Abstract
The aim of this systematic review was to examine the characteristics of Paleolithic diet (PD) interventions designed for adult patients with autoimmune thyroid disease (AITD) in order to determine if diet elements have the potential to successfully reduce thyroid antibodies (Ab) such as thyroglobulin (Tg), thyroid peroxidase (TPO), and thyroid stimulating hormone receptor (TSHR), and improve thyroid hormones (thyroxine (T4), triiodothyronine (T3) and thyroid stimulating hormone (TSH)) or resolve AITD pathogenesis. Randomized controlled trials (RCTs) with an adult population of 18 years and older, diagnosed with Hashimoto’s thyroiditis (HT) or Graves’ disease (GD) (Basedow’s), who were placed on a diet of Paleolithic or ancestral nature, and achieved reduction of AITD Abs, improvement of thyroid hormones, and, or resolution of AITD were searched. Various electronic databases were used. Bias was assessed using critical appraisal tools from the Scottish Intercollegiate Guidelines Network (SIGN) and Joanna Briggs Institute (JBI). Studies were excluded according to exclusion criteria and results analyzed. One randomized controlled trial (RCT), a pilot study, and six case studies were found. In total, eight AITD studies focusing on Paleolithic or ancestral interventions were located. In highlight, females were the predominant gender. Case studies solely focused on AITD with protocols ranging from 8–60 weeks. All studies showed clinical improvements, one had significant improvement, two showed AITD resolution. After structured evaluation of nutritional interventions utilizing the PD on the effects of AITD, it was concluded foods of ancestral nature along with the addition of specific supplements, food components, exercise and mindfulness meditation, and exclusion of modern day foods have a considerable impact on thyroid Ab and hormones. The relevant studies suggest while this dietary protocol can be useful in clinical practice, larger-scale studies need to be conducted.
There are currently no dietary interventions recommended for the treatment of autoimmune thyroid disease. The Paleo diet has been documented to improve AITD antibodies and thyroid hormones in both Hashimoto’s thyroiditis and Graves’ disease.
The Paleo diet can provide a natural source of nutrients similar to supplemental nutrients that have shown positive results on AITD.
The paleo diet provides specific macronutrient percentages that may be beneficial in reducing AITD antibodies, while improving thyroid hormones.
Methylation supplementation may be useful in AITD cases.
Key teaching points
Introduction
Background
The World Health Organization describes AITD as the most abundant organ-specific autoimmune endocrine disorder worldwide (Citation1). Currently, it affects 1-3 in every 1,000 individuals (Citation2). Both HT and GD are the major causes of AITD, with the primary factor due to the loss of immune tolerance (Citation1). Susceptibility results from genetic and environmental factors (Citation1). Although researchers have identified genetics and environment as potential risk factors, mechanisms to which these cause autoimmunity are still unclear (Citation1).
Conventional treatment
Conventional treatment of thyroid diseases are not managed with nutritional remedies, and at diagnosis, treatment guidelines begin with basic T4 therapy (Citation1, Citation2). The aim is to add or reduce thyroid hormones in the body to positively influence thyroid function (Citation1, Citation2). However, a more comprehensive approach to managing AITD is needed since the conventional treatment method does not ameliorate Abs. The approach would include diet evaluation, physical activity assessment, genetic and laboratory testing, examination of lifestyle, psychosocial assessment, and basic disease education. Although nutrition is promoted worldwide as a treatment strategy for conditions such as gastrointestinal diseases, weight reduction, diabetes, and cardiovascular complications, it is downplayed as an effective treatment option for autoimmune complications and thyroid disease. Therefore, implementing nutritional methods should be new way to deliver successful treatment methods for AITD patients.
Nutritional interventions
Thus far, there is little evidence of the impact diet and nutrients have on thyroid function, hormone production, and Ab reduction. Iodine is one micronutrient that has been proposed on numerous occasions for thyroid conditions (Citation1, Citation2), but studies present mixed results (Citation3–6). Zinc, selenium, vitamin C, and myoinositol have all been shown to have positive effects on AITD in rebalancing hormones by reducing thyroid Ab levels (Citation7–11). Additionally, potassium iodide has been shown to influence membrane activity and reduce thyroid Abs (Citation5, Citation12), while vitamin D plays a role in reducing inflammatory biomarkers and rebalancing hormone levels (Citation13–15). Furthermore, plant components, food components, and herbs have demonstrated a positive impact on Ab status in AITD (Citation16, Citation17) such as ashwagandha (Citation18), tobacco (Citation19), prunella (Citation20), cassava (Citation21, Citation22), bugleweed (Citation23, Citation24), nigella sativa (Citation25, Citation26), amino acids such as L-carnitine (Citation27), phytoestrogens (Citation28), and specific diets (Citation29–33). Despite the small amount of evidence found on nutrients, whole foods, and plant components, and the impact of their constituents on AITD, the majority of these studies have positive results, with some resulting in symptom resolution and resolution of diagnosis.
The paleolithic diet
The PD is a nutritional intervention based on the foods and eating habits that would have been utilized during the Paleolithic era (Citation34, Citation35). New evidence reports the PD includes eating lean meats and their organs, seafood, fruits, vegetables, roots, small amounts of grains and legumes, as well as nuts and seeds, mushrooms, and moss (Citation34–36). It excludes domesticated (dairy and refined foods such as sugars, flours, and fine salts) processed, genetically modified, and canned foods and ingredients (additives, preservatives, and anticaking agents) (Citation34, Citation35). This systematic review focused on gathering evidence of Paleolithic interventions in AITD.
Materials and methods
Objective
Does the PD influence reduction of Abs and improvement in thyroid hormones in adults who have autoimmune thyroid disease compared to standard of care? ().
Table 1. PICO outline.
Study inclusion criteria
Inclusion criteria is defined as adults 18 years or older, study population with either HT or GD/Basedow’s diagnosis and prescribed or not prescribed conventional T4 therapy; studies defining a diet that is Paleolithic, of ancestral in nature, or defined as Paleo; studies that are RCTs; study outcomes of an improvement of AITD, no effect on AITD, or negative effects on AITD; studies limited to English language, and studies and trials that may include standard of care/conventional T4 therapy for comparison to the PD.
Study exclusion criteria
Exclusion criteria is defined as studies whose study population are children and adolescents under 18 years of age, animal studies, duplicate records, studies that are not relevant to study population such as comorbidities describing diabetes type 2 mellitus, cardiovascular disease, mental health conditions, or other comorbidities, ongoing incomplete trials, proposed study designs or study intervention designs and unpublished doctoral papers or thesis.
Search strategy
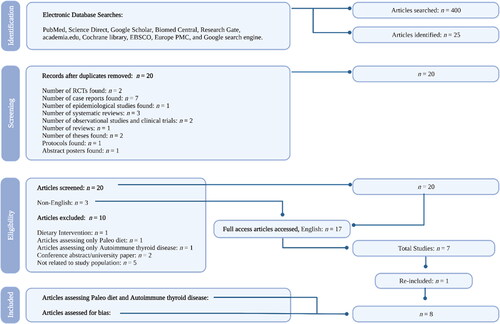
Relevant electronic databases, registers, websites, and other data sources were reviewed from inception (1st of September, 2021) to completion (31st of March, 2022) including PubMed, Science Direct, Google Scholar, Biomed Central, Research Gate, academia.edu, Cochrane library, EBSCO, Europe PMC, and Google search engine. The searches were reviewed to identify studies that might warrant inclusion. The search strategy was limited to the English language. The search terms were used in different combinations by all three researchers (JH, KD, and LJ) and results can be viewed in .
Table 2. Search terms and results.
Selection process
In total, 25 articles were identified. A summary of results and selection processes can be reviewed in .
The research team excluded a total of 17 articles. The first article excluded lacked evidence of the PD influencing AITD (Citation40), while another one only had mentions of dietary protocols similar to the Paleolithic lifestyle (Citation32) and did not actually specify Paleo as the primary diet protocol. The third article excluded was a conference abstract (Citation52) but was later identified as a peer-reviewed study and was re-included (Citation45). Another article was excluded because it was a university paper and had not yet been accepted into a preprint database or peer-reviewed journal (Citation53). Three articles were excluded because they could not be found in English (Citation37–39). Another article was excluded because it was a proposed dietary intervention (Citation54). Additionally, other articles were excluded due to having no relation to the inclusion criteria (Citation41, Citation50, Citation55–57). Furthermore, the removal of duplicate studies was conducted. In total, eight published studies met the inclusion criteria and were eligible for data extraction (Citation29, Citation42–46, Citation51, Citation58).
Quality assessment and screening
The methods used to determine which studies were eligible for inclusion and exclusion were conducted by three reviewers who compared the characteristics (participants, interventions, outcomes, and methods) of each study. The initial screen was performed by the primary screener (JH) to exclude any articles that were identified through the search that did not meet criteria. Two additional reviewers (KD and LJ) also conducted separate screens, and reviewers independently reviewed the abstract of the remaining papers, appraised papers for best evidence, and assessed for bias. Risk of bias was conducted using the SIGN checklists and JBI critical appraisal tools (Citation59, Citation60). All articles were assessed for inclusion to include the PD and improvements of Abs in AITD. Reviewers screened through each article and extracted those which the description of the PD was not aligned with a PD.
The flow of research and the screening process of the studies are provided in .
Possible causes of diversity in the literature may be the different forms of the PD being used in research. Therefore, it was important for the authors to be direct, clear, and specific on which available literature was used to evaluate the impact of the PD on AITD Ab and thyroid hormone levels. Due to the scarcity of research of PD and AITD, researchers used extreme caution and sensitivity when reviewing the available research.
Intervention characteristics
The main characteristics of the study population and intervention are listed in .
Table 3. Characteristics of study populations.
Tools for assessing and eliminating bias
The JBI uses critical appraisal tools for use in systematic reviews when analyzing available literature from case studies (Citation59). It critically appraises how case studies are judged and the effectiveness of such studies to be used in practice (Citation59). JBI tools incorporate a process of appraisal by use of questions and checklists to better assess the information being reviewed (Citation59). Hence, all case studies included in this systematic review have been critiqued by the JBI critical appraisal tool to assess for reporting quality as well as bias. The SIGN tool is a similar critical appraisal tool that addresses randomized controlled trials, their reporting quality, as well as the risk of bias (Citation60). Both JBI and SIGN tools were used to assess the literature.
Results
All studies included diet instructions given in person, measurement of lab biomarkers, and PD components. Six of the eight studies were case reports (Citation42–46, Citation58), while one was a pilot study (Citation29) and the other a prospective RCT (Citation51). The pilot study included 17 individuals and the prospective RCT included 35 individuals respectively (Citation29, Citation51). All studies consisted only of female participants who were tested for thyroid hormones and Abs with the exception of one (Citation51). Furthermore, protocols ranged from 8-60 weeks, with average protocol equating to 32.25 weeks.
Significant improvements were identified in one particular case study including a reduction of TSH by 36.4%, Tg Ab by 47.4%, TPO Ab by 28.9%, and an increase in total T4 (T-T4) by 21.5% and total T3 (T-T3) by 33.3% in just 8 weeks (Citation42). Four studies showed clinical improvement of both HT and GD Ab and resolution of diagnosis over a 12 week to 24 month period; reduction in TSH and TPO Ab (Citation43), normalized TSH and Tg Ab, and reduction of TPO Ab (Citation44), resolution of GD diagnosis via negative TSHR Abs and normalization of T4 and T3 (Citation46), and resolution of HT diagnosis via normalization of TSH, and both Tg and TPO Abs (Citation58). Arick (Citation45) found an improvement in HT Tg Abs. Manousou et al. (Citation51) stated there were not significant differences in TSH, T4, and T3 between groups, but when they did longitudinal analyses, TSH and FT4 increased in the PD group and they found T3 decreases in the PD group after the first 6 months. Additionally, Abbott et al. (Citation29) found no significant changes in HT thyroid hormones or Abs, but noted a significant improvement in health related quality of life (HRQL) and symptom burden. In total, six of the eight studies (75%) showed considerable improvements in both HT and GD Abs and resolution of both HT and GD diagnoses (Citation42–46, Citation58). Results may be associated with the level of compliance from study subjects.
There were many similar dietary components amongst interventions, which can be viewed in . Five of the protocols mentioned avoiding gluten (Citation29, Citation42–44, Citation58). Four supplemented vitamin D3 (Citation42, Citation45, Citation46, Citation58). Three studies mentioned methylation support (Citation44, Citation46, Citation58), three supplemented vitamin C (Citation44–46), and three supplemented essential fatty acids, DHA/EPA (Citation45, Citation46, Citation58). Two studies supplemented magnesium and zinc (Citation44, Citation58), two supplemented various forms of selenium (Citation44, Citation46), and two supplemented probiotics (Citation46, Citation58). Equally, additional food components were also added: three added bone broth (Citation29, Citation42, Citation44) and two added fermented foods (Citation29, Citation44). Furthermore, all protocols increased phytonutrients and promoted weight loss (Citation29, Citation42–46, Citation51, Citation58). It can be inferred that while new evidence states a PD includes small amounts of grains, avoiding glutinous grains may have tremendous benefits. Supporting AITD with supplements such as vitamin D, vitamin C, fatty acids, magnesium, zinc, selenium, methylation or B vitamins, and probiotics may also be beneficial. Finally, including bone broth, fermented foods, and higher intakes of phytonutrients may be critical for positive results. Two studies with total HT and GD resolution used a combination of seven of these recommendations, and it may be concluded that the addition of supplements, food components, and exclusion of gluten may influence AITD outcomes (Citation46, Citation58).
Table 4. Supplemental and additional food component protocols.
There were mixed opinions on the type of exercise component in these studies, which can be viewed in . While Arick (Citation45) reduced the exercise intensity and length of time, and increased strength training time, Avard and Grant (Citation44) encouraged gentle walks 3 times per week, and (Citation58) encouraged exercise for at least 20 minutes, 3 to 4 times per week. Abbott et al. (Citation29) found improvements in physical functioning components and encouraged increasing time outdoors. Brogan et al. (Citation46) encouraged yoga practice (physical exercise with meditation), while the remaining studies mentioned no exercise. None of the studies gave specific exercise routine instructions but rather suggested exercise in general minimal effort. We can conclude from this evidence the minimal recommended amount of exercise is 3 times per week at 20 minutes, while style types for GD are kundalini yoga, and HT are walks, strength training, participating in outdoor activities, and general exercise movement (Citation29, Citation44–46, Citation58). Furthermore, two more studies encouraged mindfulness meditation (Citation29, Citation58). Complete resolution of AITD was achieved in two studies and they both utilized minimal exercise and meditation (Citation46, Citation58). Despite the limited evidence, these eight studies demonstrate that the PD may contribute to developing personalized nutrition support that has a positive impact on AITD.
Table 5. Integrative protocols.
Discussion
This is the first systematic review to study the potential benefits of the PD and the effects it has on AITD. Crucial protocol elements for disease improvement and resolution are the inclusion of Paleolithic or ancestral diet components, added supplements, added food components, the exclusion of modern day foods and ingredients, participating in minimal physical activity and mindfulness meditation, increasing phytonutrient intake, and excluding gluten. Additionally, protocol length, proper evaluation of AITD clinical status, and client compliance are vital for disease improvement and resolution.
Protocol length, evaluation, and compliance
Evaluation of all thyroid biomarkers (T3, T4, RT3, TSH, TSHR, Tg, and TPO Abs) should be taken into account and it is suggested to also assess lipid panels (TC, TG, LDL, HDL, and CRP). Nutrient panels should include B vitamins, vitamin D3, vitamin C, essential fatty acids, zinc, selenium, and magnesium. Other lab markers could include a comprehensive metabolic panel, cortisol, DHEA, ferritin, copper, and CBC evaluation. Similarly, protocol length and compliance should be considered because they may alter results. We report the average protocol length should consist of a minimum of 8 months and compliance to intervention should be strictly enforced for maximum results.
Protocol elements
The addition of supplemental nutrients is proposed in many papers with positive results on AITD (Citation7–11, Citation13, Citation15). A real PD would have consisted of 100% organic, chemical free foods in their natural form to support thyroid metabolism (i.e. seafood, vegetables, and moss as a natural source for iodine; lean meats and nuts as a natural source of selenium and zinc; mushrooms as a source of vitamin D; meats, herbs, roughage, and seaweeds as a source of B vitamins; colorful vegetables and fruits as source of vitamin C and phytonutrients; and legumes and grains seasonally contributing phytoestrogens). We’ve shown that although research is limited, all the protocols that included supplements illustrate positive results ranging from general improvement of AITD hormone and Ab markers to resolution of AITD diagnosis (Citation29, Citation42–46, Citation58).
Including extra food components, increasing phytonutrients, and maintaining specific dietary macronutrient percentages have also shown to positively influence AITD. Here we report three studies that decreased carbohydrate percentage and increased protein percentage in their protocols (Citation42–44), added fermented foods (Citation29, Citation44), as well as included higher intakes of plant phytonutrients (Citation29, Citation42–46, Citation51, Citation58). During the Paleoithic era individuals would have been limited from refrigeration and research suggests Neanderthals may have resorted to fermentation as a resource to preserve foods (Citation61). Moreover, we know that Neanderthals consumed carbohydrates and were not documented to discriminate against food groups (Citation48, Citation49, Citation62). Nonetheless, they preferred to consume high ranking seasonally fresh plants that were non-domesticated in nature, high in phytonutrients, and lower in starch (low ranked) to help counteract their higher protein diets (Citation48, Citation49, Citation62).
Five of the AITD protocols mentioned gluten avoidance showing improvements on AITD outcomes (Citation29, Citation42–44, Citation58). Interestingly, gluten would have been an issue during the Paleolithic era. Researchers are now finding celiac disease haplotype rs13098911 gene variant stems from Neanderthals (Citation63). As well, during the Neolithic era wheat only existed in a quarter of the world in Eurasia (Citation64). Additionally, 1st-2nd century skeletal remains have revealed gluten intolerance (Citation65). This may indicate that celiac disease could date back even further. Thus, removing gluten is essential for a PD protocol and to rebalance AITD hormones and Abs (Citation14, Citation29–33, Citation42–46, Citation51, Citation58).
Limitations
The majority of the evidence came from case reports, limiting the number of study subjects and decreasing the ability to generalize the PD protocol in large populations. Thus, larger scale studies are needed as the current evidence does show positive results in improving thyroid hormone levels, reducing thyroid Ab biomarkers, and AITD resolution.
One concerning limitation is that all studies included only female subjects, which may be construed as prejudice, limiting the ability to generalize the benefits of the PD across both genders. Research suggests AITD predominantly develops in females (Citation66), which suggests that estrogen may be a component (Citation1). However, HT is higher in older females, which may eliminate that theory (Citation1). Studies have shown that one of the two X-chromosomes in females is randomly inactivated by methylation (Citation66), which is a normal 50/50 gene inactivation. However, skewed X-chromosome inactivation is abnormal and represents a positive selection for the mutant gene. This may suggest that methylation supplementation could be useful in AITD cases. As well, X-chromosome contains FOXP3 and CD40 genes (Citation66), which are also proposed AITD genes. (Citation67) found that inactivation of the X-chromosomes was present in 34% of subjects with AITD. Therefore, testing for genes when investigating AITD cases could be considerably significant.
Finally, two studies measured TSH, T4, T3, R-T3, and Tg and TPO Ab in relation to the PD (Citation45, Citation51). They both did not focus solely on thyroid disease. One did not measure any thyroid Abs (Citation51), while the other measured both Tg and TPO Ab, but only saw a reduction of Tg Ab (Citation45). Their inclusion may be construed as biased because it assesses T4, T3, and TSH as negative predictors to indicate the PD depletes iodine (Citation51), while the other (Citation45) measures TSH, T4, T3, R-T3, and Tg and TPO Ab, to assess chronic fatigue syndrome in addition to HT.
Conclusion
After structured evaluation of dietary interventions consisting of the PD on the effects of AITD, it was concluded that PDs of ancestral nature have a remarkable impact on thyroid Ab. The addition of specific nutraceuticals and food components, while increasing phytonutrient intake and lowering carbohydrate intake (particularly starches), and the removal of gluten can further improve AITD. The characteristics of the PD are a multicomponent lifestyle intervention that demonstrates an intervention that can be delivered to the AITD community to reduce symptoms, improve thyroid homeostasis, and improve overall quality of life. Thus, there is a strong need for future research with large-scale randomized control trials utilizing the PD to help create clinical nutrition therapy guidelines for AITD (Citation47).
Declarations
Review registration
This systematic review was registered with PROSPERO. Within the PROSPERO database there are thirteen records of systematic reviews involving Paleolithic diet, none of which focus on thyroid or autoimmune thyroid diseases. The registration number for this systematic review is CRD42022311884. The review protocol can be accessed at the National Institute for Health Research, International prospective register of systematic reviews.
During the COVID-19 pandemic, all systematic review submissions to the PROSPERO website that awaited registration more than 30 days were automatically published. For this reason, the research team had to amend the original document accepted upon registration in order to complete the study.
Ethical approval
This publication is a systematic review and therefore ethical approval was not required as no primary research was undertaken.
Registration
This systematic review was registered with PROSPERO. Registration number is CRD42022311884.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
There are no grants or funding from individuals, organizations, groups, companies or other legal entities.
References
- Franco JS, Amaya-Amaya J, Anaya JM, et al. 2013. Thyroid disease and autoimmune diseases. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, editors. Autoimmunity: from bench to bedside (Chapter 30). El Rosario University Press. https://www.ncbi.nlm.nih.gov/books/NBK459466/
- Mincer DL, Jialal I. 2021. Hashimoto thyroiditis. In: StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459262/
- Chow CC, Phillips DI, Lazarus JH, Parkes AB. Effect of low dose iodide supplementation on thyroid function in potentially susceptible subjects: are dietary iodide levels in Britain acceptable? Clin Endocrinol (Oxf). 1991;34(5):413–6. doi:10.1111/j.1365-2265.1991.tb00314.x.
- Huang H, Shi Y, Liang B, Cai H, Cai Q, Lin R. Optimal iodine supplementation during antithyroid drug therapy for Graves’ disease is associated with lower recurrence rates than iodine restriction. Clin Endocrinol (Oxf). 2018;88(3):473–8. doi:10.1111/cen.13543.
- Reinhardt W, Luster M, Rudorff KH, Heckmann C, Petrasch S, Lederbogen S, Haase R, Saller B, Reiners C, Reinwein D, et al. Effect of small doses of iodine on thyroid function in patients with Hashimoto’s thyroiditis residing in an area of mild iodine deficiency. Eur J Endocrinol. 1998;139(1):23–8. doi:10.1530/eje.0.1390023.
- Yoon SJ, Choi SR, Kim DM, Kim JU, Kim KW, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC, et al. The effect of iodine restriction on thyroid function in patients with hypothyroidism due to Hashimoto’s thyroiditis. Yonsei Med J. 2003;44(2):227–35. doi:10.3349/ymj.2003.44.2.227.
- Karimi F, Omrani GR. Effects of selenium and vitamin C on the serum level of antithyroid peroxidase antibody in patients with autoimmune thyroiditis. J Endocrinol Invest. 2019;42(4):481–7. doi:10.1007/s40618-018-0944-7.
- Kyrgios I, Giza S, Kotanidou EP, Kleisarchaki A, Tsinopoulou VR, Papadopoulou A, Markantonatou AM, Kanellidou E, Giannakou A, Galli-Tsinopoulou A. l-selenomethionine supplementation in children and adolescents with autoimmune thyroiditis: a randomized double-blind placebo-controlled clinical trial. J Clin Pharm Ther. 2019;44(1):102–8. doi:10.1111/jcpt.12765.
- Mahmoodianfard S, Vafa M, Golgiri F, Khoshniat M, Gohari M, Solati Z, Djalali M. Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: a randomized double-blind controlled trial. J Am Coll Nutr. 2015;34(5):391–9. doi:10.1080/07315724.2014.926161.
- Mazokopakis EE, Papadakis JA, Papadomanolaki MG, Batistakis AG, Giannakopoulos TG, Protopapadakis EE, Ganotakis ES. Effects of 12 months treatment with L-selenomethionine on serum anti-TPO levels in patients with Hashimoto’s thyroiditis. Thyroid. 2007;17(7):609–12. doi:10.1089/thy.2007.0040.
- Nordio M, Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto’s patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. 2017;21(2 Suppl):51–9.
- Suzuki N, Yoshimura Noh J, Sugisawa C, Hoshiyama A, Hiruma M, Kawaguchi A, Morisaki M, Ohye H, Suzuki M, Matsumoto M, et al. Therapeutic efficacy and limitations of potassium iodide for patients newly diagnosed with Graves’ disease. Endocr J. 2020;67(6):631–8. doi:10.1507/endocrj.EJ19-0379.
- Chahardoli R, Saboor-Yaraghi AA, Amouzegar A, Khalili D, Vakili AZ, Azizi F. Can supplementation with vitamin D modify thyroid autoantibodies (anti-TPO Ab, Anti-Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto’s thyroiditis? A double blind, randomized clinical trial. Horm Metab Res. 2019;51(5):296–301. doi:10.1055/a-0856-1044.
- Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D on thyroid autoimmunity in Levothyroxine-treated women with Hashimoto’s thyroiditis and normal vitamin D status. Exp Clin Endocrinol Diabetes. 2017;125(4):229–33. doi:10.1055/s-0042-123038.
- Pratita W, Arto KS, Arto NS. Efficacy of vitamin-D supplement on thyroid profile in children with Graves’ disease. Open Access Maced J Med Sci. 2020;8(B):798–801. doi:10.3889/oamjms.2020.4790.
- Yang H, Bi X, Tang H, Zeng J, Cong Y, Wu T, Chen Q. Clinical efficacy of Yingliu treatment for Graves disease. Int J Clin Exp Med. 2015;8(4):6145–53.
- Yang H, Cong Y, Wu T, Tang H, Ma M, Zeng J, Zhang W, Lian Z, Yang X. Clinical efficacy of Yingliu mixture combined with metimazole for treating diffuse goitre with hyperthyroidism and its impact on related cytokines. Pharm Biol. 2017;55(1):258–63. doi:10.1080/13880209.2016.1260595.
- Sharma AK, Basu I, Singh S. Efficacy and safety of ashwagandha root extract in subclinical hypothyroid patients: a double-blind, randomized placebo-controlled trial. J Altern Complement Med. 2018;24(3):243–8. doi:10.1089/acm.2017.0183.
- Schmeltz LR, Blevins TC, Aronoff SL, Ozer K, Leffert JD, Goldberg MA, Horowitz BS, Bertenshaw RH, Troya P, Cohen AE, et al. Anatabine supplementation decreases thyroglobulin antibodies in patients with chronic lymphocytic autoimmune (Hashimoto’s) thyroiditis: a randomized controlled clinical trial. J Clin Endocrinol Metab. 2014;99(1):E137–E142. 4th doi:10.1210/jc.2013-2951.
- Yang K, Guo KQ, Wu HY. Clinical effect of Prunellae Oral Liquid on goiter with different thyroid function. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(1):37–9.
- Biassoni P, Ravera G, Bertocchi J, Schenone F, Bourdoux P. Influence of dietary habits on thyroid status of a nomadic people, the Bororo shepherds, roaming a central African region affected by severe iodine deficiency. Eur J Endocrinol. 1998;138(6):681–5. doi:10.1530/eje.0.1380681.
- Osman BA, Ng ML, Bakar AA, Khalid BA. The effect of cassava leave intake on thyroid hormone and urinary iodine. East Afr Med J. 1993;70(5):314–5.
- Beer AM, Wiebelitz KR, Schmidt-Gayk H. Lycopus europaeus (Gypsywort): effects on the thyroidal parameters and symptoms associated with thyroid function. Phytomed Int J Phytother Phytopharmacol. 2008;15(1-2):16–22. doi:10.1016/j.phymed.2007.11.001.
- Eiling R, Wieland V, Niestroj M. Improvement of symptoms in mild hyperthyroidism with an extract of Lycopus europaeus (Thyreogutt® mono). Wien Med Wochenschr. 2013;163(3–4):95–101. doi:10.1007/s10354-012-0167-z.
- Farhangi MA, Dehghan P, Tajmiri S, Abbasi MM. The effects of Nigella sativa on thyroid function, serum Vascular Endothelial Growth Factor (VEGF) - 1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: a randomized controlled trial. BMC Complement Altern Med. 2016;16(1):471. doi:10.1186/s12906-016-1432-2.
- Farhangi MA, Dehghan P, Tajmiri S. Powdered black cumin seeds strongly improves serum lipids, atherogenic index of plasma and modulates anthropometric features in patients with Hashimoto’s thyroiditis. Lipids Health Dis. 2018;17(1):59. doi:10.1186/s12944-018-0704-x.
- An JH, Kim YJ, Kim KJ, Kim SH, Kim NH, Kim HY, Kim NH, Choi KM, Baik SH, Choi DS, et al. L-carnitine supplementation for the management of fatigue in patients with hypothyroidism on levothyroxine treatment: a randomized, double-blind, placebo-controlled trial. Endocr J. 2016;63(10):885–95. https://www.jstage.jst.go.jp/article/endocrj/63/10/63_EJ16-0109/_pdf/-char/en doi:10.1507/endocrj.EJ16-0109.
- Sathyapalan T, Dawson AJ, Rigby AS, Thatcher NJ, Kilpatrick ES, Atkin SL. The effect of phytoestrogen on thyroid in subclinical hypothyroidism: randomized, double blind, crossover study. Front Endocrinol (Lausanne). 2018;9:531. doi:10.3389/fendo.2018.00531.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto’s thyroiditis. Cureus. 2019;11(4):e4556. DOI doi:10.7759/cureus.4556.
- Esposito T, Lobaccaro JM, Esposito MG, Monda V, Messina A, Paolisso G, Varriale B, Monda M, Messina G. Effects of low-carbohydrate diet therapy in overweight subjects with autoimmune thyroiditis: possible synergism with ChREBP. Drug Des Devel Ther. 2016;10:2939–46. doi:10.2147/DDDT.S106440.
- Kuiper MW, van der Gaag EJ. Subclinical hypothyroidism in children can normalize after changes in dietary intake. Food Nutr Sci. 2012;03(03):411–6. doi:10.4236/fns.2012.33059.
- Ostrowska L, Gier D, Zyśk B. The influence of reducing diets on changes in thyroid parameters in women suffering from obesity and Hashimoto’s disease. Nutrients. 2021;13(3):862. doi:10.3390/nu13030862.
- van der Gaag E, van der Palen J, Schaap P, van Voorthuizen M, Hummel T. A lifestyle (dietary) intervention reduces tiredness in children with subclinical hypothyroidism, a randomized controlled trial. Int J Environ Res Public Health. 2020;17(10):3689. doi:10.3390/ijerph17103689.
- Challa HJ, Bandlamudi M, Uppaluri KR. 2021. Paleolithic diet. In: StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482457/. Accessed September 18, 2021
- de la O V, Zazpe I, Martínez JA, Santiago S, Carlos S, Zulet MÁ, Ruiz-Canela M. Scoping review of Paleolithic dietary patterns: a definition proposal. Nutr Res Rev. 2021;34(1):78–106. doi:10.1017/s0954422420000153.
- Weyrich LS, Duchene S, Soubrier J, Arriola L, Llamas B, Breen J, Morris AG, Alt KW, Caramelli D, Dresely V, et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature. 2017;544(7650):357–61. doi:10.1038/nature21674.
- Sabljak V. Designing and comparing Mediterranean, Paleo and vegetarian diet plans for a patient with Hashimoto’s thyroiditis [Diploma thesis]. Osijek: Josip Juraj Strossmayer University of Osijek. PTFOS Repository; 2019. https://urn.nsk.hr/urn:nbn:hr:109:363282.
- Kostiukow A, Rosołek M, Romanowski M, Ignaszak E, Samborski W. 2018. Diet as an important supporting factor in the treatment of Hashimoto’s disease. In: Medical Aspects of Cosmetology and Dietetics. Lubin: Wydawnictwo Naukowe TYGIEL. p. 200–213. https://bc.wydawnictwo-tygiel.pl/public/assets/266/Medyczne%20aspekty%20kosmetologii%20i%20dietetyki.pdf#page=200https://bc.wydawnictwo-tygiel.pl/public/assets/266/Medyczne%20aspekty%20kosmetologii%20i%20dietetyki.pdf#page=200.
- Shidlovskyi VO, Shidlovskyi OV, Sheremet MI, Shevchenko SI, Skochylo OV. Dietary nutrition in complex treatment of patients with Hashimoto thyroiditis. Bull Med Biol Res. 2020;3(5):175–84. doi:10.11603/bmbr.2706-6290.2020.3.11529.
- Hoffman R. Can the paleolithic diet meet the nutritional needs of older people? Maturitas. 2017;95:63–4. doi:10.1016/j.maturitas.2016.09.005.
- Keestra S, Derkx I, Sikka G, Chaudhary N, Salali GD. 2021. Bitter taste perception in BaYaka hunter-gatherers. BioRxiv https://www.biorxiv.org/content/biorxiv/early/2021/11/29/2021.11.25.469973.full.pdf.
- Whitfield M, Hollywood JB, Keister A. Nutritional Management of Hashimoto’s Thyroiditis: A Case Report. Elsevier SSRN; 2021. doi:10.2139/ssrn.3990138.
- Al-Bayyari N. Successful dietary intervention plan for Hashimoto’s thyroiditis: a case study. Roman J Diab Nutr Metab Dis. 2020;27(4):381–5. https://www.rjdnmd.org/index.php/RJDNMD/article/view/828
- Avard N, Grant SJ. A case report of a novel, integrative approach to Hashimoto’s thyroiditis with unexpected results. Adv Integr Med. 2018;5(2):75–9. doi:10.1016/j.aimed.2018.03.003.
- Arick CT. Chiropractic management of a patient with chronic fatigue: a case report. J Chiropr Med. 2016;15(4):314–20. doi:10.1016/j.jcm.2016.08.006.
- Brogan K, Marcelino G, Pedro C, Siefert A. Healing of Graves’ disease through lifestyle changes. Adv Mind Body Med. 2019;33(2):4–11.
- Kawakami-Tani T, Fukawa E, Tanaka H, Abe Y, Makino I. Effect of 1 alpha-hydroxyvitamin D3 on serum levels of thyroid hormones in hyperthyroid patients with untreated Graves’ disease. Metabolism. 1997;46(10):1184–8. doi:10.1016/S0026-0495(97)90214-6.
- Sistiaga A, Mallol C, Galván B, Summons RE. The Neanderthal meal: a new perspective using faecal biomarkers. PLoS One. 2014;9(6):e101045. doi:10.1371/journal.pone.0101045.
- Henry AG, Brooks AS, Piperno DR. Plant foods and the dietary ecology of Neanderthals and early modern humans. J Hum Evol. 2014;69:44–54. doi:10.1016/j.jhevol.2013.12.014.
- Liu G, Liang L, Bray GA, Qi L, Hu FB, Rood J, Sacks FM, Sun Q. Thyroid hormones and changes in body weight and metabolic parameters in response to weight loss diets: the POUNDS LOST trial. Int J Obes (Lond). 2017;41(6):878–86. doi:10.1038/ijo.2017.28.
- Manousou S, Stål M, Larsson C, Mellberg C, Lindahl B, Eggertsen R, Hulthén L, Olsson T, Ryberg M, Sandberg S, et al. A Paleolithic-type diet results in iodine deficiency: a 2-year randomized trial in postmenopausal obese women. Eur J Clin Nutr. 2018;72(1):124–9. doi:10.1038/ejcn.2017.134.
- Poster presentation abstracts. J Chiropract Educ. 2015;29(1):90–108. doi:10.7899/JCE-14-34.
- Connor T. 2014. A case study of a wheat-free diet on autoimmune disease progression [Doctoral dissertation]. Colorado State University. https://mountainscholar.org/handle/10217/88512.
- Wojtas N, Wadolowska L, Bandurska-Stankiewicz E. Evaluation of qualitative dietary protocol (Diet4Hashi) application in dietary counseling in Hashimoto thyroiditis: study protocol of a randomized controlled trial. Int J Environ Res Public Health. 2019;16(23):4841. doi:10.3390/ijerph16234841.
- de Menezes E, Sampaio H, Carioca A, Parente NA, Brito FO, Moreira T, de Souza A, Arruda S. Influence of Paleolithic diet on anthropometric markers in chronic diseases: systematic review and meta-analysis. Nutr J. 2019;18(1):41. doi:10.1186/s12937-019-0457-z.
- Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr. 2015;102(4):922–32. doi:10.3945/ajcn.115.113613.
- Zaman B, Rasool SO, Sabri SM, Raouf GA, Balatay AA, Abdulhamid MA, Hussein DS, Odisho SK, George ST, Hassan SM, et al. Prevalence of thyroid dysfunctions in a large, unselected population in Duhok city, Iraqi Kurdistan: a cross-sectional study. J Biol Res. 2021;94(2):107–15. https://plu.mx/plum/a/? doi:10.4081/jbr.2021.10067
- Dolan K, Finley H, Gasta M, Houseman S. Managing Hashimoto’s thyroiditis through personalized care: a case report. Altern Ther Health Med. 2018;24(3):56–61.
- Joanna Briggs Institute. 2017. The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Case Reports. Adelaide: University of Adelaide. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Case_Reports2017_0.pdf and https://jbi.global/critical-appraisal-tools.
- Scottish Intercollegiate Guidelines Network. 2021. Methodology Checklist 1: Systematic Reviews and Meta-Analyses. Edinburgh: SIGN. http://www.sign.ac.uk.
- Amato KR, Mallott EK, Maia PD, Sardaro MLS. Predigestion as an evolutionary impetus for human use of fermented food. Curr Anthropol. 2021;62(S24):S207–S219. doi:10.1086/715238.
- Hardy K, Bocherens H, Miller JB, Copeland L. Reconstructing Neanderthal diet: the case for carbohydrates. J Hum Evol. 2022;162:103105. doi:10.1016/j.jhevol.2021.103105.
- Taskent RO, Alioglu ND, Fer E, Donertas HM, Somel M, Gokcumen O. Variation and functional impact of Neanderthal ancestry in Western Asia. Genome Biol Evol. 2017;9(12):3516–24. doi:10.1093/gbe/evx216.
- Balfourier F, Bouchet S, Robert S, De Oliveira R, Rimbert H, Kitt J, Choulet F, Paux E, Worldwide phylogeography and history of wheat genetic diversity. Sci Adv. 2019;5(5):eaav0536. doi:10.1126/sciadv.aav0536.
- Gasbarrini G, Rickards O, Martínez-Labarga C, Pacciani E, Chilleri F, Laterza L, Marangi G, Scaldaferri F, Gasbarrini A. Origin of celiac disease: how old are predisposing haplotypes? World J Gastroenterol. 2012;18(37):5300–4. doi:10.3748/wjg.v18.i37.5300.
- Ishido N, Inoue N, Watanabe M, Hidaka Y, Iwatani Y. The relationship between skewed X chromosome inactivation and the prognosis of Graves’ and Hashimoto’s diseases. Thyroid. 2015;25(2):256–61. doi:10.1089/thy.2014.0318.
- Yin X, Latif R, Tomer Y, Davies TF. Thyroid epigenetics: X chromosome inactivation in patients with autoimmune thyroid disease. Ann N Y Acad Sci. 2007;1110:193–200. doi:10.1196/annals.1423.021.