Abstract
Objective: Growing interest in the metabolic state of ketosis has driven development of exogenous ketone products to induce ketosis without dietary changes. Bis hexanoyl (R)-1,3-butanediol (BH-BD) is a novel ketone ester which, when consumed, increases blood beta-hydroxybutyrate (BHB) concentrations. BH-BD is formulated as a powder or ready-to-drink (RTD) beverage; the relative efficacy of these formulations is unknown, but hypothesized to be equivalent.
Methods: This randomized, observer-blinded, controlled, crossover decentralized study in healthy adults (n = 15, mean age = 33.7 years, mean BMI = 23.6 kg/m2) aimed to elucidate blood BHB and glucose concentrations before and 15, 30, 45, 60, 90 and 120 minutes following two serving sizes of reconstituted BH-BD powder (POW 25 g, POW 12.5 g), compared to a RTD BH-BD beverage (RTD 12.5 g), and a non-ketogenic control, all taken with a standard meal.
Results: All BH-BD products were well tolerated and increased BHB, inducing nutritional ketosis (BHB ≥0.5 mM) after ∼15 minutes, relative to the control. BHB remained elevated 2 h post-consumption. The control did not increase BHB. Ketosis was dose responsive; peak BHB concentration and area under the curve (AUC) were two-fold greater with POW 25 g compared to POW 12.5 g and RTD 12.5 g. There were no differences in peak BHB and AUC between matched powder and RTD formulas. Blood glucose increased in all conditions following the meal but there were neither significant differences in lowest observed concentrations, nor consistent differences at each time point between conditions. These results demonstrate that both powdered and RTD BH-BD formulations similarly induce ketosis with no differences in glucose concentrations in healthy adults.
Introduction
The ketone bodies beta-hydroxybutyrate (BHB), acetoacetate, and acetone are fat-derived endogenous molecules with two broad functions. First, ketones act as a metabolic substrate that allow conversion of energy stored in lipids into a form that is readily oxidized by the brain, heart and other peripheral tissues, prolonging survival during periods of starvation (Citation1). Second, ketones are signaling metabolites that contribute to an environmentally responsive network that regulates healthspan and lifespan (Citation2). Whilst biological effects of BHB have been observed at blood concentrations as low as 0.2 mM (Citation3), a state of ketosis is often characterized by blood BHB concentrations ≥0.5 mM (Citation4–6). Physiological ketosis occurs when dietary carbohydrate intake is low, such as during fasting (Citation7), or adherence to a very low-carbohydrate, high-fat diet (Citation8), whilst pathological ketoacidosis (blood BHB >10 mM) occurs in dysregulated metabolic states such as type 1 diabetes (Citation9).
Endogenous ketosis involves adipose lipolysis to generate fatty acid substrate for the liver, hepatic ketogenesis, hyperketonemia, and increased ketone oxidation (Citation10) (). Recently, exogenous sources of ketones, such as ketone esters, have been developed for their ability to elevate blood ketone concentrations without the need for changes in dietary macronutrient intake. These ketone esters have been used to test the effects of exogenous ketosis on a variety of end points across states of health and disease, ranging from physical (Citation12–15) and cognitive (Citation16–18) performance to blood glucose regulation (Citation19–22) and cardiac function (Citation23,Citation24). The consumption of exogenous ketone products may replicate a subset of the effects of endogenous ketosis (Citation6, Citation25). However, it is still unknown to what extent ketosis achieved through exogenous means is similar to endogenous ketosis in humans.
Figure 1. Metabolic pathways involved in endogenous and exogenous ketosis. Endogenous ketosis increases lipolysis, classical hepatic ketogenesis, hyperketonemia and ketone oxidation. Exogenous ketosis with BH-BD consumption, as either a ready to drink beverage or a powder, involves intestinal hydrolysis of BH-BD, classical and non-classical hepatic ketogenesis, hyperketonemia and ketone oxidation. Abbreviations: BHB, beta-hydroxybutyrate; BH-BD, bis hexanoyl (R)-1,3-butanediol. Modifed from Crabtree et al. (Citation11) and created using BioRender.com.
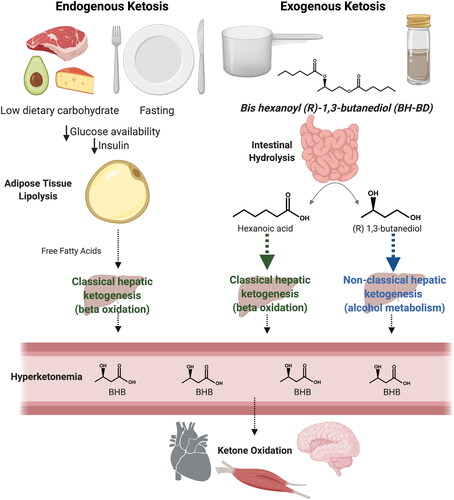
Bis-hexanoyl (R)-1,3-butanediol (BH-BD) is a novel example of a ketone ester that strongly induces hepatic ketogenesis, largely independently of dietary carbohydrate intake or circulating insulin concentrations. BH-BD is rapidly metabolized by enzymes in the small intestine to form ketogenic precursors: hexanoic acid - a medium chain fatty acid, and (R)-1,3-butanediol - a ketogenic alcohol (Citation26) (). These hydrolysis products are transported to the liver via the portal circulation where hexanoic acid acts as a constitutive substrate for ‘classical’ hepatic ketogenesis resulting in release of BHB and acetoacetate (Citation27), and (R)-1,3-butanediol is converted to BHB via a ‘non-classical’ hepatic ketogenic pathway (Citation28,Citation29). Oral administration of BH-BD in rats, mice, and humans result in rapid elevations in blood BHB concentrations that remain elevated for several hours (Citation11, Citation26, Citation30).
Understanding the bioavailability and bioequivalence of different formulations are established concepts in pharmaceutical development, and is also highly relevant to food and beverage formulations (Citation31). Initial clinical studies of BH-BD utilized a ready-to-drink (RTD) beverage formulation (Citation11, Citation30) resulted in elevated blood BHB for up to 4 hours following consumption in healthy adults (Citation30). All other studies of ketone esters to date utilize beverage formulations where the liquid ketone ester is mixed with water. A powdered formulation of BH-BD has been developed that utilizes encapsulation of BH-BD via spray-drying onto a soluble corn fiber carrier. Soluble corn fiber alone has been shown to lower post-prandial glycemic response (Citation32), and decrease postprandial lipids (Citation33). Therefore, it is possible that the soluble fiber carrier might alter the blood ketone or glucose effects of BH-BD compared to the RTD formulation. To examine this, we undertook a randomized, controlled, observer-blinded, cross-over study in healthy adults to determine the blood BHB and glucose effects, including dose responsiveness, of BH-BD powder in comparison to the BH-BD RTD beverage and a non-ketogenic control. We hypothesized that BH-BD powder would induce nutritional ketosis (BHB ≥0.5 mM) similarly to the matched amount in the RTD formulation.
Materials and methods
Study overview
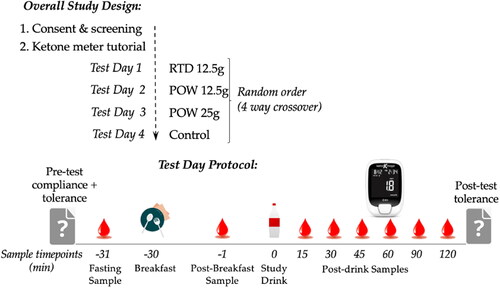
Healthy adults (n = 15) took part in a randomized, observer-blinded, controlled, four-way crossover decentralized study, in which capillary BHB and glucose concentrations were measured before and for 2 hours following ingestion of different formulations and serving sizes of BH-BD (common name, C6 ketone diester) (). Conditions studied were a powdered BH-BD formulation at two serving sizes (POW 25 g and POW 12.5 g) compared to a ready-to-drink BH-BD formulation (RTD 12.5 g) and a non-ketogenic control. This study was approved by WCG IRB (WIRB-Copernicus Group, Puyallup, WA, ID# 20213716) on July 26, 2021 and was conducted in accordance with the Declaration of Helsinki (Citation34). The study was conducted using a decentralized approach (i.e., participants did not attend any on-site study visits), using web-based conferencing and capillary blood samples collected by fingerstick at-home with a lancet and analyzed immediately using a handheld glucose/ketone meter (Keto-Mojo, Napa, CA (Citation35–37),).
Participants and screening
After providing an explanation of the study and requirements, participants provided informed consent if they remained interested in participating and prior to inclusion. Interested participants completed a medical history questionnaire to determine eligibility. Participants included healthy adults (18 − 65 years old, BMI 18.5 − 29.9 kg/m2, male, n = 7; female, n = 8). Exclusion criteria included current clinically important illness, clinically important gastrointestinal conditions, allergies to ingredients in the test products, unstable use of medications or supplements, and pregnancy, lactation, or the possibility of becoming pregnant during the study.
Study products
BH-BD (Abitec Corporation, Janesville, WI, USA) was formulated into two study products, firstly an unflavored powder (soluble corn fiber [Fibersol-2, ADM, Chicago, IL, USA] and sodium caseinate) and secondly a chocolate flavored RTD beverage (water, canola oil, whey protein concentrate, modified gum acacia, natural and artificial flavors, and cocoa powder) under aseptic conditions by The National Food Laboratory (Ithaca, NY, USA). The control beverage was matched to the RTD beverage for taste, volume and appearance; it contained the same matrix as the RTD beverage, with BH-BD replaced by canola oil (25 g), and a bitter additive (sucrose octaacetate) to approximately match the flavor of the ketone ester. The powder study product was packaged into sachets containing either 12.5 g BH-BD (19 g powder total) or 25 g BH-BD (38 g powder total). Participants were instructed to reconstitute the powder on the day of the test using 130 mL of water in a provided shaker cup. RTD beverage was aliquoted into bottles containing 12.5 g of BH-BD + 12.5 g canola oil in a total volume of 75 mL. Study products were labeled with a code to avoid explicitly identifying each condition and to blind the study team to treatment conditions; however, it was not possible to fully blind participants to the product identity given the liquid and powder forms of the study product and the difference in weight of the two powder servings. Participants were randomized to a sequence of study product consumption that was generated using an online tool (randomization.com). Study product nutritional information is shown in .
Table 1. Nutritional information of study beverage and standard meal.
Standard meal
The standardized meal consumed before study product administration on all test days was a commercially available dehydrated breakfast (Backpacker’s Pantry Boulder, OR, USA – Rocky Mountain Scramble, ), participants were instructed to rehydrate and prepare the meal according to the manufacturer’s instructions.
Study procedures
Participants completed two web-based meetings with an investigator prior to test days. The first meeting involved explanation of the study procedures to allow informed consent to be given. Following consent, participants were shipped a study kit containing all study materials. The second teleconference involved familiarization with study materials, training and practice on use the handheld ketone/glucose meter and lancet device, and how to input study data. Training included coaching on thoroughly washing and drying their hands prior to each blood draw and discarding the first blood drop after fingerstick.
Test days were scheduled with the study team to allow support, email reminders and follow-up, but participants conducted each test day independently in their home. Prior to test day 1, dietary intake data for (24-hour records) were collected and analyzed using the Automated Self-Administered 24-hour (ASA24) Dietary Assessment Tool, version (2020), developed by the National Cancer Institute, Bethesda, MD (Citation38) to collect details of the pretest evening meal so it could be replicated prior to each test day. Before each test day, participants received an email that included their product code allocation for that test day, a data input sheet and a reminder of pretest instructions. These were to start the test day fasted for 10 h, no alcohol for 10 h, no exercise for 10 h and no acute illness. Participants confirmed that they met these requirements by responding to questions on the data input sheet and completed a series of questions to assess baseline presence of gastrointestinal (GI) and systemic symptoms as previously described (Citation30). Briefly, the questionnaire features 10 symptoms (gas, nausea, vomiting, abdominal cramping, stomach rumbling, burping, reflux, diarrhea, headache, dizziness) that are scored none (0), mild (1), moderate (2) or severe (3), leading to a maximal score of 30. Participants then took a fasted BHB/glucose reading prior to consumption of the study breakfast. Participants waited for 30 minutes after breakfast consumption before taking a post-meal BHB/glucose reading and then consuming their allocated study product. BHB/glucose readings were collected 15, 30, 45, 60, 90 and 120 minutes after study product consumption (). Participants were asked to remain sedentary throughout the duration of the test and not to consume food or caloric beverages; non-caloric beverages (e.g., tea or water) were permitted ad libitum. After the last BHB/glucose reading, participants repeated the symptom questionnaire to assess any changes that occurred after the standard breakfast and study product, and adverse events were assessed using an open-ended question. Data input sheets were returned to the investigators and manually checked for possible errors, omissions, or protocol deviations prior to database entry.
Statistical methods
The sample size calculation was intended to address our primary question of whether 12.5 g of BH-BD in powder achieves nutritional ketosis (BHB ≥ 0.5 mM). Our previous study found a mean BHB Cmax after consumption of a 12.5 g BH-BD RTD beverage at 0.8 mM (SD = 0.25 mM) (Citation11). For our primary outcome BHB Cmax, an evaluable sample size of 11 was expected to provide 80% power to detect a change in mean to 0.5 mM with an alpha of 0.05, two-sided. A sample size of 15 participants were enrolled to account for an attrition rate of ∼20%.
All statistical analyses were conducted blinded using GraphPad Prism 9 for Mac (version 9.1, GraphPad Software, San Diego, CA). Data is shown as mean ± SD. The analysis was conducted on an intent-to-treat (ITT) population which was defined as those participants that consumed at least one serving of the study products. All p-values are two-sided and considered significant at the <0.05 level. Analyses were performed based on observed data, any value where the ketone meter read “LO” (below limit of detection) was recorded as 0 in the data input sheet, other missing values were not imputed. Repeated measures analysis of variance (ANOVA) models, with time and condition as factors, were used to compare the group differences between maximal BHB concentration (Cmax), total BHB delivery (area under the curve, AUC) and minimal glucose concentration (Cmin) throughout this study. Model derived pairwise comparisons were made between each of the four study groups with Tukey’s post hoc corrections.
Analysis of BHB Cmax, BHB AUC and glucose Cmin were undertaken using normalized BHB and glucose values, in order to remove variation that may be caused by intra- and inter- individual differences in baseline BHB or glucose concentrations. BHB and glucose concentrations were normalized by subtraction of the value measured 30 minutes after the study meal (immediately before consumption of the study product). Maximal BHB values and minimal glucose values for each individual on each test day were manually extracted from the normalized data. BHB AUC for each individual was calculated using normalized BHB concentrations using Graph Pad Prism’s trapezoid method.
The composite beverage tolerability intensity score was calculated as the sum of all scores of the individual symptoms for the post-product questionnaire. The post-consumption composite scores were compared between all groups with the Friedman Test.
Results
Of the 15 participants randomized, all were 100% compliant with study product intake and included in the ITT population. All participants completed all four study conditions without major protocol deviations. Thus, a per protocol population was not defined and only the ITT analysis completed. Baseline characteristics were self-reported by participants and are shown along with pretest dietary intake recorded from the ASA24 tool in .
Table 2. Baseline characteristics of study participants.
Blood BHB concentrations were significantly increased by all BH-BD drinks; no changes in BHB were seen in the control condition (). The threshold for nutritional ketosis (BHB ≥0.5 mM) was reached approximately 15 minutes after product consumption and nutritional ketosis was maintained throughout the 2 hour sampling period. Subjects with BHB ≥0.5 mM at the 2 hour timepoint was 100% in POW 25 g, 67% in POW 12.5 g and 73% in RTD 12.5 g. Normalized BHB Cmax was the highest in the condition with the largest BH-BD serving, being significantly greater in the POW 25 g (1.7 ± 0.6 mM) than in all other conditions (POW 12.5 g = 0.83 ± 0.2 mM, P < 0.0001; RTD 12.5 g = 0.91 ± 0.3 mM, P = 0.0015). Whilst there were no differences in BHB Cmax between the POW 12.5 g and RTD 12.5 g conditions (P = 0.825), BHB Cmax was significantly higher in both 12.5 g BH-BD conditions than in control (Control = 0.1 ± 0.1 mM; P < 0.0001) (). Similarly, normalized BHB AUC was significantly greater in the POW 25 g condition (2.26 ± 0.6 mM*h) compared to all other conditions (vs POW 12.5 g P = < 0.0001; vs RTD 12.5 g P = 0.0019); no differences were seen between BHB AUC with both formulations containing 12.5 g BH-BD (POW 12.5 g = 1.00 ± 0.3 mM*h; RTD 12.5 g = 1.22 ± 0.5 mM*h; P = 0.3434) and all BH-BD study products resulted in greater BHB AUC than the control study product (0.16 ± 0.2 mM*h; P < 0.0001) ().
Figure 3. Capillary blood BHB and glucose concentrations in healthy adults (n = 15) following consumption of 25 g or 12.5 g of BH-BD as powder, 12.5 g RTD beverage formulation, or a control drink. A: mean blood BHB concentrations over time, B: peak BHB concentration for each subject normalized to each individual’s baseline value, C: normalized BHB AUC, D: mean glucose concentrations over time, E: minimal normalized glucose concentration for each individual.
Abbreviations: AUC, area under the curve; BHB, beta hydroxybutyrate; BH-BD, bis hexanoyl (R)-1,3-butanediol; Cmax, peak concentration; Cmin, minimal concentration. Data are mean ± standard deviation. * = p <0.05 between group, ** = p <0.001, *** = p <0.0001 between group, NS = not significant; † = p <0.05 between control and POW 25 g groups.
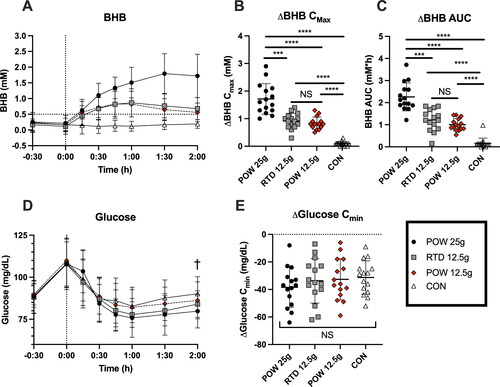
Blood glucose concentrations increased following the study meal, with the measured peak at 30 minutes post-meal, and then fell following consumption of all study products (). There was a main effect of time (P < 0.0001), but not of study product (P = 0.3878) or study product x time (P = 0.4176) on glucose. No significant differences were seen in normalized glucose Cmin between study groups (POW 25 g = −38.5 ± 14.5 mg/dL; POW 12.5 g = −32.7 ± 15 mg/dL; RTD 12.5 g = −33.7 ± 16 mg/dL; main effect P = 0.2485) ().
All study products were well tolerated. No significant differences in post-product total composite tolerability scores were detected between BH-BD vs control (). Only two participants rated tolerability symptoms at a ‘moderate’ or greater intensity (control: dizziness, n = 1; POW 12.5 g: burping, n = 1). Two subjects reported flushing after consuming the POW 25 g study product. These adverse events were judged by the Investigators as mild and probably related to study product intake. In both cases, the symptoms resolved within a few hours.
Table 3. Post-product composite beverage tolerability score.
Discussion
This study aimed to evaluate the effect of formulation on blood ketone and glucose response following consumption of the novel ketone ester BH-BD. All BH-BD study products induced a state of nutritional ketosis (blood BHB ≥ 0.5 mM) that was maintained for the 2-hour sampling period. Blood ketone responses did not differ between 12.5 g servings of BH-BD as RTD and powder formulations. Ketone concentrations were further elevated by increasing the BH-BD powder serving size to 25 g. There was no effect of formulation on glucose responses. Our findings suggest that both powder and RTD formulations of BH-BD are effective at increasing circulating ketone concentrations.
BH-BD is the first ketone ester to be formulated into a powder, therefore this study provides crucial data to demonstrate equivalency between the RTD beverage and powdered ketone ester formulations. The normalized BHB Cmax values seen in this study with 12.5 g (RTD = 0.9 mM, POW = 0.8 mM) and 25 g (POW =1.7 mM) are similar to those reported in a previous study of the same RTD BH-BD study products following a different standard meal (RTD 12.5 g = 0.8 mM; RTD 25 g = 1.7 mM) (Citation11). Together, these results demonstrate both the replicability of ketosis in different study populations and also the equivalency of the powdered and RTD formulations of BH-BD. The powder formulation may provide an advantage over the RTD formulation in a greater choice of use which may in turn accommodate taste preferences. In addition, future work can evaluate the possible metabolic effects of the soluble fiber carrier (Citation32,Citation33, Citation39).
Increasing the absolute serving size of BH-BD from 12.5 g to 25 g led to corresponding increases in both BHB Cmax and BHB AUC. Notably, most scientific research on ketone esters uses body weight adjusted servings (Citation12, Citation19–21, Citation40,Citation41). We used standard serving sizes to mimic real world use. The highest weight adjusted serving size in our study was ∼460 mg/kg, which is somewhat lower than servings used in functional studies of ketone esters for athletic performance (573 mg/kg) (Citation15), cardiac function (714 mg/kg) (Citation23), and brain function (625 mg/kg) (Citation18), therefore future work should include larger serving sizes of BH-BD to understand if the ketone response to increasing serving size continues, or if there are alterations in BHB kinetics with higher amounts.
A relatively large body of work has demonstrated that ketone esters similar to BH-BD can reduce blood glucose response when taken before either a 75 g oral glucose bolus (Citation20,Citation21) or a mixed meal containing ∼84 g of carbohydrate (Citation22, Citation40). In this study, consumption of BH-BD 30 min after a standard meal did not alter blood glucose responses compared to a non-ketogenic control beverage. The 30-min time interval was chosen to allow the carbohydrate from the study meal to blunt endogenous ketone production. It is possible that ketones must be consumed in advance of, or more closely in conjunction with dietary carbohydrate in order to modulate glycaemic response. It is also notable that the amount of carbohydrate in the standard meal used here (24 g) was low in comparison to previous studies, suggesting the effect of exogenous ketones on glycaemic response may only become detectable with greater carbohydrate load.
Limitations of the present study include the 2-hour study duration. Our primary aim was to capture peak BHB concentration, which was known to occur within one hour of RTD BH-BD product consumption, thus we chose the 2-hour time period to minimize participant burden, attrition and increase compliance, especially given the decentralized design. However, this meant that we did not see BHB concentrations return to baseline and cannot compare BHB AUC results to previous studies with longer measurement periods. While our decentralized approach allowed for minimal burden, participants were not monitored during blood draws thus their adherence to exact timing is unknown. We also did not include a 25 g RTD condition for direct comparison with the 25 g powder condition, to again minimize participant burden; and data from previous studies of 25 g in RTD beverages provides a comparison to the data we collected here (Citation11). As the participants were not blinded to their BHB and glucose concentrations, this could have affected our tolerance data, although we did not see between-group differences in this study. Additionally, whilst we closely matched calories in the POW 25 g, RTD 12.5 g and CON conditions, the caloric content of the POW 12.5 g beverage was lower, but as this was due to the lack of additional canola oil, we do not expect that this would have altered either the BHB or glucose data. Finally, these results may not be generalizable to all populations. Factors such as age, sex-differences, physical fitness, habitual diet, metabolic health status, gastric emptying rates, and ethnicity could conceivably affect metabolism of ketone esters such as BH-BD and will be the focus of future work.
In conclusion, exogenous ketone esters such as BH-BD induce nutritional ketosis independently of a ketogenic diet or fasting. Here we demonstrate that a novel powdered formulation of BH-BD is well tolerated and delivers equivalent blood ketone responses to a RTD beverage formulation, without any consistent changes in glucose concentrations. The results of this study provide evidence to support the future use of BH-BD as a tool to study the impact of exogenous ketosis on a range of health and performance outcomes.
Author contributions
Conceptualization, B.J.S, J.C.A, K.M.N.; methodology, B.J.S, K.M.N, J.C.A; formal analysis, B.J.S; investigation, B.J.S, K.M.N.; data curation, B.J.S, K.M.N.; writing—original draft preparation, B.J.S, K.M.N.; writing—review and editing, B.J.S, J.C.A, K.M.N; visualization, B.J.S; project administration, B.J.S, K.M.N.; funding acquisition, B.J.S, J.C.A. All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
This study was conducted according to the guidelines of the Declaration of Helsinki (2004), and approved by WCG IRB (WIRB-Copernicus Group, Puyallup, WA) on July 26, 2021 (ID# 20213716).
Acknowledgments
Thank you to Cosmin Beliciu at The National Food Laboratory for his assistance with packaging and shipping of study product. Thank you to Amy Boileau, Martin Ducker and Barbara Winters for their helpful comments on the protocol and manuscript.
Disclosure statement
B.J.S. has stock options in BHB Therapeutics Ltd., and Juvenescence Ltd. B.J.S. is an inventor on patents related to the use of ketone bodies. J.C.A. holds stock options in Juvenescence Limited and Juvenescence Life Sciences Limited. All other authors have no competing interests.
Data availability statement
The data presented here may be available upon reasonable request from the corresponding author and in accordance with intellectual property considerations.
Additional information
Funding
References
- Cahill GF.Jr., Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi:10.1146/annurev.nutr.26.061505.111258.
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25(1):42–52. doi:10.1016/j.tem.2013.09.002.
- Mikkelsen KH, Seifert T, Secher NH, Grøndal T, van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-beta-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab. 2015;100(2):636–43. doi:10.1210/jc.2014-2608.
- Shaw DM, Merien F, Braakhuis A, Maunder E, Dulson DK. Exogenous ketone supplementation and keto-adaptation for endurance performance: disentangling the effects of two distinct metabolic states. Sports Med. 2020;50(4):641–56. doi:10.1007/s40279-019-01246-y.
- Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15(1):13–20. doi:10.1080/17461391.2014.959564.
- Poff AM, Koutnik AP, Egan B. Nutritional ketosis with ketogenic diets or exogenous ketones: features, convergence, and divergence. Curr Sports Med Rep. 2020;19(7):251–9. doi:10.1249/JSR.0000000000000732.
- Cahill GF.Jr., Starvation in man. N Engl J Med. 1970;282(12):668–75. doi:10.1056/NEJM197003192821209.
- Hallberg SJ, McKenzie AL, Williams PT, Bhanpuri NH, Peters AL, Campbell WW, Hazbun TL, Volk BM, McCarter JP, Phinney SD, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583–612. doi:10.1007/s13300-018-0373-9.
- Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412–26. doi:10.1002/(SICI)1520-7560(199911/12)15:6<412::AID-DMRR72>3.0.CO;2-8.
- Robinson AM, Williamson DH. Physiological roles of ketone-bodies as substrates and signals in mammalian-tissues. Physiol Rev. 1980;60(1):143–87. doi:10.1152/physrev.1980.60.1.143.
- Crabtree CD, Blade T, Hyde PN, Buga A, Kackley ML, Sapper TN, Panda O, Roa-Diaz S, Anthony JC, Newman JC, et al. Bis hexanoyl (R)-1,3-butanediol, a novel ketogenic ester, acutely increases circulating R- and S- ß-hydroxybutyrate concentra-tions in healthy adults. J Am Coll Nutr. 2022:1–9. doi:10.1080/07315724.2021.2015476.
- Poffe C, et al. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019;597(12):3009–27.
- Poffe C, et al. Bicarbonate unlocks the ergogenic action of ketone monoester intake in endurance exercise. Med Sci Sports Exerc. 2021;53(2):431–41.
- EVANS MARK, MCSWINEY FT, BRADY AJ, EGAN B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sports Exerc. 2019;51(12):2506–15. doi:10.1249/MSS.0000000000002065.
- Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256–68. doi:10.1016/j.cmet.2016.07.010.
- Mujica-Parodi LR, Amgalan A, Sultan SF, Antal B, Sun X, Skiena S, Lithen A, Adra N, Ratai E-M, Weistuch C, et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc Natl Acad Sci USA. 2020;117(11):6170–7. doi:10.1073/pnas.1913042117.
- Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exerc. 2018;50(11):2330–8. doi:10.1249/MSS.0000000000001700.
- Coleman K, Phillips J, Sciarini M, Stubbs B, Jackson O, Kernagis D. A Metabolic intervention for improving human cognitive performance during hypoxia. Aerosp Med Hum Perform. 2021;92(7):556–62. doi:10.3357/AMHP.5767.2021.
- Walsh JJ, Neudorf H, Little JP. 14-Day ketone supplementation lowers glucose and improves vascular function in obesity: a randomized crossover trial. J Clin Endocrinol Metab. 2021;106(4):e1738–e1754. doi:10.1210/clinem/dgaa925.
- Myette-Cote E, et al. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol. 2018;596(8):1385–95.
- Myette-Côté É, Caldwell HG, Ainslie PN, Clarke K, Little JP. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. The American Journal of Clinical Nutrition. 2019;110(6):1491–501. doi:10.1093/ajcn/nqz232.
- Greaves G, Xiang R, Rafiei H, Malas A, Little JP. Prior ingestion of a ketone monoester supplement reduces postprandial glycemic responses in young healthy-weight individuals. Appl Physiol Nutr Metab. 2021;46(4):309–17. doi:10.1139/apnm-2020-0644.
- Selvaraj S, et al. Acute echocardiographic effects of exogenous ketone administration in healthy participants. J Am Soc Echocardiogr. 2021;35:305–311.
- Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor A, Kautzner J, Melenovsky V. Myocardial ketone body utilization in patients with heart failure: The impact of oral ketone ester. Metabolism. 2021;115:154452. doi:10.1016/j.metabol.2020.154452.
- Dearlove DJ, Harrison OK, Hodson L, Jefferson A, Clarke K, Cox PJ. The effect of blood ketone concentration and exercise intensity on exogenous ketone oxidation rates in athletes. Med Sci Sports Exerc. 2021;53(3):505–16. doi:10.1249/MSS.0000000000002502.
- Stubbs BJ, Blade T, Mills S, Thomas J, Yufei X, Nelson FR, Higley N, Nikiforov AI, Rhiner MO, Verdin E, et al. In vitro stability and in vivo pharmacokinetics of the novel ketogenic ester, bis hexanoyl (R)-1,3-butanediol. Food Chem Toxicol. 2021;147:111859. doi:10.1016/j.fct.2020.111859.
- Traul KA, Driedger A, Ingle DL, Nakhasi D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem Toxicol. 2000;38(1):79–98. doi:10.1016/S0278-6915(99)00106-4.
- Miller SA, Dymsza HA. Utilization by rat of 1,3-butanediol as a synthetic source of dietary energy. J Nutr. 1967;91(1):79–88. &. doi:10.1093/jn/91.1.79.
- Mehlman MA, Tobin RB, Hahn HK, Kleager L, Tate RL. Metabolic fate of 1,3-butanediol in rat – liver tissue slices metabolism. J Nutr. 1971;101(12):1711–8. &. doi:10.1093/jn/101.12.1711.
- Chen O, Blonquist T, Mah E, Sanoshy K, Beckman D, Nieman K, Winters B, Anthony J, Verdin E, Newman J, et al. Tolerability and safety of a novel ketogenic ester, bis-hexanoyl (R)-1,3-butanediol: a randomized controlled trial in healthy adults. Nutrients. 2021;13(6):2066. doi:10.3390/nu13062066.
- Hoag SW, Hussain AS. The impact of formulation on bioavailability: summary of workshop discussion. J Nutr. 2001;131(4 Suppl):1389S–91S. doi:10.1093/jn/131.4.1389S.
- Livesey G, Tagami H. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): meta-analysis of randomized controlled trials. Am J Clin Nutr. 2009;89(1):114–25. doi:10.3945/ajcn.26842.
- Kishimoto Y, Oga H, Tagami H, Okuma K, Gordon DT. Suppressive effect of resistant maltodextrin on postprandial blood triacylglycerol elevation. Eur J Nutr. 2007;46(3):133–8. doi:10.1007/s00394-007-0643-1.
- Assembly WG. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the: 56th WMA General Assembly, Tokyo, October 2004. 2004. Ferney-Voltaire, France: World Medical Association.
- Moore AR, Holland-Winkler AM, Ansley JK, Boone EDH, Schulte MKO. Reliability and diagnostic performance of a new blood ketone and glucose meter in humans. J Int Soc Sports Nutr. 2021;18(1):6. doi:10.1186/s12970-020-00404-2.
- How Accurate are Glucose & Ketone Meters? [accessed 2022 Aug 5]. https://keto-mojo.com/testing-how-accurate-are-glucose-ketone-meters/.
- Blood Glucose Monitoring System Comparison Report (Keto-Mojo GK+, Keto-Mojo TD-4279, Abbott Precision Xtra, Accu-Chek Performa) [accessed 2022 Aug 5]. https://keto-mojo.com/wp-content/uploads/2021/02/Blood-Glucose-Monitoring-System-Accuracy-Comparison-Report.pdf.
- NCI. Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool. [cited 2022]. Available from: https://epi.grants.cancer.gov/asa24/.
- Ye Z, Arumugam V, Haugabrooks E, Williamson P, Hendrich S. Soluble dietary fiber (Fibersol-2) decreased hunger and increased satiety hormones in humans when ingested with a meal. Nutr Res. 2015;35(5):393–400. doi:10.1016/j.nutres.2015.03.004.
- Stubbs BJ, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848.
- Shivva V, Cox PJ, Clarke K, Veech RL, Tucker IG, Duffull SB. The population pharmacokinetics of d-β-hydroxybutyrate following administration of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Aaps J. 2016;18(3):678–88. doi:10.1208/s12248-016-9879-0.