Abstract
Objective
Statin monotherapy for dyslipidemia is limited by adverse effects and limited effectiveness in certain subgroups like metabolic syndrome. Add-on therapy with an agent with a known safety profile may improve clinical outcomes, and virgin coconut oil (VCO) may be the candidate agent for improving the cardiometabolic profile. The present study was conducted to evaluate the effect of add-on VCO with atorvastatin in dyslipidemia in adults.
Methods
A randomized, double-blind clinical trial was conducted on 150 patients with dyslipidemia who were randomized into control and test groups. The control group received atorvastatin monotherapy, whereas the test group received add-on VCO with atorvastatin for 8 weeks. At baseline, demographic, clinical, and biochemical parameters were assessed and repeated after 8 weeks of therapy. The main outcome measures were lipid profile, cardiovascular risk indices, 10-year cardiovascular risk, body fat compositions, and thiobarbituric acid reactive substances (TBARS).
Results
The increase in HDL in the test group was significantly greater than in the control group (MD: 2.76; 95%CI: 2.43–3.08; p < 0.001). The changes in the atherogenic index (p = 0.003), coronary risk index (p < 0.001), cardiovascular risk index (p = 0.001), and TBARS (p < 0.001) were significantly greater in the test group. The decrease in LDL, total cholesterol and lipoprotein(a), were significantly higher in the control group. There were no significant differences between the groups with respect to the changes in triglyceride, VLDL, and 10-year cardiovascular risk.
Conclusions
Add-on VCO (1000 mg/day) with atorvastatin (10 mg/day) can achieve a better clinical outcome in patients with dyslipidemia by increasing HDL and improving oxidative stress cardiovascular risk indices.
1. Introduction
Dyslipidemia is a well-established risk factor for cardiovascular (CV) diseases (Citation1). Lipid abnormalities, including high levels of low-density lipoprotein cholesterol (LDL-C), elevated triglycerides, and low levels of high-density lipoprotein cholesterol (HDL-C), are independent predictors of CV disease (Citation2–4). The National Cholesterol Education Program Adult Treatment Panel III (ATP III) guideline and the American College of Cardiology (ACC) and American Heart Association (AHA) guidelines recommend the use of statins for primary prevention based on a patient’s cardiovascular risk profile and low-density lipoprotein cholesterol (LDL-C) level (Citation5, Citation6). Despite the proven benefits of statin therapy, many patients fail to achieve lipid goals in clinical practice, or significant residual cardiovascular risk persists especially in patients with mixed dyslipidemia and metabolic syndromes (Citation7–12). While the addition of niacin, fibrate, or ezetimibe may be useful in this setting, the combination therapy may lead to more adverse drug reactions (Citation13–15).
Virgin Coconut Oil (VCO), a nutraceutical, contains a considerable amount of medium-chain fatty acids similar to those in mother’s milk (Citation16). The beneficial effects of VCO in the reduction of cardiovascular risk have been proved from previous animal and clinical studies (Citation17). The animal studies by Nevin KG et al., Famurewa et al. and Arunima et al. demonstrated the potential hypolipidemic and antioxidant properties of VCO and was attributed to the biologically active polyphenols (Citation16, Citation18–20). The clinical trial conducted by Cardoso et al. reported that VCO increased HDL-C levels in patients with coronary artery disease (CAD) (Citation21). In another clinical trial, Chinwong et al. concluded that daily consumption of VCO in young, healthy adults significantly increased high-density lipoprotein cholesterol without any safety issues (Citation22). In a recent meta-analysis on 15 trials, Neelakantan et al. found the pooled effect size of increase in HDL and LDL by VCO are significantly higher as compared to tropical oils (Citation23).
Although different animal and human studies showed increase in HDL-C by VCO, the study by Harris et al. and Cardoso et al. also reported increase in total cholesterol, and LDL-C (Citation21, Citation24). These findings have created doubts among physicians regarding the overall hypolipidemic action of VCO when used as monotherapy. In this clinical scenario, we hypothesized that add-on therapy with VCO with first line hypolipidemic agents like statins may improve the clinical outcome in patients with dyslipidemia. Our literature search also revealed that, to date, no clinical trial evaluated the potential of VCO as an add-on hypolipidemic agent in dyslipidemia. So, the present clinical trial was designed with an objective to evaluate the effect of VCO on cardiometabolic parameters as an add-on with statins in adult patients with dyslipidemia.
2. Materials and methods
2.1. Study design
The present study was a randomized, double-blind, parallel design, add-on placebo-controlled clinical trial conducted in a single center. The study followed the ethical guidelines for biomedical research on human participants (2017) from the Indian Council of Medical Research (ICMR), and approval from the institutional ethics committee was obtained. The study has been registered with ClinicalTrials.gov (NCT03906539).
2.2. Study population
Patients attending General Medicine outpatient department of our institute with dyslipidemia were screened and enrollment was done following predefined selection criteria. The recruitment started on 22 May 2019 and was completed on 31 December 2021.
2.2.1. Inclusion criteria
Patients aged >18 years, of either gender with the clinical diagnosis of dyslipidemia [diagnosis of dyslipidemia is made when either of the lipid abnormality is present: LDL-C > 140mg/dL, HDL-C < 40mg/dL, Triglyceride >150mg/dL according to diagnostic criteria of dyslipidemia] (Citation25) were recruited for the study. All the patients were treatment-naive or did not take any treatment for at least 2 wk before inclusion.
2.2.2. Exclusion criteria
Patients with other co-morbidities like ischemic heart disease, heart failure, arrhythmias, stroke, diabetes mellitus, malignancy, musculoskeletal and hepatic diseases or with a history of hypersensitivity to statins or coconut oil or history of alcohol/drug abuse were excluded. Patients who were already under treatment for the presenting conditions, pregnant and nursing women were also excluded from the study.
2.3. Randomization and allocation concealment
The recruited patients were randomized by random block randomization into test group and control group using computer-generated random codes with an allocation ratio of 1:1 by the principal investigator who was not involved in patient recruitment. The VCO or placebo capsules were dispensed by the investigators after collection of baseline samples. The allocation concealment was implemented by supplying sequentially numbered similar looking drug bottles containing capsules of VCO or placebo.
2.4. Study procedure and data collection
After recruitment, detailed history, and clinical evaluations were done and written informed consent was taken. At baseline, serum lipoprotein levels, liver function test, cardiovascular risk indices and 10-year cardiovascular risk were assessed. The recruited patients were randomized into test and control groups using computer-generated algorithms. The patients in control groups received tablet atorvastatin (10 mg/day) and a placebo capsule for VCO. The test group received capsule VCO (1000 mg/day) as an add-on to tablet atorvastatin (10 mg/day). Apart from tab atorvastatin, all the patients in both study groups were advised for lifestyle modification including dietary modification and exercise. As per dietary instructions, the patients were advised a diet plan with a daily calorie intake between 2000 and 2200 Kcal. All patients were followed up after 8 weeks and all parameters were reevaluated. At 4 weeks, a telephonic follow-up was done to assess the occurrence of adverse drug reactions (if any).
2.5. Assessment of medication adherence
All the patients were instructed to bring the drug dispensing container and atorvastatin tablet strips during their follow-up visit at 8 weeks and medication adherence or compliance was measured by pill counting method. A patient was considered adherent if he/she had taken 90% of both prescribed medicines.
2.6. Withdrawal criteria and rescue therapy
As all the recruited patients were drug-naïve, it was decided that a starting dose of 10 mg/day of atorvastatin would be optimal and rational. It was planned to withdraw atorvastatin if any patient reported of any adverse drug reaction related to musculoskeletal system and creatinine Kinase (CK) would be estimated. If CK ≥ 10 times of the upper limit of normal, atorvastatin would be withdrawn, and fibrate (preferably fenofibrate) would be started as rescue medication and those patients were planned to be excluded from the analysis. If CK < 10 times upper limit of normal, atorvastatin was planned to be continued with regular CK monitoring.
2.7. Study outcome measures
Serum Lipoprotein level (LDL, HDL, VLDL), total cholesterol, triglyceride and serum Lipoprotein(a)were estimated by autoanalyzer.
Cardiovascular risk indices were calculated as: Atherogenic index (AI) = LDL cholesterol/HDL cholesterol; Coronary risk index (CRI) = total cholesterol/HDL cholesterol; Cardiovascular risk index (CVRI) = triglyceride/HDL cholesterol.
10-year Cardiovascular risk by using ACC/AHA atherosclerotic cardiovascular disease (ASCVD) risk algorithm (Citation26).
Body fat composition was measured by digital body fat analyzer using bioelectric impedance analysis.
Estimation of lipid peroxidation [thiobarbituric acid reactive substances (TBARS)] was done by spectrofluorometric method (Citation27).
The occurrence of adverse effects (AEs) was sought by the nondirective questioning of the patient at 4 weeks (telephonic follow-up) and at 8-week follow-up visit.
2.8. Statistical analysis
Continuous variables have been represented as a mean ± standard deviation (SD) or median (IQR) and categorical variables as a percentage. Comparison of means between the groups was performed using unpaired t-test/Mann Whitney U test and within the group by two-sided paired t-test/Wilcoxon signed rank test. Fisher’s exact test was used for comparing categorical variables between the groups. Intention to treat (ITT) analysis was conducted by replacing missing values using multiple imputations and the pooled data was used for analysis. SPSS 23.0 (IBM, NY) was used for performing statistical analyses. p < 0.05 will be considered as significant.
2.8.1. Sample size calculation
A sample size of 75 was calculated to achieve 80% power to detect a difference of 3.3 mg/dL in HDL-C between the group means with known group standard deviations of 7.2 and with a significance level (alpha) of 0.05 using a two-sided two-sample t-test.
3. Results
3.1. Patients
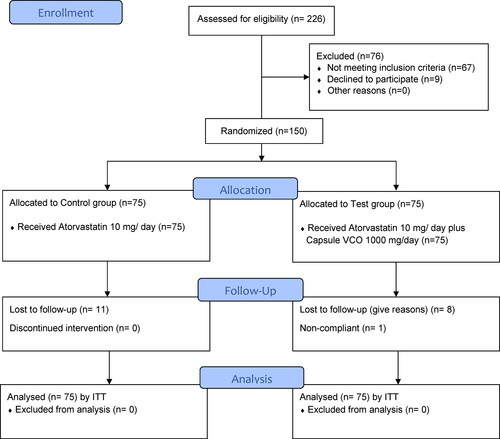
The recruitment was started in May 2019 and was completed in December 2021. Of the 226 patients with dyslipidemia screened, 67 did not meet the selection criteria and another 9 patients declined to participate. Finally, a total of 150 patients were recruited for the study and randomized into control (n = 75, received tablet atorvastatin 10 mg/day) and test (n = 75, received capsule VCO 1000 mg/day as an add-on to tablet atorvastatin 10 mg/day) groups, using computer-generated codes. In the control group, 11 patients and in the test group 8 patients were lost to follow-up. Additionally, one patient in test group was found to be non-compliant. The patients’ enrollment, allocation and follow-up has been depicted in CONSORT flow diagram. () Demographic characteristics and clinical parameters were assessed at baseline, and comparison showed no significant difference between the study groups. () Hence, the study groups were considered homogenous at baseline with minimum ethnic variation in the study population with similar food habits. Out of the total 150 patients 87 patients (58%) were male and 63 patients (42%) were female. In the present study, most of the patients (77% in control group and 76% in test group) belonged to the age group 31–60 years. All patients were assessed for metabolic syndrome as per NCEP ATP III (2005 revision) criteria (Citation28, Citation29) and 101 patients out of 150 (67.3%) were diagnosed to have metabolic syndrome.
Table 1. Baseline demographic and clinical characteristics.
3.2. Primary outcome measure
3.2.1. Change in lipid profile
The changes in lipid profile (LDL, HDL, VLDL, Total cholesterol, triglyceride, Lipoprotein(a) was assessed in individual study groups and compared between the groups. Except Lipoprotein (a), change in all parameters were statistically significant in both the study groups. There was a significant increase in HDL levels in test group when compared to control group (p < 0.001). The decrease in control group was greater than test group for LDL (p = 0.004) and total cholesterol (<0.001). There was no significant difference in change in triglyceride levels (p = 0.147) and VLDL (p = 0.145). ()
Table 2. Change in Lipid profile in study groups (intention-to-treat analysis).
3.3. Secondary outcome measures
3.3.1. Change in cardiovascular risk indices
Three cardiovascular indices (atherogenic index, coronary risk index, cardiovascular risk index) were calculated from LDL, HDL, Total cholesterol and triglyceride values. All indices were found to be decreased after the therapy in both study arms. When the mean changes in both the groups were compared, it was found that the improvements were greater in the test groups (AI: p = 0.003; CRI: p < 0.001; CVRI: p = 0.001) who received add-on VCO. ()
Table 3. Change in cardiovascular risk indices and 10-year cardiovascular risk in study groups (intention-to-treat analysis).
3.3.2. Change in 10-year cardiovascular risk
ACC/AHA atherosclerotic cardiovascular disease (ASCVD) risk algorithm has been used to calculate 10-year cardiovascular risk. In both the study groups, the change in 10-years cardiovascular risk decreased significantly, however, the difference in change between the groups was not statistically significant (p = 0.557). ()
3.3.3. Change in body fat composition
The change in body fat composition has been evaluated by bioelectrical impedance analysis (BIA). The visceral fat, total body fat, whole body subcutaneous fat, whole body skeletal muscle, subcutaneous trunk fat and trunk skeletal muscle have been measured and the results showed that there was no significant change in either of the parameters over the study period in both study groups (supplemental Table S1).
3.3.4. Change in Thiobarbituric Acid Reactive Substances (TBARS)
Thiobarbituric Acid Reactive Substances (TBARS) were measured by spectroflurometric method at baseline and follow-up at 8 wk. The changes in both control and test group individually were statistically significant (p < 0.001) and also found to be significant (p < 0.001) when the mean change between the groups were compared (supplemental Table S2).
3.3.5. Regression analysis
A binary logistic regression was performed to ascertain the effect of different covariates like age, sex, BMI, blood pressure, HDL, LDL, VLDL, TG, Lp(a), TBARS, visceral fat, total body fat, subcutaneous whole-body fat, whole-body skeletal muscle, trunk subcutaneous fat, trunk skeletal muscle on the presence or absence of metabolic syndrome. The logistic regression model was found to be statistically significant (Chi-square = 50.64, df = 4, p < 0.001) with baseline diastolic blood pressure, HDL, and serum triglyceride, skeletal muscle trunk) as independent variable. The model can be used to explain the 39.9% variation (Nagelkerke R2=0.399) and correctly classifies 81.3% of cases for the presence or absence of metabolic syndrome. The standardized beta coefficient and pvalue for the independent variables are mentioned in supplemental Table S3.
3.3.6. Evaluation of adverse drug reactions
No adverse reaction was reported during the study period from either of the study groups.
4. Discussion
In the present study, the primary outcome measure of the present study was to evaluate the effect of add-on virgin coconut oil on the change in different lipoprotein fractions. The change in HDL found in the present study is similar to the findings in the previous human studies by Chinwong et al., Cardoso et al., and Harris et al. (Citation21, Citation22, Citation24) Cardoso et al. observed that in patients with coronary artery disease, a diet high in coconut extra virgin oil raises HDL cholesterol, lowers waist circumference and body mass (Citation21). There was a trend toward increase in triglycerides in test group when compared to control group in our study, though the difference was not significant. When examining the effects of saturated fats on metabolic risk factors, distinction should be made based on chain length (Citation30). It is to be reiterated that virgin coconut oil is portrayed to increase medium chain triglycerides (MCT) which enhances the activation of the pathways for lipid and glucose metabolism as well as mitochondrial biogenesis in mice (Citation31, Citation32). Considering the overall picture of the lipid profile, the present study has found that the add-on VCO has achieved a better control of dyslipidemia profile in comparison to the monotherapy with atorvastatin.
The present study evaluated different cardiovascular risk indices like atherogenic index, coronary risk index and cardiovascular risk index which are derived parameters from the label of different lipoproteins and triglyceride. Improvement in all three cardiovascular risk indices were found to be significantly greater in test group in comparison to the control group. The present study also evaluated 10-year cardiovascular risk by using ACC/AHA atherosclerotic cardiovascular disease (ASCVD) risk algorithm and was found to decrease in both groups significantly, however, there was no significant difference between the groups. Hence, it can be concluded that the better control of lipid profile with add-on VCO may be translated to a significant decrease in different cardiovascular risk indices. This effect may be due to increase in HDL as well as MCTs which are already known to be protective for heart. Dyslipidemia is a well-established risk factor for cardiovascular diseases and the beneficial effects of VCO in reduction of cardiovascular risk have been proved from previous animal and clinical studies. The results from our study also support the previous studies in respect to the beneficial effect of add-on VCO in better control of lipid profile and reduction of cardiovascular risk.
In this study, the decrease in visceral fat in the test group was more than the control group, however, the difference was not statistically significant. The follow-up duration in the study was 8 wk and probably change in body fat composition takes longer time to reflect a significant change. The previous preclinical studies by Adeyemi et al., Famurewa et al. and Nevin et al. found antioxidant property of VCO (Citation16, Citation18, Citation33, Citation34). In the present study, the change in TBARS has been evaluated and the decrease in test group was significantly greater than control group. VCO preserves higher levels of biologically active unsaponifiable components such polyphenols and tocopherol and thus has a positive impact in enhancing antioxidant status and preventing the oxidation of lipids and proteins (Citation35). VCO demonstrates antioxidant and anti-inflammatory effects via regulation of TLR4/MAPK pathway and modulating TLR/NF-κB signaling pathways (Citation36, Citation37). A longer duration study would have been able to detect the changes in body fat compositions.
Atorvastatin is an established hypolipidemic agent and can improve the lipid profile when used as monotherapy. However, the long-term therapy with atorvastatin may lead to adverse drug reactions. The present study found that add-on VCO may achieve an additive effect with atorvastatin and lead to a better clinical outcome. The use of add-on VCO with atorvastatin may help by decreasing the requirement of dose increment of atorvastatin leading to less chance of adverse drug reactions.
To the best of our knowledge, this is the first randomized controlled trial on the effect of VCO on cardiometabolic parameters in dyslipidemia. The main strength of this study lies in its robust study design with adequate power and sample size, good compliance and follow-up of the patients. The important limitation of the study is its short follow-up period, because to detect an appreciable change in body fat composition, and 10-year cardiovascular risk, a longer follow-up period is required. Secondly, being a single center study, the generalizability of the results may be limited. Additionally, dyslipidemia being often presented with other co-morbid conditions like diabetes mellitus and hypertension, a broader inclusion criterion with more pragmatic approach would have increased the applicability of the results. Lastly, polyphenol levels could have been quantified to substantiate the evidence for the mechanism of action of VCO.
In conclusion, add-on VCO with atorvastatin can achieve a more favorable lipid profile by increasing HDL and medium chain triglycerides. The cardiovascular risk indices improve significantly with add-on VCO in comparison to atorvastatin monotherapy. The physicians may consider prescribing add-on VCO with atorvastatin to keep the dose requirement of statins at lower level and minimize potential adverse drug reactions. Though we are aware of the debate over the beneficial cardiovascular effect of VCO, the results of present study give provide an indication toward the favorable impact. In future, multicentric clinical trials with longer duration of follow-up may be conducted to establish the findings and to assess the effectiveness and safety of add-on VCO in dyslipidemia.
Author contributions
RM: conception and design of the work; the acquisition, analysis, and interpretation of data, drafting of the manuscript, final approval of the version to be published.
RRM: conception and design of the work; acquisition of data; critical revision of the manuscript; final approval of the version to be published.
AD: conception and design of the work; acquisition of data; critical revision of the manuscript; final approval of the version to be published.
SM: acquisition, analysis, and interpretation of data, drafting of the manuscript; final approval of the version to be published.
MP: design of the work; acquisition of data; drafting of the manuscript; final approval of the version to be published.
AM: acquisition, analysis, and interpretation of data, critical revision of the manuscript; final approval of the version to be published.
Ethical approval
The present study was conducted following ICMR’s ethical guidelines for biomedical research on human subjects (2017) after obtaining written approval from the institutional ethics committee (T/EMF/Pharma/18/23). The study has been registered with ClinicalTrials.gov with ID NCT03906539.
Informed consent
Written informed consent has been obtained from all subjects who participated in this study.
Supplemental Material
Download MS Word (26.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi:10.1016/S0140-6736(04)17018-9.
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi:10.1161/01.cir.79.1.8.
- Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw K-T, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–8. doi:10.1161/CIRCULATIONAHA.106.637793.
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–16. doi:10.1001/jama.298.3.309.
- Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ, American Heart Association, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39. doi:10.1161/01.CIR.0000133317.49796.0E.
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi:10.1161/01.cir.0000437738.63853.7a.
- Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med. 2000;160(4):459–67. doi:10.1001/archinte.160.4.459.
- Pearson TA. The undertreatment of LDL-cholesterol: addressing the challenge. Int J Cardiol. 2000;74 Suppl 1(1):S23–S8. doi:10.1016/s0167-5273(99)00108-4.
- Velarde GP, Choudhary N, Bravo-Jaimes K, Smotherman C, Sherazi S, Kraemer DF. Effect of atorvastatin on lipogenic, inflammatory and thrombogenic markers in women with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2021;31(2):634–40. doi:10.1016/j.numecd.2020.10.002.
- Deedwania P, Murphy SA, Scheen A, Badariene J, Pineda AL, Honarpour N, Keech AC, Sever PS, Pedersen TR, Sabatine MS, et al. Efficacy and safety of PCSK9 inhibition with evolocumab in reducing cardiovascular events in patients with metabolic syndrome receiving statin therapy: secondary analysis from the FOURIER randomized clinical trial. JAMA Cardiol. 2021;6(2):139–47. doi:10.1001/jamacardio.2020.3151.
- Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14(1):1–10. doi:10.1007/s11883-011-0219-7.
- Ridker PM, Genest J, Boekholdt SM, Libby P, Gotto AM, Nordestgaard BG, Mora S, MacFadyen JG, Glynn RJ, Kastelein JJP, et al. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 2010;376(9738):333–9. doi:10.1016/S0140-6736(10)60713-1.
- Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409–15. doi:10.1161/01.CIR.0000068312.21969.C8.
- Davidson MH, Rosenson RS, Maki KC, Nicholls SJ, Ballantyne CM, Mazzone T, Carlson DM, Williams LA, Kelly MT, Camp HS, et al. Effects of fenofibric acid on carotid intima-media thickness in patients with mixed dyslipidemia on atorvastatin therapy: randomized, placebo-controlled study (FIRST). Arterioscler Thromb Vasc Biol. 2014;34(6):1298–306. doi:10.1161/ATVBAHA.113.302926.
- McKenney JM. Combination treatment with atorvastatin plus niacin provides effective control of complex dyslipidemias: a literature review. Postgrad Med. 2012;124(1):7–20. doi:10.3810/pgm.2012.01.2513.
- Famurewa AC, Ekeleme-Egedigwe CA, Nwali SC, Agbo NN, Obi JN, Ezechukwu GC. Dietary supplementation with virgin coconut oil improves lipid profile and hepatic antioxidant status and has potential benefits on cardiovascular risk indices in normal rats. J Diet Suppl. 2018;15(3):330–42. doi:10.1080/19390211.2017.1346031.
- Babu AS, Veluswamy SK, Arena R, Guazzi M, Lavie CJ. Virgin coconut oil and its potential cardioprotective effects. Postgrad Med. 2014;126(7):76–83. doi:10.3810/pgm.2014.11.2835.
- Famurewa AC, Ejezie FE. Polyphenols isolated from virgin coconut oil attenuate cadmium-induced dyslipidemia and oxidative stress due to their antioxidant properties and potential benefits on cardiovascular risk ratios in rats. Avicenna J Phytomed. 2018;8(1):73–84.
- Arunima S, Rajamohan T. Virgin coconut oil improves hepatic lipid metabolism in rats–compared with copra oil, olive oil and sunflower oil. Indian J Exp Biol. 2012;50(11):802–9.
- Nevin KG, Rajamohan T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin Biochem. 2004;37(9):830–5. doi:10.1016/j.clinbiochem.2004.04.010.
- Cardoso DA, Moreira AS, de Oliveira GM, Raggio Luiz R, Rosa G. A coconut extra virgin oil-rich diet increases Hdl cholesterol and decreases waist circumference and body mass in coronary artery disease patients. Nutr Hosp. 2015;32(5):2144–52.
- Chinwong S, Chinwong D, Mangklabruks A. Daily consumption of virgin coconut oil increases high-density lipoprotein cholesterol levels in healthy volunteers: a randomized crossover trial. Evid Based Complement Alternat Med. 2017;2017:7251562–8. doi:10.1155/2017/7251562.
- Neelakantan N, Seah JYH, van Dam RM. The effect of coconut oil consumption on cardiovascular risk factors: a systematic review and meta-analysis of clinical trials. Circulation. 2020;141(10):803–14. doi:10.1161/CIRCULATIONAHA.119.043052.
- Harris M, Hutchins A, Fryda L. The impact of virgin coconut oil and high-oleic safflower oil on body composition, lipids, and inflammatory markers in postmenopausal women. J Med Food. 2017;20(4):345–51. doi:10.1089/jmf.2016.0114.
- Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, Daida H, Biro S, Hirobe K, Funahashi T, et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14(4):155–8. doi:10.5551/jat.e537.
- Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. 2014doi:10.1161/01.cir.0000437741.48606.98.
- Yahyavi H, Kaykhaii M, Hashemi M. A rapid spectrofluorimetric method for the determination of malondialdehyde in human plasma after its derivatization with thiobarbituric acid and vortex assisted liquid–liquid microextraction. RSC Adv. 2016;6(3):2361–7. doi:10.1039/C5RA22079C.
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231–7. doi:10.1242/dmm.001180.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi:10.1161/CIRCULATIONAHA.105.169404.
- St-Onge MP, Bosarge A, Goree LL, Darnell B. Medium chain triglyceride oil consumption as part of a weight loss diet does not lead to an adverse metabolic profile when compared to olive oil. J Am Coll Nutr. 2008;27(5):547–52. doi:10.1080/07315724.2008.10719737.
- Jadhav HB, Annapure US. Triglycerides of medium-chain fatty acids: a concise review. J Food Sci Technol. 2023;60(8):2143–52. doi:10.1007/s13197-022-05499-w.
- Wang Y, Liu Z, Han Y, Xu J, Huang W, Li Z. Medium Chain Triglycerides enhances exercise endurance through the increased mitochondrial biogenesis and metabolism. PLoS One. 2018;13(2):e0191182. doi:10.1371/journal.pone.0191182.
- Adeyemi WJ, Olayaki LA, Abdussalam TA, Toriola AP, Olowu AB, Yakub AJ, Raji AO. Investigation of the effects of dietary modification in experimental obesity: low dose of virgin coconut oil has a potent therapeutic value. Biomed Pharmacother. 2020;126:110110. doi:10.1016/j.biopha.2020.110110.
- Nevin KG, Rajamohan T. Influence of virgin coconut oil on blood coagulation factors, lipid levels and LDL oxidation in cholesterol fed Sprague–Dawley rats. e-SPEN. 2008;3(1):e1–e8. doi:10.1016/j.eclnm.2007.09.003.
- Arunima S, Rajamohan T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats - a comparative study. Food Funct. 2013;4(9):1402–9. doi:10.1039/c3fo60085h.
- Chen X, Kim DI, Moon HG, Chu M, Lee K. Coconut oil alleviates the oxidative stress-mediated inflammatory response via regulating the mapk pathway in particulate matter-stimulated alveolar macrophages. Molecules. 2022;27(9):2898. doi:10.3390/molecules27092898.
- Jose SP, K IM, Ratheesh M, Asha S, Sandya S, Rajmohan V. Polyphenolic fraction of virgin coconut oil inhibits the inflammatory response in oxidized LDL activated human peripheral blood mononuclear cells by modulating TLR/NF-κB signaling pathways. Eur J Integr Med. 2017;10:59–65. doi:10.1016/j.eujim.2017.01.004.