Abstract
Objective
The study aimed to examine the role of an Acacia catechu and Scutellaria baicalensis formulation, UP446, on supporting immune function in response to influenza vaccination.
Methods
A randomized, triple-blind, placebo-controlled, parallel study consisted of a 56-day intervention period with a 28-day pre-vaccination period, an influenza vaccination on Day 28 and 28-day post-vaccination period. Fifty healthy adults 40–80 years of age who had not received their flu vaccine were randomized to either UP446 or Placebo. At baseline, Days 28 and 56, immune and oxidative stress markers were measured in blood and a quality of life questionnaire was administered. Participants completed the Wisconsin Upper Respiratory Symptom Survey (WURSS)-24 daily.
Results
In the post-vaccination period, total IgA and IgG levels increased in participants supplemented with UP446 vs. those on Placebo (p ≤ 0.026). As well, influenza B-specific IgG increased 19.4% from Day 28 to 56 and 11.6% from baseline at Day 56 (p ≤ 0.0075). Serum glutathione peroxidase (GSH-Px) was increased in the pre-vaccination period and from baseline at Day 56 with UP446 supplementation (p ≤ 0.0270).
Conclusion
These results suggest a 56-day supplementation with UP446 was beneficial in mounting a robust humoral response following vaccination. Increasing GSH-Px in the pre-vaccination period may help improve antioxidant functions and potentially mitigate the oxidative stress induced following vaccination.
Introduction
Acute respiratory tract infections are one of the leading causes of morbidity and mortality. According to the World Health Organization (WHO), an estimated 3–5 million severe cases of seasonal influenza require hospitalization and account for 290,000–650,000 deaths per year globally (Citation1). To mitigate the impact of respiratory viruses, several public health strategies are available including annual vaccination programs for influenza. In recent years including the past 2020/2021 season, vaccination rates in Canada and the US ranged from 27 to 50% of the adult population (Citation2). In Canada, the national influenza vaccination coverage goal is 80% of those at risk of influenza-related complications or hospitalization. Every year, these programs must predict which strain to vaccinate against due to the virus’s ability to rapidly evolve and mutate. In the United States (US), the 2019/20 flu vaccine effectiveness was estimated at only 34–39% for adults 18 years of age or older (Citation3). This is consistent with previous years, with effectiveness varying between 19 and 54% (Citation4). Strategies that may increase the efficacy of the flu vaccine and reduce the severity and duration of upper respiratory tract infections (URTIs) are needed.
Immunomodulatory nutraceuticals with a well-established safety profile with proven efficacy in boosting the immune response to influenza vaccine while maintaining innate and adaptive immune functions are certainly warranted. Various nutrients including vitamin A, vitamin B12, folate, selenium, iron, copper and omega-3 fatty acids play an important role in supporting the immune system, and optimal nutritional status is key in protection against viral infections (Citation5). The addition of supplements or nutritional interventions have been investigated to improve the immune response to influenza and efficacy of the influenza vaccine (Citation6–9) and more recently the COVID-19 vaccine (Citation10). The flavonoid extracts of Acacia catechu and Scutellaria baicalensis have been shown to reduce pro-inflammatory gene expression in vitro (Citation11) and Acacia catechu supports anti-inflammatory activity and increases antibody titers after immunization in rodent models (Citation12). While these in vitro and in vivo studies suggest these ingredients have effects on immune function, there is a dearth of evidence on their immunomodulatory properties using human vaccine models in clinical trials.
The objective of this study was to investigate a formulation, UP446, containing Acacia catechu and Scutellaria baicalensis compared to Placebo on supporting immune function in a healthy adult population. A vaccine model using the quadrivalent influenza vaccine (QIV) was used to examine if prophylactic supplementation of UP446 modulates the immune response that is induced after administering a vaccine (Citation8, Citation13). Modulation of immune function focused on immune cell populations and humoral immunity as they are the direct effectors of the influenza vaccination immune response (Citation14, Citation15).
Materials and methods
Study design
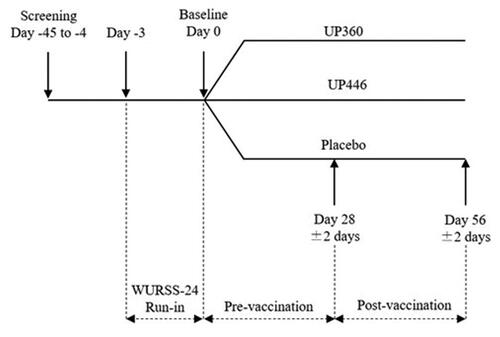
The design was a randomized, triple-blind, placebo-controlled, parallel study conducted at the KGK Science Inc. clinic site (London, Ontario, Canada) from February 17 to May 21, 2021. The complete study design is shown in which includes three groups: UP446, UP360 and Placebo. The UP360 formulation contains a mixture of aloe vera gel powder, rosemary and Poria cocos extracts. The current manuscript will report on UP446 vs. Placebo only as UP446 has a distinct mechanism of action and differentially affects immune function compared to UP360.
In-clinic study visits occurred at screening, baseline (Day 0), Day 28 and Day 56, where baseline to Day 28 was considered the “pre-vaccination” period, and Day 28 to Day 56 was “post-vaccination.” At screening, potential participants were assessed for eligibility using medical history, inclusion/exclusion criteria, clinical chemistry and hematology, concomitant therapies, and current health and vaccination status. Prior to baseline, eligible participants completed a 3-day run-in period during which the Wisconsin Upper Respiratory Symptom Survey (WURSS)-24 and study diary was completed daily to identify any participants who self-reported COVID-19 or flu, and the WURRS-24 assessed by the Medical Director (MD). Any participant with COVID-19 or flu prior to baseline was removed from the study and followed up appropriately. Study products were consumed daily for a total of 56 days including 28 days before and 28 days after a challenge with a seasonal QIV.
Ethics and participants
This study was approved by the Natural and Non-Prescription Health Products Directorate (NNHPD), Health Canada, Ottawa, Ontario on February 1, 2021. Research ethics board approval was granted on February 2, 2021 from the Institutional Review Board (IRB) Services, Aurora, Ontario (Pro00049388). The study was conducted in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP) and in accordance with the Declaration of Helsinki guidelines and its subsequent amendments. The trial was registered on the International Clinical Trials Registry Platform on September 10, 2021 (ISRCTN15838713) and followed the CONSORT guidelines for randomized controlled trials (Citation16) (Supplementary Table S1). Written informed consent was obtained from all participants prior to any study procedures being initiated.
Table 1. Baseline demographic characteristics for participants in the intent-to-treat (ITT) population (n = 50).
Participants who met all inclusion without meeting any exclusion criteria at screening and baseline were randomized into study groups. Participants were male and female between the ages of 40 and 80 years, had not yet but were willing to receive the influenza vaccine and agreed to provide verbal history of flu vaccination. Participants were required to complete questionnaires and diaries, and maintain current lifestyle habits of diet, exercise, and sleep as well to maintain their current medications, and supplement routine.
Individuals were excluded if they had a known allergy to the investigational products (IPs) or QIV; were unvaccinated and had the flu prior to baseline from September 2020, or prior to study vaccination; had a diagnosis of COVID-19 prior to baseline or study vaccination; had received the COVID-19 vaccine; used dietary supplements or herbal medicines to boost or modulate the immune system unless willing to washout; used immunomodulators within 4 weeks of baseline; were cognitively impaired and/or unable to give informed consent; or had any other condition, chronic disease, or lifestyle factor, that, in the opinion of the MD, may have adversely affected the participant’s ability to complete the study or its measures or posed significant risk to the participant.
Investigational products and vaccines
The IP, UP446, contained a formulation of Acacia catechu (Senegalia catechu) and Scutellaria baicalensis for which preparation details have been disclosed in a US patent (Citation17) and the preparation of extracts and full composition using High Performance Liquid Chromatography (HPLC) has been previously described by Yimam et al. (Citation18) and Yimam et al. (Citation19). Briefly, UP446 is a greenish yellow to brown powder containing standardized extracts from Acacia catechu and Scutellaria baicalensis. The Scutellaria baicalensis extract has a baicalin content of no less than 60% as the major component (Citation18). Catechins were extracted from Senegalia catechu from stem heartwood at a ratio of 20:1. The major component in the Senegalia catechu extract is (+)-catechin at no less than 10% (Citation18). The IP contained excipients maltodextrin, microcrystalline cellulose (MCC), and magnesium stearate.
The Placebo contained MCC and magnesium stearate. All products were manufactured by Acenzia (Tecumseh, Ontario, Canada).
Participants were instructed to take two capsules of study product (either UP446 or placebo) per day, one in the morning and one in the evening around mealtimes with food and 4–6 ounces of water for 56 days. If a dose was missed, participants were instructed to take the dose as soon as possible and advised not to exceed four capsules daily.
At Day 28, all participants received an intramuscular injection of influenza vaccine specific for the 2020/2021 flu season (FLUCELVAX® QUAD, Seqirus, Kirkland, QC). The strains present in the vaccine were Hemagglutinin A/Hawaii/70/2019 (H1N1) pdm09-like virus (A/Nebraska/14/2019), Hemagglutinin A/Hong Kong/45/2019 (H3N2)-like virus (A/Delaware/39/2019), Hemagglutinin B/Washington/02/2019-like virus (B/Darwin/7/2019) and Hemagglutinin B/Phuket/3073/2013-like virus (B/Singapore/INFTT-16-0610/2016).
Randomization and blinding
Eligible participants were assigned a randomization number by a blinded investigator according to the order of the randomization list (www.randomization.com). A randomization schedule was created and provided to the investigator indicating the order of randomization. Investigators, other site personnel, statisticians and participants were blinded to the products.
The Placebo was matched to the IP with similar excipients and appearance to ensure blinding. The IP and Placebo were sealed in bottles that were identical in appearance and labeled according to the requirements of ICH GCP guidelines and applicable local regulatory guidelines. Unblinded personnel not involved in any study assessments labeled the study products.
Compliance
Participants were instructed to save all unused and open packages and bring them to each study visit and product compliance was calculated by determining the number of dosage units taken divided by the number of dosage units expected to be taken, multiplied by 100. In the event of a discrepancy between the information in the study diary and the amount of study product returned, compliance was based on the product returned unless an explanation for the loss was provided.
Outcomes
The objective of this study was to investigate the efficacy of UP446 on supporting immune function in healthy adults following influenza vaccination. The primary outcome tested the difference between UP446 and Placebo in the increase in blood lymphocyte populations (CD3+, CD4+, CD8+, CD45+, TCRγδ+, CD3−CD16+56+) and immunoglobulins (IgG, IgM, and IgA) from baseline at Day 28 and 56. Secondary outcomes included erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), hematology, complement C3 and C4 proteins, influenza-specific antibodies, advanced glycation end products (AGEs), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD). The incidence, frequency, and severity of URTI symptoms, COVID-19 and flu cases and hospitalizations, use of over-the-counter cold and flu medication and quality of life were other secondary outcomes. Safety outcomes included clinical chemistry, pre- and post-emergent adverse events, and vitals.
Laboratory analysis
Blood drawn at baseline, Day 28 and Day 56 by venipuncture was used to assess primary, secondary and safety outcomes. Lymphocyte populations were analyzed by flow cytometry (London Health Sciences Center Laboratory, London, ON). A whole blood sample was incubated with fluorescent-tagged monoclonal antibodies specific for T and B lymphocytes and Natural Killer cells. The flow cytometry instrument was a 10-colour Navios and Kaluza software was used for analysis, both from Beckman Coulter. Analysis of two and four-colour tubes with CD45 and antigen gating were used to determine the populations of interest and the absolute counts were determined using a bead-based method also known as single platform testing. Immunoglobulins were analyzed using Roche Cobas c701 analyzer (LifeLabs, London, ON). The reference ranges for adults over 19 years of age were between 0.5–4.17 g/L, 6–16 g/L, and 0.3–2.30 for IgA, IgG, and IgM, respectively.
ESR, CRP, hematology, complement C3 and C4 protein were measured by Roche Cobas c701 with reference ranges for C3 and C4 proteins of 0.9–1.80 and 0.15–0.53 g/L, respectively. Hematology including hemoglobin, hematocrit, platelet count, red blood cell count (RBC), red blood cell indices, red cell distribution width (RDW), white blood cell count (WBC), differentials (neutrophils, lymphocytes, monocytes, eosinophils, basophils) was analyzed (LifeLabs) using standardized procedures. Influenza-specific antibodies were analyzed by Qualitative ELISAs as follows (Unigen, Tacoma, WA): Human Influenza A IgA ELISA (Abcam # ab108743), Human Influenza A IgG ELISA (Abcam # ab108745), Human Influenza A IgM ELISA (Abcam # ab108747), Human Influenza B IgA ELISA (Abcam # ab108744), Human Influenza B IgG ELISA (Abcam # ab108746), Human Influenza B IgM ELISA (Abcam # ab108748). The observed ranges were as follows: 0–28 for Influenza A IgA, 0–73 for Influenza A IgG, 0–51 for Influenza A IgM, 0–18 for Influenza B IgA, 0–29 for Influenza B IgG, 0 − 26 for Influenza B IgM. AGE Assay Kit (Abcam # ab273298), a semi-quantitative fluorescent method was used for the analysis of AGEs. The observed range was 60–110 AU/mg serum protein. SOD was analyzed using the SOD Assay Kit (Cayman Chem # 706002) for which the analytical range was 0–5.75 U/mL. Glutathione Peroxidase Assay Kit (Abcam # ab102530), a quantitative colorimetric method was used to analyze GSH-Px. The amount of GSH-Px enzyme was proportional to the amount of glutathione disulfide (GSSG) produced from reduced glutathione (GSH) as measured by the NADPH consumed in the reduction of GSSG to GSH. The analytical range for GSH-Px was 0–333 mU/mL.
Modified WURSS-24 questionnaire
The WURSS-24 Questionnaire captured the incidence, frequency, and severity of URTI symptoms (Citation20). The modified WURSS-24 used in this study included a revision that referred to “symptoms” rather than “cold” to clarify that symptoms may be due to any URTI not specific to a cold. A new URTI episode was defined as the sudden appearance of one or more symptoms captured by questions 2 to 5 of the WURSS-24 Questionnaire, not attributed to allergies (as reported by participants) with at least two prior days of “not sick” as defined by Murdoch et al. (Citation21). The WURSS-24 was completed daily during the 56-day intervention period, as well as during the three-day run-in period to ensure participants did not have a cold, flu, or COVID-19 at baseline prior to starting on study products.
Prior to the Day 28 vaccination, responses on the WURSS-24 were reviewed and participants were assessed on a case-by-case basis by the MD to determine and confirm status as flu or COVID-19. Any participant presenting with flu or COVID-19 was not eligible to receive the influenza vaccination at Day 28 and was removed from the study with appropriate follow-up. Prior to Day 56, the WURSS-24 was reviewed by a study coordinator and participants with a self-reported or with a diagnosis of COVID-19 were assessed by the MD and removed from the study.
Study diaries
The daily study diary was used to capture outcomes of rates of influenza and COVID-19 infections, hospitalizations due to influenza or COVID-19 and over-the-counter cold and flu medication use. The study diary was also used to monitor any changes in participants health or lifestyle. Participants were required to record daily during the 3-day run-in period and 56-day study period, any AEs, use of concomitant therapies, hours of sleep, alcohol, tobacco and cannabis use, hours and description of exercise and any changes to their health. Study diaries were reviewed at each study visit to ensure the health of participants and any outcomes related to influenza or COVID-19 were assessed.
Vitality and quality of life
The Vitality and Quality of Life (QoL) Questionnaire was used to assess the energy levels and quality of life of participants and was designed to be used in populations with no associated pathologies. The questionnaire consisted of 31-items assessed on a 7-point Likert scale where 1 represented “Never,” and 7 represented “Always.” A higher summative score indicated that more vitality was subjectively perceived by the participants and individual questions were also analyzed. This questionnaire has been previously used to assess QoL parameters in over 273 healthy participants.
Safety and adverse events
Blood drawn at baseline, Day 28 and 56 was analyzed for clinical chemistry which included alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, creatinine, electrolytes (Na+, K+, Cl−), estimated glomerular filtration rate (eGFR) and glucose (LifeLabs). Blood pressure and heart rate were measured at baseline, Day 28 and 56.
Participants recorded any AEs in their study diary and were classified based on the description, duration, intensity, frequency, and outcome. All AEs were assessed by the MD for causality and intensity. The Medical Dictionary for Regulatory Activities (MEDRA) terminology version 22.0 was used for coding. For women of childbearing potential, urine pregnancy tests were conducted at the KGK clinic at baseline and Day 56.
Statistical analysis
A power calculation was performed to determine the planned total sample size of 75 participants, with 25 participants randomized to each study group (Citation13, Citation22, Citation23). This sample size enabled detection of a difference in CD3+ of 10.8%, difference in CD4+ of 6.5%, difference in CD8+ of 4.9%, difference in CD45+ of 16.7% and difference in NK cells (CD56+) of 4.4% for lymphocytes; difference in IgA of 67 mg/dL (0.67 g/L), difference in IgG of 440 mg/dL (4.4 g/L), and difference in IgM of 133 mg/dL (1.33 g/L) at an overall 5% significance level and 80% power. This sample size accounted for a loss to follow up rate of 20%.
All the primary and secondary endpoints were analyzed as continuous variables. For each primary and secondary endpoint, descriptive statistics including number of subjects, arithmetic mean, standard deviation, median, minimum, and maximum values, where appropriate, were presented for each study visit and for the changes from baseline (Day 0) to Day 28 (pre-vaccination), Day 28 to Day 56 (post-vaccination) and baseline (Day 0) to end-of-study (Day 56). Endpoints gathered by study diaries in the intervals between study visits were reported as pre-vaccination (Day 0 to Day 28) and post-vaccination (Day 28 to Day 56). Differences of outcomes at visits, and differences in change of outcomes between visits, were assessed by linear mixed model, with study group and visit as the fixed effects. Each model included the baseline value as a covariate. An interaction term for study group and visit was included where appropriate. Within-group changes were assessed by pairwise comparison of estimated marginal means.
For outcomes captured by WURSS-24, the area under the curve (AUC) for total daily symptom scores was a measure of global illness severity over a defined time period, where higher values indicate greater symptom severity and frequency. The mean global severity index was defined as the total global severity score divided by the number of symptoms. Mean symptom severity scores were defined as AUC for mean symptom severity is a measure of URTI symptom severity over a defined time period, where higher values indicate greater symptom severity. A well day was defined as a day for which the score of question 1 was zero (“Not sick”) and a sick day was defined as a day for which the score of question 1 “How sick do you feel today?” was between 1 and 7.
The intent-to-treat (ITT) population consisted of all participants who received capsules and with any available post-randomization efficacy information. The per protocol (PP) population included all participants who consumed at least 80% of either study product, did not have any major protocol violations, and completed all study visits and procedures connected with measurement of the primary variables.
All statistical analysis were completed using the R Statistical Software Package Version 3.6.3 or newer for Microsoft Windows (Citation24). All tests of significance for primary outcomes were performed as one-sided, and for secondary and future analysis outcomes were performed at two-sided, alpha level = 0.05 unless otherwise specified. All data are presented as mean ± standard deviation (SD) unless noted.
Results
Study population
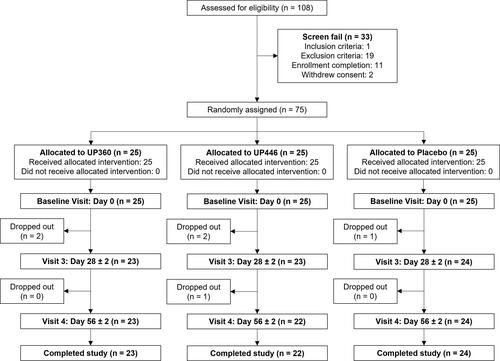
A total of 108 individuals were consented and screened with 75 participants enrolled in the study (). In the UP446 and Placebo groups, 50 participants were included in the Intent-to-Treat (ITT) population and a total of 46 in the per-protocol (PP) population. Four participants in the UP446 group were removed from the PP population due to booking a COVID-19 vaccine during the study period (n = 1), flu symptoms experienced prior to Day 28 (n = 2), and hospitalization for pneumonia prior to Day 56 (n = 1). The disposition of participants throughout the study is shown in . The mean compliance was >96% for both groups.
Enrolled participants were 40 to 79 years old with 64–68% females, 50–54 years of age, predominately Western and Eastern European, and employed full-time (). There was no difference in demographics or body mass index (BMI) between UP446 and Placebo groups at baseline.
Total immunoglobulin response
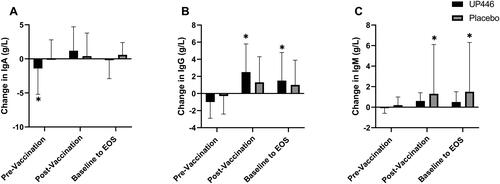
In the ITT population, the Placebo group had a significant increase in total IgG concentrations in the 28-day pre-vaccination period, from baseline to Day 28 (p = 0.031) (). There were no other changes in total immunoglobulin concentrations before vaccination.
Figure 3. Change in total (A) IgA, (B) IgG, and (C) IgM concentrations between UP446 and Placebo in the pre-vaccination period (baseline to Day 28), post-vaccination period (Day 28 to Day 56) and from baseline to end-of-study (EOS, Day 56) in the ITT population (n = 50). All values presented are mean ± standard deviation (SD). * indicates a significant within-group difference at the specific timepoint and ** indicates a significant difference between UP446 and Placebo.
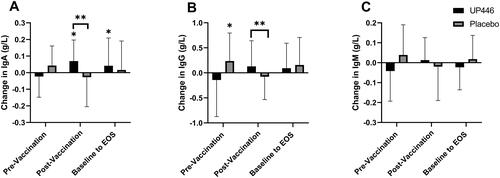
In the post-vaccination period (Day 28 to Day 56), IgA (p = 0.026) and IgG (p = 0.016) increased in the UP446 group compared to Placebo (). A total of 63.6% of participants supplemented with UP446 had an increase in IgA post-vaccination compared to only 37.5% of those on Placebo. There was also a significant increase in IgA levels with UP446 from baseline to end of study (Day 56) ().
Vaccine-specific immunoglobulin response
There were no significant differences in influenza-A or influenza-B specific antibodies between UP446 and Placebo groups ( and ).
Figure 4. Change in influenza A-specific (A) IgA, (B) IgG, and (C) IgM concentrations between UP446 and Placebo in the pre-vaccination period (baseline to Day 28), post-vaccination period (Day 28 to Day 56) and from baseline to end-of-study (EOS, Day 56) in the ITT population (n = 50). All values presented are mean ± standard deviation (SD). * indicates a significant within-group difference at the specific timepoint.
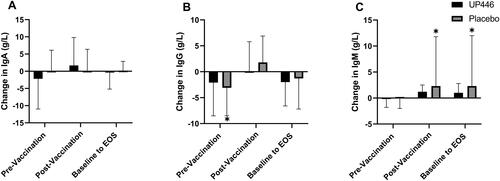
Figure 5. Change in influenza B-specific (A) IgA, (B) IgG, and (C) IgM concentrations between UP446 and Placebo in the pre-vaccination period (baseline to Day 28), post-vaccination period (Day 28 to Day 56) and from baseline to end-of-study (EOS, Day 56) in the ITT population (n = 50). All values presented are mean ± standard deviation (SD). * indicates a significant within-group difference at the specific timepoint.
However, there was a decrease from baseline in the pre-vaccination period in influenza A-specific IgG in the Placebo group (p = 0.0048) (). Participants on Placebo had an increase in influenza A-specific IgM in the post-vaccination period and from baseline to Day 56 (p ≤ 0.0252) ().
In the case of influenza B, there was an increase of 19.4% in IgG levels in the post-vaccination period with UP446 supplementation (p < 0.001) (). There was also a 11.6% increase in influenza B IgG-specific levels in the UP446 group from baseline at Day 56 (p = 0.0075). The Placebo group had increases in influenza B-specific IgM levels in the post-vaccination period and from baseline to Day 56 (p ≤ 0.0107) which was not seen with UP446 supplementation ().
Immune cell phenotypes and other inflammatory and hematological markers
There were no significant differences between UP446 and Placebo in the percentage of CD3+CD4+, CD3+CD8+, TCR γδ+, and CD3−CD56+ populations (). There was a significantly lower percentage of total lymphocytes (CD45+) in the UP446 group compared to Placebo at Day 28, but this did not result in a significantly different change from baseline. Participants on Placebo had a reduction in NK cells (CD3−CD56+) from baseline at Day 56 (p = 0.025) ().
Table 2. Immune cell phenotypes in blood of participants in the ITT population (n = 50).
There were no significant differences in CRP, ESR and complement proteins C3 and C4 between UP446 and Placebo groups in the pre- or post-vaccination or from baseline at Day 56 (data not shown). Hematology parameters including WBC count, neutrophils, lymphocytes, monocytes, basophils, reticulocyte count, RBC count, hemoglobin, hematocrit, platelet count and RBC indices were not significantly different between UP446 and Placebo groups pre- or post-vaccination or from baseline at Day 56 (Supplementary Table S2). There was a significant 0.10 × 109/L increase in eosinophils in the pre-vaccination period in the UP446 group. There was only one participant whose eosinophil count was outside the laboratory range but this was not clinically relevant as assessed by the MD.
Oxidative stress markers
In the pre-vaccination period, there was a greater increase in SOD in the Placebo group compared to UP446 (p = 0.0392) (). However, in the post-vaccination period, participants on Placebo had a significant reduction in SOD.
Table 3. Serum concentrations of antioxidant and oxidative stress markers in participants in the ITT population (n = 50).
Participants supplemented with UP446 had a significant 13.6% increase in GSH-Px in the pre-vaccination period and a significant 15.7% increase from baseline at Day 56. Concentrations of GSH-Px were not significantly different between UP446 and Placebo groups during the study.
Participants supplemented with UP446 as well as those on Placebo had significant increases in AGEs in the pre-vaccination period and from baseline at Day 56. There was a reduction in AGEs in the post-vaccination period with UP446 and Placebo, however this was not statistically significant ().
Upper respiratory tract infections
There were no significant differences in mean global severity index between UP446 and Placebo groups in the pre- vs. post-vaccination period (). There were no significant differences in other subjective measures of URTI illness between UP446 and Placebo groups during the study period including mean symptom severity index, duration, frequency, or severity of URTI symptoms. The mean number of well days ranged from 97.5 to 99.6% of total days in both the pre- and post-vaccination periods. There were also no significant differences in use of over-the-counter cold and flu medication. There were no hospitalizations due to COVID-19 or flu.
Table 4. Upper respiratory tract infection (URTI) symptom severity, well and sick days from the Modified Wisconsin Upper Respiratory Symptom Survey (WURSS)-24 in the ITT population (n = 50).
Quality of life and safety
There were no significant differences in individual questions of vitality and QoL between UP446 and Placebo groups in the pre-vaccination or post-vaccination periods, or from baseline at Day 56 (Supplementary Table S3). This aligns with the number of well days reported by participants during the 56-day study period.
In addition to the maintenance of QoL during the study, UP446 supplementation was found to be safe and well tolerated. There was a total of eight AEs reported by seven participants in the UP446 group. One participant reported a urinary tract infection and pneumonia and dropped out of the study and six were AEs reported by six participants: two reports of headache, and one report of each back pain, urinary tract infection, vertigo, and diarrhea. In the Placebo group there were six AEs reported by six participants: one report of each nausea, heart burn, stomach ache, ALT increase, headache, and hot flashes. All AEs were either unlikely to be, or not, related to the study products. All AEs were resolved by the end of the study period except the one participant who was hospitalized with pneumonia. Follow-up of this participant was done, and their recovery reported.
There were no significant changes in clinical chemistry parameters or vitals during the study (Supplementary Table S4). All clinical chemistry values that were outside the laboratory range were deemed not clinically relevant when assessed by the MD except for ALT, AST, and potassium. For ALT and AST, one participant in the Placebo group had values outside the laboratory range at Day 28 that were deemed clinically relevant, however at Day 56, both ALT and AST levels had returned to the normal range. There were four participants who had potassium levels outside the laboratory range and that were deemed clinically relevant. Potassium was repeated and were within the normal laboratory range for all participants except for one who did not complete a repeat measurement therefore advised to follow-up with their general practitioner.
Differences in the PP population
There were few differences in the PP population compared to the ITT with the exception of results discussed below.
The changes in total immunoglobulin responses were similar in the PP population, except for the UP446 group having a reduction in IgM levels compared to Placebo in the pre-vaccination period (p = 0.039) (data not shown). Though similar patterns were seen in the ITT population, they did not reach statistical significance ().
Similar to the ITT population, there were no significant differences in other subjective measures of URTI illness between UP446 and Placebo groups during the study period including mean symptom severity index, duration, frequency, or severity of URTI symptoms in the PP population (). The participant hospitalized with pneumonia was removed from the PP population analysis, therefore the variability in mean symptom severity scores and number of sick days was not observed ().
Table 5. URTI symptom severity, well and sick days from the Modified WURSS-24 in the per protocol (PP) population (n = 46).
The overall incidence of influenza infection was low, with two participants in the UP446 group reporting flu-like symptoms as assessed by the WURSS-24 and confirmed by the MD. These participants were removed from the PP population, but their removal did not result in any differences between the ITT and PP population.
Discussion
Acacia catechu and Scutellaria baicalensis possess unique immunomodulatory properties including antioxidant and anti-inflammatory functions (Citation11) and have been reported to increase antibody titers after immunization (Citation12). However, to date there have been no studies examining the immunomodulatory effects of this formulation with human vaccine models.
This study was conducted during the 2021 winter season at the time of the COVID-19 pandemic and was associated with a low incidence of flu. This was likely the result of COVID-19 mitigation strategies, contributing to a decline in flu illness and resulting hospitalizations. While this is beneficial from a public health perspective, this made it challenging to differentiate the efficacy of UP446 compared to Placebo on cold and flu like symptoms during what would normally be considered the “flu season.” This was an unavoidable limitation of the study due to the pandemic.
Despite these challenges, there were significant improvements in immune markers with UP446 supplementation. Participants supplemented with UP446 had significant increases in total IgA concentrations in the 28-day post-vaccination period and influenza B-specific IgA levels were significantly decreased in the pre-vaccination period. However, in the post-vaccination period and over the 56-day study period, influenza B-specific IgG was increased. Total IgG levels were also significantly increased compared to Placebo in the post-vaccination period. In response to influenza vaccination, the humoral immune response produces IgA, IgG, and IgM antibodies approximately 14-days after immunization (Citation25). Vaccine-induced IgA antibodies have been shown to be important novel immune markers correlated with other immune measures such as IgG titers and hemagglutination inhibition (HI) response (Citation26). In both adults and children, high influenza-specific IgA levels were found to be negatively associated with viral transmission and infection (Citation27–29). Further, IgG constitutes approximately 75% of antibodies found in serum (Citation30) and both IgA and IgG are critical in the response in influenza infection (Citation31).Therefore, increases in IgA and IgG in the 28-day post-vaccination period support a beneficial humoral response to vaccination with UP446 supplementation.
Prior to vaccination, there was minimal effects on antibody response except that participants supplemented with UP446 had a significant reduction in total serum IgM antibodies compared to those on Placebo in the PP population. It is possible this difference was driven by two participants in the UP446 group with reductions in IgM of 0.45 g/L from baseline. Interestingly, there were no significant effects of UP446 supplementation on lymphocyte populations including helper or cytotoxic T cells, TCRγδ+ T cells, NK cells or total lymphocytes. This suggests that UP446 may preferentially influence the activity of certain aspects of immunity, such as humoral immunity, over cell-mediated or innate immunity. Response of the humoral immune system through production of antibodies following vaccination is considered a useful measure that correlates with specific protection. For studies on probiotics and vaccine models, this has been defined as the “gold standard” to determine the influence on immune function (Citation32).
There were no significant differences in the severity, frequency, or duration of URTI symptoms. The incidence of influenza infection was low, and only three participants in the UP446 group were identified and removed from the PP population analysis due to reporting flu-like symptoms or pneumonia. One participant began experiencing cold and flu symptoms five days following their Day 28 visit and was hospitalized for pneumonia 11 days later. This participant reported 15 sick days which likely contributed to the variability in mean global severity index, mean symptom severity scores and number of sick days in the ITT population in the UP446 group. In the Placebo group there was one participant reporting five sick days in the post-vaccination period. For both groups, the mean number of well days ranged from 97.5 to 99.6% of total days in the pre- and post-vaccination periods. Participants in both groups reported “remained well” for the majority of the 56-day study period. This aligns with the low rates of influenza activity reported in the United States for the 2020–2021 season, for which 0.2% of the respiratory samples tested were positive for the influenza virus (Citation2). By comparison, the rates of positive influenza tests in the previous three flu seasons prior to the COVID-19 pandemic peaked between 26.2 and 30.3%.
The individual ingredients in UP446 have been shown to have antioxidant and anti-inflammatory effects (Citation11, Citation12). In the pre-vaccination period UP446 supplementation was found to increase serum GSH-Px levels. The enzyme GSH-Px is part of the antioxidant defense system that maintains redox homeostasis and plays a critical role in mitigating the oxidative stress generated with influenza infection (Citation33). Administration of live attenuated influenza vaccine has been shown to elicit an increase in biomarkers of oxidative stress in the breath of healthy participants (Citation34). Therefore, increasing GSH-Px improved the antioxidant properties of UP446. As GSH-Px plays a key role in modulating overall oxidative and reductive stress (Citation35, Citation36), UP446 may be beneficial in mitigating the oxidative stress response following vaccination. However, to evaluate the direct role of UP446 in oxidative stress, levels of reduced glutathione (GSH) or protein carbonyl should be measured in future. It is unknown why there were significantly increased AGEs at Day 56 with both groups. However AGE accumulation has been proposed to be associated with increased age (Citation37), therefore this may be a reflection of the older population in this study.
All AEs were resolved by the end of the study period except for the participant who was hospitalized with pneumonia for which their recovery was reported. There were no significant differences between UP446 and Placebo in clinical chemistry parameters or vitals during the study. There were participants with spurious, high potassium levels during the study, however, upon repeating this blood work, these levels had returned to normal. This pseudohyperkalemia often occurs as a result of laboratory procedures or measurements (Citation38). Therefore, UP446 supplementation was found to be safe during the 56-day study.
This study presents several areas for future research. While UP446 supplementation significantly increased total IgA concentrations in serum, the majority of IgA is produced by cells residing in the lamina propria of the small and large intestine (Citation39). IgA also plays a key role in the regulation of gut-associated immunity (Citation40). Therefore, future research should examine whether supplementation of UP446 may be used in other indications such as gut health. Moreover, given the effects of UP446 on immune cell populations, future studies should consider measuring the production of cytokines related to these populations including interleukin (IL)-4 and IL-17. Further, considering the results of this study in healthy adults, future studies on UP446 are warranted in populations at increased risk of URTI illness such as older adults, teachers, and individuals with asthma and allergic rhinitis (Citation41).
The results of this study indicate that supplementation with UP446 significantly increased total IgA and IgG concentrations in the post-vaccination period compared to Placebo in healthy adults. These findings were supported by the significant increase in influenza B-specific IgG levels in the post-vaccination period and suggests that UP446 was beneficial in mounting a robust humoral response following vaccination. UP446 supplementation increased GSH-Px levels in the pre-vaccination period which may be beneficial in improving antioxidant functions, and potentially mitigating oxidative stress induced following influenza vaccination. There were no differences in clinical chemistry or vitals, and all AEs were either unlikely or not related to the study products confirming the good safety of UP446 during the 56-day study period.
Supplemental Material
Download MS Word (79.8 KB)Acknowledgments
The authors wish to thank the participants in this study and for their compliance to the conduct of the study.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Health Canada. Flu (influenza): for health professionals; [accessed 2021 Aug 18]. https://www.canada.ca/en/public-health/services/diseases/flu-influenza/health-professionals.html#a8.
- CDC. Influenza (Flu); [accessed 2021 Aug 18]. https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm.
- Dawood FS, Chung JR, Kim SS, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Martin ET, Belongia EA, et al. Interim estimates of 2019–20 seasonal influenza vaccine effectiveness—United States, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(7):177–82. doi:10.15585/mmwr.mm6907a1
- Flannery B, Kondor RJG, Chung JR, Gaglani M, Reis M, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, et al. Spread of antigenically drifted influenza A (H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis. 2020;221(1):8–15. doi:10.1093/infdis/jiz543
- Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. doi:10.3390/nu12041181
- Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012;3(1):91–129.
- Yeh T-L, Shih P-C, Liu S-J, Lin C-H, Liu J-M, Lei W-T, Lin C-Y. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:217–30. doi:10.2147/DDDT.S155110
- Rizzardini G, Eskesen D, Calder PC, Capetti A, Jespersen L, Clerici M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2012;107(6):876–84. doi:10.1017/s000711451100420x
- Xiao L, Engen PA, Leusink-Muis T, van Ark I, Stahl B, Overbeek SA, Garssen J, Naqib A, Green SJ, Keshavarzian A, et al. The combination of 2’-fucosyllactose with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides that enhance influenza vaccine responses is associated with mucosal immune regulation in mice. J Nutr. 2019;149(5):856–69. doi:10.1093/jn/nxz006
- Fernández-Ferreiro A, Formigo-Couceiro FJ, Veiga-Gutierrez R, Maldonado-Lobón JA, Hermida-Cao AM, Rodriguez C, Bañuelos O, Olivares M, Blanco-Rojo R. Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the immune response of elderly subjects to COVID-19 vaccination: a randomized controlled trial. Nutrients. 2022;14(1):228. doi:10.3390/nu14010228
- Tseng-Crank J, Sung S, Jia Q, Zhao Y, Burnett B, Park DR, Woo SS. A medicinal plant extract of Scutellaria baicalensis and Acacia catechu reduced LPS-stimulated gene expression in immune cells: a comprehensive genomic study using QPCR, ELISA, and microarray. J Diet Suppl. 2010;7(3):253–72. doi:10.3109/19390211.2010.493169
- Sunil MA, Sunitha VS, Radhakrishnan EK, Jyothis M. Immunomodulatory activities of Acacia catechu, a traditional thirst quencher of South India. J Ayurveda Integr Med. 2019;10(3):185–91. doi:10.1016/j.jaim.2017.10.010
- Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, Rodriguez JM, Xaus J. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23(3):254–60. doi:10.1016/j.nut.2007.01.004
- Wiggins KB, Smith MA, Schultz-Cherry S. The nature of immune responses to influenza vaccination in high-risk populations. Viruses. 2021;13(6):1109. doi:10.3390/v13061109
- Rosendahl Huber SK, Hendriks M, Jacobi RHJ, van de Kassteele J, Mandersloot-Oskam JC, van Boxtel RAJ, Wensing AMJ, Rots NY, Luytjes W, van Beek J. Immunogenicity of influenza vaccines: evidence for differential effect of secondary vaccination on humoral and cellular immunity. Front Immunol. 2019;9. doi:10.3389/fimmu.2018.03103
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG, Consort. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi:10.1016/j.ijsu.2011.10.001.
- Jia Q. Formulation of a mixture of free-B-ring flavonoids and flavans as a therapeutic agent. Patent number US7514469B2. USA, 2009.
- Yimam M, Zhao Y, Ma W, Jia Q, Do S-G, Shin J-H. 90-day oral toxicity study of UP446, a combination of defined extracts of Scutellaria baicalensis and Acacia catechu, in rats. Food Chem Toxicol. 2010;48(5):1202–9. doi:10.1016/j.fct.2010.02.011
- Yimam M, Lee YC, Jia Q. 26-week repeated oral dose toxicity study of UP446, a combination of defined extracts of Scutellaria baicalensis and Acacia catechu, in beagle dogs. Regul Toxicol Pharmacol. 2016;78:66–77. doi:10.1016/j.yrtph.2016.04.007
- Barrett B, Brown RL, Mundt MP, Thomas GR, Barlow SK, Highstrom AD, Bahrainian M. Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS-21). Health Qual Life Outcomes. 2009;7:76. doi:10.1186/1477-7525-7-76
- Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, Florkowski CM, Livesey JH, Camargo CA, Scragg R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333–9. doi:10.1001/jama.2012.12505
- Nantz MP, Rowe CA, Nieves C, Jr, Percival SS. Immunity and antioxidant capacity in humans is enhanced by consumption of a dried, encapsulated fruit and vegetable juice concentrate. J Nutr. 2006;136(10):2606–10. doi:10.1093/jn/136.10.2606
- de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K, et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24(44-46):6670–4. doi:10.1016/j.vaccine.2006.05.048
- R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2019.
- Moldoveanu Z, Clements ML, Prince SJ, Murphy BR, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13(11):1006–12. doi:10.1016/0264-410x(95)00016-t
- Abreu RB, Clutter EF, Attari S, Sautto GA, Ross TM. IgA responses following recurrent influenza virus vaccination. Front Immunol. 2020;11:902. doi:10.3389/fimmu.2020.00902
- Clements M, Betts R, Tierney E, Murphy B. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24(1):157–60. doi:10.1128/jcm.24.1.157-160.1986
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, Treanor J, Zangwill K, Hayden FG, Bernstein DI, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181(3):1133–7. doi:10.1086/315323
- Ambrose CS, Wu X, Jones T, Mallory RM. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine. 2012;30(48):6794–801. doi:10.1016/j.vaccine.2012.09.018
- Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41–S52. doi:10.1016/j.jaci.2009.09.046
- Li Z-N, Lin S-C, Carney PJ, Li J, Liu F, Lu X, Liu M, Stevens J, Levine M, Katz JM, et al. IgM, IgG, and IgA antibody responses to influenza A(H1N1)pdm09 hemagglutinin in infected persons during the first wave of the 2009 pandemic in the United States. Clin Vaccine Immunol. 2014;21(8):1054–60. doi: 10.1128/CVI.00129-14.
- MacDonald TT, Bell I. Probiotics and the immune response to vaccines. Proc Nutr Soc. 2010;69(3):442–6. doi:10.1017/s0029665110001758
- Chen K-K, Minakuchi M, Wuputra K, Ku C-C, Pan J-B, Kuo K-K, Lin Y-C, Saito S, Lin C-S, Yokoyama KK. Redox control in the pathophysiology of influenza virus infection. BMC Microbiol. 2020;20(1):214. doi:10.1186/s12866-020-01890-9
- Phillips M, Cataneo RN, Chaturvedi A, Danaher PJ, Devadiga A, Legendre DA, Nail KL, Schmitt P, Wai J. Effect of influenza vaccination on oxidative stress products in breath. J Breath Res. 2010;4(2):026001. doi:10.1088/1752-7155/4/2/026001
- Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15(7):1957–97. doi:10.1089/ars.2010.3586
- Handy DE, Loscalzo J. The role of glutathione peroxidase-1 in health and disease. Free Radic Biol Med. 2022;188:146–61. doi:10.1016/j.freeradbiomed.2022.06.004
- Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65(9):963–75. doi:10.1093/gerona/glq074
- Asirvatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255–9. doi:10.4103/1947-2714.110426
- Suzuki K, Nakajima A. New aspects of IgA synthesis in the gut. Int Immunol. 2014;26(9):489–94. doi:10.1093/intimm/dxu059
- Suzuki K, Kawamoto S, Maruya M, Fagarasan S. GALT: organization and dynamics leading to IgA synthesis. Adv Immunol. 2010;107:153–85. doi:10.1016/b978-0-12-381300-8.00006-x
- Thomas M, Bomar PA. Upper respiratory tract infection. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.