 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Biocultural diversity embraces the dynamic, place-based and complex relationship between biological and cultural diversity. Several studies describe a direct, positive relationship between biological and cultural diversity; however, this relationship is usually entwined within a particular socio-ecological context. We explored the relationship between cultural diversity and agrobiodiversity in smallholder farming systems in a rural landscape in south-central Chile considered as Indigenous Mapuche ancestral territory. We hypothesized a positive correlation between cultural diversity and agrobiodiversity in this context. We estimated three levels of agrobiodiversity: (i) subsystems (vegetable garden, orchard, chacra, annual crops and natural places), (ii) plant species and (iii) plant landraces. In our study area, smallholders form three distinctive groups based on their cultural origin: (i) Indigenous Mapuche, (ii) Chileans and (iii) foreigners. Using diversity indices, we explored patterns across 15 focal landscapes (3.14 km2). Contrary to our hypothesis, we found a negative correlation between cultural diversity and agrobiodiversity, as while Mapuche farms presented the highest agrobiodiversity and were dominant in most focal landscapes, Chilean and foreign-owned farms were mostly dominated by monocultures. This negative link highlights the need to further study this relationship considering different socio-ecological aspects from a historical perspective, as well as from a socio-political point of view. Understanding the complex interactions between culture and biodiversity could help us in facing current challenges such as biodiversity loss, cultural homogenization and reducing conventional agriculture impacts.
1. Introduction
The Food and Agriculture Organization of the United Nations (FAO) estimated that 75% of the agrobiodiversity worldwide was lost during the 20th century (FAO Citation2010). Agrobiodiversity, considered as the variety of animals, plants and microorganisms used directly or indirectly in agricultural systems (Brookfield Citation2001) is the result of several millennia of agriculture, and represents not only an immense library of genetic diversity and valuable traits (Castañeda-Álvarez et al. Citation2016) but also an invaluable amount of knowledge in terms of associated agricultural practices, culinary traditions, medicinal tools, and other cultural benefits (Tutwiler, Bailey, Attwood, Remans, Ramirez Citation2017). In the current context of climate change, biodiversity loss, social conflicts and pandemic events, agrobiodiversity is a crucial issue for food security and sovereignty (Altieri, Nicholls, Henao, Lana Citation2015; Figueroa-Helland, Thomas, Aguilera Citation2018), not only because it represents a source of good nutrition but also because the productivity and stability of farming systems are tightly linked to its functional biodiversity (e.g. pollination, nutrient cycling, etc.) (Altieri, Nicholls, Henao, Lana Citation2015; Dainese et al. Citation2019; Tamburini et al. Citation2020). Understanding how biodiversity relates to agroecosystems functions could help at designing sustainable agricultural systems at local, regional and global scales (Zimmerer et al. Citation2019).
The relationship between ethnic groups and their environment is mediated by culture (Berkes, Folke, Colding Citation2000; Perino et al. Citation2019). The concept of biocultural diversity embraces the dynamic, place-based and complex relationship arising from links and feedbacks between biological diversity (genetic, populations, species, ecosystems) and human cultural diversity (covering from individual ideas to entire cultures, including linguistic diversity) (Bridgewater, Rotherham, Rozzi Citation2019; Maffi Citation2007), as these concepts are entwined within a particular socio-ecological context (Ibarra et al. Citation2021; Liu et al. Citation2007). Tilley (Citation2006) asserted that landscapes are “always in process, rather than static, being and becoming” and contested by people in line with individual, social or political circumstances. The application of the biocultural diversity framework in the context of socioecological resilience and biodiversity conservation offers an opportunity to reduce biodiversity loss by understanding how nature and culture interacts to create the main ecological processes driving landscape changes (Agnoletti & Rotherham, Citation2015; Bridgewater, Rotherham, Rozzi Citation2019; Rotherham Citation2015). Currently, both urbanization of rural areas and migration of rural people to urban centres are the major landscape drivers changing the biocultural diversity of a territory (Hatab, Cavinato, Lagerkvist Citation2019). Rotherham (Citation2013) described this process as “cultural severance”, leading to long-term biodiversity loss and thus, a decrease in landscape quality, which usually occurs as a result of a loss in traditional-ethnic knowledge, sometimes replaced by conventional management practices, leading to the concomitant loss of strategies to deal with future global challenges (Massawe, Mayes, Cheng Citation2016; Mijatović, Van Oudenhoven, Eyzaguirre, Hodgkin Citation2013).
Several studies illustrated this merge of biological and cultural diversity in landscape scales, describing a positive relationship between them (Loh & Harmon, Citation2005; Maffi & Woodley, Citation2012; Pretty et al., Citation2009; Ricciardi, Mehrabi, Wittman, James, Ramankutty Citation2021), and different hypotheses have been proposed to explain it. On one hand, the positive association between cultural and biological diversity has been proposed to respond merely to the geographic location (Loh & Harmon, Citation2005; Stepp et al. Citation2004). Thus, this positive correlation has been reported at global or regional scales finding that higher linguistic diversity co-occurs with higher biodiversity (Gorenflo, Romaine, Mittermeier, Walker-Painemilla Citation2012; Moore et al. Citation2002). On the other hand, positive correlation of cultural and biological diversity could be explained from an ethnoecological perspective, exploring how distinct ethnic groups perceive and appropriate nature through their beliefs (i.e. cosmovision), knowledge or cognitive systems, manifested in agricultural practices that can promote and maintain high levels of biodiversity (Berkes Citation2017; Pilgrim & Pretty, Citation2010; Ricciardi, Mehrabi, Wittman, James, Ramankutty Citation2021). Other hypotheses propose the isolation between cultural groups as the main mechanism behind culture and biodiversity correlation (Gorenflo, Romaine, Mittermeier, Walker-Painemilla Citation2012). As high biologically diverse areas could reduce the need of different ethnic/cultural groups to communicate and share resources, it might explain the positive correlation between linguistic (as proxy of cultural diversity) and biological diversity (Sutherland Citation2003). Biodiversity can also increase in human dominated landscapes, by selecting specific species traits in specific locations, thus increasing diversity at genetic, species and landscape levels (Ibarra et al. Citation2021). Thus, the correlation between cultural diversity and biodiversity can also be related to the available local knowledge that promotes biodiversity in agroecosystems (Wyckhuys et al. Citation2020).
The Araucanía region, in south-central Chile, was part of the area dominated by the Indigenous Mapuche people, and was the last geopolitical region to be incorporated into the Chilean State in 1883 (Bengoa Citation2011). Currently, this region is recognized as ancestral Mapuche land (De la Maza Citation2014) and hosts the highest proportion of Mapuche rural population in the country (INE (Instituto Nacional de Estadísticas) Citation2017). Between 1883 and 1901, the Chilean State promoted migration to the region, mainly for European settlers, granting land titles with the condition that settlers farm the land. In the 1890s, the State began to give land to officers and soldiers from the Chilean army, and later, to married Chilean men without a criminal record (Bengoa Citation2011). During that period, only 10% of ancestral Mapuche land (approx. 500,000 ha) was assigned to the Mapuche by the State, in the form of reservations located in different areas distributed across the less productive land of the region (Di Giminiani Citation2015). Since then, Mapuche Indigenous people have undergone into processes of land retribution by the State, which are still taking place nowadays (Bauer Citation2018). As a result, increases the number of cultures that coexisted in the region, such as Mapuche, Chilean, Spanish, German, Dutch, French, Suisse, Italians, amongst others (Montalba, Vieli, Vallejos Romero, Zunino, Vera Citation2017). Therefore, the Araucanía region represents an excellent opportunity to study the complex and dynamic relationships between culture and biodiversity in agroecosystems. Here, we hypothesize that the arrival of new cultural groups to the Araucanía region, also introduced new culinary traditional and cultivated species, which increased the agrobiodiversity of the territory, and therefore higher levels of cultural diversity, represented by three different cultural groups (i.e. “Mapuche”, “Chilean” and “Foreigner”), positively correlates with higher agrobiodiversity. By exploring the relationship of cultural diversity and agrobiodiversity, we will provide evidence to create appropriate public policies to conserve agrobiodiversity in a landscape highly threatened by global change and food security.
2. Materials and methods
2.1. Study site
We conducted our study in the drylands (Secano interior) of the Coastal Range within the Araucanía region (38.20S; 72.78 W), located in south-central Chile (). The climate is temperate Mediterranean, characterized by a long dry season of 5-6 months per year, and 800–900 mm of annual precipitation (Uribe, Cabrera, De la Fuente, Paneque Citation2012). The Secano Interior area was one of the first colonized in 1883 by Chilean and European settlers (mainly Swiss, Italian, German, and French) (Di Giminiani, Fonck, Perasso Citation2021). After the colonization process, under a human vs. nature socio-political paradigm (Nahuelpán Citation2012), in less than 20 years non-Mapuche people burned more than 500,000 ha of native forests and converted it to agricultural land (Henríquez Jaramillo Citation2013). This long-term depletion of vegetation and soil organic matter led to unproductive agricultural lands, which later led to forest plantations with exotic species (e.g. Pinus and Eucalyptus spp.). The Chilean law define smallholding agriculture those farm with maximum 12 ha of basic irrigation system. In our study area, the landscape is dominated by small agricultural farms (less than 15 ha), and to a less extent forest plantations owned by large private companies. The crop species in the area include mainly cereals (wheat, oat, barley, rye), legumes and potatoes. Livestock (cattle and sheep) for meat production is typically implemented in uncultivated farm areas. Due to the historic process that determined the actual land ownership, Mapuche smallholders’ farms are usually smaller than the non-Mapuche. In this context, the farms located in our study area have poor productive capacity and water scarcity, which, in addition to climate change, is one of the main threats that these farmers currently face (Little, Lara, McPhee, Urrutia Citation2009; Montalba, Fonseca, García, Vieli, Altieri Citation2015).
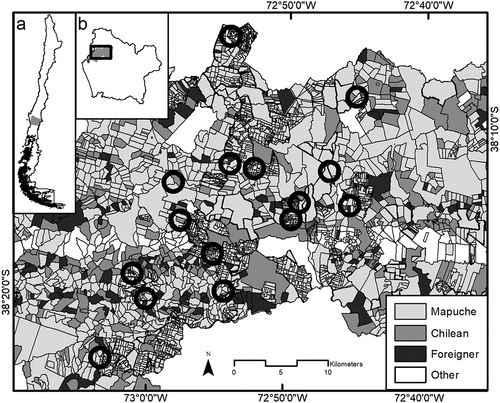
Figure 1. Location of the 15 focal landscapes in the study area. Inset maps show location in south-central Chile (a) and in Araucanía region (b). Each focal landscape is depicted by circles of 3.141 km2 each, and farms are classified according to different cultural groups (Mapuche, Chilean, Foreigner); “Other” category refers to any other type of property that does not account as an agricultural farm, e.g. forest plantation or natural conservation areas, school, etc. Coordinates according to WGS84.
2.2. Characterization of cultural diversity
We mapped land ownership in our study area based on superposition of two layers of data: (i) from the Chilean Internal Revenue Service (SII) registers for 1999 (the newest version available), which include land property rights, and (ii) from the database of Mapuche communities from the State National Corporation of Indigenous Development (CONADI) for 2001, which describes Mapuche settlements in southern Chile. In our study area, we defined the following cultural groups based on the socio-political history of colonization in southern Chile: (1) Mapuche: Local Indigenous community, including those living on the area for several generations and the ones who immigrated to the area incentivized by land restitution promoted by Chilean State since 1994 (Vergara, Foerster, Gundermann Citation2004); (2) Chilean: non-indigenous that are mainly descendants of Chilean settlers after the geopolitical incorporation of the Araucanía Region to the Chilean State at the end of the 19th century; and (3) Foreigner: descendants of European settlers that established in the area at end of the 19th century onwards (Di Giminiani, Fonck, Perasso Citation2021). Based on the first database, we listed each farm according to the first and second surnames of the owner (in Chile, the Hispanic dual surname system is used; Barrai et al. Citation2012). If one of the surnames was considered Mapuche, we classified it as Mapuche. If one surname was identified as European, then the farm was classified as Foreigner. European surnames found were of German, Italian, French, English or Swiss origin, since they represent the settlers that established in Araucanía region. When one surname was Mapuche and the other European, then it was still classified as Foreigner, however this situation occurred only in <1% of the farms analysed (data not shown). Whereas no surname was classified as Mapuche or European, it was typically of Spanish origin and it was considered as Chilean. We applied questionnaires to a representative subsample of farmers to evaluate the adequacy of using surnames as indicator of cultural group (see next paragraphs for further details).
After we characterized land ownership in the study area, we randomly select 15 circular areas of 1000 m radius (3.141 km2, thereafter denoted as focal landscape) (). This area size was determined based on to criteria: (1) the inclusion of sufficient number of farms in each focal landscape in order to represent the local culture diversity and (2) a number of farms that was feasible to study in each focal landscape. All manipulations with cartographic materials were performed with ArcGIS version 10.1 (ESRI).
In order to obtain data of the farms for each cultural group we selected a representative sample of farms using a stratified random sampling within each focal landscape, allowing us to select 10% or more of the farms of each cultural group within each focal landscape. On each of these sampled farms we applied a questionnaire in February–April 2016 by visiting each of these sampled farms. The questionnaire was administered to the head of the household (woman or man) and participation was voluntary. In this questionnaire, we asked the interviewee to self-identify themselves with any of the cultural groups defined here (Mapuche, Chilean, European, other). After performing the interviews, we were able to confirm that surnames were an adequate indicator to identify the cultural group of each farmer.
This research was authorized by the Research Ethics Committee of the Universidad de La Frontera and was conducted following the code of ethics of the International Society of Ethnobiology. All farmers who decided to participate signed an informed consent forms and were interviewed according to mutually agreed conditions, with guaranteed anonymity and confidentiality. Farmers were also given the option to discontinue their participation at any time.
2.3. Characterization of agrobiodiversity
We estimated agrobiodiversity by quantifying the species that are cultivated for food purposes, according to the following nested levels of diversity: (1) Subsystem: vegetable garden, orchard, chacra,Footnote1 annual crops, and natural areas; (2) Species: cultivated species richness for each subsystem; and (3) Landraces: different distinctive genetic entities within a cultivated species, which can correspond to different landraces or commercial varieties, but we will represent them as landraces along the text. The information about the cultivated species and landraces in each farm was informed by the interviewee during the application of the questionnaires and then confirmed visually by the interviewer, which also was a knowledgeable agricultural professional. This expert’s visual inspection also ensured to avoid duplicity (different names for the same species or variety) or homonymous (two different species or varieties having the same name) of species and landraces in the data.
2.4. Diversity index estimation
2.4.1. Cultural diversity and dominance indices
We estimated cultural diversity based on the Shannon index of diversity (H´; Shannon & Weaver, Citation1949) defined as
where N is the number of cultural groups presented per focal landscape (maximum value N = 3), and pi represents the proportional area of the ith cultural group on the total area occupied by the sum of all cultural groups in the focal landscape.
Additionally, we calculated the dominance of each cultural group at the landscape scale for each focal landscape using the Simpson (Citation1949) index (D), which defines the probability that two selected farms at random belong to the same group. It is the inverse of diversity and ranges from 0 to 1, where 1 represents maximum dominance. D is defined as
where N is the number of cultural groups presented on each focal landscape and pi represents the proportional area of the ith group on the total area occupied by the sum of all groups in the focal landscape.
We calculated the cultural diversity and dominance indices using the package “vegan” (Oksanen et al. Citation2019) in R software (version 4.0.2, R Development Core Team 2020).
2.4.2. Agrobiodiversity index (AI)
We created an agrobiodiversity index (AI) for each ith cultural group, calculated as
where i represents each cultural group interviewed per focal landscape; subsystemi represents the number of different subsystems in the farm of the ith cultural group in the focal landscape; speciesi represents the number of different species in each subsystem of the farm of the ith cultural group in the focal landscape; and landrace represents the number of different landraces (or varieties) for each cultivated species of the ith cultural group in the focal landscape.
Based on this AI, we calculated the Total Agrobiodiversity Index (TA) for each focal landscape as:
where N is the total number of cultural groups presented on each focal landscape, and pi represents the proportional area of the ith cultural group on the total area occupied by all cultural groups considered together on the focal landscape.
2.5. Statistical analysis
To test for differences on AI between cultural groups and the proportional area occupied by each group on the focal landscape, we used Kruskal–Wallis rank test (Zar Citation2019) followed by Dunn’s non-parametric post-hoc mean comparison as data was non-normally distributed. Correlations were tested using Spearman rank test. Linear Regression Models were used to test the association between proportion of area occupied by different cultural groups and TA or AI. All statistical analysis were performed in R (version 4.0.2, R Development Core Team 2020). We used p values < 0.10 as a general guide in identifying significant differences, to reduce the probability of committing Type II errors (i.e. concluding no difference when in fact an ecological difference existed), which was more likely given our small sample sizes (Spirito, Rowland, Wisdom, Tabeni Citation2020; Zar Citation2019).
3. Results
Most of the focal landscapes evaluated presented all their area (100%) occupied by agricultural farms, only three focal landscapes have 68–88% of their area occupied by agricultural farms, and the rest is occupied by exotic forest plantations and urban areas (Suppl Table S1). All focal landscapes presented Mapuche farms, while Chilean farms were absent in two of them. Foreigner farms were less frequent, occurring in 9 out of the 15 focal landscapes evaluated. Considering the total farmed area of each focal landscape, 59–100% is occupied by Mapuche farms, 0–37% by Chilean farms, and 0–20% by Foreigner farms (H = 26.3; p < 0.001; ), Suppl Table S1). According to our data, each Mapuche farm holds an average of 4.2 ha (0.95 SD), which is lower than the area of Chilean and Foreigner farms, with an average of 9.3 (9.8 SD) and 14.8 ha (16.8 SD), respectively (Suppl Table S1).
Figure 2. (a) Proportion of agricultural area for each cultural group (Indigenous Mapuche, Chilean, Foreigner) and (b) Agrobiodiversity index (AI) for each cultural group (Mapuche, Chilean, Foreigner), at each focal landscape (n = 15) in Araucanía region, south-central Chile. Horizontal lines represent the mean values ± SD. Significant differences (p < 0.1) are denoted by different letters (Dunn’s test).
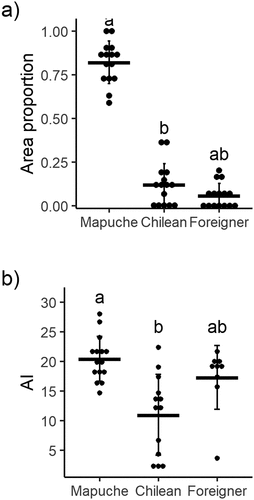
We found significantly higher levels of AI in Mapuche farms compared to Chilean farms, and Foreigner farms were not significantly different from Chilean or Mapuche farms (H = 26.3, p < 0.01; )). Mapuche farms hold on average the highest values for the three nested levels of agrobiodiversity (). All Mapuche farms presented four or five subsystem categories considered in this study, and on average 26 plant species and 31 landraces. In contrast, several Chilean farms presented fewer numbers of subsystems and on average had 14 species and 15 different landraces. Foreigner farms reached higher values of agrobiodiversity than Chilean but lower than Mapuche farms, as none presented the five subsystems, having on average 23 species and 25 landraces (). Several cultivated species and landraces were only present in the Mapuche farms. Among the distinctive landraces in Mapuche farms, we found cereals such as wheat and rye, and vegetables such as tomatoes, potatoes, beans and chard. Also, quinoa was only cultivated in Mapuche farms (data not shown).
Table 1. Characterization of agrobiodiversity on each focal landscape (n = 15) for each cultural group (Mapuche, Chilean and Foreigner) at Araucanía region, in south-central Chile. The data were obtained from a survey questionnaire. See Supplementary Table 1 for more details on farm characterization
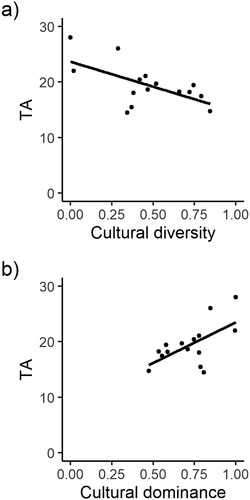
Cultural diversity index ranged from 0 to 1 at focal landscapes, and this index was negatively correlated with TA (Spearman rho = −0.47, p = 0.08; )). This index typically ranges between 1.5 and 3.5 in most ecological studies, and the index is rarely greater than 4 (Magurran Citation2013). Also, this relationship was also evident by a positive correlation between TA and the cultural dominance index (Spearman rho = 0.49, p = 0.07; )). However, the cultural dominance index ranged from 0.4 to 1, denoting the dominance of one cultural group (the Mapuche) in most focal landscapes.
Figure 3. Relationship between total agrobiodiversity index (TA) and (a) cultural diversity index, and (b) cultural dominance index at each focal landscape (n = 15) in Araucanía region, south-central Chile. For visual purposes, a solid line shows fitted linear regressions.
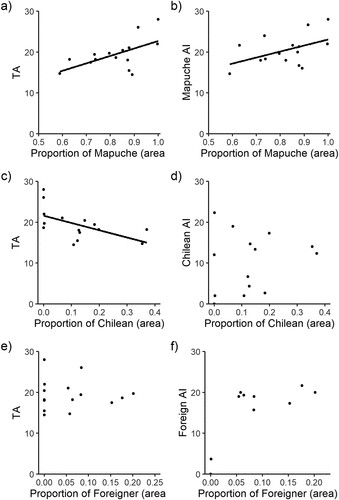
We further explored the relationship between cultural dominance and agrobiodiversity for each cultural group (). The proportion of Mapuche area had a weak but positive effect on TA (R2 = 0.34, p = 0.02; )) and Mapuche AI (R2 = 0.22, p = 0.08; )). We found the opposite pattern for the Chilean group, since TA was negatively affected by proportion of Chilean area (R2 = 0.32, p = 0.03; )), but Chilean AI was not affected by it ()). Also, we found no effects for Foreigner farms ()). Finally, we found no relationship between the Mapuche AI and the proportion of Chilean or Foreigner area. Similarly, no relationship was found for Chilean AI nor Foreigner AI, and the area occupied by farms from different cultural groups.
Figure 4. On the left panel, relationship between Total Agrobiodiversity index (TA) and proportional area occupied by (a) Mapuche, (c) Chilean and (e) Foreigner cultural groups. On the right panel, relationship between Agrobiodiversity index (AI) and proportional area occupied by (a) Mapuche, (c) Chilean and (e) Foreigner cultural groups. A solid line shows fitted linear regressions. Note the differences in x-axis scales among plots.
4. Discussion
Biocultural diversity results from the interaction, at a given time and place, between people and nature. Contrary to our hypothesis, we found that higher cultural diversity correlated with lower levels of agrobiodiversity. This result reflects the fact that higher cultivated diversity was mainly determined by higher proportion of Mapuche farms in our focal landscapes and that the presence of non-Mapuche farms did not presented novel species (i.e. not present in Mapuche farms) or landraces in the landscapes.
Mapuche farms in our study area presented higher agrobiodiversity when compared to the other cultural groups. Previous research highlights the role of Mapuche´s own ontology and cosmology attached to biodiversity (Santiago, Acuña, Luks, Ibarra Citation2020; Urra & Ibarra, Citation2018). For example, Mapuche Indigenous people perceives nature in a holistic way, rather than a utilitarian one (Ñanculef Citation2016). From a historical perspective, before the incorporation of Mapuche into the Chilean State (1881), Bengoa (Citation2011) defined them as “proto-agrarian”, arguing that agriculture was not the central way of life but merely supplementary to other activities such as huntering and gathering. After this socio-political process, the Mapuche were incorporated into modern society, which was described by Saavedra Pelaéz (Citation2002) as a process of “peasant-izing” of Mapuche. From this historical perspective towards the present, Mapuche´s agrobiodiversity could be explained by a mix of planned choices (e.g. wheat cultivation as a response to global demand; Villalobos, Silva, Silva, Estellé Citation1982) and by default explained by local conditions. For example, Mapuche farmers have been adopted several cultivated species from foreign cultures that have been present in their territory at different stages in their history (i.e. Indigenous Inca, Spanish, other Europeans, Chileans) (Barreau, Ibarra, Wyndham, Kozak Citation2019; Montalba, Fonseca, García, Vieli, Altieri Citation2015). An important portion of those adopted species or landraces have been conserved because of their specific culinary uses, medicinal or spiritual purposes through different rituals (Montalba & Stephens, Citation2014). Additionally, many of these landraces are adapted to the local edaphoclimatic conditions, such as water scarcity and low soil fertility (e.g. old wheat landraces, quinoa landraces, chilli pepper landraces), making them successful in farms that have no access to conventional agricultural inputs (e.g. chemical fertilizers, pesticides, irrigation systems) (Montalba, Fonseca, García, Vieli, Altieri Citation2015). Previous research of Durán (Citation1992), evidencing how Mapuche Indigenous people have generated a culture -or in this case a “horti-culture”- bonded with their territory over decades. Nowadays, these species and landraces have still persisted in several Mapuche farms in our study area, explaining the higher cultivated diversity index in these farms. Previous studies have found an average of 51 cultivated plant species in Mapuche farms in south-central Chile (Urra & Ibarra, Citation2018). The pronounced range of variation in AI for Mapuche small-farms could partly respond to their farm-develop strategy based on diversification of practices and species (Ibarra, Caviedes, Antonia, Pessa Citation2018). The conservation of traditional species and landraces in family gardens allows the maintenance and enhancement of local traditions and trades, the latter being part of the biocultural heritage of localities and territories in Chile (Castro & Romo, Citation2008).
In general, all farms in our study area were influenced by conventional agricultural practices, such as monocultures and use of pesticides, with the concomitant agrobiodiversity reduction (Bellisario Citation2007; Kay Citation2002). In Chile, agriculture development was highly influenced by the Green Revolution during the second half of the 20th century, particularly during the dictatorship regime (1973-1990). In this period, Chile became a neoliberal economy-based country, emphasizing the progressive liberalization of the economy (Bellisario Citation2007), favouring open export markets and agribusiness companies (Murray Citation2002). In this context, non-Mapuche farmers adopted conventional agricultural practices to a higher extent than Mapuche farmers (Montalba, Vera, Vieli Citation2011). Along this historical process, agrobiodiversity loss is attached to demographic and cultural changes: (i) fewer farmers; (ii) less land used for cultivation; and (iii) less crop varieties cultivated, resulting in shifts from multi-species systems to monocultures, with the concomitant simplification of resources used and farm management practices (Pingali Citation2012).
At the landscape scale, in our study area, agrobiodiversity increases with higher proportion of Mapuche small-farms, suggesting the positive influence of each other’s agrobiodiversity. One of the mechanism under this pattern could be seed exchange networks, which is a common practice in Mapuche communities (Ibarra, Caviedes, Antonia, Pessa Citation2018), mainly performed by woman (Núñez Citation2014). In this sense, Labeyrie et al. (Citation2016) analysed the social processes involved in crop diversity dynamics and human cultural diversity in central Africa, highlighting the crucial role of homophily (i.e. preferential interaction among members of the same social group) as a key factor in determining seed exchange networks. Homophily imply trust and, at the same time, it is consequently constrained by social barriers (Leclerc et al., Citation2012). In this sense, the non-positive relationship between Mapuche and non-Mapuche (Chilean and Foreigner) people could be influenced by a socio-political long-term historical conflict between them in the Araucanía region (Bauer Citation2016; Merino & Mellor, Citation2009; Ranjan, Castillo, Morales Citation2021).
In the past, the Mapuche, compared to non-Indigenous, have had less access to land, less irrigation possibilities, are were vulnerable to higher levels of poverty (19% Mapuche under poverty vs 11% non-Mapuche) and received less support from Chilean State (López Citation2016). This socio-political context can be seen as a cultural isolation (Alvarado Lincopi Citation2016), which transcends the agricultural dimension to permeate other spheres of life, for example, school racialization (Becerra, Mansilla, Merino, Rivera Citation2015) or employment opportunities (Cabrera et al., Citation2020). This isolation could partly explain the differences between cultural diversity and agrobiodiversity found here, but the great range of variation in AI indicates that Mapuche farmers are not homogeneous along their agricultural practices. In addition, we have to consider that Indigenous people are not always like a homogenous mass (sensu Craven Citation2020), rather they can interact within the socio-ecological system to support local agriculture. Therefore, we can distinguish some Mapuche and non-Mapuche people willing to share cultural elements (Figueiredo et al. Citation2019), blending into a hybrid-type-of agriculture, in which, most of the cases, Indigenous agrobiodiversity has been replaced by conventional inputs (Parada & Salas, Citation2019). This depiction of cultural mixing is further corroborated by studies of cultural hybridity in agroecosystem in indigenous-modern living in Andean systems (Zimmerer et al. Citation2020) and also in rural Senegal (Faye Citation2020).
Advancing towards unravelling the dynamics involved in the relationships between culture and agrobiodiversity needs to explore in depth the complexity arising from this interaction. In our study case, a negative link between cultural group diversity and agrobiodiversity evidence this intricate, not straightforward relation. It is worth to notice that our results are somewhat constrained by the reduced spatial variability of cultural diversity found in our study area, which is mainly dominated by Mapuche farms, presenting low proportions of Chilean and even less of Foreigner farms. Thus, we were not able to assess diversity interactions with scarce presence or absence of Mapuche farms, therefore the effects of having only Chilean or Foreigner farmers in a focal landscape cannot be empirically obtained. Further research should try to reach more diversified combination of cultural scenarios and farmers from different cultural backgrounds. For example, despite Mapuche farms were dominant in our study area, this is not the case of other areas of southern Chile. Because culture diversity is a fluid, dynamic process, future research should also investigate when individuals identify with two or more cultural groups, such as those who identify in this area as both Mapuche and Chilean or Foreigner (e.g. Italymapu denomination to those descendants from Mapuche and Italian progenitors; Montalba, Fonseca, García, Vieli, Altieri Citation2015). Under the global scenario of decline of small-holder’s agriculture (Blanckaert, Swennen, Flores, López, Saade Citation2004; Burkitbayeva & Swinnen, Citation2018), encouraging socio-political discourses and land policy involving all the cultural and biological diversity inhabiting the landscape could prevent from biocultural homogenization, which has been considered by Rozzi (Citation2018) “as a major, but little perceived, global driver of biological and cultural diversity loss that frequently entail social and environmental injustices”. Understanding the complex interactions between culture and biodiversity could help us in facing current challenges such as biodiversity loss, cultural homogenization, climate change and reducing conventional agriculture impacts.
5. Conclusion
Opposite to our hypothesis, we found a negative correlation between cultural diversity and agrobiodiversity. This negative result highlights the complexity and dynamic under the biocultural diversity framework, denoting the need to further study this relationship considering different socio-ecological aspects from an historical perspective, as well as, from a socio-political point of view.
By analysing agrobiodiversity patterns at the landscape scale, a broader perspective of agrobiodiversity conservation can be addressed. Our results suggest that higher aggregation levels of Mapuche small-farms are related to higher agrobiodiversity levels. The current policy of the Chilean State consisting in buying land for Mapuche as a restitution process for historical land loss (Bauer Citation2018) should consider the importance of the location of the land acquired by the Mapuche communities, since our results suggest that neighbouring Mapuche Indigenous farms could increases the levels of agrobiodiversity presented in the landscape.
Ethical approval
The study protocol was approved by the Research Ethics Committee of Universidad de La Frontera.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Supplemental Material
Download MS Word (14.9 KB)Acknowledgements
The authors are grateful to all the farmers that collaborated by giving us information and access to their farms. Many thanks to Bárbara Herrera Tamaya and the people that collaborated in the field sampling.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/27685241.2022.2083987.
Additional information
Funding
Notes
1. Chacra is a term originally from Quechua language and refers to an area that is intensively managed to produce several annual crop species such as potato, corn, quinoa, faba bean, among others, with the purpose of producing food for family consumption.
References
- Agnoletti, M., & Rotherham, I. D. (2015). Landscape and biocultural diversity. Biodiversity and Conservation, 24, 3155. https://doi.org/10.1007/s10531-015-1003-8
- Altieri, M. A., Nicholls, C. I., Henao, A., & Lana, M. A. (2015). Agroecology and the design of climate change-resilient farming systems. Agronomy for Sustainable Development, 35(3), 869–890. https://doi.org/10.1007/s13593-015-0285-2
- Alvarado Lincopi, C. A. (2016). Mapurbekistán: de indios a mapurbes en la capital del reyno. Racismo, segregación urbana y agencias mapuche en Santiago de Chile (Master’s thesis, Universidad Nacional de La Plata. Facultad de Humanidades y Ciencias de la Educación).
- Barrai, I., Rodriguez-Larralde, A., Dipierri, J., Alfaro, E., Acevedo, N., Mamolini, E., Scapoli, C., Carrieri, A., & Scapoli, C. (2012). Surnames in Chile: A study of the population of Chile through isonymy. American Journal of Physical Anthropology, 147(3), 380–388. https://doi.org/10.1002/ajpa.22000
- Barreau, A., Ibarra, J. T., Wyndham, F. S., & Kozak, R. A. (2019). Shifts in Mapuche food systems in Southern andean forest landscapes: historical processes and current trends of biocultural homogenization. Mountain Research and Development, 39(1),R12–R23. https://doi.org/10.1659/MRD-JOURNAL-D-18-00015.1
- Bauer, K. (2016). Land versus territory: Evaluating indigenous land policy for the Mapuche in Chile. Journal of Agrarian Change, 16(4), 627–645. https://doi.org/10.1111/joac.12103
- Bauer, K. (2018). Not-so-neoliberal governance: Chile’s response to Mapuche territorial demands. Latin American and Caribbean Ethnic Studies, 13(3), 214–236. https://doi.org/10.1080/17442222.2018.1457007
- Becerra, S., Mansilla, J., Merino, M. E., & Rivera, P. (2015). School violence against Mapuche indigenous students in Chilean secondary schools. Procedia-Social and Behavioral Sciences, 197, 1538–1543. https://doi.org/10.1016/j.sbspro.2015.07.107
- Bellisario, A. (2007). The chilean agrarian transformation: Agrarian reform and capitalist ‘Partial’Counter-agrarian reform, 1964–1980: Part 1: Reformism, socialism and free-market neoliberalism. Journal of Agrarian Change, 7(1), 1–34. https://doi.org/10.1111/j.1471-0366.2007.00138.x
- Bengoa, J. (2011). Los Mapuches: Historia, cultura y conflicto. Cahiers des Amériques latines, 2011(68), 89–107.
- Berkes, F. (2017). Sacred ecology. Abingdon. Routledge.
- Berkes, F., Folke, C., & Colding, J. (Eds.). (2000). Linking social and ecological systems: Management practices and social mechanisms for building resilience. Cambridge University Press.
- Blanckaert, I., Swennen, R. L., Flores, M. P., López, R. R., & Saade, R. L. (2004). Floristic composition, plant uses and management practices in homegardens of San Rafael Coxcatlán, Valley of Tehuacán-Cuicatlán, Mexico. Journal of Arid Environments, 57(2), 179–202. https://doi.org/10.1016/S0140-1963(03)00100-9
- Bridgewater, P., Rotherham, I. D., & Rozzi, R. (2019). A critical perspective on the concept of biocultural diversity and its emerging role in nature and heritage conservation. People and Nature, 1(3), 291–304. https://doi.org/10.1002/pan3.10040
- Brookfield, H. (2001). Exploring agrodiversity. Columbia University Press.
- Burkitbayeva, S., & Swinnen, J. (2018). Smallholder agriculture in transition economies. Journal of Agrarian Change, 18(4), 882–892. https://doi.org/10.1111/joac.12284
- Cabrera, F. D. L. M., & Bolomey Córdova, C. F. (2020). Persistence and changes in state dependence in a Mapuche indigenous territory, Chile. Critique of Anthropology, 40(1), 146–168.
- Castañeda-Álvarez, N. P., Khoury, C. K., Achicanoy, H. A., Bernau, V., Dempewolf, H., Eastwood, R. J., Toll, J., Harker, R. H., Jarvis, A., Maxted, N., Müller, J. V., Ramirez-Villegas, J., Sosa, C. C., Struik, P. C., Vincent, H., & Toll, J. (2016). Global conservation priorities for crop wild relatives. Nature Plants, 2(4), 1–6. https://doi.org/10.1038/nplants.2016.22
- Castro, V., & Romo, M. (2008). Tradiciones culturales y biodiversidad. Biodiversidad de Chile, Patrimonio y Desafíos. Ed. En CONAMA. 468–493.Ocho Libros.
- Craven, J. M. (2020). Indigenous approaches to economic development and sustainability. International Critical Thought, 10(1), 126–137. https://doi.org/10.1080/21598282.2020.1742529
- Dainese, M., Martin, E. A., Aizen, M. A., Albrecht, M., Bartomeus, I., Bommarco, R., Steffan-Dewenter, I., Chaplin-Kramer, R., Gagic, V., Garibaldi, L. A., Ghazoul, J., Grab, H., Jonsson, M., Karp, D. S., Kennedy, C. M., Kleijn, D., Kremen, C., Landis, D. A., Letourneau, D. K. andSteffan-Dewenter, I. (2019). A global synthesis reveals biodiversity-mediated benefits for crop production. Science Advances, 5(10), eaax0121. https://doi.org/10.1126/sciadv.aax0121
- De la Maza, F. (2014). Between conflict and recognition: The construction of Chilean indigenous policy in the Araucanía region. Critique of Anthropology, 34(3), 346–366. https://doi.org/10.1177/0308275X14531836
- Di Giminiani, P. (2015). The becoming of ancestral land: Place and property in Mapuche land claims. American Ethnologist, 42(3), 490–503. https://doi.org/10.1111/amet.12143
- Di Giminiani, P. D., Fonck, M., & Perasso, P. (2021). Can natives be settlers? Emptiness, settlement and indigeneity on the settler colonial frontier in Chile. Anthropological Theory, 21(1), 82–106. https://doi.org/10.1177/1463499619868088
- FAO. Second report on the state of the world’s plant genetic resources for food and agriculture. Commission on Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: 2010.
- Faye, J. B. (2020). Indigenous farming transitions, sociocultural hybridity and sustainability in rural Senegal. NJAS: Wageningen Journal of Life Sciences, 92(1), 1–8. https://doi.org/10.1016/j.njas.2020.100338
- Figueroa-Helland, L., Thomas, C., & Aguilera, A. P. (2018). Decolonizing food systems: Food sovereignty, indigenous revitalization, and agroecology as counter-hegemonic movements. Perspectives on Global Development and Technology, 17(1–2), 173–201. https://doi:10.1163/15691497-12341473
- Gorenflo, L. J., Romaine, S., Mittermeier, R. A., & Walker-Painemilla, K. (2012). Co-occurrence of linguistic and biological diversity in biodiversity hotspots and high biodiversity wilderness areas. Proceedings of the national academy of sciences, 109( 21), 8032–8037. https://doi.org/10.1073/pnas.1117511109
- Hatab, A. A., Cavinato, M. E. R., & Lagerkvist, C. J. (2019). Urbanization, livestock systems and food security in developing countries: A systematic review of the literature. Food Security, 11(2), 279–299. https://doi.org/10.1007/s12571-019-00906-1
- Henríquez Jaramillo, L. (2013). Cinco décadas de transformaciones en La Araucanía Rural. Polis. Revista Latinoamericana, 34. http://journals.openedition.org/polis/8802
- Ibarra, J. T., Caviedes, J., Altamirano, T. A., Urra, R., Barreau, A., & Santana, F. (2021). Social-ecological filters drive the functional diversity of beetles in homegardens of campesinos and migrants in the southern Andes. Scientific Reports, 11(1), 1–14. https://doi.org/10.1038/s41598-021-91185-4
- Ibarra, J. T., Caviedes, J., Antonia, B., & Pessa, N. (Eds.). (2018). Huertas familiares y comunitarias: Cultivando soberanía alimentaria. Ediciones UC.
- INE (Instituto Nacional de Estadísticas) (2017) Ministerio de Economía, Chile. https://regiones.ine.cl/araucania/estadisticas
- Kay, C. (2002). Chile’s neoliberal agrarian transformation and the peasantry. Journal of Agrarian Change, 2(4), 464–501. https://doi.org/10.1111/1471-0366.00043
- Labeyrie, V., Thomas, M., Muthamia, Z. K., & Leclerc, C. (2016). Seed exchange networks, ethnicity, and sorghum diversity. Proceedings of the national academy of sciences, 113( 1), 98–103. https://doi.org/10.1073/pnas.1513238112
- Leclerc, C., & Coppens d’Eeckenbrugge, G. (2012). Social organization of crop genetic diversity. The G× E× S interaction model. Diversity, 4(1), 1–32. https://doi.org/10.3390/d4010001
- Little, C., Lara, A., McPhee, J., & Urrutia, R. (2009). Revealing the impact of forest exotic plantations on water yield in large scale watersheds in South-Central Chile. Journal of Hydrology, 374(1–2), 162–170. https://doi.org/10.1016/j.jhydrol.2009.06.011
- Liu, J., Dietz, T., Carpenter, S. R., Alberti, M., Folke, C., Moran, E., Taylor, W. W., Deadman, P., Kratz, T., Lubchenco, J., Ostrom, E., Ouyang, Z., Provencher, W., Redman, C. L., Schneider, S. H., & Taylor, W. W. (2007). Complexity of coupled human and natural systems. science, 317(5844), 1513–1516. https://doi.org/10.1126/science.1144004
- López, D. (2016). Discriminación y exclusión: Tendencias en las brechas étnicas de ingresos urbanos y rurales en Chile. Serie Documentos de Trabajo N°200. Grupo de Trabajo “Cohesión Territorial para el Desarrollo.” Rimisp Santiago Chile.
- Loh, J., & Harmon, D. (2005). A global index of biocultural diversity. Ecological Indicators, 5(3), 231–241. https://doi.org/10.1016/j.ecolind.2005.02.005
- Maffi, L. (2007). Biocultural diversity and sustainability. The Sage handbook of environment and society. SLE Pound, 267–277.
- Maffi, L., & Woodley, E. (2012). Biocultural diversity conservation: A global sourcebook. Routledge.
- Magurran, A. E. (2013). Measuring biological diversity. John Wiley & Sons.
- Massawe, F., Mayes, S., & Cheng, A. (2016). Crop diversity: An unexploited treasure trove for food security. Trends in Plant Science, 21(5), 365–368. https://doi.org/10.1016/j.tplants.2016.02.006
- Merino, M. E., & Mellor, D. J. (2009). Perceived discrimination in Mapuche discourse: Contemporary racism in Chilean society. Critical Discourse Studies, 6(3), 215–226. https://doi.org/10.1080/17405900902974902
- Mijatović, D., Van Oudenhoven, F., Eyzaguirre, P., & Hodgkin, T. (2013). The role of agricultural biodiversity in strengthening resilience to climate change: Towards an analytical framework. International Journal of Agricultural Sustainability, 11(2), 95–107. https://doi.org/10.1080/14735903.2012.691221
- Montalba, R., & Stephens, N. (2014). Ecological change and the “ecological Mapuche”: A historical sketch of the human ecology of Chile’s Araucania region. Human Ecology, 42(4), 637–643. http://dx.doi.org/10.1007/s10745-014-9678-0
- Montalba, R., Fonseca, F., García, M., Vieli, L., & Altieri, M. (2015). Determinación de los niveles de riesgo socioecológico ante sequías en sistemas agrícolas campesinos de La Araucanía chilena: Influencia de la diversidad cultural y la agrobiodiversidad. Papers: Revista de Sociologia, 100(4), 0607–624. https://papers.uab.cat/article/view/v100-n4-montalba-fonseca-garcia-vieli-altieri/pdf_1
- Montalba, R., Vera, L., & Vieli, L. (2011). Historia ecológica de la degradación de los bosques y recursos naturales en La Araucanía Chilena. In H. En Bernal, C. Sierra, M. Onaindia, & T. González (Eds.), Bosques del Mundo, Cambio Climático y Amazonía. País Vasco (pp. 97–117). Cátedra Unesco-EHU de Desarrollo Sostenible y Educación Ambiental.
- Montalba, R., Vieli, L., Vallejos Romero, A., Zunino, H., & Vera, L. (2017). Determinación de las fuerzas conductoras de la transformación ambiental de La Araucanía chilena: El “paisaje cultural” como marco de análisis. Diálogo Andino, (54), 51–61. http://dx.doi.org/10.4067/S0719-26812017000300051
- Moore, J. L., Manne, L., Brooks, T., Burgess, N. D., Davies, R., Rahbek, C., & Balmford, A. (2002). The distribution of cultural and biological diversity in Africa. Proceedings of the royal society of London. Series B: Biological sciences, 269 ( 1501), 1645–1653. https://doi.org/10.1098/rspb.2002.2075
- Murray, W. E. (2002). The neoliberal inheritance: Agrarian policy and rural differentiation in democratic Chile. Bulletin of Latin American Research, 21(3), 425–441. https://doi.org/10.1111/1470-9856.00052
- Nahuelpán, H. (2012). Formación colonial del Estado y desposesión en Ngulumapu. Ta iñ fijke xipa rakizuameluwün. Historia, colonialismo y resistencia desde el país Mapuche, 123–156.
- Ñanculef, J. (2016). Tayiñ mapuche kimün. Epistemología mapuche–Sabiduría y conocimientos. Universidad de Chile.
- Núñez, R. D. (2014). Malen ka anümkanwe, las mujeres pewenche y sus huertas. Tesis Agronomía, Facultad de Agronomía, Universidad de Chile.
- Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., & Wagner, H. (2019). Package ‘vegan’. Community Ecology Package, 2 (9). version.
- Parada, S. P., & Salas, C. B. (2019). Evaluación participativa de la sustentabilidad entre un sistema campesino bajo manejo convencional y uno agroecológico de una comunidad Mapuche de la Región de la Araucanía (Chile). Revista de la Facultad de Ciencias Agrarias UNCuyo, 51(1), 323–336. https://revistas.uncu.edu.ar/ojs3/index.php/RFCA/article/view/2454
- Perino, A., Pereira, H. M., Navarro, L. M., Fernández, N., Bullock, J. M., Ceaușu, S., Pe’er, G., van Klink, R., Kuemmerle, T., Lomba, A., Pe’er, G., Plieninger, T., Rey Benayas, J. M., Sandom, C. J., Svenning, J.-C., & Wheeler, H. C. (2019). Rewilding complex ecosystems. Science, 364(6438), eaav5570. https://doi.org/10.1126/science.aav5570
- Pilgrim, S., & Pretty, J. N. (Eds.). (2010). Nature and culture: Rebuilding lost connections. Earthscan.
- Pingali, P. L. (2012). Green revolution: Impacts, limits, and the path ahead. Proceedings of the national academy of sciences, 109( 31), 12302–12308. https://doi.org/10.1073/pnas.0912953109
- Ranjan, R., Castillo, A., & Morales, K. (2021). Mapuche cosmovision and territorial rights: An interdisciplinary approach to understand the conflict of Wallmapu, Chile. Rupkatha Journal on Interdisciplinary Studies in Humanities, 13(1),1–20. https://dx.doi.org/10.21659/rupkatha.v13n1.17.
- Ricciardi, V., Mehrabi, Z., Wittman, H., James, D., & Ramankutty, N. (2021). Higher yields and more biodiversity on smaller farms. Nature Sustainability, 4(7),651–657. https://doi.org/10.1038/s41893-021-00699-2
- Rotherham, I. D. (2013). Cultural severance and the end of tradition. In Cultural severance and the environment (pp. 11–30). Springer. https://doi.org/10.1007/978-94-007-6159-9_2
- Rotherham, I. D. (2015). Bio-cultural heritage and biodiversity: Emerging paradigms in conservation and planning. Biodiversity and Conservation, 24(13), 3405–3429. https://doi.org/10.1007/s10531-015-1006-5
- Rozzi, R. (2018). Biocultural homogenization: A wicked problem in the anthropocene. In Rozzi, R, May, Jr RH, Chapin, FS III, Massardo, F, GavinKlaver, M, Klaver, I, Pauchard, A, Nuñez, MA, Simberloff, D (Eds.), From biocultural homogenization to biocultural conservation (pp. 21–48). Springer.
- Saavedra Pelaéz, A. (2002). Los Mapuche en la sociedad chilena actual. LOM Ediciones.
- Santiago, C. M., Acuña, N. F., Luks, S. K., & Ibarra, J. T. (2020). Local knowledge in montane homegardens in the southern Andes: A refuge of Mapuche pewenche biocultural memory. In Revista de Ecología de Montaña (Vol. 175, pp. 0373–2568). e060 -lhttps://doi.org/10.3989/pirineos.2020.175010
- Shannon, C. E., & Weaver, W. (1949). The mathematical theory of communication–Univ. Illinois press. I. Vol. 11 ( 117). Urbana, IL.
- Simpson, E. H. (1949). Measurement of diversity. Nature, 163(4148), 688. https://doi.org/10.1038/163688a0
- Spirito, F., Rowland, M., Wisdom, M., & Tabeni, S. (2020). Tracking native small mammals to measure fine-scale space use in grazed and restored dry woodlands. Global Ecology and Conservation, 24, e01348. https://doi.org/10.1016/j.gecco.2020.e01348
- Stepp, J. R., Cervone, S., Castaneda, H., Lasseter, A., Stocks, G., & Gichon, Y. (2004). Development of a GIS for global biocultural diversity. Policy Matters, 13(267–270), 6.
- Sutherland, W. J. (2003). Parallel extinction risk and global distribution of languages and species. Nature, 423(6937), 276. https://doi.org/10.1038/nature01607
- Tamburini, G., Bommarco, R., Wanger, T. C., Kremen, C., van der Heijden, M. G., Liebman, M., & Hallin, S. (2020). Agricultural diversification promotes multiple ecosystem services without compromising yield. Science Advances, 6(45), eaba1715. https://doi.org/10.1126/sciadv.aba1715
- Tilley, C. (2006). Introduction: Identity, place, landscape and heritage. Journal of Material Culture, 11(1–2), 7–32. https://doi.org/10.1177/1359183506062990
- Tutwiler, M. A., Bailey, A., Attwood, S., Remans, R., & Ramirez, M. (2017). Agricultural biodiversity and food system sustainability. In W. D. Boef, M. Haga, L. Sibanda, M. S. Swaminathan, & P. Winters (Eds.), Mainstreaming agrobiodiversity in sustainable food systems: Scientific foundations for an agrobiodiversity index (pp. 2–21). Bioversity International.
- Uribe, J., Cabrera, R., De la Fuente, A., & Paneque, M. (2012). Atlas Bioclimático De Chile (pp. 228). Adros impresores.
- Urra, R., & Ibarra, J. T. (2018). Estado del Conocimiento Sobre Huertas Familiares en Chile: Agrobiodiversidad y cultura en un mismo espacio. Etnobiología, 16(1), 31–46.
- Vergara, J. I., Foerster, R., & Gundermann, H. (2004). Más acá de la legalidad. La CONADI, la ley indígena y el pueblo mapuche (1989-2004). Polis. Revista Latinoamericana, 3, 8.
- Villalobos, S., Silva, O., Silva, F., & Estellé, P. (1982). Historia de Chile. Editorial Universitaria.
- Wyckhuys, K. A., González-Chang, M., Adriani, E., Albaytar, A. B., Albertini, A., Avila, G., Tiwari, S., Boreros, A. D., Fanani, M. Z., Nguyen, D. T., Nguyen, G., Nurkomar, I., & Tiwari, S. (2020). Delivering on the promise of biological control in Asia’s food systems: A humboldtian perspective. Frontiers in Sustainable Food Systems, 4, 140. https://doi.org/10.3389/fsufs.2020.00140
- Zar, J. H. (2019). Biostatistical analysis. Pearson Education, Incorporated.
- Zimmerer, K. S., de Haan, S., Jones, A. D., Creed-Kanashiro, H., Tello, M., Carrasco, M., Olivencia, Y. J., Plasencia Amaya, F., Cruz-Garcia, G. S., Tubbeh, R., & Jiménez Olivencia, Y. (2019). The biodiversity of food and agriculture (Agrobiodiversity) in the anthropocene: Research advances and conceptual framework. Anthropocene, 25, 100192. https://doi.org/10.1016/j.ancene.2019.100192
- Zimmerer, K. S., De Haan, S., Jones, A. D., Creed-Kanashiro, H., Tello, M., Amaya, F. P., Hultquist, C., Meza, K., Tubbeh, R. M., Nguyen, K. T., & Hultquist, C. (2020). Indigenous smallholder struggles in Peru: Nutrition security, agrobiodiversity, and food sovereignty amid transforming global systems and climate change. Journal of Latin American Geography, 19(3), 74–111. https://doi.org/10.1353/lag.2020.0072
- Pretty, J., Adams, B., Berkes, F., De Athayde, S. F., Dudley, N., Hunn, E., & Pilgrim, S. (2009). The intersections of biological diversity and cultural diversity: Towards integration. Conservation and Society, 7(2), 100–112.
- Durán, T. (1992). Horticultura entre los Mapuches, condiciones sociales y culturales de su vigencia. En Centro Interdisciplinario de investigación y desarrollo; Sociedad Mapuche Lonko Kilapan. In Sociedad y Cultura Mapuche: undefined cambio y la resistencia cultural. Temuco, Chile.
- Figueiredo, A., Rocha, C., Montagna, P., Ferreiro, T., Guerrero, C., Varela, M., & Licata, L. (2019). Representations of history and present-day intergroup relations between Indigenous and non-Indigenous people: The Mapuche in Chile. In P. Salter, S. Mukherjee (Eds.), History, collective memory and national identities. Nova Science Publishers.
