?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Low mental energy can contribute to decreased productivity, altered life balance, decreased physical performance, and ultimately affect quality of life. As such, there is a great demand for food and beverage products that positively impact mental energy. Numerous products claim to alter mental energy making continued review of the scientific evidence critical. The objective of this study was to conduct a scoping review of randomized controlled trials to evaluate the effect of 18 dietary ingredients on mental energy outcomes in adults without severe disease. Methods: A literature search, completed using PubMed, resulted in the identification of 2261 articles, 190 of which met eligibility from initial abstract review. Full-text review was completed on the 190 studies which resulted in 101 articles that fully met eligibility for inclusion in this study. The search strategy for two ingredients did not yield any eligible studies, leaving studies for 16 ingredients that were extracted and summarized by reported significantly improved outcomes for cognition, mood and perceived feelings, and sleep assessments. The preliminary results for several dietary ingredients directionally suggested a mental energy benefit (≥20% of outcomes), including ashwagandha, chamomile, dark chocolate, ginseng, green tea, lavender, lion’s mane mushroom, maca, tart cherries, turmeric, and valerian root. The results of this scoping review suggest that of the 16 dietary ingredients reviewed, 11 may be promising for further exploration on their potential benefits in supporting mental energy. Given consumer demand and market growth for food and beverage products that positively impact mental energy; continued efforts in assessment method alignment and additional evaluation in well-designed trials is warranted.
Of the 16 dietary ingredients reviewed, 11 (ashwagandha, chamomile, dark chocolate, ginseng, green tea, lavender, lion’s mane mushroom, maca, melatonin foods, turmeric, and valerian root) may be promising for further exploration on their potential mental energy benefits.
Dark chocolate, ginseng, ashwagandha, and lion’s mane mushroom were the most promising ingredients for further evaluation in the cognition domain of the ingredients evaluated.
Turmeric, maca, lavendar, and ashwagandha were the most promising ingredients for further evaluation in the mood and perceived feelings domain of the ingredients evaluated.
Ashwagandha, chamomile, green tea, melatonin foods, valerian root were the most promising ingredients for further evaluation in the sleep domain of the ingredients evaluated.
Additional, well-designed, consistent, clinical trials and systematic reviews are warranted as the challenge of heterogeneity in mental energy study design remains.
KEY TEACHING POINTS
Introduction
A definition and model of mental energy have been developed by the International Life Sciences Committee (ILSI) (Citation1–3). Mental energy was defined as “the ability to perform mental tasks, the intensity of feelings of energy and fatigue, and the motivation to accomplish mental and physical tasks”. According to the ILSI committee, mental energy encompasses three dimensions cognition, the mood of energy, and motivation, and assessment tools for these constructs have been previously reviewed (Citation4–6). A number of methods exist that are reportedly linked to positive impacts on mental energy including physical activity, mindfulness and meditation, cognitive behavioral therapy, and improving sleep quality (Citation7–13). The addition of mental energy enhancing ingredients in foods and beverages have resulted in numerous consumer products that claim to positively impact mental energy in a rapidly growing market (Citation14).
Feelings of persistent reduced mental energy is a regular occurrence among individuals worldwide (Citation15). This “lack of energy” prevalence is common with studies suggesting ∼30% and affecting younger working adults, older individuals, and particularly women the most (Citation16, Citation17). Low mental energy can contribute to decreased personal and professional productivity, altered life balance, decreased physical performance, and ultimately effect quality of life (Citation1, Citation18, Citation19). In addition to the individual burden, the economic burden in the United States of lost productivity as a result of fatigue are costly and highlights the need for methods to address mental energy to support overall wellness (Citation20).
Given the rapid expansion and expected growth of this market and the need for evidenced-based product development, the purpose of this review was to assess the scientific evidence on specific dietary ingredients and their effects on mental energy. A literature search strategy was developed and implemented for 18 ingredients identified from consumer research with perceived benefits on mental energy by consumers. The potential bioactive compounds and mechanisms of action in mental energy are summarized in . Given the heterogeneity of the identified randomized controlled trials (RCT), the data was summarized providing a scoping state of the science regarding dietary ingredients and mental energy but also highlighting the need for both consistency in methodology and continued research.
Table 1. Potential mode of action in mental energy for the dietary ingredients evaluated.
Methods
This scoping review was conducted in alignment with the field of nutrition (Citation60–63). A review strategy was developed and refined by all investigators prior to implementing the search and reviewing the records returned. A list of 18 ingredients () was identified based on findings from consumer research that examined perceptions of mental energy benefits associated with food ingredients. Caffeine, gingko biloba, and omega-3 fatty acids were also identified from the consumer research findings but were not included in the study because regulatory bodies have previously assessed this scientific evidence (Citation1, Citation64–68).
Table 2. Search term strategy and records result summary.
Literature search
The scoping literature search was conducted up to February 9, 2021, using the PubMed database for relevant studies. The search term strategy included combinations of the ingredient terms with mental energy terms described in (see also Supplemental Material) and developed using published reviews on each ingredient. Human, randomized controlled trials, and best match filters were applied during the PubMed search. The identification of studies eligible for review was performed by scanning titles and abstracts using Abstrackr (Citation69).
Study eligibility criteria
Potentially relevant studies were exported from Abstrackr and full-text articles were obtained. The review included RCT published in English that evaluated the effects of dietary ingredients on mental energy outcomes. Animals and topical terms were excluded and no restrictions on publication date were imposed. The population of interest included apparently healthy and non-healthy adults, 18 years of age, and without any diagnosis of severe disease (e.g., cancer, autoimmune diseases, neurologic disorders, major mental illness; and Supplemental Material). Additionally, studies were considered exclusionary if it: included a dietary ingredient measured as part of a dietary pattern or without a proper control, evaluated dietary ingredients used as a disease therapeutic, included pregnant or lactating women, evaluated ingredients that were administered via inhalation, or studies that did not assess an endpoint related to mental energy.
Table 3. PICOS table for inclusion of studies.
Abstraction of data and descriptive statistical analysis
Data was extracted from manuscripts that met all of inclusion criteria and none of the exclusion criteria. Data extracted from eligible studies included:
General information – PMID, title, authors, journal, year of publication
Study design – population (healthy or unhealthy); disease/condition (if unhealthy), trial type, arm
Participant characteristics – age, sample size (randomized, evaluable)
Intervention – ingredient intervention, comparator, intervention form, intervention dose, intervention duration
Results – sample type, results summary
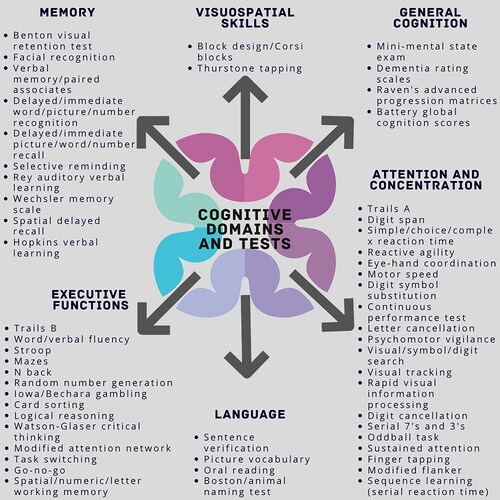
An abbreviated version of the full extraction is included in Supplementary Table 1. If multiple dose levels were evaluated in the study, each dose level was reported separately. In trials that evaluated multiple timepoints, only results from the final timepoint were included in the descriptive statistics. All relevant mental energy outcomes (subjective and objective) were extracted and categorized as either (i) cognition which included outcomes evaluated by individual cognitive tests, test batteries, or brain scanning methods, (ii) sleep including subjective and objective assessments, or (iii) mood and perceived feelings which also included subjective and objective assessments. Cognitive outcomes were additionally subcategorized into six domains as outlined in and initially described by Reger et al. (Citation70). Additional references were utilized to categorize outcomes not originally described. Due to the heterogeneity in trial designs and risk of misinterpretation, a meta-analysis was not completed. The total number of improved outcomes and the total number of outcomes (Citation71) are summarized by subcategory of cognition for each ingredient and by category (mood and perceived feelings, sleep, and overall cognition). Summary statistics for overall cognition are the result of the sum of the six domains. Percentages of improved outcomes are also provided for ease of review and a cutoff of ≥20% of outcomes was applied (Citation72); however, overinterpretation caution is warranted given the heterogeneity of the trial designs.
Results
Study selection and characteristics
The initial database search retrieved 2261 articles. After screening these abstracts, 190 of these met eligibility criteria. Full-text articles were retrieved and reviewed in detail resulting in identification of a total of 101 studies for inclusion. The search strategy for cordyceps mushroom and licorice root did not result in any eligible articles and are not described further. The study selection is outlined by ingredient in and the extracted data in . In addition, the tables include intervention, intervention duration, mean age of the sample, sample size, mental energy outcomes evaluated, the total number of improved outcome results, and the total number of outcomes reported by category (Supplementary Table 1). A summary of the number of improved mental energy outcomes by ingredient are included in .
Table 4a. Total number of improved mental energy outcomes for each dietary ingredient.
Ashwagandha
Five trials (Citation73–77) were identified which evaluated the effects of ashwagandha (Withania somnifera) extract on mental energy outcomes (Supplementary Table 1). The extract was administered daily in all studies for as little as 2 wk (Citation73) and as long as 16 wk (Citation74) at dosages ranging from 120 mg/day to 1000 mg/day in a range of 20–150 participants. The mean age of the sample population ranged from 25–51 years. Mental energy outcomes evaluated in the identified trials included cognitive tests within the attention and concentration and executive functions domain with a majority of the outcomes evaluating mood and perceived feelings and one study which evaluated sleep (Citation77). Ashwagandha interventions resulted in improvement in 69%, 63%, and 80% of the mood and perceived feelings, sleep, and cognitive function outcomes evaluated, respectively ().
Blueberry
The search strategy resulted in four trials (Citation78–81) that met the criteria and evaluated the effects of blueberry powder, extract, or concentrate (liquid) on outcomes of mental energy (Supplementary Table 1). Blueberry was administered chronically at dosages ranging from 24 g/day to 1000 mg/day, or 30 mL/day in one trial which evaluated blueberry concentrate, for 12–24 wk in 21–112 participants (mean age range = 20–71 years). One trial looked at acute administration of freeze-dried blueberry (Citation80) with evaluation of outcomes 2 h after consumption (n = 21; mean age = 20 years). Mental energy outcomes evaluated included mood and perceived feelings and a majority of the outcomes assessed cognitive function including tests of attention and concentration, executive functions, and memory. Blueberry interventions resulted in improvement in 13% and 8% of the mood and perceived feelings and cognitive function outcomes evaluated, respectively ().
Chamomile
Two trials (Citation82, Citation83) were identified which evaluated the effects of chamomile extract or tea on mental energy outcomes (Supplementary Table 1). The extract was administered daily in both studies for four weeks at 400 mg/day extract (n = 60; mean age = 70 years) (Citation82) or 2 g/day dried flowers (n = 72; mean age = 33 years) (Citation83) steeped in 300 mL water. Mental energy outcomes evaluated in the identified trials included mood and perceived feelings and a majority of outcomes evaluated sleep. Chamomile interventions resulted in improvement in 60% of the sleep outcomes but none of the mood and perceived feelings outcomes evaluated ().
Citrus
The citrus search strategy resulted in eight trials (Citation84–91) that met inclusion/exclusion criteria and evaluated the effects of bitter orange extract, dried flowers, or orange juice (liquid) on outcomes of mental energy (Supplementary Table 1). Orange was administered chronically at dosages of 1000 mg/day orange (bitter) dried flower (Citation87, Citation88) or 125–500 mL/day orange juice (Citation85, Citation86). Outcomes were evaluated following chronic administration of orange for 8–24 wk in 36–156 participants (mean age range = 53–71 years). Four of the trials (Citation84, Citation89–91) also evaluated acute administration of 20–103 mg orange (bitter) extract or 240 mL orange juice with evaluation of outcomes 1.25–6 h after consumption in 16–50 participants (mean age range = 22–48 years). Mental energy outcomes evaluated included mood and perceived feelings, one sleep outcome, and a majority of the outcomes assessed cognitive function including tests of attention and concentration, executive functions, general cognition, and memory. Orange interventions resulted in improvement in the sleep outcome and 11 and 15% of the mood and perceived feelings and cognitive function outcomes evaluated, respectively ().
Dark chocolate
Sixteen studies (Citation92–107) were identified which evaluated the effects of dark chocolate on mental energy outcomes (Supplementary Table 1). The extract was administered both acutely in seven trials (Citation93–95, Citation100, Citation104, Citation105, Citation107) and chronically in nine trials (Citation92, Citation96–99, Citation101–103, Citation106). Chronic administration occurred at dosages ranging from 0.25–100 g/day dark chocolate or cocoa for 2–12 wk (n = 18–90; mean age range = 23–73 years). Acute administration was provided at 0.25–1 g theobromine with a 4 h follow-up (n = 84; mean age = 23) (Citation100) and 0.4–50 g dark chocolate or cocoa with a 1– 2.5 h follow-up (n = 20–258; mean age range = 19–36 years) (Citation93–95, Citation104, Citation105, Citation107). Mental energy outcomes evaluated in the identified trials included mood and perceived feelings and cognitive function. These interventions resulted in improvement in 33% of the cognitive function outcomes, a majority in attention and concentration and executive functions. In addition, 11% of the mood and perceived feelings outcomes evaluated showed improvement ().
Ginseng
The ginseng search strategy identified 13 studies (Citation108–120) that met inclusion/exclusion criteria and evaluated the effects of ginseng extract on mental energy (Supplementary Table 1). Ginseng was administered at doses ranging from 100–1200 mg for acute administration with 1–6 h follow-up (n = 20–52; mean age range = 20–52 years) (Citation108, Citation110–112, Citation115–117). Outcomes were evaluated following chronic administration of ginseng at 200–1000 mg/day for 1–16 wk in 23–284 participants (mean age range = 22–61 years) (Citation109, Citation113, Citation118–120). Mental energy outcomes evaluated included mood and perceived feelings, one sleep outcome, and a majority of the outcomes assessed cognitive function including tests of attention and concentration, executive functions, memory, and visuospatial skills. Ginseng interventions resulted in no significant improvement in the single sleep outcome and 15% and 23% of the mood and perceived feelings and cognitive function outcomes evaluated, respectively ().
Green tea
A total of 13 studies (Citation121–133) were identified which evaluated the effects of green tea on mental energy outcomes (Supplementary Table 1). The extract was administered acutely in a majority of the trials ranging from 0.3–5 h follow-up and a dose of 50 mg to 200 mg theanine (Citation122, Citation123, Citation125, Citation126, Citation128, Citation131–133), 270 mg epigallocatechin gallate (EGCG) (Citation127), or 4 g matcha powder (Citation124)(n = 9–27; mean age range = 22–28 years). Chronic administration occurred in three trials (Citation121, Citation129, Citation130) at 200 mg/day theanine (Citation121), 500 mg/day green tea polyphenols (Citation129), or 800 mg/day EGCG (Citation130) for 4, 24, and 8 wk, respectively (n = 30–150 participants; mean age range = 48–58 years). Mental energy outcomes evaluated in the identified trials included cognitive tests within the attention and concentration, executive functions, language, and memory domains, mood and perceived feelings, and sleep. These interventions resulted in improvement in 9%, 43%, and 11% of the mood and perceived feelings, sleep, and cognitive function outcomes evaluated, respectively ().
Guarana
Three trials (Citation134–136) were identified which evaluated the effects of guarana on mental energy outcomes (Supplementary Table 1). The extract was administered daily in all studies at ∼1 g/day (n = 27–45; mean age range = 25–65 years) for 3 (Citation136), 5 (Citation134), or 150 (Citation135) days. Mental energy outcomes evaluated in the identified trials included mood and perceived feelings, sleep, and cognitive outcomes within attention and concentration, memory, and visuospatial skills domains. Guarana interventions resulted in no improvement in any of the reported outcomes ().
Lavender
Four studies (Citation87, Citation88, Citation137, Citation138) were identified which evaluated the effects of lavender oil, dried flowers, or tea on mental energy outcomes (Supplementary Table 1). The extract was administered daily in three of the studies (Citation87, Citation88, Citation137) for 2–8 wk at 1–4 g/day (n = 60–156; mean age range = 53–67 years). Bradely et al. (Citation138) evaluated the acute effects of 100 or 200 μL lavender oil with a 0.5 h follow-up assessment (n = 96; mean age = 36 years). Mental energy outcomes evaluated in the identified trials included mood and perceived feelings and one sleep outcome. Lavender interventions resulted in improvement of 63% of the mood and perceived feeling outcomes but not the reported sleep outcome ().
Table 4b. Total number of improved mental energy outcomes for each dietary ingredient (continued).
Lion’s mane mushroom
Three studies (Citation139–141) were identified which evaluated the effects of lion’s mane mushroom on mental energy outcomes (Supplementary Table 1). The mushroom extract was administered daily in all studies for 4–16 wk at 2–3.2 g/day (n = 26–31; mean age range = 41–70 years). Mental energy outcomes evaluated in the identified trials included cognitive function outcomes within general cognition and memory domains, two mood and perceived feelings outcomes, and one sleep outcome. Lion’s mane mushroom interventions resulted in improvement of 50% of the cognitive function outcomes. None of the mood and perceived feelings and sleep outcomes were reported as improved ().
Maca
Two studies (Citation142, Citation143) evaluated the effects of maca on mental energy outcomes (Supplementary Table 1). The mushroom extract was provided at 3.3 (Citation143) or 3.5 (Citation142) g/day (n = 52–54) for 12 wk in both studies. Mental energy outcomes evaluated in the identified trials included only mood and perceived feelings outcomes. Maca interventions resulted in improvement of 86% of the seven mood and perceived feelings outcomes assessed ().
Melatonin foods
The melatonin foods search strategy identified five studies (Citation144–148) that met the criteria and evaluated the effects of cherry (juice and powder) (Citation145, Citation147, Citation148) and walnuts (Citation144, Citation146) on outcomes of mental energy (Supplementary Table 1). Cherry juice concentrate (16 oz/day or 60 mL/day) and cherry powder (37.7 g/day) were administered for 5–14 days (n = 15–30; mean age range = 27–72 years) in three studies (Citation145, Citation147, Citation148). Two studies (Citation144, Citation146) evaluated the effects of walnut consumption at 60 g/day for 8 wk (n = 47 in both studies). Mental energy outcomes evaluated included mood and perceived feelings, sleep, and cognitive function including tests of executive functions, general cognition, and memory. Walnut and cherry interventions resulted in improvement in 46% and 4% of the sleep and cognitive function outcomes evaluated, respectively. There were no significantly improved outcomes reported for mood and perceived feelings ().
Mint
Six studies (Citation149–154) were identified which evaluated the mint (oil) or spearmint (extract) on mental energy outcomes (Supplementary Table 1). Ingredient interventions were administered chronically at 600–900 mg/day for spearmint for 17–90 days in 10–106 participants (mean age range = 28–59 years) (Citation149, Citation150, Citation152, Citation153). Acute administration was provided at 50 μL or 100 μL peppermint oil (Citation151) or 100 mg menthol (Citation154) (n = 22, 22, and 12, respectively) with assessment at 1.5–6 h. Mental energy outcomes evaluated in the identified trials included mood and perceived feelings, sleep, and cognitive function which included tests of attention and concentration, executive functions, memory, and visuospatial skills. These interventions resulted in improvement in 11% of the cognitive function outcomes. In addition, 6% of the mood and perceived feelings and 10% of the sleep outcomes evaluated showed improvement ().
Reishi mushroom
Only one study resulted from the search strategy which evaluated the effects of reishi mushroom on mental energy outcomes (Supplementary Table 1). Chu et al. (Citation155) administered the mushroom extract at 1.44 g/day for 12 wk in 23 participants (mean age = 55 years). The study evaluated a single measure of mood and perceived feelings which did not improve with the intervention ().
Turmeric
Seven trials (Citation156–162) were identified which evaluated the effects of turmeric on mental energy (Supplementary Table 1). The extract was administered daily in all studies for as little as 10 days and as long as 18 months at dosages ranging from 200 mg/day to 1000 mg/day in 30–134 participants (mean age range = 25–69 years). Mental energy outcomes evaluated in the identified trials included cognitive tests within all six domains with a majority of the outcomes evaluating mood and perceived feelings. Turmeric interventions resulted in improvement in 45% of the mood and perceived feelings and 14% cognitive function outcomes evaluated ().
Valerian root
The valerian root search strategy resulted in nine studies (Citation59, Citation163–170) that met the criteria and evaluated the effects of valerian root on outcomes of mental energy (Supplementary Table 1). Valerian root was evaluated chronically at dosages ranging 6.2 to 600 mg/day for 3–28 days (n = 57–405; mean age range = 26–44 years) in four studies (Citation163, Citation165, Citation166, Citation170). Five studies (Citation59, Citation164, Citation167–169) evaluated the effects of acute intake of valerian root at dosages ranging from 300–1800 mg (n = 9–40; mean age range = 22–56 years). Mental energy outcomes evaluated included mood and perceived feelings, sleep, and cognitive function including tests of attention and concentration and memory. Valerian root interventions resulted in improvement in 24% of the sleep outcomes evaluated. There were no significantly improved outcomes reported for cognitive function or mood and perceived feelings ().
Discussion
The results of this scoping review suggest that some dietary ingredients may be promising for further evaluation on improving aspects of mental energy in individuals. Although, improvements varied by ingredient and category but according to the current definition of mental energy, there is not a need to alter all domains to affect mental energy (Citation1). To our knowledge, one scoping review has been previously completed evaluating the effect of dietary ingredients (gingko biloba, ginseng, glucose, and omega-3 fatty acids), on mental energy and published more than a decade ago. In addition, a recent expert narrative review provided an overview of the evidence on glucose, ginseng, sage, rosemary, peppermint, and gingko biloba (Citation171). Similarly, our review provides additional evaluation of ginseng and mint and spearmint but further evaluated the scientific evidence on 16 additional ingredients based on consumer perceptions of dietary ingredients that provide focus or energy.
Cognition
The largest evidence-base for trials on cognition evaluated the effects of dark chocolate in 21 trials with 22 out of 67 outcomes (33%) reporting improved effects. Although, the results here should be met with caution given the heterogeneity in administration of the chocolate intervention. Chocolate contains a wide range of compounds with the potential to alter cognition including antioxidants and methylxanthines. Flavanols, a flavonoid subclass, has been proposed to deter age-related decline in cognitive performance by increasing the quantity and interaction between neurons and on opposing neuron degeneration in regions of the brain responsible for memory (Citation172). Theobromine is a methylxanthine compound, like caffeine, that is naturally found in cocoa and has also been hypothesized for its role in cognition and mood. This effect may occur through inhibition of adenosine receptors, similarly to caffeine but with less affinity, speeding up neural activity (Citation32).
Similarly, ginseng was evaluated in 17 trials with a total of 19 out of 84 outcomes (23%) reporting improved effects. Ginsenoside compounds in ginseng are hypothesized to be responsible for its bioactivity through the cholinergic system (Citation112). A number of additional studies have been published since the prior review (Citation1), supporting improved mental energy from ginseng interventions consistent with the recent narrative review (Citation171). A limited number of studies were identified which evaluated the effects of ashwagandha (5 trials) and lions mane mushroom (3 trials) on cognition, however a majority of outcomes indicated improvement of cognition outcomes 80% (4 of 5 outcomes) and 50% (2 of 4 outcomes), respectively. The mechanism of action associated with ashwagandha may be through cyclooxygenase‐2 enzyme inhibition, α2‐macroglobulin reduction, and increasing the expression of brain‐derived neurotrophic factor (Citation21).
Blueberry, citrus, melatonin foods, mint, and turmeric resulted in improvements in <20% of the outcomes evaluated. The remaining ingredients, chamomile, guarana, lavender, maca, reishi mushroom, and valerian root require further evaluation as the search strategy identified no improved outcomes or cognitive outcomes were not evaluated.
Mood and perceived feelings
Among the dietary ingredients most promising for their effect on mood and perceived feelings, turmeric were evaluated in 6 trials with 45% (17 of 38) of outcomes reported as improved. Curcumin is the main curcuminoid of the spice turmeric with a wide array of reported effects in vivo and in vitro including antidepressant, anti-inflammatory, and antioxidant properties. Specifically, in vivo studies suggest curcumin may regulate the monoaminergic pathways by influencing levels of serotonin, dopamaine, and noradrenaline in the central nervous system (Citation56).
Maca was evaluated two trials and 86% (6 of 7) of mood and perceived outcomes were reported as improved, respectively. Maca is the root of the plant Lepidium meyenii and may modulate androgenic and estrogenic activity in vitro (Citation142). The mechanism by which Maca may alter mood and perceived feelings in humans has yet to be elucidated. Lavender was evaluated in four trials and 63% (5 of 8 outcomes) were reported as improved. The bioactive constituents of lavender including linalool, linalyl acetate, ocimene, camphor, and flavonoids may exert their effect through anxiolytic, antioxidant, and antidepressant activities (Citation87, Citation173). Finally, the search strategy suggests ashwagandha may also be a promising ingredient to support mood and perceived feelings. Ashwagandha was evaluated in four trials and 69% of outcomes (9 of 13 outcomes) were reported as improved following treatment.
Finally, blueberry, citrus/orange, dark chocolate, ginseng, green tea, and mint, resulted in improvements in <20% of the outcomes evaluated. The remaining ingredients, chamomile, guarana, lion’s mane mushroom, lavender, melatonin foods, reishi mushroom, and valerian root require further evaluation as the search strategy identified no improvement or mood and perceived feelings outcomes were not evaluated.
Sleep
Given the importance of the role of sleep for overall wellness and particularly cognition, energy, and emotional health, key facets of mental energy, sleep outcomes were also evaluated as part of the search strategy (Citation174–177). The search strategy suggested the role of the 18 dietary ingredient interventions in sleep was the least evaluated of the three categories. Ashwaghanda, chamomile, green tea, melatonin foods, and valerian root provided the most promising effects.
Ashwaghanda improved in 63% (5 of 8) of reported outcomes but was only evaluated in a single study, although a mechanism of action for the effect of ashwaghanda on sleep in humans is not understood. Chamomile interventions improved 60% (6 of 10) of sleep outcomes evaluated in two trials. Chamomile may produce tranquilizing effects as a result of flavonoid compounds like apigenin, which bind benzodiazepine receptors in the brain (Citation178). Green tea interventions improved 43% (3 of 7) of sleep outcomes but was evaluated in only one trial. L-theanine is an amino acid found in green tea and may be responsible for its sleep effects by monoaminergic modulation through glutamate, serotonin, and dopamine transmission (Citation37). The effect of melatonin foods on sleep outcomes was evaluated in three trials, all evaluated cherry as an ingredient, and 46% (11 of 24) sleep outcomes improved. Cherries contain high amounts of melatonin, serotonin, and tryptophan which are hypothesized to be responsible for the sleep-promoting properties (Citation145). Valerian root was the most studied ingredient (7 trials) for sleep outcomes. The valerian root search strategy resulted in improvement in 24% (9 of 37) of sleep outcomes evaluated. Mechanisms of action for the effects of valerian root on sleep are not well defined and may be attributed to valepotriates and sesquiterpenes compounds inducing sedative and tranquilizing effects (Citation59).
Mint resulted in improvements in <20% (2 of 20) of the sleep outcomes evaluated and citrus/orange intervention was evaluated in one study which reported improvement in one sleep outcome. The remaining ingredients, blueberry, dark chocolate, ginseng, guarana, lavender, lion’s mane mushroom, maca, reishi mushroom, and turmeric, require further evaluation as the search strategy identified no improvement or sleep outcomes were not evaluated.
Strengths and limitations
A major strength of this study is the broad inclusivity of ingredients which allows a picture of the state of the evidence on the role of dietary ingredients in mental energy for further exploration. Although with this strength comes the challenge of the heterogeneity of the studies in a limited mental energy field with inconsistent, often nonobjective methods, resulting in the need for caution in overinterpreting the results. Moreover, the heterogeneity of the studies continues to limit the interpretation of the science, particularly as a result of differing study durations, dose and form of dietary ingredients, participant adherence/compliance, and the lack of consistent objective methods. This is particularly difficult for cognition assessments. There is not only a vast array of tests utilized and control variation (practice effects, for example) but the methods of analysis (individual tests vs. domains for example) make comparisons between studies difficult. The ILSI mental energy committee suggested that sustained attention was the best cognitive measure (Citation1). Previous work by O’Connor and Lieberman suggests cognitive tests that evaluate vigilance, sustained attention, and choice reaction time, and for mood, the Profile of Mood State vigor scale were ideal for the evaluation of mental energy (Citation6, Citation179). Future studies should focus on objective outcomes, and potentially set recommended cognitive tests for nutrition research to reduce heterogeneity (Citation180), and may also benefit from mechanistic evaluation using multi-omics technologies (Citation181). Inherent bias cannot be ruled out; although Abstrackr is a powerful tool for abstract screening, an oversight of eligible studies was possible. In addition, given the design of this study as a scoping review to allow for further hypothesis-driven exploration, the search strategy was designed for one database and did not include grey literature which would be beneficial in future investigation. Finally, the study did not include a review of interactions and potential adverse effects associated with consumption of these dietary ingredients which should be considered in mental energy product formulations.
Conclusion
Consumer demand as well as consumer belief in the effectiveness of mental energy ingredients that have yet to be proven, drives the need for continued evaluation of the evidence-base on dietary ingredients that positively impact mental energy. Of the 16 dietary ingredients reviewed, 11 show promise for further exploration on their mental energy effects including ashwagandha, chamomile, dark chocolate, ginseng, green tea, lavender, lion’s mane mushroom, maca, melatonin foods, turmeric, and valerian root. The challenge still remains in interpreting the mental energy evidence base due to the heterogeneity in the study designs and methods of assessment and analysis. Additional, well-designed, consistent, clinical trials and systematic reviews are warranted given the limited number of studies on some of these ingredients to determine the mental energy effects in individuals without severe disease.
Author contributions
All authors contributed to the study design and manuscript development. KMN lead and developed the search strategy, conducted the search, reviewed abstracts and full-text articles, completed the data extraction and descriptive statistics, and lead manuscript development. YZ, MT, and KK acquired funding. YZ directed the study. All authors read and approved the final manuscript.
Supplemental Material
Download MS Excel (126.5 KB)Disclosure statement
KMN has no relevant interests to declare. MT and KK are currently employed by General Mills, Inc. YZ was employed by General Mills, Inc. during the study.
Additional information
Funding
References
- Gorby HE, Brownawell AM, Falk MC. Do specific dietary constituents and supplements affect mental energy? Review of the evidence. Nutr Rev. 2010;68(12):697–718. doi:10.1111/j.1753-4887.2010.00340.x.
- O’Connor PJ. Mental energy: developing a model for examining nutrition-related claims. Nutr Rev. 2006;64(7 Pt 2):S2–S6. doi:10.1301/nr.2006.jul.s2-s6.
- Cook DB, Davis JM. Mental energy: defining the science. Nutr Rev. 2006;64(7 Pt 2):S1. doi:10.1111/j.1753-4887.2006.tb00251.x.
- Lieberman HR. Mental energy: assessing the cognition dimension. Nutr Rev. 2006;64(7 Pt 2):S10–S13. doi:10.1111/j.1753-4887.2006.tb00252.x.
- Barbuto JE.Jr. Mental energy: assessing the motivation dimension. Nutr Rev. 2006;64(7 Pt 2):S14–S16. doi:10.1111/j.1753-4887.2006.tb00253.x.
- O’Connor PJ. Mental energy: assessing the mood dimension. Nutr Rev. 2006;64(7 Pt 2):S7–S9. doi:10.1111/j.1753-4887.2006.tb00256.x.
- O’Connor PJ, Puetz TW. Chronic physical activity and feelings of energy and fatigue. Med Sci Sports Exerc. 2005;37(2):299–305. doi:10.1249/01.mss.0000152802.89770.cf.
- Pastier N, Jansen E, Boolani A. Sleep quality in relation to trait energy and fatigue: an exploratory study of healthy young adults. Sleep Sci. 2022;15(Spec 2):375–9. doi:10.5935/1984-0063.20210002.
- Puetz TW. Physical activity and feelings of energy and fatigue: epidemiological evidence. Sports Med. 2006;36(9):767–80. doi:10.2165/00007256-200636090-00004.
- Puetz TW, O’Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: a quantitative synthesis. Psychol Bull. 2006;132(6):866–76. doi:10.1037/0033-2909.132.6.866.
- Johns SA, Tarver WL, Secinti E, Mosher CE, Stutz PV, Carnahan JL, Talib TL, Shanahan ML, Faidley MT, Kidwell KM, et al. Effects of mindfulness-based interventions on fatigue in cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol. 2021;160:103290. doi:10.1016/j.critrevonc.2021.103290.
- Xiang Y, Lu L, Chen X, Wen Z. Does Tai Chi relieve fatigue? A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2017;12(4):e0174872. doi:10.1371/journal.pone.0174872.
- Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev. 2008;2008(3):Cd001027. doi:10.1002/14651858.CD001027.pub2.
- Heckman MA, Sherry K, De Mejia EG. Energy Drinks: an Assessment of Their Market Size, Consumer Demographics, Ingredient Profile, Functionality, and Regulations in the United States. Compr Rev Food Sci Food Saf. 2010;9(3):303–17. doi:10.1111/j.1541-4337.2010.00111.x.
- Puetz TW, Flowers SS, O’Connor PJ. A randomized controlled trial of the effect of aerobic exercise training on feelings of energy and fatigue in sedentary young adults with persistent fatigue. Psychother Psychosom. 2008;77(3):167–74. doi:10.1159/000116610.
- Meng H, Hale L, Friedberg F. Prevalence and predictors of fatigue in middle-aged and older adults: evidence from the health and retirement study. J Am Geriatr Soc. 2010;58(10):2033–4. doi:10.1111/j.1532-5415.2010.03088.x.
- Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative. J Psychosom Res. 2011;71(3):117–23. doi:10.1016/j.jpsychores.2011.04.007.
- Brahms M, Heinzel S, Rapp M, Mückstein M, Hortobágyi T, Stelzel C, Granacher U. The acute effects of mental fatigue on balance performance in healthy young and older adults – A systematic review and meta-analysis. Acta Psychol (Amst). 2022;225:103540. doi:10.1016/j.actpsy.2022.103540.
- Clemente FM, Ramirez-Campillo R, Castillo D, Raya-González J, Silva AF, Afonso J, Sarmento H, Rosemann T, Knechtle B. Effects of mental fatigue in total running distance and tactical behavior during small-sided games: a systematic review with a meta-analysis in youth and young adult’s soccer players. Front Psychol. 2021;12:656445. doi:10.3389/fpsyg.2021.656445.
- Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the U.S. workforce: prevalence and implications for lost productive work time. J Occup Environ Med. 2007;49(1):1–10. doi:10.1097/01.jom.0000249782.60321.2a.
- Ng QX, Loke W, Foo NX, Tan WJ, Chan HW, Lim DY, Yeo WS. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother Res. 2020;34(3):583–90. doi:10.1002/ptr.6552.
- Hein S, Whyte AR, Wood E, Rodriguez-Mateos A, Williams CM. Systematic review of the effects of blueberry on cognitive performance as we age. J Gerontol A Biol Sci Med Sci. 2019;74(7):984–95. doi:10.1093/gerona/glz082.
- Spencer JP. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4(4):243–50. doi:10.1007/s12263-009-0136-3.
- Henriques JF, Serra D, Dinis TCP, Almeida LM. The anti-neuroinflammatory role of anthocyanins and their metabolites for the prevention and treatment of brain disorders. Int J Mol Sci. 2020;21(22):8653. doi:10.3390/ijms21228653.
- Avallone R, Zanoli P, Corsi L, Cannazza G, Baraldi M. Benzodiazepine-like compounds and GABA in flower heads of Matricaria chamomilla. 1996.
- Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3(6):895–901. doi:10.3892/mmr.2010.377.
- Galluzzi S, Zanardini R, Ferrari C, Gipponi S, Passeggia I, Rampini M, Sgrò G, Genovese S, Fiorito S, Palumbo L, et al. Cognitive and biological effects of citrus phytochemicals in subjective cognitive decline: a 36-week, randomized, placebo-controlled trial. Nutr J. 2022;21(1):64. doi:10.1186/s12937-022-00817-6.
- Matsuzaki K, Nakajima A, Guo Y, Ohizumi Y. A narrative review of the effects of citrus peels and extracts on human brain health and metabolism. Nutrients. 2022;14(9):1847. doi:10.3390/nu14091847.
- Cai ZL, Wang CY, Jiang ZJ, Li HH, Liu WX, Gong LW, Xiao P, Li CH. Effects of cordycepin on Y-maze learning task in mice. Eur J Pharmacol. 2013;714(1-3):249–53. doi:10.1016/j.ejphar.2013.05.049.
- Yoneda M, Sugimoto N, Katakura M, Matsuzaki K, Tanigami H, Yachie A, Ohno-Shosaku T, Shido O. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J Nutr Biochem. 2017;39:110–6. doi:10.1016/j.jnutbio.2016.10.002.
- Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15(10):2779–811. doi:10.1089/ars.2010.3697.
- Smit HJ. Theobromine and the pharmacology of cocoa. Handb Exp Pharmacol. 2011;(200):201–34. doi:10.1007/978-3-642-13443-2_7.
- Lee SH, Hur J, Lee EH, Kim SY. Ginsenoside rb1 modulates level of monoamine neurotransmitters in mice frontal cortex and cerebellum in response to immobilization stress. Biomol Ther (Seoul). 2012;20(5):482–6. doi:10.4062/biomolther.2012.20.5.482.
- Feng H, Xue M, Deng H, Cheng S, Hu Y, Zhou C. Ginsenoside and its therapeutic potential for cognitive impairment. Biomolecules. 2022;12(9):1310. doi:10.3390/biom12091310.
- Schmidt A, Hammann F, Wölnerhanssen B, Meyer-Gerspach AC, Drewe J, Beglinger C, Borgwardt S. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231(19):3879–88. doi:10.1007/s00213-014-3526-1.
- Afzal O, Dalhat MH, Altamimi ASA, Rasool R, Alzarea SI, Almalki WH, Murtaza BN, Iftikhar S, Nadeem S, Nadeem MS, et al. Green tea catechins attenuate neurodegenerative diseases and cognitive deficits. Molecules. 2022;27(21):7604. doi:10.3390/molecules27217604.
- Rao TP, Ozeki M, Juneja LR. In search of a safe natural sleep aid. J Am Coll Nutr. 2015;34(5):436–47. doi:10.1080/07315724.2014.926153.
- Torres E, Pinaffi-Langley A, Figueira MS, Cordeiro KS, Negrão LD, Soares MJ, da Silva CP, Alfino MCZ, Sampaio GR, de Camargo AC. Effects of the consumption of guarana on human health: A narrative review. Compr Rev Food Sci Food Saf. 2022;21(1):272–95. doi:10.1111/1541-4337.12862.
- López V, Nielsen B, Solas M, Ramírez MJ, Jäger AK. Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front Pharmacol. 2017;8:280. doi:10.3389/fphar.2017.00280.
- Jiang R, Gao J, Shen J, Zhu X, Wang H, Feng S, Huang C, Shen H, Liu H. Glycyrrhizic acid improves cognitive levels of aging mice by regulating T/B cell proliferation. Front Aging Neurosci. 2020;12:570116. doi:10.3389/fnagi.2020.570116.
- Cho MJ, Kim JH, Park CH, Lee AY, Shin YS, Lee JH, Park CG, Cho EJ. Comparison of the effect of three licorice varieties on cognitive improvement via an amelioration of neuroinflammation in lipopolysaccharide-induced mice. Nutr Res Pract. 2018;12(3):191–8. doi:10.4162/nrp.2018.12.3.191.
- Kawagishi H, Shimada A, Shirai R, Okamoto K, Ojima F, Sakamoto H, Ishiguro Y, Furukawa S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994;35(10):1569–72. doi:10.1016/S0040-4039(00)76760-8.
- Ma B-J, Shen J-W, Yu H-Y, Ruan Y, Wu T-T, Zhao X. Hericenones and erinacines: stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology. 2010;1(2):92–8. doi:10.1080/21501201003735556.
- Guo SS, Gao XF, Gu YR, Wan ZX, Lu AM, Qin ZH, Luo L. Preservation of cognitive function by lepidium meyenii (maca) is associated with improvement of mitochondrial activity and upregulation of autophagy-related proteins in middle-aged mouse cortex. Evid Based Complement Alternat Med. 2016;2016:4394261. doi:10.1155/2016/4394261.
- Zha S, Zhao Q, Chen J, Wang L, Zhang G, Zhang H, Zhao B. Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohydr Polym. 2014;111(111584):584–7. 587 doi:10.1016/j.carbpol.2014.05.017.
- Pino-Figueroa A, Huyen V, Kelley CJ, Maher TJ. Mechanism of action of lepidium meyenii (maca): an explanation for its neuroprotective activity. Am J Neuroprotect Neuroregen. 2011;3(1):87–92. doi:10.1166/ajnn.2011.1035.
- Salehi B, Sharopov F, Fokou PVT, Kobylinska A, Jonge L, Tadio K, Sharifi-Rad J, Posmyk MM, Martorell M, Martins N, et al. Melatonin in medicinal and food plants: occurrence, bioavailability, and health potential for humans. Cells. 2019;8(7):681. doi:10.3390/cells8070681.
- Meng X, Li Y, Li S, Zhou Y, Gan R-Y, Xu D-P, Li H-B. Dietary sources and bioactivities of melatonin. Nutrients. 2017;9(4):367. doi:10.3390/nu9040367.
- Fallarini S, Miglio G, Paoletti T, Minassi A, Amoruso A, Bardelli C, Brunelleschi S, Lombardi G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br J Pharmacol. 2009;157(6):1072–84. doi:10.1111/j.1476-5381.2009.00213.x.
- Mushtaq N, Schmatz R, Pereira LB, Ahmad M, Stefanello N, Vieira JM, Abdalla F, Rodrigues MV, Baldissarelli J, Pelinson LP, et al. Rosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic rats. Cell Biochem Funct. 2014;32(3):287–93. doi:10.1002/cbf.3014.
- Rocha J, Eduardo-Figueira M, Barateiro A, Fernandes A, Brites D, Bronze R, Duarte CM, Serra AT, Pinto R, Freitas M, et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin Pharmacol Toxicol. 2015;116(5):398–413. doi:10.1111/bcpt.12335.
- Lee JM, Kwon H, Jeong H, Lee JW, Lee SY, Baek SJ, Surh YJ. Inhibition of lipid peroxidation and oxidative DNA damage by Ganoderma lucidum. Phytother Res. 2001;15(3):245–9. doi:10.1002/ptr.830.
- Huang S, Mao J, Ding K, Zhou Y, Zeng X, Yang W, Wang P, Zhao C, Yao J, Xia P, et al. Polysaccharides from ganoderma lucidum promote cognitive function and neural progenitor proliferation in mouse model of alzheimer’s disease. Stem Cell Reports. 2017;8(1):84–94. doi:10.1016/j.stemcr.2016.12.007.
- D’Cunha NM, Seddon N, Mellor DD, Georgousopoulou EN, McKune AJ, Panagiotakos DB, Kellett J, Naumovski N. Curcumin for cognition: is it just hype, based on current data? Adv Nutr. 2019;10(1):179–81. doi:10.1093/advances/nmy066.
- Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27–37. doi:10.1016/j.cbi.2008.05.003.
- Lopresti AL, Hood SD, Drummond PD. Multiple antidepressant potential modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J Psychopharmacol. 2012;26(12):1512–24. doi:10.1177/0269881112458732.
- Yuan CS, Mehendale S, Xiao Y, Aung HH, Xie JT, Ang-Lee MK. The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anesth Analg. 2004;98(2):353–8. doi:10.1213/01.Ane.0000096189.70405.A5.
- Dietz BM, Mahady GB, Pauli GF, Farnsworth NR. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res. 2005;138(2):191–7. doi:10.1016/j.molbrainres.2005.04.009.
- Diaper A, Hindmarch I. A double-blind, placebo-controlled investigation of the effects of two doses of a valerian preparation on the sleep, cognitive and psychomotor function of sleep-disturbed older adults. Phytother Res. 2004;18(10):831–6. doi:10.1002/ptr.1574.
- Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition: nutritional research series. Vol. 1. AHRQ Technical Reviews. Rockville (MD): agency for Healthcare Research and Quality (US); 2009.
- Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138(12):2297–306. doi:10.3945/jn.108.097154.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to caffeine and increased fat oxidation leading to a reduction in body fat mass (ID 735, 1484), increased energy expenditure leading to a reduction in body weight (ID 1487), increased alertness (ID 736, 1101, 1187, 1485, 1491, 2063, 2103) and increased attention (ID 736, 1485, 1491, 2375) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011;9(4):2054. doi:10.2903/j.efsa.2011.2054.
- Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40(9):1243–55. doi:10.1016/s0278-6915(02)00096-0.
- European Food Safety Authority Scientific Opinion on the substantiation of health claims related to docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and brain, eye and nerve development (ID 501, 513, 540), maintenance of normal brain function (ID 497, 501, 510, 513, 519, 521, 534, 540, 688, 1323, 1360, 4294), maintenance of normal vision (ID 508, 510, 513, 519, 529, 540, 688, 2905, 4294), maintenance of normal cardiac function (ID 510, 688, 1360), “maternal health; pregnancy and nursing” (ID 514), “to fulfil increased omega-3 fatty acids need during pregnancy” (ID 539), “skin and digestive tract epithelial cells maintenance” (ID 525), enhancement of mood (ID 536), “membranes cell structure” (ID 4295), “anti- inflammatory action” (ID 4688) and maintenance of normal blood LDL-cholesterol concentrations (ID 4719) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. Efsa J. 2011;9(4):2078. doi:10.2903/j.efsa.2011.2078.
- European Food Safety Authority DHA and improvement of memory function: evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006. Efsa J. 2016;14(5):e04455. doi:10.2903/j.efsa.2016.4455.
- Assessment report on Ginkgo biloba L., folium. 2014. https://www.ema.europa.eu/en/medicines/herbal/ginkgo-folium#documents-section
- Wallace BC, Trikalinos TA, Lau J, Brodley C, Schmid CH. Semi-automated screening of biomedical citations for systematic reviews. BMC Bioinformatics. 2010;11:55. doi:10.1186/1471-2105-11-55.
- Reger MA, Welsh RK, Watson GS, Cholerton B, Baker LD, Craft S. The relationship between neuropsychological functioning and driving ability in dementia: a meta-analysis. Neuropsychology. 2004;18(1):85–93. doi:10.1037/0894-4105.18.1.85.
- Lamport DJ, Saunders C, Butler LT, Spencer JP. Fruits, vegetables, 100% juices, and cognitive function. Nutr Rev. 2014;72(12):774–89. doi:10.1111/nure.12149.
- Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273–86. doi:10.1093/biostatistics/kxx069.
- Pingali U, Pilli R, Fatima N. Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacognosy Res. 2014;6(1):12–8. doi:10.4103/0974-8490.122912.
- Lopresti AL, Drummond PD, Smith SJ. A Randomized, Double-Blind, Placebo-Controlled, Crossover Study Examining the Hormonal and Vitality Effects of Ashwagandha (Withania somnifera) in Aging, Overweight Males. Am J Mens Health. 2019;13(2):1557988319835985. doi:10.1177/1557988319835985.
- Lopresti AL, Smith SJ, Malvi H, Kodgule R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: a randomized, double-blind, placebo-controlled study. Medicine (Baltimore). 2019;98(37):e17186. doi:10.1097/md.0000000000017186.
- Choudhary D, Bhattacharyya S, Joshi K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract: a Double-Blind, Randomized, Placebo-Controlled Trial. J Evid Based Complementary Altern Med. 2017;22(1):96–106. doi:10.1177/2156587216641830.
- Deshpande A, Irani N, Balkrishnan R, Benny IR. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020;72:28–36. doi:10.1016/j.sleep.2020.03.012.
- Whyte AR, Cheng N, Fromentin E, Williams CM. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (thinkblue™) in maintenance of episodic and working memory in older adults. Nutrients. 2018;10(6):660. doi:10.3390/nu10060660.
- Bowtell JL, Aboo-Bakkar Z, Conway ME, Adlam AR, Fulford J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl Physiol Nutr Metab. 2017;42(7):773–9. doi:10.1139/apnm-2016-0550.
- Khalid S, Barfoot KL, May G, Lamport DJ, Reynolds SA, Williams CM. Effects of acute blueberry flavonoids on mood in children and young adults. Nutrients. 2017;9(2):158. doi:10.3390/nu9020158.
- Miller MG, Hamilton DA, Joseph JA, Shukitt-Hale B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur J Nutr. 2018;57(3):1169–80. doi:10.1007/s00394-017-1400-8.
- Adib-Hajbaghery M, Mousavi SN. The effects of chamomile extract on sleep quality among elderly people: a clinical trial. Complement Ther Med. 2017;35(35109):109–14. 114 doi:10.1016/j.ctim.2017.09.010.
- Chang SM, Chen CH. Effects of an intervention with drinking chamomile tea on sleep quality and depression in sleep disturbed postnatal women: a randomized controlled trial. J Adv Nurs. 2016;72(2):306–15. doi:10.1111/jan.12836.
- Bush JA, Ratamess NA, Stohs SJ, Ellis NL, Vought IT, O’Grady EA, Kuper JD, Kang J, Faigenbaum AD. Acute hematological and mood perception effects of bitter orange extract (p-synephrine) consumed alone and in combination with caffeine: a placebo-controlled, double-blind study. Phytother Res. 2018;32(8):1593–607. doi:10.1002/ptr.6090.
- Kean RJ, Lamport DJ, Dodd GF, Freeman JE, Williams CM, Ellis JA, Butler LT, Spencer JP. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: an 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am J Clin Nutr. 2015;101(3):506–14. doi:10.3945/ajcn.114.088518.
- Igase M, Okada Y, Ochi M, Igase K, Ochi H, Okuyama S, Furukawa Y, Ohyagi Y. Auraptene in the peels of citrus Kawachiensis (Kawachibankan) contributes to the preservation of cognitive function: a randomized, placebo-controlled, double-blind study in healthy volunteers. J Prev Alzheimers Dis. 2018;5(3):197–201. doi:10.14283/jpad.2017.47.
- Farshbaf-Khalili A, Kamalifard M, Namadian M. Comparison of the effect of lavender and bitter orange on anxiety in postmenopausal women: a triple-blind, randomized, controlled clinical trial. Complement Ther Clin Pract. 2018;31:132–8. doi:10.1016/j.ctcp.2018.02.004.
- Kamalifard M, Farshbaf-Khalili A, Namadian M, Ranjbar Y, Herizchi S. Comparison of the effect of lavender and bitter orange on sleep quality in postmenopausal women: a triple-blind, randomized, controlled clinical trial. Women Health. 2018;58(8):851–65. doi:10.1080/03630242.2017.1353575.
- Stohs SJ, Preuss HG, Keith SC, Keith PL, Miller H, Kaats GR. Effects of p-synephrine alone and in combination with selected bioflavonoids on resting metabolism, blood pressure, heart rate and self-reported mood changes. Int J Med Sci. 2011;8(4):295–301. doi:10.7150/ijms.8.295.
- Alharbi MH, Lamport DJ, Dodd GF, Saunders C, Harkness L, Butler LT, Spencer JP. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur J Nutr. 2016;55(6):2021–9. doi:10.1007/s00394-015-1016-9.
- Jung YP, Earnest CP, Koozehchian M, Galvan E, Dalton R, Walker D, Rasmussen C, Murano PS, Greenwood M, Kreider RB. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J Int Soc Sports Nutr. 2017;14:3. doi:10.1186/s12970-016-0159-2.
- Sumiyoshi E, Matsuzaki K, Sugimoto N, Tanabe Y, Hara T, Katakura M, Miyamoto M, Mishima S, Shido O. Sub-chronic consumption of dark chocolate enhances cognitive function and releases nerve growth factors: a parallel-group randomized trial. Nutrients. 2019;11(11):2800. doi:10.3390/nu11112800.
- Karabay A, Saija JD, Field DT, Akyürek EG. The acute effects of cocoa flavanols on temporal and spatial attention. Psychopharmacology (Berl). 2018;235(5):1497–511. doi:10.1007/s00213-018-4861-4.
- Meier BP, Noll SW, Molokwu OJ. The sweet life: the effect of mindful chocolate consumption on mood. Appetite. 2017;108:21–7. doi:10.1016/j.appet.2016.09.018.
- Kuebler U, Arpagaus A, Meister RE, von Känel R, Huber S, Ehlert U, Wirtz PH. Dark chocolate attenuates intracellular pro-inflammatory reactivity to acute psychosocial stress in men: a randomized controlled trial. Brain Behav Immun. 2016;57:200–8. doi:10.1016/j.bbi.2016.04.006.
- Mastroiacovo D, Kwik-Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, Righetti R, Bocale R, Lechiara MC, Marini C, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study–a randomized controlled trial. Am J Clin Nutr. 2015;101(3):538–48. doi:10.3945/ajcn.114.092189.
- Brickman AM, Khan UA, Provenzano FA, Yeung LK, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17(12):1798–803. doi:10.1038/nn.3850.
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81(10):904–9. doi:10.1212/WNL.0b013e3182a351aa.
- Pase MP, Scholey AB, Pipingas A, Kras M, Nolidin K, Gibbs A, Wesnes K, Stough C. Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J Psychopharmacol. 2013;27(5):451–8. doi:10.1177/0269881112473791.
- Baggott MJ, Childs E, Hart AB, de Bruin E, Palmer AA, Wilkinson JE, de Wit H. Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology (Berl). 2013;228(1):109–18. doi:10.1007/s00213-013-3021-0.
- Martin FP, Antille N, Rezzi S, Kochhar S. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4(6):554–67. doi:10.3390/nu4060554.
- Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, Raffaele A, Ferri L, Bocale R, Lechiara MC, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012;60(3):794–801. doi:10.1161/hypertensionaha.112.193060.
- Camfield DA, Scholey A, Pipingas A, Silberstein R, Kras M, Nolidin K, Wesnes K, Pase M, Stough C. Steady state visually evoked potential (SSVEP) topography changes associated with cocoa flavanol consumption. Physiol Behav. 2012;105(4):948–57. doi:10.1016/j.physbeh.2011.11.013.
- Field DT, Williams CM, Butler LT. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol Behav. 2011;103(3-4):255–60. doi:10.1016/j.physbeh.2011.02.013.
- Scholey AB, French SJ, Morris PJ, Kennedy DO, Milne AL, Haskell CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. 2010;24(10):1505–14. doi:10.1177/0269881109106923.
- Crews WD, Jr., Harrison DW, Wright JW. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. Am J Clin Nutr. 2008;87(4):872–80. doi:10.1093/ajcn/87.4.872.
- Smit HJ, Gaffan EA, Rogers PJ. Methylxanthines are the psycho-pharmacologically active constituents of chocolate. Psychopharmacology (Berl). 2004;176(3-4):412–9. doi:10.1007/s00213-004-1898-3.
- Ong Lai Teik D, Lee XS, Lim CJ, Low CM, Muslima M, Aquili L. Ginseng and ginkgo biloba effects on cognition as modulated by cardiovascular reactivity: a randomised trial. PLoS One. 2016;11(3):e0150447. doi:10.1371/journal.pone.0150447.
- Lee N, Lee SH, Yoo HR, Yoo HS. Anti-fatigue effects of enzyme-modified ginseng extract: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2016;22(11):859–64. doi:10.1089/acm.2016.0057.
- Ossoukhova A, Owen L, Savage K, Meyer M, Ibarra A, Roller M, Pipingas A, Wesnes K, Scholey A. Improved working memory performance following administration of a single dose of American ginseng (Panax quinquefolius L.) to healthy middle-age adults. Hum Psychopharmacol. 2015;30(2):108–22. doi:10.1002/hup.2463.
- LaSala GS, McKeever RG, Patel U, Okaneku J, Vearrier D, Greenberg MI. Effect of single-dose Ginkgo biloba and Panax ginseng on driving performance. Clin Toxicol (Phila). 2015;53(2):108–12. doi:10.3109/15563650.2014.999159.
- Scholey A, Ossoukhova A, Owen L, Ibarra A, Pipingas A, He K, Roller M, Stough C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology (Berl). 2010;212(3):345–56. doi:10.1007/s00213-010-1964-y.
- Reay JL, Scholey AB, Kennedy DO. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol. 2010;25(6):462–71. doi:10.1002/hup.1138.
- Reay JL, Kennedy DO, Scholey AB. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J Psychopharmacol. 2006;20(6):771–81. doi:10.1177/0269881106061516.
- Reay JL, Kennedy DO, Scholey AB. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19(4):357–65. doi:10.1177/0269881105053286.
- Scholey AB, Kennedy DO. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol. 2002;17(1):35–44. doi:10.1002/hup.352.
- Kennedy DO, Scholey AB, Wesnes KA. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol Behav. 2002;75(5):739–51. doi:10.1016/s0031-9384(02)00665-0.
- Ellis JM, Reddy P. Effects of Panax ginseng on quality of life. Ann Pharmacother. 2002;36(3):375–9. doi:10.1345/aph.1A245.
- Cardinal BJ, Engels HJ. Ginseng does not enhance psychological well-being in healthy, young adults: results of a double-blind, placebo-controlled, randomized clinical trial. J Am Diet Assoc. 2001;101(6):655–60. doi:10.1016/s0002-8223(01)00165-1.
- Wiklund IK, Mattsson LA, Lindgren R, Limoni C. Effects of a standardized ginseng extract on quality of life and physiological parameters in symptomatic postmenopausal women: a double-blind, placebo-controlled trial. Swedish Alternative Medicine Group. Int J Clin Pharmacol Res. 1999;19(3):89–99.
- Hidese S, Ogawa S, Ota M, Ishida I, Yasukawa Z, Ozeki M, Kunugi H. Effects of L-theanine administration on stress-related symptoms and cognitive functions in healthy adults: a randomized controlled trial. Nutrients. 2019;11(10):2362. doi:10.3390/nu11102362.
- Kahathuduwa CN, Dhanasekara CS, Chin SH, Davis T, Weerasinghe VS, Dassanayake TL, Binks M. l-Theanine and caffeine improve target-specific attention to visual stimuli by decreasing mind wandering: a human functional magnetic resonance imaging study. Nutr Res. 2018;49:67–78. doi:10.1016/j.nutres.2017.11.002.
- Kahathuduwa CN, Dassanayake TL, Amarakoon AMT, Weerasinghe VS. Acute effects of theanine, caffeine and theanine-caffeine combination on attention. Nutr Neurosci. 2017;20(6):369–77. doi:10.1080/1028415x.2016.1144845.
- Dietz C, Dekker M, Piqueras-Fiszman B. An intervention study on the effect of matcha tea, in drink and snack bar formats, on mood and cognitive performance. Food Res Int. 2017;99(Pt 1):72–83. doi:10.1016/j.foodres.2017.05.002.
- Dodd FL, Kennedy DO, Riby LM, Haskell-Ramsay CF. A double-blind, placebo-controlled study evaluating the effects of caffeine and L-theanine both alone and in combination on cerebral blood flow, cognition and mood. Psychopharmacology (Berl). 2015;232(14):2563–76. doi:10.1007/s00213-015-3895-0.
- Yoto A, Motoki M, Murao S, Yokogoshi H. Effects of L-theanine or caffeine intake on changes in blood pressure under physical and psychological stresses. J Physiol Anthropol. 2012;31(1):28. doi:10.1186/1880-6805-31-28.
- Wightman EL, Haskell CF, Forster JS, Veasey RC, Kennedy DO. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: a double-blind, placebo-controlled, crossover investigation. Hum Psychopharmacol. 2012;27(2):177–86. doi:10.1002/hup.1263.
- Foxe JJ, Morie KP, Laud PJ, Rowson MJ, de Bruin EA, Kelly SP. Assessing the effects of caffeine and theanine on the maintenance of vigilance during a sustained attention task. Neuropharmacology. 2012;62(7):2320–7. doi:10.1016/j.neuropharm.2012.01.020.
- Shen CL, Chyu MC, Pence BC, Yeh JK, Zhang Y, Felton CK, Doctolero S, Wang JS. Green tea polyphenols supplementation and Tai Chi exercise for postmenopausal osteopenic women: safety and quality of life report. BMC Complement Altern Med. 2010;10:76–1076. doi:10.1186/1472-6882-10-76.
- Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, Bluck L, Coward A, Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. 2009;101(6):886–94. doi:10.1017/s0007114508047727.
- Haskell CF, Kennedy DO, Milne AL, Wesnes KA, Scholey AB. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol Psychol. 2008;77(2):113–22. doi:10.1016/j.biopsycho.2007.09.008.
- Kimura K, Ozeki M, Juneja LR, Ohira H. L-Theanine reduces psychological and physiological stress responses. Biol Psychol. 2007;74(1):39–45. doi:10.1016/j.biopsycho.2006.06.006.
- Lu K, Gray MA, Oliver C, Liley DT, Harrison BJ, Bartholomeusz CF, Phan KL, Nathan PJ. The acute effects of L-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum Psychopharmacol. 2004;19(7):457–65. doi:10.1002/hup.611.
- Silvestrini GI, Marino F, Cosentino M. Effects of a commercial product containing guaraná on psychological well-being, anxiety and mood: a single-blind, placebo-controlled study in healthy subjects. J Negat Results Biomed. 2013;12:9. doi:10.1186/1477-5751-12-9.
- Galduróz JC, Carlini EA. The effects of long-term administration of guarana on the cognition of normal, elderly volunteers. Sao Paulo Med J. 1996;114(1):1073–8. doi:10.1590/s1516-31801996000100003.
- Galduróz JC, Carlini EdA Acute effects of the Paulinia cupana, "Guaraná" on the cognition of normal volunteers. Sao Paulo Med J. 1994;112(3):607–11. doi:10.1590/s1516-31801994000300007.
- Bazrafshan MR, Jokar M, Shokrpour N, Delam H. The effect of lavender herbal tea on the anxiety and depression of the elderly: a randomized clinical trial. Complement Ther Med. 2020;50:102393. doi:10.1016/j.ctim.2020.102393.
- Bradley BF, Brown SL, Chu S, Lea RW. Effects of orally administered lavender essential oil on responses to anxiety-provoking film clips. Hum Psychopharmacol. 2009;24(4):319–30. doi:10.1002/hup.1016.
- Saitsu Y, Nishide A, Kikushima K, Shimizu K, Ohnuki K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed Res. 2019;40(4):125–31. doi:10.2220/biomedres.40.125.
- Nagano M, Shimizu K, Kondo R, Hayashi C, Sato D, Kitagawa K, Ohnuki K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed Res. 2010;31(4):231–7. doi:10.2220/biomedres.31.231.
- Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother Res. 2009;23(3):367–72. doi:10.1002/ptr.2634.
- Brooks NA, Wilcox G, Walker KZ, Ashton JF, Cox MB, Stojanovska L. Beneficial effects of Lepidium meyenii (Maca) on psychological symptoms and measures of sexual dysfunction in postmenopausal women are not related to estrogen or androgen content. Menopause. 2008;15(6):1157–62. doi:10.1097/gme.0b013e3181732953.
- Stojanovska L, Law C, Lai B, Chung T, Nelson K, Day S, Apostolopoulos V, Haines C. Maca reduces blood pressure and depression, in a pilot study in postmenopausal women. Climacteric. 2015;18(1):69–78. doi:10.3109/13697137.2014.929649.
- Pribis P. Effects of walnut consumption on mood in young adults-a randomized controlled trial. Nutrients. 2016;8(11):668. doi:10.3390/nu8110668.
- Garrido M, González-Gómez D, Lozano M, Barriga C, Paredes SD, Rodríguez AB. A Jerte valley cherry product provides beneficial effects on sleep quality. Influence on aging. J Nutr Health Aging. 2013;17(6):553–60. doi:10.1007/s12603-013-0029-4.
- Pribis P, Bailey RN, Russell AA, Kilsby MA, Hernandez M, Craig WJ, Grajales T, Shavlik DJ, Sabatè J. Effects of walnut consumption on cognitive performance in young adults. Br J Nutr. 2012;107(9):1393–401. doi:10.1017/s0007114511004302.
- Howatson G, Bell PG, Tallent J, Middleton B, McHugh MP, Ellis J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr. 2012;51(8):909–16. doi:10.1007/s00394-011-0263-7.
- Pigeon WR, Carr M, Gorman C, Perlis ML. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J Med Food. 2010;13(3):579–83. doi:10.1089/jmf.2009.0096.
- Falcone PH, Nieman KM, Tribby AC, Vogel RM, Joy JM, Moon JR, Slayton CA, Henigman MM, Lasrado JA, Lewis BJ, et al. The attention-enhancing effects of spearmint extract supplementation in healthy men and women: a randomized, double-blind, placebo-controlled, parallel trial. Nutr Res. 2019;64:24–38. doi:10.1016/j.nutres.2018.11.012.
- Ostfeld I, Ben-Moshe Y, Hoffman MW, Shalev H, Hoffman JR. Effect of spearmint extract containing rosmarinic acid on physical and executive functioning after a tactical operation. J Spec Oper Med. 2018;18(4):92–6. doi:10.55460/HVYN-6PAG.
- Kennedy D, Okello E, Chazot P, Howes MJ, Ohiomokhare S, Jackson P, Haskell-Ramsay C, Khan J, Forster J, Wightman E. Volatile terpenes and brain function: investigation of the cognitive and mood effects of mentha × piperita l. essential oil with in vitro properties relevant to central nervous system function. Nutrients. 2018;10(8):1029. doi:10.3390/nu10081029.
- Herrlinger KA, Nieman KM, Sanoshy KD, Fonseca BA, Lasrado JA, Schild AL, Maki KC, Wesnes KA, Ceddia MA. Spearmint extract improves working memory in men and women with age-associated memory impairment. J Altern Complement Med. 2018;24(1):37–47. doi:10.1089/acm.2016.0379.
- Falcone PH, Tribby AC, Vogel RM, Joy JM, Moon JR, Slayton CA, Henigman MM, Lasrado JA, Lewis BJ, Fonseca BA, et al. Efficacy of a nootropic spearmint extract on reactive agility: a randomized, double-blind, placebo-controlled, parallel trial. J Int Soc Sports Nutr. 2018;15(1):58. doi:10.1186/s12970-018-0264-5.
- Gelal A, Jacob P, 3rd, Yu L, Benowitz NL. Disposition kinetics and effects of menthol. Clin Pharmacol Ther. 1999;66(2):128–35. doi:10.1053/cp.1999.v66.100455001.
- Chu TT, Benzie IF, Lam CW, Fok BS, Lee KK, Tomlinson B. Study of potential cardioprotective effects of Ganoderma lucidum (Lingzhi): results of a controlled human intervention trial. Br J Nutr. 2012;107(7):1017–27. doi:10.1017/s0007114511003795.
- Kuszewski JC, Howe PRC, Wong RHX. Evaluation of cognitive performance following fish-oil and curcumin supplementation in middle-aged and older adults with overweight or obesity. J Nutr. 2020;150(12):3190–9. doi:10.1093/jn/nxaa299.
- Ataei-Almanghadim K, Farshbaf-Khalili A, Ostadrahimi AR, Shaseb E, Mirghafourvand M. The effect of oral capsule of curcumin and vitamin E on the hot flashes and anxiety in postmenopausal women: a triple blind randomised controlled trial. Complement Ther Med. 2020;48:102267. doi:10.1016/j.ctim.2019.102267.
- Uchio R, Muroyama K, Okuda-Hanafusa C, Kawasaki K, Yamamoto Y, Murosaki S. Hot water extract of curcuma longa L. improves serum inflammatory markers and general health in subjects with overweight or prehypertension/mild hypertension: a randomized, double-blind, placebo-controlled trial. Nutrients. 2019;11(8):1822. doi:10.3390/nu11081822.
- Small GW, Siddarth P, Li Z, Miller KJ, Ercoli L, Emerson ND, Martinez J, Wong KP, Liu J, Merrill DA, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266–77. doi:10.1016/j.jagp.2017.10.010.
- Fanaei H, Khayat S, Kasaeian A, Javadimehr M. Effect of curcumin on serum brain-derived neurotrophic factor levels in women with premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. Neuropeptides. 2016;56(5625):25–31. 31 doi:10.1016/j.npep.2015.11.003.
- Esmaily H, Sahebkar A, Iranshahi M, Ganjali S, Mohammadi A, Ferns G, Ghayour-Mobarhan M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: a randomized controlled trial. Chin J Integr Med. 2015;21(5):332–8. doi:10.1007/s11655-015-2160-z.
- Cox KH, Pipingas A, Scholey AB. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol. 2015;29(5):642–51. doi:10.1177/0269881114552744.
- Roh D, Jung JH, Yoon KH, Lee CH, Kang LY, Lee SK, Shin K, Kim DH. Valerian extract alters functional brain connectivity: A randomized double-blind placebo-controlled trial. Phytother Res. 2019;33(4):939–48. doi:10.1002/ptr.6286.
- Thomas K, Canedo J, Perry PJ, Doroudgar S, Lopes I, Chuang HM, Bohnert K. Effects of valerian on subjective sedation, field sobriety testing and driving simulator performance. Accid Anal Prev. 2016;92:240–4. doi:10.1016/j.aap.2016.01.019.
- Oxman AD, Flottorp S, Håvelsrud K, Fretheim A, Odgaard-Jensen J, Austvoll-Dahlgren A, Carling C, Pallesen S, Bjorvatn B. A televised, web-based randomised trial of an herbal remedy (valerian) for insomnia. PLoS One. 2007;2(10):e1040. doi:10.1371/journal.pone.0001040.
- Jacobs BP, Bent S, Tice JA, Blackwell T, Cummings SR. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine (Baltimore). 2005;84(4):197–207. doi:10.1097/01.md.0000172299.72364.95.
- Gutierrez S, Ang-Lee MK, Walker DJ, Zacny JP. Assessing subjective and psychomotor effects of the herbal medication valerian in healthy volunteers. Pharmacol Biochem Behav. 2004;78(1):57–64. doi:10.1016/j.pbb.2004.02.011.
- Hallam KT, Olver JS, McGrath C, Norman TR. Comparative cognitive and psychomotor effects of single doses of Valeriana officianalis and triazolam in healthy volunteers. Hum Psychopharmacol. 2003;18(8):619–25. doi:10.1002/hup.542.
- Balderer G, Borbély AA. Effect of valerian on human sleep. Psychopharmacology (Berl). 1985;87(4):406–9. doi:10.1007/bf00432503.
- Leathwood PD, Chauffard F, Heck E, Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav. 1982;17(1):65–71. doi:10.1016/0091-3057(82)90264-7.
- O’Connor PJ, Kennedy DO, Stahl S. Mental energy: plausible neurological mechanisms and emerging research on the effects of natural dietary compounds. Nutr Neurosci. 2021;24(11):850–64. doi:10.1080/1028415x.2019.1684688.
- Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med. 2012;52(1):35–45. doi:10.1016/j.freeradbiomed.2011.09.010.
- Fißler M, Quante A. A case series on the use of lavendula oil capsules in patients suffering from major depressive disorder and symptoms of psychomotor agitation, insomnia and anxiety. Complement Ther Med. 2014;22(1):63–9. doi:10.1016/j.ctim.2013.11.008.
- Chaput JP, Dutil C, Featherstone R, Ross R, Giangregorio L, Saunders TJ, Janssen I, Poitras VJ, Kho ME, Ross-White A, et al. Sleep duration and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45(10 (Suppl. 2):S218–s231. doi:10.1139/apnm-2020-0034.
- Sharma S, Kavuru M. Sleep and metabolism: an overview. Int J Endocrinol. 2010;2010:270832. doi:10.1155/2010/270832.
- Scott AJ, Webb TL, Martyn-St James M, Rowse G, Weich S. Improving sleep quality leads to better mental health: A meta-analysis of randomised controlled trials. Sleep Med Rev. 2021;60:101556. doi:10.1016/j.smrv.2021.101556.
- Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–67.
- Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21(12):841–60. doi:10.1016/j.euroneuro.2011.04.002.
- Lieberman HR. Cognitive methods for assessing mental energy. Nutr Neurosci. 2007;10(5-6):229–42. doi:10.1080/10284150701722273.
- Romijn AR, Latulippe ME, Snetselaar L, Willatts P, Melanson L, Gershon R, Tangney CA, Young H. Perspective: advancing dietary guidance for cognitive health—focus on solutions to harmonize test selection, implementation, and evaluation. Adv Nutr. 2023;14(3):366–78. doi:10.1016/j.advnut.2023.03.010.
- Pinu FR, Beale DJ, Paten AM, Kouremenos K, Swarup S, Schirra HJ, Wishart D. Systems biology and multi-omics integration: viewpoints from the metabolomics research community. Metabolites. 2019;9(4):76. doi:10.3390/metabo9040076.