK, P. (2021). Ecotoxicological assessment of sewage sludge-derived biochars-amended soil. Environmental Pollution, 275, 116484. https://doi.org/https://doi.org/10.1016/j.envpol.2021.116484
- Wang, B., Gao, B., & Fang, J. (2017). Recent advances in engineered biochar productions and applications. Critical Reviews in Environmental Science and Technology, 47(22), 2158–2207. https://doi.org/https://doi.org/10.1080/10643389.2017.1418580
- Wang, G., Li, Q., Gao, X., & Wang, X. C. (2019a). Sawdust-derived biochar much mitigates VFAs accumulation and Improves microbial activities to enhance Methane production in thermophilic anaerobic digestion. ACS Sustainable Chemistry & Engineering, 7(2), 2141–2150. https://doi.org/https://doi.org/10.1021/acssuschemeng.8b04789
- Wang, G., Li, Q., Li, Y., Xing, Y., YAO, G., LIU, Y., Chen, R., & Wang, X. C. (2020a). Redox-active biochar facilitates potential electron tranfer between syntrophic partners to enhance anaerobic digestion under high organic loading rate. Bioresource Technology, 298, 122524. https://doi.org/https://doi.org/10.1016/j.biortech.2019.122524
- Wang, L., Ok, Y. S., TSANG, D. C. W., ALESSI, D. S., RINKLEBE, J., Wang, H., MAŠEK, O., HOU, R., O’CONNOR, D., & HOU, D. (2020b). New trends in biochar pyrolysis and modification strategies: Feedstock, pyrolysis conditions, sustainability concerns and implications for soil amendment. Soil Use and Management, 36(3), 358–386. https://doi.org/https://doi.org/10.1111/sum.12592
- Wang, Y., & LIU, R. (2018). H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions. Science of the Total Environment, 628-629, 1139–1148. https://doi.org/https://doi.org/10.1016/j.scitotenv.2018.02.137
- Wang, Y., Qiu, L., ZHU, M., Sun, G., ZHANG, T., & KANG, K. (2019b). Comparative evaluation of Hydrothermal Carbonization and low temperature pyrolysis of eucommia ulmoides oliver for the production of Solid biofuel. Scientific Reports, 9(1), 5535. https://doi.org/https://doi.org/10.1038/s41598-019-38849-4
- WESTERHOLM, M., MOESTEDT, J., & SCHNÜRER, A. (2016). Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Applied Energy, 179, 124–135. https://doi.org/https://doi.org/10.1016/j.apenergy.2016.06.061
- Wong, J. W. C., KAUR, G., MEHARIYA, S., KARTHIKEYAN, O. P., & Chen, G. (2018). Food waste treatment by anaerobic co-digestion with saline sludge and its implications for energy recovery in Hong Kong. Bioresource Technology, 268, 824–828. https://doi.org/https://doi.org/10.1016/j.biortech.2018.07.113
- Xing, J., Li, L., Li, G., & Xu, G. (2019). Feasibility of sludge-based biochar for soil remediation: characteristics and safety performance of heavy metals influenced by pyrolysis temperatures. Ecotoxicology and Environmental Safety, 180, 457–465. https://doi.org/https://doi.org/10.1016/j.ecoenv.2019.05.034
- Xu, S., Wang, C., DUAN, Y., & Wong, J. W. C. (2020). Impact of pyrochar and hydrochar derived from digestate on the co-digestion of sewage sludge and swine manure. Bioresource Technology, 314, 123730. https://doi.org/https://doi.org/10.1016/j.biortech.2020.123730
- YAASHIKAA, P. R., KUMAR, S. E. N. T. H. I. L., VARJANI, P., & Saravanan, A. (2020). A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnology Reports, 28, e00570. https://doi.org/https://doi.org/10.1016/j.btre.2020.e00570
- YANG, X., NG, W., Wong, B. S. E., Baeg, G. H., Wang, C.-H., & Ok, Y. S. (2019). Characterization and ecotoxicological investigation of biochar produced via slow pyrolysis: effect of feedstock composition and pyrolysis conditions. Journal of Hazardous Materials, 365, 178–185. https://doi.org/https://doi.org/10.1016/j.jhazmat.2018.10.047
- Yuan, H. Y., Ding, L. J., Zama, E. F., LIU, P. P., HOZZEIN, W. N., & ZHU, Y. G. (2018). Biochar modulates methanogenesis through electron syntrophy of microorganisms with ethanol as a substrate. Environmental Science & Technology, 52(21), 12198–12207. https://doi.org/https://doi.org/10.1021/acs.est.8b04121
- ZHANG, L., LIM, E. Y., LOH, K.-C., Ok, Y. S., Lee, J. T. E., SHEN, Y., Wang, C.-H., DAI, Y., & Tong, Y. W. (2020). Biochar enhanced thermophilic anaerobic digestion of food waste: focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Conversion and Management, 209, 112654. https://doi.org/https://doi.org/10.1016/j.enconman.2020.112654
- ZHANG, M., & ZANG, L. (2019). A review of interspecies electron transfer in anaerobic digestion. IOP Conf. Series: Earth and Environmental Science, 310, 042026. https://doi.org/https://doi.org/10.1088/1755-1315/310/4/042026
- ZHAO, Z., Li, Y., ZHANG, Y., & Lovley, D. R. (2020). Sparking anaerobic digestion: promoting direct interspecies Electron transfer to enhance Methane production. iScience, 23(12), 101794. https://doi.org/https://doi.org/10.1016/j.isci.2020.101794
- ZHOU, Y., ENGLER, N., Li, Y., & NELLES, M. (2020). The influence of hydrothermal operation on the surface properties of kitchen waste-derived hydrochar: biogas upgrading. Journal of Cleaner Production, 259, 121020. https://doi.org/https://doi.org/10.1016/j.jclepro.2020.121020