Abstract
The aim of this meta-analysis was to investigate the association between the use of glucocorticoids and the mortality of covid-19 patients. Trials were identified through a comprehensive systematic search of four medical databases including PubMed, Cochrane Library, Web of Science, and Embase. We also searched for relevant papers using the Google search engine and major Preprint platforms including Medrix, bioRxiv, and SSRN. A meta-analysis was performed on the pooled results of these studies. Fourteen studies were enrolled. Five studies were Chinese, and nine were foreign. In the foreign studies, we found use of steroids was associated with a decrease in mortality (RR, 0.81; 95% CI 0.68–0.97; p = 0.02) whilst in the Chinese studies the use of steroids was associated with an increase in mortality (RR, 1.70; 95% CI 1.10–2.63; p = 0.02). The foreign studies included high-dose and medium-dose groups. The medium-dose glucocorticoid group (0.5 mg/kg/d ≤ Prednisone ≤ 1.0 mg/kg/d) showed an association with decreased mortality of covid-19 patients (RR 0.86; 95% CI 0.75–1.00; p = 0.05). We also found an association with decreasing mortality of covid-19 patients (RR 0.65; 95% CI 0.43–0.98; heterogeneity p = 0.04) in patients treated for ≤ five days. In summary, this meta-analysis demonstrated that the use of a medium dose of glucocorticoids for a short time is likely to decrease mortality, although this needs clinical confirmation.
PUBLIC INTEREST STATEMENT
The aim of this meta-analysis was to investigate the association between the use of glucocorticoids and the mortality of covid-19 patients. We did a comprehensive systematic search of four medical databases including PubMed, Cochrane Library, Web of Science, Embase, and major preprint platforms including Medrix, bioRxiv, and SSRN. Fourteen studies were enrolled. We demonstrated a positive correlation between survival of covid-19 patients and use of steroids: a medium dose use of glucocorticoids for a short time being more likely to decrease mortality. At the same time, giving a medium dose use of steroids for a short time has little risk of side effects for covid-19 patients. But, questions such as indication, time of onset and duration of steroids need to be better clarified in well-designed quantitative trials.
1. Introduction
In mid-December 2019, patients with infectious pneumonia of unknown pathogen appeared successively in Wuhan City, Hubei Province, China. The main clinical manifestations were fever ≥38°C, dry cough, low or normal white blood cell count and low lymphocyte count, and was given the name novel coronavirus (2019-nCoV)–infected pneumonia (NCIP) (Li et al., Citation2020). Subsequently, the epidemic spread rapidly to the whole of China and other countries in the world. At present, the main clinical treatment measures include: 1) Symptomatic and supportive treatment such as mechanical ventilation. 2) Antiviral treatment: antiviral drugs, antimalarials, interferons, although no specific drugs for the new coronavirus have been found (Yan et al., Citation2020). 3) Glucocorticoids, which can inhibit the inflammatory reaction (Russell et al., Citation2020; Zhao et al., Citation2020). According to some reports, due to anti-inflammatory effects, glucocorticoids can quickly improve lung injury, improve oxygenation, and thereby reduce mortality, which is beneficial to the prognosis of viral pneumonia (Shang et al., Citation2020; Sterne et al., Citation2020). For patients with progressive deterioration of oxygenation indicators, rapid imaging progress, and excessive activation of the body’s inflammatory response, glucocorticoids should be used in a short period of time (3–5 days), and the recommending dose should not be over the equivalent of 1–2 mg/kg/day of methylprednisolone. Other studies suggest that the use of glucocorticoids will increase the chance of nosocomial infection in patients and prolong the clearance time of the virus, resulting in an increase in the mortality of patients with viral pneumonia (Russell et al., Citation2020; Zhao et al., Citation2020). Even WHO strongly recommends the use of systemic corticosteroids in patients with severe and critical COVID-19 while not recommending to use corticosteroids in patients with non-severe COVID-19 infection (https://wenku.baidu.com/view/920053946394dd88d0d233d4b14e852459fb3902.html 2021). However, the use of glucocorticoids still remains extremely controversial for viral pneumonia including COVID-19. Whether glucocorticoids can be used as an adjuvant treatment for viral pneumonia is inconclusive. Therefore, we conducted a literature search on the efficacy of glucocorticoids for the adjuvant treatment of new coronavirus pneumonia, and screened relevant available literature (Chen et al., Citation2020; Deng et al., Citation2020; Dequin et al., Citation2020; Edalatifard et al., Citation2020; Horby et al., Citation2021; Jeronimo et al., Citation2021; Keller et al., Citation2020; Nelson et al., Citation2021; Ramiro et al., Citation2020; Tomazini et al., Citation2020; Wu et al., Citation2020; https://wenku.baidu.com/view/920053946394dd88d0d233d4b14e852459fb3902.html 2020; Yang et al., Citation2020; Zhou et al., Citation2020) for meta-analysis to explore its impact on the prognosis of viral pneumonia.
2. Material and methods
2.1. Search strategy
Trials were identified through a comprehensive systematic search of four medical databases including PubMed, Cochrane Library, Web of Science, and Embase. We also searched relevant papers using the Google search engine and major Preprint platforms including Medrix, bioRxiv, and SSRN. The search strategy used the terms “2019 novel coronavirus disease” OR “COVID19” OR “COVID-19 pandemic” OR “SARS-CoV-2 infection” OR “COVID-19 virus disease” OR “2019 novel coronavirus infection” OR “2019-nCoV infection” OR “coronavirus disease 2019” OR “coronavirus disease-19” OR “2019-nCoV disease” OR “COVID-19 virus infection” AND “glucocorticoid” OR “adrenal cortex hormones” OR “betamethasone valerate” OR “glucocorticoids” OR “methylprednisolone” OR “Cortisone” OR “adrenal cortex hormones” OR “betamethasone valerate” OR “Dexamethasone” OR “Cortodoxone” OR “Hydrocortisone”. No language restriction or publication status criteria were set. Reference lists of relevant articles were also screened for eligible studies. The last search was performed on December 5, 2020.
2.2. Inclusion and exclusion criteria
Two researchers, ZE and LY, individually screened manuscripts for potentially qualifying studies. Another investigator, HD, checked the results, and differences were resolved by consensus. The inclusion criteria were as follows: 1) Randomized controlled trials (RCTs), cohort studies, case–control studies, and cross-sectional studies. 2) Study settings and patient characteristics were provided. 3) Detailed data on glucocorticoids and outcomes were available. Results refer to all-cause mortality at the end of follow-up for each study. The exclusion criteria were as follows: (1) duplicate reports and (2) preliminary studies including patient groups that overlapped with later reports.
2.3. Definition of intervention and outcomes
We took the all-cause mortality at the end of follow-up of each study as the main result. A pharmacological intervention was considered to be a situation where the patient received at least glucocorticoids and/or other antiviral drugs. According to the research definition, a meta-analysis was performed to evaluate the correlation between intervention effects and patient outcomes.
2.4. Data extraction
Standard strategies were used to extract the following data from each study: study characteristics (authors, publication date, study design, follow-up duration, and sample size), participants (age and gender), dose of COVID 19 patients receiving glucocorticoid therapy, duration, and results (all-caused ethics). The data were extracted by two researchers (ZE and LY) and screened separately by a third researcher (HD).
2.5. Quality assessment
An independent researcher, DY, used the Newcastle–Ottawa quality assessment scale to assess study quality and risk of bias of retrospective studies. According to this scale, the number of stars indicates the quality of the research. 1 to 3 stars are considered low-quality; 4 to 6 stars, medium-quality; and 7 to 9 stars, high-quality. The improved Jadad score (7 points) was used to evaluate the quality of RCTs, and the classification criteria were high quality (6–7 points), medium quality (4–5 points) and low quality (1–3 points). Two investigators independently conducted quality assessment, and the third investigator (WL) checked the results and resolved any disagreements.
2.6. Statistical analysis
Statistical analyses were performed using Review Manager 5.4 software to synthesize data and generate a forest map, and calculate the odds ratio (OR) and 95% confidence interval (CI) of the research results. The total effect and subgroup analysis were used with the Z test. If P < 0.05, the difference was statistically significant. Heterogeneity of the included studies was assessed using the I2 statistic. This study uses a random effects model for analysis. Sensitivity analysis was carried out by excluding documents one by one or changing the effect models and methods. The publication bias detection uses Review Manager5.4 to draw a funnel chart for analysis (Figure ). According to the time and dose of glucocorticoid use, a subgroup analysis and sensitivity analysis plan was proposed. If more than 10 studies were included, a funnel chart was used to assess publication bias. A 2-sided P value less than 0.05 was considered significant.
3. Results
Search results and study selection
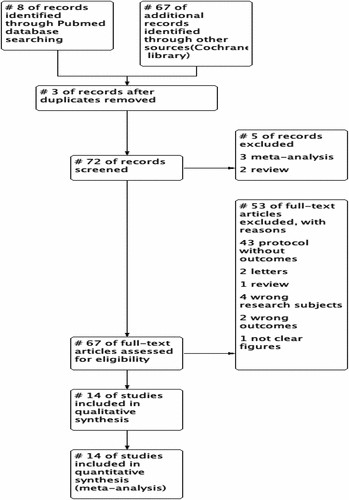
The flowchart of research selection is shown in Figure . We identified 75 references through the initial database queries and manual searches. Among them, three were excluded as duplicates and five were deleted after the title and abstract were posted. In the end, 67 articles were included into full-text review. Fifty three studies were deleted for the following reasons: 43 protocols with no results; two letters; one review article; four involving irrelevant research topics; two with irrelevant results and one with no clear numbers. Fourteen studies that provided data and results of patient medication were included in the systematic review and meta-analysis.
Characteristics of the included studies
The main characteristics of the selected studies are shown in Table . In total they include 10,954 patients. Five retrospective studies, comprising 943 patients, are from China; the non-Chinese studies (9812 patients) are six RCTs, one prospective historically controlled comparison study, and two observational studies. The types of glucocorticoids are given in the RCTs, but not in the retrospective studies (Chen et al., Citation2020; Deng et al., Citation2020; Dequin et al., Citation2020; Edalatifard et al., Citation2020; Horby et al., Citation2021; Jeronimo et al., Citation2021; Keller et al., Citation2020; Nelson et al., Citation2021; Ramiro et al., Citation2020; Tomazini et al., Citation2020; Wu et al., Citation2020; https://wenku.baidu.com/view/920053946394dd88d0d233d4b14e852459fb3902.html 2020; Yang et al., Citation2020; Zhou et al., Citation2020).
Table 1. Characteristics of included studies
Study quality and publication bias
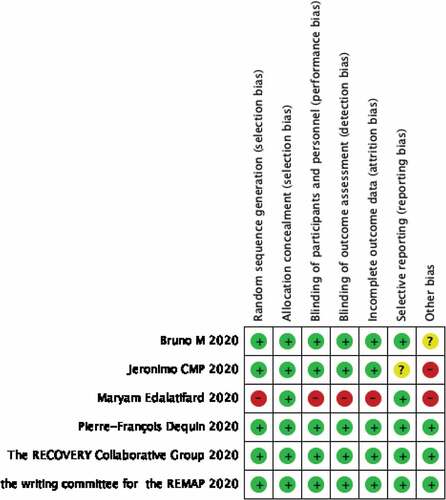
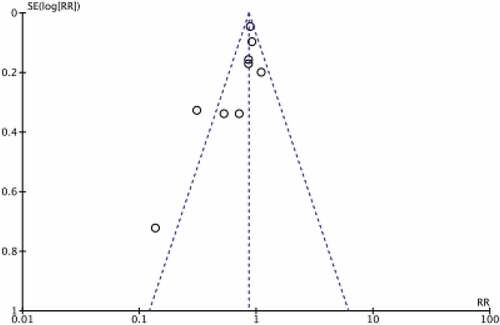
The evaluation quality of the included studies is shown in Figure . The Newcastle–Ottawa quality assessment scale was used to assess study quality and risk of bias for retrospective studies. These are shown in Table . The scale consists of three elements (selection, comparability, and exposure) and is covered by eight items. According to this scale, the number of stars was used to evaluate study quality. A total of four stars can be awarded for selection, two for comparability, and three for exposure. Studies were categorized as low quality (1–3 stars); moderate quality (4–6 stars); and high quality (7–9 stars>). The modified Jadad score (7 points) was used to assess the quality of RCTs, with classification criteria of high quality (6–7 points), moderate quality (4–5 points), and low quality (1–3 points). The publication bias is shown in Figure . In Figure from Funnel plot for publication bias detection, It showed that there existed little publication bias. Because most of those studies with large clinical samples were the upper area of funnel plot, which represented little publication bias.
Table 2. The study quality and risk of bias for retrospective studies
3.0.1. Sensitivity analysis
We found the heterogeneity of the Chinese studies to be 79% and we executed sensitivity analysis by omitting single studies one after another. The heterogeneity was reduced to 0% (P < 0.00001) after omitting Yang et al. (Citation2020). This study is the main reason for heterogeneity in the Chinese group. The heterogeneity in the western group was 63% which decreased to 38% (P = 0.07) after omitted Ramiro et al., (Citation2020), which represents the origin of heterogeneity in the western group. Sensitivity analysis is carried out by excluding studies one by one. Every time one study was excluded, the heterogeneity changed. In this way, we could find which study affected heterogeneity of those studies mostly.
3.0.2. Main effect
Of the 14 studies, five are studies from China. They did not give the type and time of use of glucocorticoids because they were retrospective studies. The other seven studies were prospective studies, and two were retrospective cohort studies. Their characteristics are shown in Table which also shows what type of glucocorticoids they used and time of treatment.
Table 3. The specific characteristics of nine prospective and retrospective cohort studies (excluding studies from China)
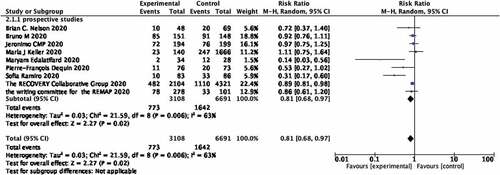
The use of glucocorticoids and mortality of covid-19 patients in studies from foreign countries Figure provides a summary of the results of a meta-analysis on the relationship between glucocorticoid use and mortality in covid-19 patients. There are nine trials that show an association between glucocorticoid use and mortality in covid-19 patients. In the nine studies, the crude death rate of covid-19 patients was 24.87% in the glucocorticoid group (773 out of 3108 patients) and 24.54% in the non-steroid group (1642 out of 6691 patients). In the cohort studies group from abroad, we found a significant association between use of steroid and decreasing mortality of covid-19 patients (RR, 0.81; 95% CI 0.68–0.97; p = 0.02; Figure ). High heterogeneity was observed (I2 = 63%).
Figure 3. Forest plot of the relationship between the use of glucocorticoids and mortality rate of covid-19 patients in studies from western countries.
The use of glucocorticoids and mortality of covid-19 patients in studies from China
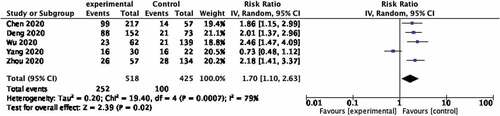
In this group, we found an association between use of steroid and increasing mortality of covid-19 patients (RR, 1.70; 95% CI 1.10–2.63; p = 0.0007; Figure ). High heterogeneity was observed (I2 = 79%).
Figure 4. Forest plot of the relationship between the use of glucocorticoids and mortality rate of covid-19 patients in studies from China.
Mortality of covid-19 patients associated with the different doses of glucocorticoids
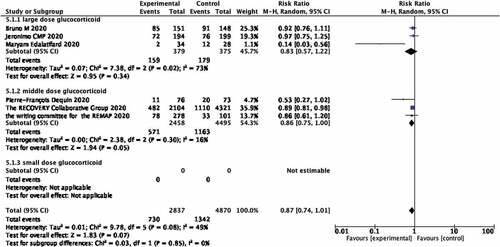
Since the RCT studies involved different steroid usage, subgroup analyses were performed next for different doses. According to clinical guidelines for glucocorticoids in China (https://wenku.baidu.com/view/920053946394dd88d0d233d4b14e852459fb3902.html), three RCTs assessed high-dose glucocorticoid (Prednisone >1.0 mg/kg/d) whilst three RCTs assessed medium dose glucocorticoid (0.5 mg/kg/d ≤ Prednisone ≤ 1.0 mg/kg/d). Use of high dose glucocorticoids was not related to a decrease in mortality of covid-19 patients (RR 0.83; 95% CI 0.57–1.22; p = 0.34; Figure ) whilst the middle dose glucocorticoid group showed an association with decreased mortality of covid-19 patients (RR 0.86; 95% CI 0.75–1.00; p = 0.05; Figure ). No studies used low dose glucocorticoid.
Figure 5. Forest plot of the association between the different doses of glucocorticoids and mortality rate of covid-19 patients in RCTs studies.
Mortality of covid-19 patients associated with the different types of studies
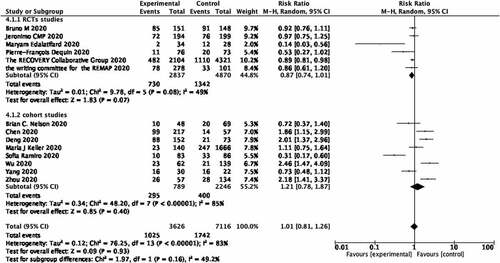
The research studies were divided into two groups according to the types of study; RCTs and cohort studies. In RCTs, the use of glucocorticoids may decrease the mortality of covid-19 patients, which did not show a statistical difference (RR 0.87; 95% CI 0.74–1.01; heterogeneity I2 = 49%; p = 0.07; Figure ). In the Cohort studies, the use of glucocorticoids may increase the mortality of covid-19 patients, which also did not show a statistical difference (RR 1.21; 95% CI 0.78–1.87; heterogeneity I2 = 85%; p = 0.40; Figure ).
Figure 6. Forest plot of the relationship between the use of glucocorticoids and mortality rate of covid-19 patients in different type of studies.
Mortality of covid-19 patients associated with the different time of using glucocorticoids
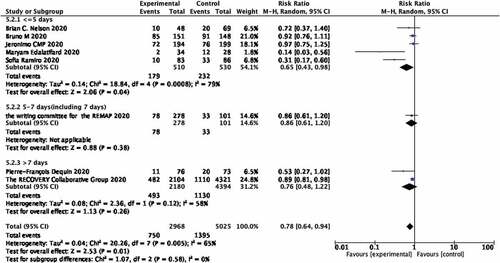
After checking all times of using steroids, we divided research studies clearly showing the time of glucocorticoid use into three groups (≤5 days, >5 and ≤7 days, >7 days). Five studies belonged to the ≤5 days group, which showed an association with decreasing mortality of covid-19 patients (RR 0.65; 95% CI 0.43–0.98; heterogeneity I2 = 79%; p = 0.04; Figure ). There was only one study each in the >5 and ≤7 days groups which showed no relationship with the decrease mortality of covid-19 patients (RR 0.86; 95% CI 0.61–1.20; p = 0.38; Figure ). There were two studies in the >7 days group, which also did not show a relationship with the decrease mortality of covid-19 patients (RR 0.76; 95% CI 0.48–1.22; p = 0.26; Figure ).
4. Discussion
At present, 2019-nCoV is still epidemic worldwide with increasing numbers of deaths and with no specific anti-virus drug therapy. The treatments for COVID-19 patients are mainly supportive treatments. 2019-nCoV is likely to cause an inflammatory cytokine storm, and therefore REACT (Sterne et al., Citation2020) holds the view that the effect of steroids is to inhibit the inflammation, quickly improving lung injury, improving oxygenation, and thereby reduce mortality. Zhao et al. (Citation2020) concluded an opposing view that the use of glucocorticoids will increase the chance of nosocomial infection in patients and prolong the clearance time of the virus, resulting in an increase in the mortality of patients with viral pneumonia. Interestingly, the foreign and China have different views about this topic, which suggests that the use of steroids is controversial. We have found that in Chinese studies, steroids increase mortality of covid-19 patients, whilst steroids decrease mortality in foreign studies. Whether the use of glucocorticoids will decrease the mortality of covid-19 patients therefore needs further discussion.
Why would results be so different between Chinese studies and non-Chinese studies? We think that primarily the types of those studies contribute to the final results. In the Chinese studies, all studies are retrospective studies with small number of patients and lacked a strict and organized design. Second, those studies were published at the early time of the emergence of SARS-CoV-2, and some treatments were limited at that time. The glucocorticoid treatments were not normative. In addition to this, in Chinese retrospective studies, patients received multiple treatments, for example, some patients received anti-virus treatment, Chinese medicine and also glucocorticoid treatments. We cannot therefore know which treatment worked successfully in those patients. Another important reason is that in the Chinese retrospective studies the starting time for using glucocorticoids and the covid-19 stages at which glucocorticoids were used are different. In addition, there is no record for what kind of glucocorticoids and the dose of steroids they used. This may lead to the view that the use of glucocorticoids to covid-19 patients will increase the mortality.
Regarding the foreign studies, most of them conclude steroids will decrease the mortality of covid-19 patients. The REMAP-CAP Researcher Writing Committee (https://doi.org/10.1001/jama.2020.17022 2020) concluded that compared with not using hydrocortisone, treatment with a 7-day fixed-dose course of hydrocortisone or shock-dependent dosing of hydrocortisone, resulted in 93% and 80% probabilities of superiority with regard to the odds of improvement in organ support-free days within 21 days. However, some researchers have reached the opposite conclusion. Keller et al., (Citation2020) showed that early use of glucocorticoid therapy had nothing to do with the mortality of covid-19 patients, and (Pei et al., Citation2020) showed that steroid use was associated with an increased risk of death (OR, 2.43; 95% CI, 1.44–4.1; P = 0.001; I2 = 61.9%). However, there are some factors that may be influencing the result in Western studies. For example, the Recovery, Jeromino, Brain, and Bruno set a 28-day mortality rate, while the Pierre-Francois and REMAP-CAP investigator writing committees set a 21-day mortality rate. Sofia, Marla and Maryam did not mention the timing of mortality. Through our meta-analysis, most western studies show that the use of steroids will decrease the mortality of covid-19 patients.
Among foreign studies, different types of steroids and dosages were used. We have to do some classification by conversion of glucocorticoid dose. This suggests that in the high-dose group (Prednisone>1.0 mg/kg/d) there is no statistical differences between the use of steroids and mortality of covid-19 patients, while there is a statistical difference for the medium-dose group (0.5 mg/kg/d ≤ Prednisone ≤ 1.0 mg/kg/d). We also divided them into three groups according to the time of using glucocorticoids, the <5 days group also showed a close relationship between decrease of covid-19 patients’ mortality and using steroids while in the 5–7 days group and >7 days group there are no statistically significant associations. In our meta-analysis, a positive correlation between mortality of covid-19 patients and use of steroids was confirmed, and a medium dose of glucocorticoids for a short period will be more likely to decrease mortality. This result is convincing and objective based on the large numbers of people in these studies.
Although the mechanisms of the relationship between the use of steroids and mortality of covid-19 are still unclear, several hypotheses may be proposed. SARS-CoV-2 causes a delay in the innate immune response and it disarranges the immune system leading to an overwhelming inflammatory reaction (the “cytokine storm”). In this scenario, high levels of interleukins (IL), notably IL-6 and IL-1, create a positive feedback of chemokines and immune responses, and powers pulmonary and systemic tissue damage, leading to capillary leakage and SARS (severe acute respiratory syndrome), the main cause of death in patients with COVID-19 (Silva Júnior ML et al., Citation2021). The emergence of a cytokine storm will cause systemic inflammatory response syndrome, acute respiratory distress syndrome, even multiple organ failure. Especially some researchers (Ellinghaus et al., Citation2020, Oct. 15) showed respiratory failure caused by COVID-19 with a multi-gene cluster on chromosome 3, which indicated that more further researches about potential genetic treatment could be done in the future. Corticosteroids have a wide and nonspecific anti-inflammatory action; they may influence the transcription of mRNA of inflammatory cytokines and thus decrease the production of inflammatory mediators (Silva Júnior ML et al., Citation2021). The use of glucocorticoids will therefore decrease cytokine storm-induced complications. On the other hand, immunosuppression in a fragile respiratory epithelium may delay viral clearance and predispose to secondary infections and clinical deterioration. The WHO therefore currently recommends against the routine use of corticosteroids in the treatment of patients with COVID-19. Corticosteroids may be used if indicated for refractory septic shock or severe ARDS (https://wenku.baidu.com/view/920053946394dd88d0d233d4b14e852459fb3902.html 2021), This recommendation is based on largest RECOVERY trial. But Anant (Parasher, Citation2021) recommended that steroids can be used for a short period of time, that is, 3–5 days in patients who show progressive deterioration of oxygen saturation, increased activation of the pro-inflammatory response and rapid worsening of features on chest imaging. Our results are consistent with this since there is little risk of side effects in covid-19 patients with short-term treatment and medium steroid doses. Questions such as indication, time of onset and duration of corticosteroids still need to be better clarified in well-designed quantitative trials.
Through sensitivity analysis, we identified (Yang et al., Citation2020) as the main cause of heterogeneity in Chinese studies, and Ramiro et al. (Citation2020) as the main cause in foreign studies. Yang et al. (Citation2020) only included critically ill patients from the intensive care unit (ICU) who required mechanical ventilation or had a fraction of inspired oxygen (FiO2) of at least 60% or more. These criteria is partial, excluding early-stage patients without need to ICU and only including critically ill patients in the final stage of disease who are more likely to develop multi-organ failure even with glucocorticoid treatment. The main source of heterogeneity in foreign studies is from Ramiro et al. (Citation2020). In this study, the immunosuppressant Tocilizumab was given to patients in the treated group in addition to Methylprednisolone. Immunosuppressants are somewhat functionally similar to steroids and their use will influence the prognosis of COVID-19, and this may be the main source of high heterogeneity in foreign studies.
For this study, we chose a controversial topic in the care of covid patients and our objective analysis has now guided our clinical practice. Our studies included both foreign and Chinese studies, and we have been able to explain why they appear to draw opposite conclusions. The limitations of our work are that heterogeneity was high, the adjustments for potential confounders differed between studies and the influence of possible confounders on the meta-analysis could not be entirely excluded.
5. Conclusion
In summary, this meta-analysis demonstrated a positive correlation between survival of covid-19 patients and use of steroids; a medium dose use of glucocorticoids for a short time being more likely to decrease mortality. At the same time, giving a medium dose use of steroids for a short time has little risk of side effects for covid-19 patients. However, questions such as indication, time of onset and duration of steroids need to be better clarified in well-designed quantitative trials.
Correction
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgements
The authors thank the First Affiliated Hospital of Chongqing Medical University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article.
Statement of ethics
This study is a meta-analysis, and all analyses are based on previously published studies; thus, no ethical approval and patient consent are required.
Additional information
Funding
Notes on contributors
Zhou Enhao
Zhou Enhao, Li You, and Yang Chun designed this research, Zhou Enhao, Li You, Hu Danlan, and Dai Yuchi searched clinical articles from four medical databases including PubMed, Cochrane Library, Web of Science, and Embase and also researched relevant papers using the Google search engine and major Preprint platforms including Medrix, bioRxiv, and SSRN. Zhou Enhao and Li You did some meta-analysis work via Revman 5.4 and wrote this article. Yang Chun revised this article and gave some useful suggestions.
References
- Chen, T., Wu, D., Chen, H., Yan, W., Yang, D., Chen, G., Ma, K., Xu, D., Yu, H., Wang, H., Wang, T., Guo, W., Chen, J., Ding, C., Zhang, X., Huang, J., Han, M., Li, S., Luo, X., Zhao, J., Ning, Q. . Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ, (2020, March, 26). 368, m1091. Erratum in: BMJ. 2020 Mar 31;368:m1295. PMID: 32217556; PMCID: PMC7190011. https://doi.org/10.1136/bmj.m1091
- clinical guideline. https://wenku.baidu.com/view/920053946394dd88d0d233d4b14e852459fb3902.html
- Deng, Y., Liu, W., Liu, K., Fang, Y.Y., Shang, J., Zhou, L., Wang, K., Leng, F., Wei, S., Chen, L., Liu, H.G. . (2020, June, 5). Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: A retrospective study. Chinese Medical Journal, 133(11), 1261–19. PMID: 32209890; PMCID: PMC7289311. https://doi.org/10.1097/CM9.0000000000000824
- Dequin, P. F., Heming, N., Meziani, F., Plantefeve, G., Voiriot, G., Badie,J. ,Francois, B., Aubron, C., Ricard, J.D., Ehrmann, S., Jouan, Y., Guillon, A., Leclerc, M., Coffre, C., Bourgoin, H., Lengelle, C., Caille-Fenerol, C., Tavernier, E., Zohar, S., Giraudeau, B., Annane, D. et al . (2020, October, 6). Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients with COVID-19: A randomized clinical trial. JAMA, 324(13), 1298–1306. PMID: 32876689; PMCID: PMC7489432. https://doi.org/10.1001/jama.2020.16761
- Edalatifard, M., Akhtari, M., Salehi, M., Naderi, Z., Jamshidi, A., Mostafaei, S., Najafizadeh, S.R., Farhadi, E., Jalili, N., Esfahani, M., Rahimi, B., Kazemzadeh, H., Mahmoodi, Aliabadi M., Ghazanfari, T., Sattarian, M., Ebrahimi, Louyeh H., Raeeskarami, S.R., Jamalimoghadamsiahkali, S., Khajavirad, N., Mahmoudi, M., Rostamian, A. . (2020, December, 24). Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. European Respiratory Journal, 56(6), 2002808. PMID: 32943404; PMCID: PMC7758541. https://doi.org/10.1183/13993003.02808-2020
- Ellinghaus, D., Degenhardt, F., Bujanda, L., Buti, M., Albilos, A., Invernizzi, P., Fernandez, J., Prati, D., Baselli, G., Asselta, R., Grimsrud, M.M., Milani, C., Aziz, F., Kassens, J., May, S., Wendorff, M., Wienbrandt, L., Uellendahl-Werth, F., Zheng, T., Yi, X., de Pablo, R. et al . (2020, Oct. 15). Genomewide association study of severe COVID-19 with respiratory failure. The New England Journal of Medicine 383(16), 1522–1534. https://doi.org/10.1056/NEJMoa2020283
- Jeronimo, C. M. P., Farias, M. E. L., Val, F. F. A., Sampaio, V.S., Alexandre, M.A.A., Melo, G.C., Safe, I.P., Borba, M.G.S., Netto, R.L.A., Maciel, A.B.S., Neto, J.R.S., Oliveira, LB., Figueiredo, E.FG, Oliveira, Dinelly K.M., de Almeida, Rodrigues M.G., Brito, M., Mourao, M.P.G., Pivoto, Joao G.A., Hajjar, L.A., Bassat, Q., Romero, G.A.S. et al . (2021, May, 4). Methylprednisolone as adjunctive therapy for patients hospitalized with Coronavirus disease 2019 (COVID-19; Metcovid): A randomized, double-blind, phase IIb, placebo-controlled trial. Clinical Infectious Diseases, 72(9), e373–e381. PMID: 32785710; PMCID: PMC7454320. https://doi.org/10.1093/cid/ciaa1177
- Keller, M. J., Kitsis, E. A., Arora, S., Chen, J.-T., Agarwal, S., Ross, M. J., Tomer, Y., & Southern, W. (2020, August). Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. Journal of Hospital Medicine, 15 (8), 489–493. PMID: 32804611; PMCID: PMC7518134 https://doi.org/10.12788/jhm.3497
- Li, Q, Guan, X, Wu, P, Wang, X., Zhou, L., Tong, Y., Ren, R., Leung, K.S.M., Lau, E.H.Y., Wong, J.Y., Xing, X., Xiang, N., Wu, Y., Li, C., Chen, Q., Li, D., Liu, T., Zhao, J., Liu, M., Tu, W., Chen, C. et al . (2020). Early transmission dynamics in Wuhan, china, of novel Coronavirus–infected pneumonia. The New England Journal of Medicine, 382(13), 1199–1207. https://doi.org/10.1056/NEJMoa2001316
- Nelson, B. C., Laracy, J., Shoucri, S., Dietz, D., Zucker, J., Patel, N., Sobieszczyk, M.E., Kubin, C.J., Gomez-Simmonds, A. . (2021, May, 4). Clinical outcomes associated with methylprednisolone in mechanically ventilated patients with COVID-19. Clinical Infectious Diseases, 72(9), e367–e372. PMID: 32772069; PMCID: PMC7454332. https://doi.org/10.1093/cid/ciaa1163
- Parasher, A. (2021, May). COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgraduate Medical Journal, 97 (1147), 312–320. Epub 2020 Sep 25. PMID: 32978337 https://doi.org/10.1136/postgradmedj-2020-138577
- Pei, L., Zhang, S., Huang, L., Geng, X., Ma, L., Jiang, W., Li, W., Chen, D. . (2020, September, 30). Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: A systematic review and meta-analysis. Polish Archives of Internal Medicine, 130(9), 726–733. Epub 2020 Aug 4. PMID: 32749826. https://doi.org/10.20452/pamw.15543
- Ramiro, S., Mostard, R. L. M., Magro-Checa, C., van Dongen, C. M. P., Dormans, T., Buijs, J., Gronenschild , M., de Kruif, M. D., van Haren, E. H. J., van Kraaij, T., Leers, M. P. G., Peeters, R., Wong, D. R., Landewe, R. B. M. . (2020, September). Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: Results of the CHIC study. Annals of the Rheumatic Diseases, 79(9), 1143–1151. Epub 2020 Jul 20. PMID: 32719045; PMCID: PMC7456552. https://doi.org/10.1136/annrheumdis-2020-218479
- RECOVERY Collaborative Group . (2021, February, 25). Dexamethasone in hospitalized patients with Covid-19. The New England Journal of Medicine, 384(8), 693–704. Epub 2020 Jul 17. PMID: 32678530; PMCID: PMC7383595. https://doi.org/10.1056/NEJMoa2021436
- Russell, C. D., Millar, J. E., & Baillie, J. K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. (2020). The Lancet, 395(10223), 473–475. British edition. https://doi.org/10.1016/S0140-6736(20)30317-2
- Shang, L., Zhao, J., Hu, Y., Du, R., & Cao, B. (2020). On the use of corticosteroids for 2019-nCoV pneumonia. The Lancet, 395(10225), 683–684. https://doi.org/10.1016/S0140-6736(20)30361-5
- Silva Júnior ML, M., Souza, L. M. A., Remc, D., Valente, R. G. D. M., & Melo, T. S. (2021, June). Review on therapeutic targets for COVID-19: Insights from cytokine storm. Postgraduate Medical Journal, 97 (1148), 391–398. Epub 2020 Oct 2. PMID: 33008960 https://doi.org/10.1136/postgradmedj-2020-138791
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working GroupSterne, J. A. C., Murthy, S., Diaz, J. V., Slutsky, A. .S, Villar, J., Angus, D. C., Annane, D., Azevedo, L. C. P., Berwanger, O., Cavalcanti, A. B., Dequin, P. F., Du, B., Emberson, J., Fisher, D., Giraudeau, B., Gordon, A. C., Granholm, A., Green, C., Haynes, R., Heming, N., Higgins, J. P. .T et al . (2020, October, 6). Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA, 324(13), 1330–1341. PMID: 32876694; PMCID: PMC7489434. https://doi.org/10.1001/jama.2020.17023
- The writing committee for the REMAP-CAP investigators. (2020). Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA, 324(13), 1317–1329. https://doi.org/10.1001/jama.2020.17022
- Tomazini, B. M., Maia, I. S., Cavalcanti, A. B., Berwanger, O., Rosa, R. G., Veiga, V. C., Avezum, A., Lopes, R. D., Bueno, F. R., Silva, M. V. A. .O, Baldassare, F. P., Costa, E. L. V., Moura, R. A. B., Honorato, M. O., Costa, A. N., Damiani, L. P., Lisboa, T., Kawano-Dourado, L., Zampieri, F. G., Olivato, G. B., Righy, C. et al . (2020, October, 6). COALITION COVID-19 Brazil III investigators. Effect of dexamethasone on days Alive and Ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA, 324(13), 1307–1316. PMID: 32876695; PMCID: PMC7489411. https://doi.org/10.1001/jama.2020.17021
- WHO, WHO/2019-nCoV/therapeutics/2021.3, 24 September 2021
- Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., Huang, H., Zhang, L., Zhou, X., Du, C., Zhang, Y., Song, J., Wang, S., Chao, Y., Yang, Z., Xu, J., Zhou, X., Chen, D., Xiong, W., Xu, L., Zhou, F. et al . (2020). Risk factors associated with acute respiratory distress syndrome and death in patients With Coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine, 180(7), 934–943. https://doi.org/10.1001/jamainternmed.2020.0994
- Yan, J., Liu, A., Huang, J., Wu, J., & Fan, H. (2020, May, 13). Research progress of drug treatment in novel Coronavirus Pneumonia. AAPS Pharmaceutical Science and Technology, 21(4), PMID: 32405780; PMCID: PMC7220569, 130. https://doi.org/10.1208/s12249-020-01679-z
- Yang, X., Yu, Y., Xu, J., Shu, H., Xia, J., Liu, H., Wu, Y., Zhang, L., Yu, Z., Fang, M., Yu, T., Wang, Y., Pan, S., Zou, X., Yuan, S., Shang, Y. . (2020, May). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine, 8(5), 475–481. Epub 2020 Feb 24. Erratum in: Lancet Respir Med. 2020 Apr;8(4):e26. PMID: 32105632; PMCID: PMC7102538. https://doi.org/10.1016/S2213-2600(20)30079-5
- Zhao, J. P., Hu, Y., Du, R. H., Chen, Z. S., Jin, Y., Zhou, M., Zhang, J., Qu, J. M., Cao, B. . (2020). Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases, 43(3), 183–184. https://doi.org/10.3760/cma.j.1001-0939.2020.03.008
- Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., Xiang, J., Wang, Y., Song, B., Gu, X., Guan, L., Wei, Y., Li, H., Wu, X., Xu, J., Tu, S., Zhang, Y., Chen, H., & Cao, B. (2020, March, 28). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. The Lancet, 395(10229), Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. PMID: 32171076; PMCID: PMC7270627, 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3