?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Food waste can be converted to a useful product such as biochar as a way of recycling waste to retain nutrients in the soil, which in turn contributes to carbon sequestration and offset some greenhouse gas emissions in the struggle to achieve carbon neutrality. Mixed food waste-derived biochars (FWB1–300°C, FWB2–450°C and FWB3–600°C) were pyrolysed at 300°C, 450°C and 600°C, respectively, using an electric kiln. Tests for physiochemical parameters and germination tests were performed. It was realized that at 300°C biochars produced had high nitrogen, organic matter, bulk density, biochar yield, and longer root lengths. The results indicate that municipal food waste biochars produced at three temperatures were suitable for use as fertilizer. However, biochar produced at a moderately lower temperature is favourable for agriculture purposes, FWB1–300°C and FWB2–450°C obtained moderate pH and ash levels and so are less toxic to the growth of plants.
1. Introduction
A report from the UN Food and Agriculture Organization found that the total impact of food waste disposal has a huge effect on biodiversity, land, and water use, and particularly worldwide environmental change (Mazac, Citation2016). Landfill gases are a source of anthropogenic methane emanations, due to a high proportion of the waste being food waste. Techniques for the effective and efficient utilization of food waste are essential to alleviate future effects on the biosphere (Mazac, Citation2016). Food, agricultural, and landscaping by-products as well as municipal waste products are common waste streams that end up in landfills. The disposal of food waste in landfill is an unsustainable and ecologically unsound practice. Luckily, these difficulties can be alleviated by producing biochar from mixed organic solid waste, to be utilized as soil upgrades and long-haul carbon sequestration. Many studies conducted demonstrate that biochar has great potential to be used as a soil amendment and bioremediation of polluted soils. Biochar leads to a decrease in N emissions in the soil (Yanai et al., Citation2007). Biochar can likewise sequester carbon from the environment and transfer it to the soil to contribute to carbon neutrality. Ren et al. (Citation2016) reported that for soils that are highly polluted by pesticides, biochar could serve as a bioremediation material.
Biochar application has been accounted for to enhance soil quality by raising soil pH, increasing the water-holding capacity, enhancing the activities of more beneficial organisms and microorganisms in the soil, enhancing the cation exchange capacity, and holding supplements. During pyrolysis, the pH of the biochar increases due to the release of alkali salts from the organic matrix of the feedstock (Ahmad et al., Citation2012). The pH of corn straw biochar increased from 9.37 to 11.32 when the pyrolysis temperature was increased from 300°C to 600°C (Yuan et al., Citation2011). Biochar can enhance the retention of cations such as Ca2+, Mg2+ and K+ (Novak et al., Citation2009; Sohi et al., Citation2010). A field amended with biochar was shown to have caused an increase of 28% in maize grain yields, additionally, there was an increase in nutrients such as calcium (Ca), magnesium (Mg), phosphorus (P), and potassium (K) availability in the soil that was amended by 17%–60% biochar (Major et al., Citation2010).
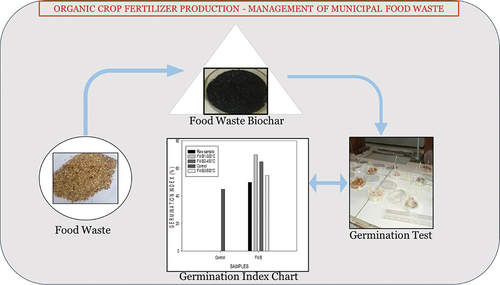
In this paper, we examined the potency of food waste being a potential feedstock for biochar production. The main objective of the study was to examine a productive way food waste could be utilized to limit the pressure on landfill sites, thereby reducing the generation of greenhouse gases. Families and institutions can adopt this, so food waste can be made useful. The specific questions include as follows: (1) Is biochar from mixed food waste a good fertilizer for farm application? (2) Did biochar enhance the growth of cucumber seedlings compared to the raw samples and the control?
Physiochemical analyses were necessary to be carried out on all samples so that a critical conclusion is drawn. After germination of the seedlings, growth measurements were carried out to know which sample of produced biochar enhanced more growth, in terms of root and shoot lengths of the cucumber seedlings. Longer root and shoot lengths are expected in biochar-amended cotton wools compared to the control and raw samples.
2. Materials and methods
2.1. Materials and food waste collection
Cooked food waste (fried rice, vegetables, and egg shells) were collected from a restaurant; uncooked food waste (cassava and plantain peels) were collected from a chop bar, and fruit waste (pineapple, orange, and mango peels) were collected from sellers around the Kotei vicinity around the Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana. Sorting was done, to remove certain unwanted materials that might have mixed up with the food waste. The food waste was then solar-dried for 4 days and was transported to the Department of Animal Science, KNUST for milling, to obtain a homogenous mix of all the food wastes. The milled food wastes were then sent to the Ceramics Laboratory, KNUST, and pyrolyzed at temperatures 300°C, 450°C, and 600°C using an electric kiln (HT13T7, Kiln, and Furnace Limited Keele St. Tunstall, Stoke-On-Trant) at a heating rate of 15°C/min with a residence time of 60 min that was held right from start to finish (). A precursor weight of 2.1 kg was maintained throughout the production process.
Empty metal containers were filled with the feedstock which were carefully placed in an electrical kiln (HT13T7, Kiln and Furnace Limited Keele St. Tunstall, Stoke-On-Trant) of Height = 50 cm, Length = 30 cm and Breadth = 35 cm) at temperatures 300°C, 450°C and 600°C. The door of the electric kiln was firmly sealed to avoid the possible penetration of oxygen. Noting the weight of the empty tin containers before the heating process, W1 was key since this will help in determining the biochar yield, cooling was done in a desiccator. Final weights were taken W2, and then the biochar yield was found. During the production of biochar, uniformity was very key since the comparison was going to be done amongst the various temperatures.
Elemental analyses were performed on each sample; these included Total Nitrogen (TN) and Total Potassium (TK) by the UDK 129 Kjeldahl distillation unit method, Total Phosphorous (TP) (Motsa & Roy, Citation2008; Moss, Citation1961), Calcium (Ca) and Magnesium (Mg) (Okalebo et al., Citation1993), Cadmium (Cd) and Arsenic (As) were determined using the Atomic absorption spectroscopy (Model 210 VGP), Organic Carbon (OC) was obtained by the Walkley—Black wet oxidation method, Organic Matter (OM), Ash and moisture content analyses were done according to Okalebo etal. (Citation1993) Cyberscan waterproof pH meter and Hach EC METER (HQ30d) were used to find pH and Electrical Conductivity (EC) levels, respectively, and then lastly porosity (Das & Sobhan, Citation2013) and bulk density (Nolan et al., Citation2011) were examined.
2.2. Germination experiment and assay
Per trial, 5 g of biochar was considered and noted (Sun et al., Citation2017) and 100 ml of deionized water was added to the sample and agitated for 2 h at 120 rev min−1. Biochars were strained utilizing a 2 mm sieve (Oh et al., Citation2012). Exactly 10 cucumber seeds were planted on cotton and carefully put in decontaminated disposable petri dishes. Cucumber seeds were employed in this study because they can thrive and germinate even in areas concentrated with high salinity contents. They are also highly responsive to ammonia and organic acid toxicity (Ofosu-Budu et al., Citation2010). The salinity nature of food waste also encouraged the choice of cucumber seedlings. On a daily basis for 5 days, 5 ml portions of distilled water and biochar raw samples and distilled water extracts were added to all the petri dishes (Oh et al., Citation2012). Each petri dish received a total of 10 seeds (Sujeeun & Thomas, Citation2017). Incubation was done at 26°C under dark conditions. Growth assessment was carried out by measuring the shoot and radicle lengths of the various cucumber plants.
shows germination at day 5, shoot and radicle lengths were measured and then the germination (%) was recorded.
2.3. Laboratory analysis
One (1.0) gram of biochar sample was weighed into a dirt-free ceramic crucible. For each batch of five tests, an unfilled crucible was incorporated for a blank. Tests were put in a cool muffle furnace, and the temperature was increased to 500°C for 2 h. The temperature was stabilized for an additional 2 h. Samples were allowed to cool down in the furnace. The ashed samples were transferred into already labeled 50 ml centrifuge tubes. The crucibles were rinsed with 10 ml of both deionized water and aqua regia into the rotator tubes. Proper mixing of the samples was undertaken for 5 min utilizing a mechanical reciprocating shaker. Dissolved samples were then centrifuged for 10 min at 3000 rpm and then afterward moved into a 100 ml volumetric flask and again made up to the 100 ml mark. Decantation of the clear supernatant digests was done into clean reagent bottles for P, Ca, Mg, and K determinations. The flame photometer was used in the determination of the NPK levels.
The organic carbon content was obtained by the Walkley—Black wet oxidation method.
The organic matter content was found by
Five (5 g) of the air-dried biochar sample (grounded to less than 2 mm) was moved into a centrifuge tube and then 50 ml of deionized water was added. The centrifuge tube was closed, shaken very well by hand, and then placed on the shaker for an hour at 200 rpm. The suspension was then allowed to stand for 30 min. A calibrated pH and EC meters were used to take the various readings.
Analyses were carried out in duplicates. The mean difference was examined by one-way ANOVA. Statistical test was considered significant at P < 0.05. Graphs were plotted by means of Sigma Plot.
3. Results and discussion
3.1. Effect of temperature on properties of biochar
The yield of food waste biochar, FWB is as follows (71.4%, 42.9%, and 28.6% at 300°C, 450°C and 600°C respectively). The decrease in yield as the temperature increased could be attributed to the volatilization of some organic fractions (Boakye et al., Citation2018). Both temperature and precursor or feedstock type mainly influences biochar yield. It was noted that as the biochar yield reduced the ash content increased as observed by Jia et al. (Citation2018). Based on the results from the pyrolysis of various materials, a reduction in yield could be due to the loss of chemically bound moisture and the breakdown of organic substances (Alghashm et al., Citation2018).
It was reported that low-temperature biochars usually record higher yields because of the slow rate of condensation of aliphatic compounds and lower loss of CH4, H2, CO2 whiles at higher temperatures biochar yields are low due to the breakdown of hydroxyl groups and mostly thermal degradation of lignocellulosic assemblies (Rehrah et al., Citation2014). McHenry (Citation2009) reported that in biochars produced at temperatures equal to or greater than 400°C, the feedstock undergoes a transformation into a fused aromatic ring structure with losses of CO2, H20, and H2. The quantity of biochar obtained is highly dependent on the precursor material regardless of its solidity.
An increase in pyrolysis temperature resulted in a decrease in biochar yield as reported also by Hossain et al. (Citation2011). It was mentioned that the content of nitrogenous substances decreased, while trace elements such as (Ca, Fe, Mg S, Cu, and Zn) increased. Sun et al. (Citation2017) performed analysis on agricultural wastes, forest litter, and natural plants and recorded a low yield as pyrolysis temperature increased. However, it was realized that the yield decrease was smaller at higher temperatures; they noticed that for corn stalk biochar, there was a decrease of 27.7% from 300°C to 400°C whiles a smaller decrease was noticed from 500°C to 600°C at a percentage of 8.9%.
Zhao et al. (Citation2017) explained that with increasing pyrolysis temperature from 300°C to 500°C, a biochar decrease was observed at a range of 47.94% to 31.71%; it was noticed that this could be due to the decomposition of lignocellulosic substances that could have occurred within such temperature range. However, at temperatures of 500°C to 600°C; the biochar yield rather occurred at a lower range of 31.7% to 28.48%. It could be inferred that most of the volatile fraction got removed at lower temperatures. A report by Yuan et al. (Citation2011) explained that as pyrolysis temperature increased a decrease in biochar yield was noticed but, on the other hand, there was an increase in the conversion of volatile materials into bio-oil and syngas.
According to Novak et al. (Citation2009) a higher biochar yield was realized at lower temperatures (250°C) because of the lower rate of condensation of aliphatic compounds, minor volatilization and minor loss of organics evolved as CO2, CH4, H2, and CO2. Lower biochar yields are recorded at higher temperatures due to the fast breakdown of organic materials at higher temperatures enhancing the release of volatiles as well. The release of volatiles could be explained by the degree of decomposition of cellulose, hemicellulose, and lignin (Crombie et al., Citation2013). Glazunova et al. (Citation2018) discovered that biochar yield reduced when pyrolysis temperature increased, giving way to the stability of pH, ash, and carbon. An increase in biochar yield has been reported by Domingues et al. (Citation2017), who indicated the effect of temperature on biochar yield using precursors such as chicken manure, eucalyptus sawdust, coffee husk, sugar bagasse, and pine bark; at temperatures 350°C, 450°C and 750°C.
The physiochemical properties of raw food waste biomass and its biochars are represented in .
Table 1. Characteristics of food waste raw (FWR) and food waste biochar (FWB)
3.1.1. Porosity
Porosity levels increased as temperatures increased for FWB (89.1%, 90.4%, and 91.1%, at 300°C, 450°C, and 600°C respectively). Liu et al. (Citation2018) reported that biochar produced at lower pyrolysis temperatures is more suitable for agriculture purposes, whereas at higher temperatures porosity is more enhanced making such biochar suitable for absorbing contaminants. The porous nature of biochar makes it possess the ability to increase plant available water. Biomass characteristics, pyrolysis temperature, and residence time affect biochar porosity; biochars with low intraporosity such as wastewater sludge biochar and poultry litter sludge will not work well for water storage in water-lacking areas; because they have low internal porosity. Biochars produced from grass have shown to exhibit good water-holding capacities compared to sewage sludge biochar. This is because grass biochar has a higher intrapore volume compared to wastewater sludge biochar. Sun and Lu (Citation2014) realized that straw biochar has a higher plant available water compared to sewage sludge biochar. Straw biochar encouraged an increase in the pore volume of pores by <10 µm, but there was no significant pore volume for sewage sludge biochar in this range. Biochar production at a higher temperature enhances the development of more pores during the pyrolysis process. An appropriate pyrolysis temperature and residence time should be selected in order to produce biochar with high intraporosity.
The bulk density of biochars decreased as temperature increased (0.23 g/cm3, 0.22 g/cm3, 0.21 g/cm3 at 300°C, 450°C, and 600°C respectively) giving biochars a very porous structure. Bulk density levels mostly depend on the properties of the precursor biomass. Bulk density is an important parameter to consider when it comes to soil physical properties since it affects soil properties and plant growth. Soils with high bulk density do not have the potential to absorb water as well as give a good ground for easy penetration of the roots of plants so growth is impeded. Mukherjee & Zimmerman (Citation2013) reported that the application of biochar reduced the bulk density of soils because biochar tends to have high porosity, so when applied decreases soil bulk density and pore volume. Githinji (Citation2013) also noticed that the continual application of biochar to soil decreases the bulk density of soils.
The ash contents increased as pyrolytic temperatures increased. Similar results were recorded by Boakye et al (Citation2018, Citation2019). Also, Domene et al. (Citation2015) carried out an analysis of food wastes (discarded from food preparation, unconsumed food, and paper plates and napkins), and found that as temperature increased ash contents increased in biochars. High ash content ensures biochars are rich in nutrients with high alkalizing capacity. A significant difference was recorded across the various temperatures in this study (ρ<0.05). Animal manures contain a substantial blend of organics and inorganics and when used in the production of biochar results in biochar rich in nutrients since the feedstock in itself is rich in nutrients (Cantrell et al., Citation2012; Wan et al., Citation2014). A high percentage of nutrients has been observed in poultry litter compared to peanut hull and pecan shell biochars. High nutrient contents in biochars indicate a high ash content (Wan et al., Citation2014). The high ash content of biochar gives it the ability to correct soil acidity (Novak et al., Citation2009). Domingues et al. (Citation2017) reported an increase in ash content as temperature increased on feedstock such as chicken manure, coffee husk, sugarcane bagasse, and eucalyptus sawdust. An analysis done by Khanmohammadi et al. (Citation2015) on sewage sludge at temperatures 300°C, 400°C, 500°C, 600°C, and 700°C; revealed that with an increase in pyrolysis temperature, ash levels increased. Thus, in this study, the biochar obtained at 300°C had the least ash content. According to Claoston et al. (Citation2014), an increase in ash content was due to the fast removal of volatiles during pyrolysis, thereby producing char rich in carbon. High ash increase could also be due to a reduction in contents of elements such as C, O, H, N, and S which are lost during heating, inorganic salts like quartz and calcite are not completely lost. Tsai et al. (Citation2012) reported an increase in ash content with an increase in pyrolysis temperature, this happened due to the loss of lignocellulosic compounds.
Also, pH (10.32–12.0) and EC (3300 μS/cm-6990 μS/cm) values increased in the biochars as the temperature was increased. According to Tsai et al. (Citation2012), an increase in the pH of rice husks and empty fruit bunch was recorded with an increment in temperature. It was mentioned that this could be due to the dissociation of minerals from the organic matrix at a temperature above 350°C at which ashes are formed. A study carried out by Yuan et al. (Citation2011) on straws of canola, corn, soybean, and peanut revealed an increase in pH with a rise in pyrolysis temperature. Generally, the pH of biochar correlates with the presence of carbonates and inorganic alkalis. Having these present in biochar is the main cause for the rise in pH (Ding et al., Citation2014). Basic cations and carbonates have been reported to rise with an increase in pyrolysis temperature leading to a rise in pH as well that is ranging from 6.5 to 10.8 (Yuan et al., Citation2011). The increase in ash and O2 functional groups which rise with the increase in temperature are associated with pH as well (Ronsse et al., Citation2013). The breaking off of acidic functional groups and the coming in of basic functional groups are some reasons why pH increases, and an increase in pH also occurs due to the separation of alkali salts away from the organic matrix thereby increasing the pH (Ding et al., Citation2014; Yuan et al., Citation2011). López-Cano et al. (Citation2018) also reported an increase in EC with a rise in pyrolysis temperature when feedstock such as holm oak and some organic waste like greenhouse wastes and green wastes were subjected to pyrolysis at different temperatures. Alghashm et al. (Citation2018) reported an increase in EC as temperature increased, it was mentioned that the EC level gives an idea of the salinity levels of the various biochar obtained, biochar with high EC level must be carefully applied to the soil so that plant growth is not impeded. Rehrah et al. (Citation2014) also recorded an increase in EC content with an increase in pyrolysis temperature. It was mentioned that there could be a possible relation between pH, EC, and ash contents as temperature increases. A significant difference was observed (ρ<0.05) for both pH and EC values, and the pyrolysis process led to a significant difference. Moreso, C:N ratios increased as temperatures were increased, FWB3–600°C recorded the highest C:N ratio. The occurrence of C:N ratio has an influence on the C and N levels during their conversion from inorganic to organic compounds. High C: N values are responsible for the stable nature of N and decreasing its loss from the soil (Alghashm et al., Citation2018).
3.2. Effect of temperature on nutrient levels of biochars
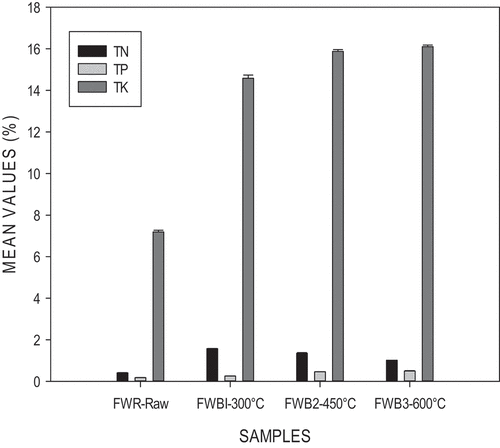
shows an illustration of the chemical properties of FWR-Raw and FWB. The results obtained showed that with increasing pyrolysis temperature TK and TP levels increased, whereas TN levels decreased.
After pyrolysis, total nitrogen levels decreased as temperatures increased with FWB1–300°C (1.576%) being the highest. A significant difference was observed across all the temperatures (ρ<0.05). Alghashm et al. (Citation2018) reported a decrease in N content in biochar as temperature increased, it was mentioned that this could have occurred due to an increase in feedstock combustion and organic compounds being volatised. Since pyrolysis temperatures occurred at 300°C, 450°C, and 600°C, most of the nitrogen-containing groups were being lost. Elnour et al. (Citation2019) also reported a decrease in biochar N content as temperature increased. It was explained that this could have occurred due to the quick level of heating with high pyrolysis temperature, with this fast and intense heating of the biomass, the interior of the biomass is exposed as well its functional groups to quick cracking.
However, for the phosphorous levels, there was an increase with rising temperature resulting in biochars rich in phosphorous at higher temperatures. Unlike Carbon and Nitrogen which are usually lost by volatilization during pyrolysis phosphorous tends to be in biochar. Phosphorous is usually dominant in the inorganic fraction. It can be volatilized at temperatures above 700°C (Gaskin et al., Citation2008; Hossain et al., Citation2011). A significant difference was observed (ρ<0.05) across the temperatures. A similar trend was observed by Naeem et al. (Citation2014) in the analysis of rice straw biochar. Potassium levels increased as temperatures were increased with the highest being in FWB3–600°C. Potassium occurs as an inorganic alkali (Zhao et al., Citation2017) and is hence a contributing factor to high pH values in food wastes biochars. A significant difference was observed (ρ<0.05). Due to the presence of salts in the feedstock used, it resulted in very high potassium content. Food waste biochars would be very helpful in soils deficient in potassium, which happens to be an issue to soils all over the world (Moody, Government and Bell, Moody & Bell, Citation2006).
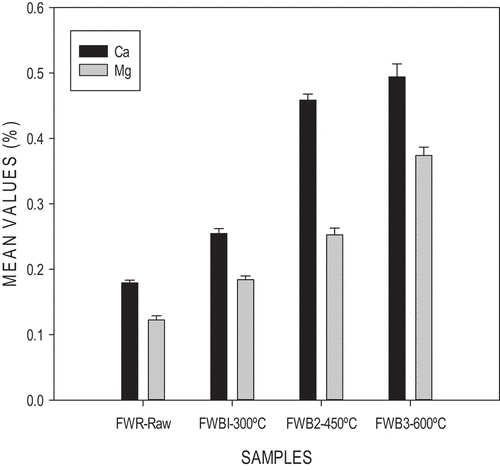
Ca levels increased as temperature increased as shown in . Food wastes biochar generally contained high Ca contents (2.017%–3.2115%) compared to the other biochar. Mostly green waste biochars contain high Ca levels due to the presence of minerals such as calcite, anorthite, augite, and hornblende (Smider & Singh, Citation2014). Gaskin et al. (Citation2008) and Singh et al. (Citation2010) reported that biochars produced from various biomass feedstock are normally rich in Ca, during the pyrolysis process, the concentration of this nutrient in biochar is enhanced by the increasing volatilization of C, H, O, and N. A significant difference was obtained across the temperatures (ρ<0.05). Naeem et al. (Citation2014) recorded similar increments in wheat straw biochar.
Mg levels increased as temperatures increased (0.18%–0.37%). The alkaline nature of biochar leads to a buildup of carbonates; Mg ions become concentrated as well. Biochar contains an assemblage of inorganic components such as Mg (Novak et al., Citation2018). During pyrolysis, minerals such as hydroxides, phosphates, sulfates, carbonates, chlorides, and nitrates are introduced due to the thermal breakdown process (Boakye et al., Citation2018), as well as causes an increase in magnesium content. A significant difference was observed across the temperatures (ρ<0.05).
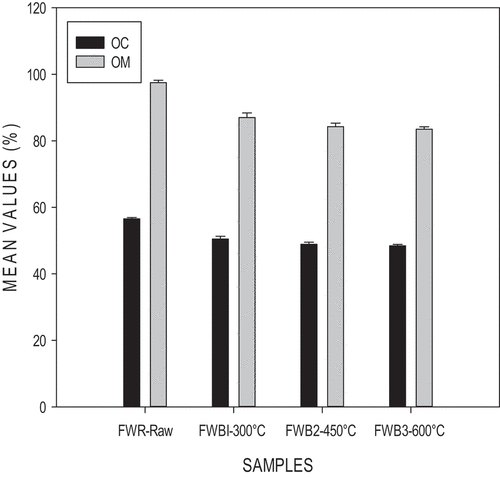
There was no significant difference (ρ>0.05) for the organic carbon content. Values obtained were 50.464%, 48.869%, and 48.434%. The volatilization of C was not rapid but at a slow process. The organic carbon content decreased as temperature increased. A similar trend was observed by Domene et al. (Citation2015) who performed an analysis of food waste. This is due to C losses which are likely due to the increased volatility of C during pyrolysis. The decrease of carbon and nitrogen resulted from the increasing biomass degradation and organic volatilization with increasing temperature. Also, OM contents decreased as temperature increased as seen in . This could be due to the loss of the organic-containing compounds. Between 130°C and 190°C, lignin and hemicellulose begin to degrade. Above 200°C, the carbonization process starts (Chandler & Dodds, Citation1983). A significant difference was observed (ρ<0.05).
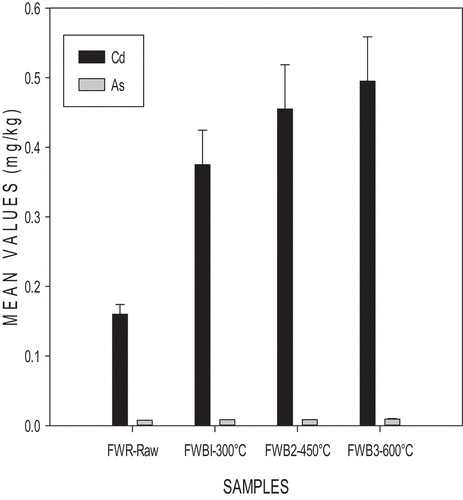
presents the heavy metal concentration in FWR-Raw and its biochar. As pyrolysis temperature was increased, the Cd and As levels in the various biochars increased. Heavy metal concentration increased as temperatures increased [FWB1–300°C (As-0.00846 mg/kg, Cd-0.375 mg/kg), FWB2–450°C (As-0.0087 mg/kg, Cd-0.455 mg/kg) and FWB3–600°C (As-0.0102 mg/kg, Cd-0.495 mg/kg)].
A similar trend was observed by Beneireh et al. (Citation2020) in the analysis of faecal sludge biochar and faecal sludge with sawdust. Increasing pyrolysis temperature rather causes a buildup of heavy metals. Liu et al. (Citation2016) upon analysis of raw sewage sludge and its biochar drew a conclusion that the pyrolysis process causes an increase in heavy metal concentration as temperatures increase. The risk involved in the usage of biochar is low compared to raw feedstock. Heavy metals tend to be immobile in biochar so making them harmless when used as soil amendments (Zhou et al., Citation2017). All biochars contained heavy metals below the permissible range given by the international biochar initiative [(As 13–100 mg/kg; Cd 1.4–39 mg/kg)] (International Biochar Initiative, Citation2012), making them safe for farm application.
4. Germination
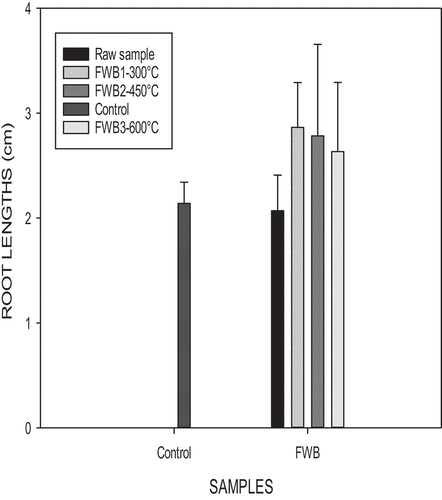
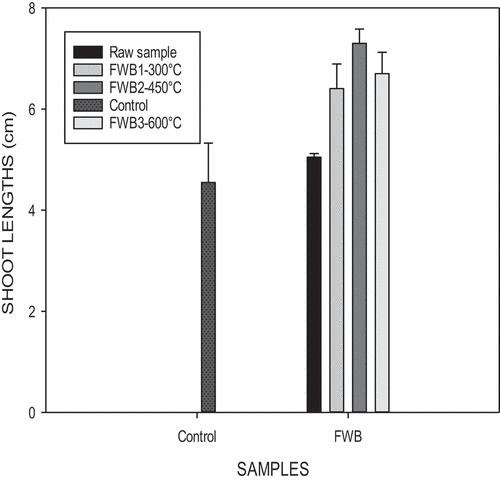
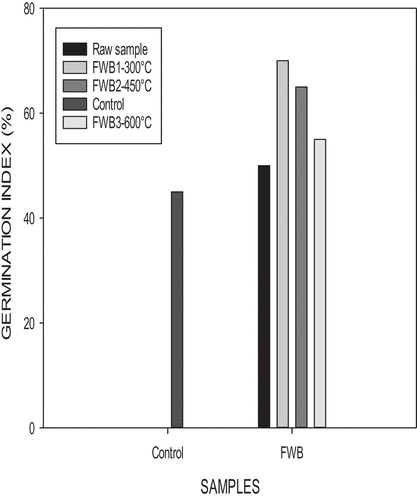
Food waste pyrolyzed at 300°C enhanced a higher germination index as well as longer root lengths (). At a temperature of 450°C longer shoot lengths were enhanced (Figure ). During pyrolysis, nutrients contained in the biochars go through a change in their chemical structures. A portion of nutrients in the biochar are not soluble in water. Biochars contain water-soluble phototoxic compounds, especially in biochars produced at higher pyrolytic temperatures (600°C) (Rogovska et al., Citation2012).
The introduction of the produced biochar to soil can therefore enhance its physical, chemical, and biological properties, leading to improved water retention, and nutrient availability. The resultant positive impact on crop yields and plant health has transformative implications for sustainable food production.
5. The cost analysis of producing the biochar
The cost analysis of producing the biochar in this study is as shown below ().
Table 2. Cost analysis of producing biochar
The cost of producing a kilogram mass of biochar. Assuming that a batch of sourced food waste can be used to produce a kilogram mass of biochar. See analysis in the table (Table ).
Hence, the cost of producing a kilogram mass of biochar was GHS 513.97 (USD 45.52).
6. Conclusion
Pyrolysis enhanced the presence of essential plant nutrients such as TP, TK, Ca, and Mg. These are major nutrients needed for plant growth, and when not present in large quantities in the soil will cause farmers to go in for inorganic fertilizers which cause global warming and pollute surface water bodies. Higher temperature leads to the enrichment of TP, TK, Ca, and Mg contents. The yield of biochar and total nitrogen levels decreased while ash content increased with increasing pyrolysis temperature. Also, pH and porosity increased with increasing temperature for all biochars; furthermore, the heavy metal concentration was within the acceptable limits provided by the International Biochar Initiative [(As 13–100 mg/kg; Cd 1.4–39 mg/kg)] (International Biochar Initiative, Citation2012). Biochars produced at lower temperatures are less phototoxic and so are encouraged for agricultural purposes.
The significance of the study is that the conversion of food wastes into biochar has a potential application for solid waste management and agriculture relevance. So, biochar production can be seen as an option for solid food waste management and organic fertilizer production. Research should be carried out on the combination and the categories of food waste feedstock that were used in the pyrolysis process.
Highlights
Increased generation of food waste due to growing population.
Disposal of food wastes into landfills reduces landfill lifespan.
Gases and leachate generated during landfilling, a threat to the ecosystem.
Food waste biochar production—waste management and organic fertilizer production.
Abbreviation
FWB represents Food Waste biochar, all biochars produced from Food Waste constitute a mixture of cooked and uncooked food wastes which were dried and milled; FWB1–300°C, FWB2–450°C and FWB3–600°C; pyrolysis temperature at which production was achieved include 300°C, 450°C and 600°C, respectively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Ahmad, M., Lee, S. S., Dou, X., Mohan, D., Sung, J. K., Yang, J. E., & Ok, Y. S. (2012). Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technology, 118, 536–14.
- Aissaoui, M. H., Trabelsi, A. B. H., Abidi, S., Zaafouri, K., Haddad, K., Jamaaoui, F., & Kwapinski, W. (2023). Sustainable biofuels and biochar production from olive mill wastes via co-pyrolysis process. Biomass Conversion and Biorefinery, 13(10), 8877–8890.
- Alghashm, S., Qian, S., Hua, Y., Wu, J., Zhang, H., Chen, W., & Shen, G. (2018). Properties of biochar from anaerobically digested food waste and its potential use in phosphorus recovery and soil amendment. Sustainability, 10(12), 4692. https://doi.org/10.3390/su10124692
- Beneireh, N. M., Boakye, P., Oduro-Kwarteng, S., & Sokama-Neuyam, Y. A. (2020). Valorization of faecal and sewage sludge via pyrolysis for application as crop organic fertilizer. Journal of Analytical and Applied Pyrolysis, 151, 104903. https://doi.org/10.1016/j.jaap.2020.104903
- Boakye, P., Sewu, D. D., & Woo, S. H. (2018). Effect of thermal pretreatment on the extraction of potassium salt from alga Saccharina japonica. Journal of Analytical and Applied Pyrolysis, 133, 68–75. https://doi.org/10.1016/j.jaap.2018.04.019
- Boakye, P., Tran, H. N., Lee, D. S., & Woo, S. H. (2019). Effect of water washing pretreatment on property and adsorption capacity of macroalgae-derived biochar. Journal of Environmental Management, 233, 165–174. https://doi.org/10.1016/j.jenvman.2018.12.031
- Cantrell, K. B., Hunt, P. G., Uchimiya, M., Novak, J. M., & Ro, K. S. (2012). Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresource Technology, 107, 419–428. https://doi.org/10.1016/j.biortech.2011.11.084
- Chandler, S. F., & Dodds, J. H. (1983). The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Reports, 2(4), 205–208. https://doi.org/10.1007/BF00270105
- Claoston, N., Samsuri, A. W., Ahmad Husni, M. H., & Mohd Amran, M. S. (2014). Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Management & Research, 32(4), 331–339. https://doi.org/10.1177/0734242X14525822
- Crombie, K., Mašek, O., Sohi, S. P., Brownsort, P., & Cross, A. (2013). The effect of pyrolysis conditions on biochar stability as determined by three methods. Global Change Biology Bioenergy, 5(2), 122–131. https://doi.org/10.1111/gcbb.12030
- Das, B. M., & Sobhan, K. (2013). Principles of geotechnical engineering. Cengage learning.
- Ding, W., Dong, X., Ime, I. M., Gao, B., & Ma, L. Q. (2014). Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere, 105, 68–74. https://doi.org/10.1016/j.chemosphere.2013.12.042
- Domene, X., Enders, A., Hanley, K., & Lehmann, J. (2015). Ecotoxicological characterization of biochars: Role of feedstock and pyrolysis temperature. Science of the Total Environment, 512, 552–561. https://doi.org/10.1016/j.scitotenv.2014.12.035
- Domingues, R. R., Trugilho, P. F., Silva, C. A., Melo, I. C. N. D., Melo, L. C., Magriotis, Z. M., & Sanchez-Monedero, M. A. (2017). Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PloS One, 12(5), e0176884. https://doi.org/10.1371/journal.pone.0176884
- Elnour, A. Y., Alghyamah, A. A., Shaikh, H. M., Poulose, A. M., Al-Zahrani, S. M., Anis, A., & Al-Wabel, M. I. (2019). Effect of pyrolysis temperature on biochar microstructural evolution, physicochemical characteristics, and its influence on biochar/polypropylene composites. Applied Sciences, 9(6), 1149. https://doi.org/10.3390/app9061149
- Gaskin, J. W., Steiner, C., Harris, K., Das, K. C., & Bibens, B. (2008). Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Transactions of the ASABE, 51(6), 2061–2069. https://doi.org/10.13031/2013.25409
- Githinji, L. (2013). Effect of biochar application rate on soil physical properties of a sandy loam. Archives of Agronomy & Soil Science, 60(4), 457–470. https://doi.org/10.1080/03650340.2013.821698
- Glazunova, D. M., Kuryntseva, P. A., Selivanovskaya, S. Y., & Galitskaya, P. Y. (2018). Assessing the potential of using biochar as a soil conditioner. IOP Conference Series: Earth and Environmental Science, 107, 012059. https://doi.org/10.1088/1755-1315/107/1/012059
- Hossain, M. K., Strezov, V., Chan, K. Y., Ziolkowski, A., & Nelson, P. F. (2011). Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. Journal of Environmental Management, 92(1), 223–228. https://doi.org/10.1016/j.jenvman.2010.09.008
- International Biochar Initiative. (2012). Standardized product definition and product testing guidelines for Biochar That is Used in Soil| International Biochar Initiative. International Biochar Initiative, 47. www.biochar-international.org/characterizationstandard
- Jia, Y., Shi, S., Liu, J., Su, S., Liang, Q., Zeng, X., & Li, T. (2018). Study of the effect of pyrolysis temperature on the Cd2+ adsorption characteristics of biochar. Applied Sciences, 8(7), 1019. https://doi.org/10.3390/app8071019
- Khanmohammadi, Z., Afyuni, M., & Mosaddeghi, M. R. (2015). Effect of pyrolysis temperature on chemical and physical properties of sewage sludge biochar. Waste Management & Research, 33(3), 275–283. https://doi.org/10.1177/0734242X14565210
- Liu, Z., Singer, S., Tong, Y., Kimbell, L., Anderson, E., Hughes, M., Zitomer, D., & McNamara, P. (2018). Characteristics and applications of biochars derived from wastewater solids. Renewable and Sustainable Energy Reviews, 90, 650–664. https://doi.org/10.1016/j.rser.2018.02.040
- Liu, X., Wang, Y., Gui, C., Li, P., Zhang, J., Zhong, H., & Wei, Y. (2016). Chemical forms and risk assessment of heavy metals in sludge-biochar produced by microwave-induced low temperature pyrolysis. RSC Advances, 6(104), 101960–101967. https://doi.org/10.1039/C6RA22511J
- López-Cano, I., Cayuela, M. L., Mondini, C., Takaya, C. A., Ross, A. B., & Sánchez-Monedero, M. A. (2018). Suitability of different agricultural and urban organic wastes as feedstocks for the production of biochar—part 1: Physicochemical characterisation. Sustainability, 10(7), 2265. https://doi.org/10.3390/su10072265
- Major, J., Rondon, M., Molina, D., Riha, S. J., & Lehmann, J. (2010). Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant and Soil, 333, 117–128.
- Mazac, R. (2016). Assessing the use of food waste biochar as a Biodynamic plant fertilizer. Biology Department and Program in Environmental Studies, Hamline University.
- McHenry, M. P. (2009). Agricultural bio-char production, renewable energy generation and farm carbon sequestration in Western Australia: Certainty, uncertainty and risk. Agriculture, Ecosystems & Environment, 129(1–3), 1–7. https://doi.org/10.1016/j.agee.2008.08.006
- Moody, P. W., & Bell, M. J. (2006). Availability of soil potassium and diagnostic soil tests. Soil Research, 44(3), 265–275. https://doi.org/10.1071/SR05154
- Moss, P. (1961). Limits of interference by fe, mn, Al and phosphate in the EDTA determination of calcium in the presence of mg using calcon-red as indicator. Journal of the Science of Food and Agriculture, 12, 30–34.
- Motsa, M. R., & Roy, R. N. (2008). Guide to Laboratory Establishment for Plant Nutrient Analysis. FAO.
- Mukherjee, A., & Zimmerman, A. R. (2013). Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar soil mixtures. Geoderma, 193, 122–130.
- Naeem, M. A., Khalid, M., Arshad, M., & Ahmad, R. (2014). Yield and nutrient composition of biochar produced from different feedstocks at varying pyrolytic temperatures. Pakistan Journal of Agricultural Sciences, 51(1), 75–82.
- Nolan, T., Troy, S. M., Healy, M. G., Kwapinski, W., Leahy, J. J., & Lawlor, P. G. (2011). Characterization of compost produced from separated pig manure and a variety of bulking agents at low initial C/N ratios. Bioresource Technology, 102(14), 7131–7138.
- Novak, J. M., Johnson, M. G., & Spokas, K. A. (2018). Concentration and release of phosphorus and potassium from lignocellulosic-and manure-based biochars for fertilizer reuse. Frontiers in Sustainable Food Systems, 2, 54. https://doi.org/10.3389/fsufs.2018.00054
- Novak, J. M., Lima, I., Xing, B., Gaskin, J. W., Steiner, C., Das, K. C., Ahmedna, M., Rehrah, D., Watts, D. W., Busscher, W. J., & Schomberg, H. (2009). Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Annals of Environmental Science, 3, 195–206.
- Ofosu-Budu, G. K., Hogarh, J. N., Fobil, J. N., Quaye, A., Danso, S. K. A., & Carboo, D. (2010). Harmonizing procedures for the evaluation of compost maturity in two compost types in Ghana. Resources, Conservation and Recycling, 54(3), 205–209. https://doi.org/10.1016/j.resconrec.2009.08.001
- Oh, T. K., Choi, B., Shinogi, Y., & Chikushi, J. (2012). Effect of pH conditions on actual and apparent fluoride adsorption by biochar in aqueous phase. Water, Air, & Soil Pollution, 223(7), 3729–3738. https://doi.org/10.1007/s11270-012-1144-2
- Okalebo, J. R., Gathua, K. W., & Woomer, P. L. (1993). Laboratory Methods of Soil and Plant Analysis: A working manual. Tropical Soil biology and fertility, Soil Science Society of East Africa.
- Rehrah, D., Reddy, M. R., Novak, J. M., Bansode, R. R., Schimmel, K. A., Yu, J., Watts, D. W., & Ahmedna, M. (2014). Production and characterization of biochars from agricultural by-products for use in soil quality enhancement. Journal of Analytical and Applied Pyrolysis, 108, 301–309. https://doi.org/10.1016/j.jaap.2014.03.008
- Ren, X. H., Zhang, P., Zhao, L. J., & Sun, H. W. (2016). Sorption and degradation of carbaryl in soils amended with biochars: Influence of biochar type and content. Environmental Science and Pollution Research, 23(3), 2724–2734. https://doi.org/10.1007/s11356-015-5518-z
- Rogovska, N., Laird, D., Cruse, R. M., Trabue, S., & Heaton, E. (2012). Germination tests for assessing biochar quality. Journal of Environmental Quality, 41(4), 1014–1022. https://doi.org/10.2134/jeq2011.0103
- Ronsse, F., Van Hecke, S., Dickinson, D., & Prins, W. (2013). Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Global Change Biology Bioenergy, 5(2), 104–115. https://doi.org/10.1111/gcbb.12018
- Singh, B., Singh, B. P., & Cowie, A. L. (2010). Characterization and evaluation of biochars for their application as a soil amendment. Soil Research, 48(7), 516–525. https://doi.org/10.1071/SR10058
- Smider, B., & Singh, B. (2014). Agronomic performance of a high ash biochar in two contrasting soils. Agriculture, Ecosystems & Environment, 191, 99–107. https://doi.org/10.1016/j.agee.2014.01.024
- Sohi, S. P., Krull, E., Lopez-Capel, E., & Bol, R. (2010). A review of biochar and its use and function in soil. In Advances in agronomy (Vol. 105, pp. 47–82). Academic Press. https://doi.org/10.1016/S0065-2113(10)05002-9
- Sujeeun, L., & Thomas, S. C. (2017). Potential of biochar to mitigate allelopathic effects in tropical island invasive plants: Evidence from seed germination trials. Tropical Conservation Science, 10, 1940082917697264. https://doi.org/10.1177/1940082917697264
- Sun, J., Drosos, M., Mazzei, P., Savy, D., Todisco, D., Vinci, G., Pan, G., & Piccolo, A. (2017). The molecular properties of biochar carbon released in dilute acidic solution and its effects on maize seed germination. Science of the Total Environment, 576, 858–867. https://doi.org/10.1016/j.scitotenv.2016.10.095
- Sun, F., & Lu, S. (2014). Biochars improve aggregate stability, water retention, and pore‐space properties of clayey soil. Journal of Plant Nutrition and Soil Science, 177(1), 26–33. https://doi.org/10.1002/jpln.201200639
- Tsai, W. T., Liu, S. C., Chen, H. R., Chang, Y. M., & Tsai, Y. L. (2012). Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere, 89(2), 198–203. https://doi.org/10.1016/j.chemosphere.2012.05.085
- Wan, Q., Yuan, J. H., Xu, R. K., & Li, X. H. (2014). Pyrolysis temperature influences ameliorating effects of biochars on acidic soil. Environmental Science and Pollution Research, 21(4), 2486–2495. https://doi.org/10.1007/s11356-013-2183-y
- Yanai, Y., Toyota, K., & Okazaki, M. (2007). Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Science & Plant Nutrition, 53(2), 181–188. https://doi.org/10.1111/j.1747-0765.2007.00123.x
- Yuan, J. H., Xu, R. K., & Zhang, H. (2011). The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technology, 102(3), 3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018
- Zhao, S. X., Ta, N., & Wang, X. D. (2017). Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies, 10(9), 1293. https://doi.org/10.3390/en10091293
- Zhou, D., Liu, D., Gao, F., Li, M., & Luo, X. (2017). Effects of biochar-derived sewage sludge on heavy metal adsorption and immobilization in soils. International Journal of Environmental Research and Public Health, 14(7), 681. https://doi.org/10.3390/ijerph14070681