Abstract
: Nearly one-third of U.S. adults do not get enough sleep regularly. Poor sleep quantity and quality are associated with many chronic diseases and conditions. Sleep outcomes are likely to deteriorate after a stroke and could delay recovery. In this cross-sectional analysis, we examined the association between a history of stroke and sleep disturbances. Multivariate logistic regression models were developed for each outcome accounting for the multiple-stage sampling design. After adjustment for confounders, a history of stroke was significantly associated with taking sleep medication (OR = 1.8, 95% CI: 1.1–3.0) but not with trouble staying asleep (OR = 1.3; 95% CI: 0.9–1.9) regardless of age, sex, and race. However, sex difference was observed for waking up not feeling well-rested, in which the association was significant for females (OR: 2.0, 95% CI = 1.1–3.6) and not for males (OR: 0.9; 95% CI = 0.6–1.5). Similarly, race difference was observed for trouble falling asleep, in which the association was significant for Non-Hispanic White (OR = 2.0; 95% CI: 1.3–3.0) and not for Non-Hispanic Black (OR = 0.4; 95% CI: 0.1–2.0). The association between a history of stroke and sleep disturbances varies by sex and race, indicating a significant association among females and Non-Hispanic Whites. A holistic approach to stroke rehabilitation that includes targeted sleep interventions is warranted.
1. Introduction
Sleep is an essential component of well-being and quality of life for people of all ages. Yet millions of people do not get enough sleep. Nearly one-third of U.S. adults do not get the recommended 7 or more hours of sleep daily (Sheehan et al., Citation2019). Inadequate sleep is associated with many chronic diseases and conditions such as type 2 diabetes, Shan et al. (Citation2015) heart disease, Wang et al. (Citation2016), H Li et al. (Citation2019) obesity, Itani et al. (Citation2017), Jike et al. (Citation2018) and depression (Itani et al., Citation2017; Jike et al., Citation2018). It could also increase the risk of accidents, injuries (Uehli et al., Citation2014) and all-cause mortality (Cappuccio et al., Citation2010)
Stroke is another important public health issue in the U.S. resulting in more than 795,000 new cases per year (Benjamin et al., Citation2018). There is a bidirectional relationship between stroke and sleep outcomes (Gottlieb et al., Citation2019). Sleep disorders can contribute to stroke occurrence and a history of stroke may affect sleep outcome. History of stroke has been associated with a high prevalence of sleep disturbances. A meta-analysis indicated a 40.7% pooled prevalence of insomnia among stroke survivors which is above the approximately 10–30% in the general population (Baylan et al., Citation2020; Bhaskar et al., Citation2016). In a Japanese study of community-dwelling elderly 65 years and older, a history of stroke was independently associated with 44.0% increases in sleep disturbance (Kishimoto et al., Citation2016). Another study found that patients with stroke took longer time to fall asleep and had poorer sleep efficiency, measured as the ratio of time spent asleep compared to the time spent in bed, than those without stroke (Sterr et al., Citation2018). However, those findings are not consistent across studies. In a Multi-Country study, for example, stroke was associated with self-reported sleep problems in only 3 of the 9 countries, which included China, Ghana, and India (Koyanagi et al., Citation2014)
The association between stroke and sleep disturbance was often reported in the elderly. Whether this association will remain in patients of younger age is not established. Additionally, the disparity of stroke incidence by age, sex, and race (Howard et al., Citation2019) could be similarly reflected in the association between history of stroke and sleep outcome. Among patients with a first-time stroke in Korea, Kim et al. reported that patients with insomnia were more likely to be older and female (Kim et al., Citation2017). In another study, shorter sleep duration was associated with higher stroke risk in females while longer duration was associated with higher stroke risk in males (Ji et al., Citation2020). Racial differences in post-stroke disability, specifically in the U.S., are of interest given the large racial disparity in stroke risk in black individuals compared with white individuals (Bushnell et al., Citation2014; Howard et al., Citation2019)
Overall, current literature regarding the association between history of stroke and sleep disturbance is sparse and yields inconclusive results (Kim et al., Citation2017; Kishimoto et al., Citation2016; Koyanagi et al., Citation2014; Sterr et al., Citation2018). Most previous studies were conducted outside of the U.S. with a focus on the early stage of rehabilitation after stroke. This study sought to evaluate the prevalence of sleep disturbances, the association between a history of stroke and sleep disturbances within the general population, and whether the association is modified by age, sex, and race/ethnicity.
2. Materials and methods
2.1. Data source and study population
This study used data from the 2018 National Health Interview Survey (NHIS). The NHIS is an annual cross-sectional household survey of the civilian noninstitutionalized population in the U.S. conducted by the Centers for Disease Control and Prevention (CDC). The main objective of the NHIS is to monitor the health of the U.S. population on a broad range of health topics. Details of the survey design and data collection process are described elsewhere (National Center for Health Statistics, Citation2015). The 2018 NHIS publicly released data files contain data collected from 29,839 households and 72,831 individual participants. This study included 25,392 participants with and without stroke history who were aged 18 years or older. Analytic variables were drawn from the Person and Sample Adult core components. The deidentified NHIS data are exempt from federal regulation for the protection of human research participants, and local IRB approval is not required.
2.2. Outcome—Sleep disturbances during the past week
The outcome was self-reported sleep disturbances during the past week, including four dichotomous measures (yes or no) regarding “trouble falling asleep”, “trouble staying asleep”, “taking sleep medication” and “not feeling well-rested”. For the first three measures, participants were asked: “in the past week, how many times did you have trouble falling asleep?”, “have trouble staying asleep”, and “take medication to help you fall asleep or stay asleep?”. For each question, the range of possible responses was 0 to 7 times. These responses were combined to create an indicator for ≥4 times of sleep disturbances in the past week. The fourth measure of sleep disturbances was an indicator variable for whether an adult reported waking up not feeling well-rested ≥4 days in the past week. This was created by subtracting from 7 the response to the survey question that asked, “In the past week, on how many days did you wake up feeling well-rested?”. Consistent with prior research, the threshold of ≥4 times in the past week was used as a conservative measure of poorer sleep quality (Galinsky et al., Citation2018; Nugent & Black, Citation2016)
2.3. Exposure—Self-reported stroke history
The variable for stroke was self-reported and based on the dichotomous response to the question: “Have you ever been told by a doctor or other health professional that you had a stroke?”
2.4. Covariates
Based on the literature review and the available data we used the following variables as covariates: age, sex, race, education, marital status, Body Mass Index (BMI), annual income, smoking status, alcohol consumption, hypertension, diabetes, hypercholesterolemia, and taking medication for depression. Age was categorized as 18 to 35, 36 to 50, 51 to 65, and 66 + years. Race/ethnicity was categorized as Hispanic, Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian, and Non-Hispanic Other. BMI (kg/m2) was categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9) and obese (≥30.0). Education was categorized as below high school diploma, high school graduate, and college or more. Marital status was categorized as in couple, widow/divorced, and single. Annual income was categorized as less than $10,000, $10,000 to $34,999, $35,000 to $54,999, $55,000 to $74,999 and $75,000 + . Alcohol consumption was categorized as abstainer, moderate and heavy drinkers. Smoking status was categorized as never, former, and current smoker.
2.5. Statistical analysis
Descriptive statistics were used to summarize the participants’ baseline characteristics. To observe the relationship between the outcome variables (sleep disturbances) and independent variable (self-reported history of stroke) logistic regression models were developed for each outcome. The covariates were assessed through multivariable logistic regression models. Multicollinearity was tested for the covariates using the variance inflation factor (VIF). A VIF of 10 or greater was used to signify multicollinearity. Model 1 assessed the unadjusted association between a history of stroke and sleep disturbances. Model 2 was adjusted for age, sex, race, education, marital status, and annual income. Model 3 was adjusted for variables included in model 2 plus Body Mass Index (BMI), smoking status, and alcohol consumption. Model 4 was adjusted for variables included in model 3 plus hypertension, diabetes, hypercholesterolemia, and taking medication for depression. To assess the effect measure modification by age, sex, and race/ethnicity, an interaction term was included in the model and the analysis was further stratified by these variables. To explore potential outcome misclassification due to the definition adopted for the study (i.e., sleep disturbance ≥4 times in the past week), a sensitivity analysis was conducted exploring all possible cut points on the occurrence of sleep disturbances during a week for each outcome measure. Another sensitivity analysis was conducted for parsimony where the manual backward procedure was applied by screening and deleting covariates one at a time until a satisfactory model was achieved (i.e., a model in which all the covariates are statistically significant). A p-value <0.05 was considered statistically significant in this study. All statistical analyses were weighted to account for the multiple-stage sampling design and performed in SAS® 9.4 software (SAS Institute Inc. Cary, NC.).
3. Result
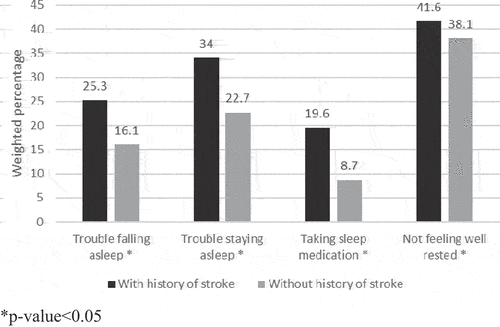
The prevalence of sleep disturbances was significantly higher among people with a history of stroke compared to those without a history of stroke (Figure , Supplemental Table S1). The prevalence of trouble falling asleep, trouble staying asleep, taking sleep medication, and not feeling well-rested were 25.3%, 34.0%, 19.6%, and 41.6% for participants with a history of stroke and 16.1%, 22.7%, 8.7%, and 38.1% for participants without a history of stroke, respectively.
The characteristics of the study participants with and without a history of stroke are shown in Table . History of stroke was equally reported by females and males. The proportions of participants with a history of stroke were higher in the elderly (age ≥66 years), Non-Hispanic Black, those in the extreme BMI groups (i.e., BMI <18.5 and BMI ≥ 30.0), and those with low income. Similarly, participants with hypertension, diabetes, hypercholesterolemia, and depression were more likely to report having a history of stroke.
Table 1. Characteristics of study participants with and without a history of stroke (n = 25,392)
Odds ratios (ORs) for the association between a history of stroke and sleep disturbances are presented in Table . In the unadjusted model, a history of stroke was significantly associated with trouble falling asleep [OR = 1.8; 95% confidence interval (95% CI): 1.5–2.1], trouble staying asleep (OR = 1.7; 95% CI: 1.5–2.0), taking sleep medication (OR = 2.6; 95% CI: 2.2–3.0) and waking up not feeling rested (OR = 1.2; 95% CI: 1.0–1.3). However, after full adjustment, a history of stroke was significantly associated with trouble falling asleep [Model 4: adjusted OR (aOR) = 1.7; 95% CI: 1.1–2.6], taking medication (aOR = 1.8; 95% CI: 1.1–3.0), and not feeling well-rested (aOR = 1.4; 95% CI: 1.0–2.0) but not with trouble staying asleep (aOR = 1.3; 95% CI: 0.9–1.9). The aORs were slightly higher in Model 2 (adjusted for socio-demographic factors) and model 3 (adjusted for socio-demographic and behavioral risk factors) compared to the fully adjusted model (Model 4).
Table 2. Association between history of stroke and sleep disturbances among U.S. adults
There was an effect measure modification by sex (p-value = 0.0457) for not feeling well-rested (Table ). While females with a history of stroke were twice as likely to report not feeling well-rested (aOR = 2.0; 95% CI: 1.1–3.6) than females without a history of stroke, such association was not found among males (aOR = 0.9; 95% CI = 0.6–1.5). Similarly, there was an effect measure modification by race/ethnicity (p-value = 0.0259) for trouble falling asleep. While Non-Hispanic Whites with a history of stroke were twice as likely to report “trouble falling asleep” (aOR = 2.0; 95% CI: 1.3–3.0) than Non-Hispanic Whites without a history of stroke, such association was not found among Hispanic (aOR = 0.5; 95% CI: 0.1–2.6) and Non-Hispanic Black (aOR = 0.4; 95% CI: 0.1–2.0).
Table 3. Association between history of stroke and sleep disturbances stratified by age group, sex, and race/ethnicity
The result of the sensitivity analysis showed that the association between self-reported history of stroke and all measures of sleep disturbances remained consistent, regardless of the cut point on the frequency of the sleep disturbances during the week (Table ). Similarly, the results of the manual backward selection were consistent with the main analysis (Supplemental Table 3)
Table 4. Association between history of stroke and sleep disturbances using different cut-points of sleep disturbances
4. Discussion
In this nationally representative sample of the U.S. population, sleep disturbance was consistently higher among participants with a history of stroke than those without. History of stroke was found to be associated with trouble falling asleep, taking sleep medication, and not feeling rested. We also reported differences in these associations by sex and race/ethnicity.
The prevalence of sleep disturbance goes from 19.6% for taking sleep medication to 41.6% for not feeling well-rested among participants with a history of stroke. Similar to previous studies, these prevalences’s were high when compared to the general population (Baylan et al., Citation2020). In their study, Li et al. reported a prevalence of 46.05% which is slightly above our individual measurement of sleep disturbances (Li et al., Citation2018).
We found in the unstratified model that a history of stroke was significantly related to trouble falling asleep but not trouble staying asleep which suggests that sleep disorders induced by stroke could be within the sleep initiation process. A recent study reported that stroke patients had poorer sleep with longer sleep latencies and lower sleep efficiency (Sterr et al., Citation2018). Sleep physiology is complex and involves almost all parts of the brain. Within the hypothalamus, the suprachiasmatic nucleus receives information about light exposure directly from the eyes and controls the circadian rhythm. Brain lesions caused by stroke may result in visual disturbances which can subsequently reduce the transmission of light exposure to the brain. Furthermore, the pineal gland, located within the brain’s two hemispheres, receives signals from the suprachiasmatic nucleus and increases the production of the hormone melatonin, which induces sleep once the lights go down (Roehrs, Citation2000). The secretion of melatonin is expected to be altered in stroke patients, influencing the melatonin/circadian rhythms which could lead to sleep disturbances (Beloosesky et al., Citation2002; Uddin et al., Citation2015). Moreover, fatigue and a lower level of physical activity are highly prevalent in the acute and chronic phases of stroke, and both aspects can negatively affect and/or interact with sleep (Wu et al., Citation2015). A further possible contributor to poor sleep quality could be greater pain or discomfort in people with history of stroke (Naess et al., Citation2012).
There are several potential explanations to our finding of effect measure modification by sex and race/ethnicity. Females with history of stroke may have a higher likelihood of disability and worse quality of life compared to males (Carcel et al., Citation2019). Additionally, hormones and genetic mechanisms in conjunction with cultural and societal demands are thought to drive the sex differences in sleep (Mallampalli & Carter, Citation2014). The racial difference in the association with trouble falling asleep could be driven by the severity of stroke and access to health care. Non-Hispanic Black and Hispanic have higher stroke mortality compared to Non-Hispanic White (CitationCenters for Disease Control and Prevention National Center for Health Statistics). One could argue that those who survived a stroke may have milder disease and therefore less complication. Also, Black stroke survivors are more likely than White stroke survivors to have a caregiver and receive more hours of help (Skolarus et al., Citation2017). Social supports could be an important factor in improving sleep outcomes.
The main limitation of this study is that the history of stroke was self-reported. However, previous studies did not find a difference between a self-reported stroke and a hospital-coded stroke suggesting the use of self-reported stroke as a fair measure to assess a history of stroke (Engstad et al., Citation2000; Jamrozik et al., Citation2014). We were not able to assess directly for depression because the variable for depression was not publicly available. We assumed that depression medication should be a reliable proxy for depression. The structure of stroke and sleep-related questions in the survey indicate that stroke potentially occurred before sleep disturbances. Nevertheless, this is a cross-sectional study, and further research in prospective design is warranted. Furthermore, since the prevalence of sleep disturbances (outcome variables) is quite high in this population, the OR may not approximate well the relative risk (Szklo & Nieto, Citation2014). In this study, however, although the prevalence was high, the ORs were relatively low, and thus discrepancies between the OR and the relative risk may not be substantial. Finally, we were not able to adjust for time since stroke diagnosis, other sleep disorders such as obstructive sleep apnea, and restless leg syndrome. Therefore, we cannot rule out the possibility of unmeasured/residual confounding.
Despite these limitations, the NHIS is a large sample, and the design allows for making inferences about the U.S. population. Stroke history was not limited to recent occurrences as it is common in the literature. We used four questions to assess sleep disturbances and conducted a sensitivity analysis which strengthens our outcome definitions. It has been reported that those who suffer from insomnia do not usually discuss the issue with their physicians (Nugent & Black, Citation2016). Therefore, sleep disturbance requires systematic screening in the care pathway for stroke. A holistic approach to rehabilitation and care provision that includes targeted sleep interventions will likely enhance sleep outcomes and quality of life in those living with chronic deficits after stroke.
Acknowledgements
We thank Michael Corrieri who assisted in proofreading the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available from the Centers for Disease Control and Prevention (CDC) at https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
Additional information
Funding
References
- Baylan, S., Griffiths, S., Grant, N., Broomfield, N. M., Evans, J. J., & Gardani, M. (2020). Incidence and prevalence of post-stroke insomnia: A systematic review and meta-analysis. Sleep Medicine Reviews, 49, 101222. https://doi.org/10.1016/j.smrv.2019.101222
- Beloosesky, Y., Grinblat, J., Laudon, M., Grosman, B., Streifler, J. Y., & Zisapel, N. (2002). Melatonin rhythms in stroke patients. Neuroscience Letters, 319(2), 103–12. https://doi.org/10.1016/S0304-3940(01)02568-X
- Benjamin, E. J., Virani, S. S., Callaway, C. W. et al. (2018). American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. https://doi.org/10.1161/CIR.0000000000000558
- Bhaskar, S., Hemavathy, D., & Prasad, S. (2016). Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. Journal of Family Medicine and Primary Care, 5(4), 780. https://doi.org/10.4103/2249-4863.201153
- Bushnell, C., McCullough, L. D., Awad, I. A., Chireau, M. V., Fedder, W. N., Furie, K. L., Howard, V. J., Lichtman, J. H., Lisabeth, L. D., Piña, I. L., Reeves, M. J., Rexrode, K. M., Saposnik, G., Singh, V., Towfighi, A., Vaccarino, V., & Walters, M. R. (2014). Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American heart association/American stroke association. Stroke, 45(5), 1545–1588. https://doi.org/10.1161/01.str.0000442009.06663.48
- Cappuccio, F. P., D’Elia, L., Strazzullo, P., & Miller, M. A. (2010). Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep, 33(5), 585–592. https://doi.org/10.1093/sleep/33.5.585
- Carcel, C., Wang, X., Sandset, E. C., Delcourt, C., Arima, H., Lindley, R., Hackett, M. L., Lavados, P., Robinson, T. G., Muñoz Venturelli, P., Olavarría, V. V., Brunser, A., Berge, E., Chalmers, J., Woodward, M., & Anderson, C. S. (2019). Sex differences in treatment and outcome after stroke: Pooled analysis including 19,000 participants. Neurology, 93(24), E2170–E2180. https://doi.org/10.1212/WNL.0000000000008615
- Centers for Disease Control and Prevention National Center for Health Statistics. Underlying cause of death 1999-2017 MN. CDC WONDER Online Database.
- Engstad, T., Bønaa, K. H., & Viitanen, M. (2000). Validity of self-reported stroke: The Tromso study. Stroke, 31(7), 1602–1607. https://doi.org/10.1161/01.STR.31.7.1602
- Galinsky, A. M., Ward, B. W., Joestl, S. S., & Dahlhamer, J. M. (2018). Sleep duration, sleep quality, and sexual orientation: Findings from the 2013-2015 national health interview survey. Sleep Health, 4(1), 56–62. https://doi.org/10.1016/j.sleh.2017.10.004
- Gottlieb, E., Landau, E., Baxter, H., Werden, E., Howard, M. E., & Brodtmann, A. (2019). The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Medicine Reviews, 45, 54–69. https://doi.org/10.1016/j.smrv.2019.03.003
- Howard, V. J., Madsen, T. E., Kleindorfer, D. O., Judd, S. E., Rhodes, J. D., Soliman, E. Z., Kissela, B. M., Safford , M. M., Moy, C. S., McClure, L. A., Howard, G. (2019). Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurology, 76(2), 179. https://doi.org/10.1001/jamaneurol.2018.3862
- Itani, O., Jike, M., Watanabe, N., & Kaneita, Y. (2017). Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Medicine, 32, 246–256. https://doi.org/10.1016/j.sleep.2016.08.006
- Jamrozik, E., Hyde, Z., Alfonso, H., Flicker, L., Almeida, O., Yeap, B., Norman, P., Hankey, G., & Jamrozik, K. (2014). Validity of self-reported versus hospital-coded diagnosis of stroke: A cross-sectional and longitudinal study. Cerebrovascular Diseases, 37(4), 256–262. https://doi.org/10.1159/000358583
- Jike, M., Itani, O., Watanabe, N., Buysse, D. J., & Kaneita, Y. (2018). Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Medicine Reviews, 39, 25–36. https://doi.org/10.1016/j.smrv.2017.06.011
- Ji, A., Lou, H., Lou, P., Xu , C., Zhang, P., Iao, C., Yang, Q. (2020). Interactive effect of sleep duration and sleep quality on risk of stroke: An 8-year follow-up study in China. Sci Rep, https://doi.org/10.1038/s41598-020-65611-y.
- Kim, W. H., Jung, H. Y., Choi, H. Y., Park, C. H., Kim, E. S., Lee, S. J., Ko, S. H., Kim, S. Y., Joa, K. L. (2017). The associations between insomnia and health-related quality of life in rehabilitation units at 1 month after stroke. Journal of Psychosomatic Research, 96, 10–14. https://doi.org/10.1016/j.jpsychores.2017.02.008
- Kishimoto, Y., Okamoto, N., Saeki, K., Tomioka, K., Obayashi, K., Komatsu, M., & Kurumatani, N. (2016). Bodily pain, social support, depression symptoms and stroke history are independently associated with sleep disturbance among the elderly: A cross-sectional analysis of the Fujiwara-kyo study. Environmental Health and Preventive Medicine, 21(5), 295–303. https://doi.org/10.1007/s12199-016-0529-z
- Koyanagi, A., Garin, N., Olaya, B., Ayuso-Mateos, J. L., Chatterji, S., Leonardi, M., Koskinen, S., Tobiasz-Adamczyk, B., & Haro, J. M. (2014). Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: A multi-country study. PLoS One, 9(12), e114742. https://doi.org/10.1371/journal.pone.0114742
- Li, H., Ren, Y., Wu, Y., & Zhao, X. (2019). Correlation between sleep duration and hypertension: A dose-response meta-analysis. Journal of Human Hypertension, 33(3), 218–228. https://doi.org/10.1038/s41371-018-0135-1
- Li, L. J., Yang, Y., Guan, B. Y., Chen, Q., Wang, A.-X., Wang, Y.-J., Zhang, N., & Wang, C.-X. (2018). Insomnia is associated with increased mortality in patients with first-ever stroke: A 6-year follow-up in a Chinese cohort study. Stroke and Vascular Neurology, 3(4), 197–202. https://doi.org/10.1136/svn-2017-000136
- Mallampalli, M. P., & Carter, C. L. (2014). Exploring sex and gender differences in sleep health: A society for women’s health research report. Journal of Women’s Health, 23(7), 553–562. https://doi.org/10.1089/jwh.2014.4816
- Naess, H., Lunde, L., & Brogger, J. (2012). The effects of fatigue, pain, and depression on quality of life in ischemic stroke patients: The Bergen stroke study. Vascular Health and Risk Management, 407. https://doi.org/10.2147/VHRM.S32780
- National Center for Health Statistics. (2015, June). Survey description. Surv Descr Natl Heal Interview Surv 2014. papers2://publication/uuid/7CD55057-5429-462A-9E38-C7255FC76B6D
- Nugent, C. N., & Black, L. I. (2016). Sleep duration, quality of sleep, and use of sleep medication, by sex and family type, 2013-2014. NCHS Data Brief.
- Roehrs, T. (2000). Sleep physiology and pathophysiology. Clinical Cornerstone, 2(5), 1–15. https://doi.org/10.1016/S1098-3597(00)90036-X
- Shan, Z., Ma, H., Xie, M., Yan, P., Guo, Y., Bao, W., Rong, Y., Jackson, C. L., Hu, F. B., & Liu, L. (2015). Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care, 38(3), 529–537. https://doi.org/10.2337/dc14-2073
- Sheehan, C. M., Frochen, S. E., Walsemann, K. M., & Ailshire, J. A. (2019). Are U.S. adults reporting less sleep?: Findings from sleep duration trends in the national health interview survey, 2004-2017. Sleep, 42(2). https://doi.org/10.1093/sleep/zsy221
- Skolarus, L. E., Freedman, V. A., Feng, C., & Burke, J. F. (2017). African American stroke survivors: more caregiving time, but less caregiving burden. Circulation: Cardiovascular Quality and Outcomes, 10(2). https://doi.org/10.1161/CIRCOUTCOMES.116.003160
- Sterr, A., Kuhn, M., Nissen, C., Ettine, D., Funk, S., Feige, B., Umarova, R., Urbach, H., Weiller, C., & Riemann, D. (2018). Post-stroke insomnia in community-dwelling patients with chronic motor stroke: Physiological evidence and implications for stroke care. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-26630-y
- Szklo, M. (Moyses), & Nieto, F. J. (2014). Understanding lack of validity: Bias. Epidemiol beyond Basics.
- Uddin, M. S., Hoque, M. I., Uddin, M. K., Kamol, S. A., & Chowdhury, R. H. (2015). Circadian rhythm of onset of stroke - In 50 cases of ischemic stroke. Mymensingh Medical Journal: MMJ, 24(1), 121–126.
- Uehli, K., Mehta, A. J., Miedinger, D., Hug, K., Schindler, C., Holsboer-Trachsler, E., Leuppi, J. D., & Künzli, N. (2014). Sleep problems and work injuries: A systematic review and meta-analysis. Sleep Medicine Reviews, 18(1), 61–73. https://doi.org/10.1016/j.smrv.2013.01.004
- Wang, D., Li, W., Cui, X., Meng, Y., Zhou, M., Xiao, L., Ma, J., Yi, G., Chen, W. (2016). Sleep duration and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Int J Cardiol, https://doi.org/10.1016/j.ijcard.2016.06.027.
- Wu, S., Mead, G., Macleod, M., & Chalder, T. (2015). Model of understanding fatigue after stroke. Stroke, 46(3), 893–898. https://doi.org/10.1161/STROKEAHA.114.006647