Abstract
Malaria is an infectious disease caused protozoa in genus Plasmodium. In spite of the efforts made in the fight against malaria, this tropic infectious disease is still one of the most common vector borne disease in the WHO African region. Therefore, this systematic review focuses on current malaria control interventions, treatment options and elimination in Africa with specific focus on Zimbabwe. The literature was searched in electronic databases such as PubMed, MEDLINE, ClinicalTrials.gov, DOAJ, Europe PubMed Central, Web of Science and Google Scholar. Furthermore, the literature search was expanded to include reference lists in peer-reviewed scientific publications. Some of the key phrases chosen in the literature search were ‘malaria control interventions’, ‘Zimbabwe and malaria’, ‘Malaria treatment’, ‘Malaria prevalence in Zimbabwe’, ‘Malaria prevalence in Africa’, ‘malaria and Africa’, and ‘Africa and malaria therapy’. In this study, 185 articles were reviewed and literature was summarized in line with the objectives of the study. Based on literature survey, it was noted that intensification of malaria control interventions and treatment has led to a remarkable decline in malaria morbidity and mortality. However, malaria remains a public health concern in most African countries including Zimbabwe. This has been attributed to the (1) development of physiological and behavioral resistance among malaria vectors in response to insecticides overuse, (2) development of resistance in P. falciparum to antimalarial drugs, (3) migration of malaria tolerant and positive individuals from malaria endemic areas to settings where malaria is less common, (4) emergence of genetically distinct malaria parasites which has limited the development of an effective malaria vaccine and protective immunity.
REVIEWING EDITOR:
1. Introduction
Malaria is an infectious vector borne disease caused by Apicomplexan protozoans of genus of Plasmodium (Mulaw et al., Citation2019). Plasmodia species are transmitted between human beings through mosquito bites. However, the other vertebrate secondary hosts for Plasmodium species include simians, rodents, birds, and reptiles (Krettli et al., Citation2001; Lima et al., Citation2015). Of the several species of Plasmodium, only five species infect humans and these are Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae and Plasmodium knowlesi. Plasmodium vivax and Plasmodium falciparum are the most common malaria pathogens and the latter is the most deadly form that dominates in Africa (Klooster et al., Citation2015; Mulaw et al., Citation2019). It has been confirmed that 98% of malaria cases reported in Zimbabwe are as a result of P. falciparum and the remainder of the cases, about 2%, are caused by Plasmodium ovale and Plasmodium malariae (Klooster et al., Citation2015). According to the research carried out between 2013 and 2016, it was concluded that Anopheles (An.) gambiae s.l. and Anopheles funestus were the most widely distributed malaria vector species in Zimbabwe (President’s Malaria Initiative, Citation2018; President’s Malaria Initiative, Zimbabwe, Citation2020)
A lot of investments and efforts in the fight against malaria have led to a significant reduction in malaria cases and malaria related deaths globally. However, in spite of these efforts, malaria is still the most common vector borne disease worldwide (Mugwagwa et al., Citation2015). Malaria is endemic in tropical and subtropical countries and approximately 50% of the world’s population is at risk of infection (Bekono et al., Citation2020; Mulaw et al., Citation2019; Quanquin et al., Citation2020; Tahghighi et al., Citation2020, Mugwagwa et al., Citation2015). According to a report released in April, 2024, there were 249 million malaria cases in 2022 coupled with about 608, 000 malaria related deaths in 85 countries (Cohen, Citation2024). The malarial burden is highest in Africa, accounting for 93% of all malaria deaths recorded globally (Mulaw et al., Citation2019). Up to 80% of Children under the age of five died due to malaria and pregnant women exposed to malaria can contributed significantly to neonatal and maternal deaths (Cohen, Citation2024). In 2015 alone, nearly 28 million pregnant women in sub-Saharan Africa were at risk of malaria (Quanquin et al., Citation2020). According to Mulaw et al., Citation2019, children aged less than 5 years accounted for 61% of all deaths in Africa.
Although malaria remains a health burden in endemic countries, malaria morbidity and mortality declined over the last 15 years due to increased vector control, improved diagnosis, and treatment (Kemibala et al., Citation2020). In 2018, malaria incidence rate, cases and number of deaths dropped by 18%, 20 million and 172,000 between 2010 and 2017, respectively (Abamecha et al., Citation2020). The decline in malaria cases and the related deaths is attributed to the introduction of prompt and effective treatment with artemisinin-based combination therapy (ACT), distribution and use of long-lasting insecticidal nets (LLINs) and nationwide coverage of indoor residual spraying (IRS) (Beavogui et al., Citation2020; Kemibala et al., Citation2020). Moreover, strategies such as intermittent preventive treatment with pyrimethaminesulfadoxine in the second and third trimesters of pregnancy (IPTp) and seasonal malaria chemoprevention (SMC) for children less than 5 years of age and environmental management have contributed significantly to the decline of malaria related deaths and cases (Gutman et al., Citation2020; Somé et al., Citation2020).
Accessibility of vector control strategies is influenced by the cost of vector control products and this is a major impediment in the implementation of strategies to prevent and manage insecticide resistance and to reduce residual transmission of malaria (Global Technical Strategy for Malaria 2016–2030, Citation2016; Winskill et al., Citation2020). Vector control interventions can be well implemented if countries come up with more accurate predictions of vector control product requirements. Accessibility of vector control interventions are also underpinned on human resource, organizational and infrastructural development (Global Technical Strategy for Malaria 2016–2030, Citation2016). Despite extensive malaria control intervention campaigns, approximately 60% of the population in malaria-infested settings is still to gain access to effective malaria interventions (Odufuwa et al., Citation2020).
Containment of malaria is hampered by the emergence of multidrug-resistant strains of Plasmodium (). Of particular importance is the development of resistance by Plasmodium falciparum, and this presenting a handful of challenges including prophylaxis and limitation on the choice of drugs used (Verde et al., Citation2011). Antimalarial drugs such as chloroquine (CQ) and antifolates/sulfadoxine-pyrimethamine are no longer effective in most endemic areas (Bekono et al., Citation2020). Introduction of combination therapy has emerged which involves the use of artemisinin-based combinations including artesunate–amodiaquine (ASAQ), artemether–lumefantrine (AL), and dihydroartemisinin–piperaquine (DP)(Beavogui et al., Citation2020). Generally, artemisinin-based combination therapy (ACT) includes artemisinin or one of its derivatives and a complimentary drug. The partner or complimentary drugs include lumefantrine, piperaquine, mefloquine, amodiaquine,artemether, arteether, artesunate and pyronaridine (Pornputtapong et al., Citation2020; Wu et al., Citation2020). The adoption of artemisinin-based combination therapy (ACT) since 2001 has reduced the impact of malaria globally. However, recently the development of resistance in P. falciparum to ACT has been reported and this has emerged to be a major impediment in the control and elimination of malaria () (Wu et al., Citation2020). Resistance of P. falciparum to ACT was initially detected in western Cambodia six years after the introduction of ACT as the first line of malaria treatment and since then ACT induced resistance of P. falciparum has spread to all countries of the Greater Mekong Sub-region (GMS) (Wu et al., Citation2020). In Africa, resistance of P. falciparum to ACT has been in countries such as Uganda, Rwanda and Eritrea. However, resistance to ACT partner therapies is yet to be confirmed in the continent (WHO, Citation2022b). Unfortunately, there is limited reported data and extensive studies should be done to assess efficacy of ACT in Africa.
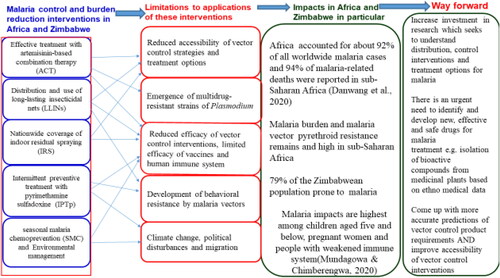
Figure 1. A synopsis of malaria control, treatment interventions, impacts, and mitigation in African countries including Zimbabwe.
Elimination and reduction of malaria is in line with the Global Technical Strategy (GTS) for Malaria 2016–2030, that targets the elimination of malaria from at least 35 countries and reduction of malaria cases and mortality by at least 90% by 2030 (Global Technical Strategy for Malaria 2016–2030, Citation2016). Current global malaria control strategies are anchored on three pillars associated with elements that direct global endeavors in reducing malaria transmission. According Global Technical Strategy for Malaria 2016–2030, (Citation2016), the first pillar ensures universal access to malaria prevention, diagnosis and treatment. The second and the third pillars involve acceleration of efforts towards elimination and attainment of malaria free status through transformation of malaria surveillance, respectively (World Health Organization, Citation2020; Premaratne et al., Citation2019).
2. Materials and methods
Literature analysis and data mining from primary and secondary databases were applied in order to extract relevant data. The literature was searched in scholarly electronic databases such as PubMed, MEDLINE, ClinicalTrials.gov, DOAJ, Europe PubMed Central, Web of Science and Google Scholar. Furthermore, the literature search was expanded to include reference lists in peer-reviewed scientific publications (snowballing literature review), government reports, reports from international organisations, literature from international bodies, dissertations, theses, and conference proceedings.
The literature search in this current study was based on (1) Boolean search using ‘OR’, ‘AND NOT’, ‘AND’, and ‘NOT’. Additionally, qualitative analysis of the evidence was applied. Notably, the Boolean search in this particular instance was categorized into two basic levels: (a) general search based on general information about ‘Malaria situation and elimination in Africa with specific focus on Zimbabwe’ and (b) specific objective search which focused on specific sub-sections: (1) The current state of malaria prevention and control, as well as their accessibility (in Africa with specific focus in Zimbabwe), (2) Antimalarial medications, resistance to antimalarial drugs, and the use of medicinal herbs in malaria therapy (in Africa with specific focus in Zimbabwe), (3) Malaria vector resistance to currently available control strategies (in Africa with specific focus in Zimbabwe), (4) Limitations of vaccines and human immune system in the fight against malaria (in Africa with specific focus in Zimbabwe), (5) Evidence of high malaria burden in Africa (in Africa with specific focus in Zimbabwe), (6) Malaria in the face of COVID-19 outbreak (infectious disease co-infection) (in Africa with specific focus in Zimbabwe), (7) Prevalence and contributing factors of malaria in Zimbabwe, (8) Steps to malaria elimination in Africa with specific focus on Zimbabwe.
A summary of the search strings used in this study include ‘malaria control interventions’, ‘Zimbabwe and malaria’, ‘Malaria treatment’, ‘Malaria prevalence in Zimbabwe’, ‘Malaria prevalence in Africa’, ‘malaria and Africa’, and ‘Africa and malaria therapy’. ‘Malaria and COVID 19 in Africa’, ‘malaria elimination and Africa’, ‘malaria elimination and Zimbabwe’, ‘COVID 19 and malaria in Zimbabwe’, ‘antimalarial drugs and resistance’, ‘resistance of malaria vectors and Zimbabwe or Africa’.
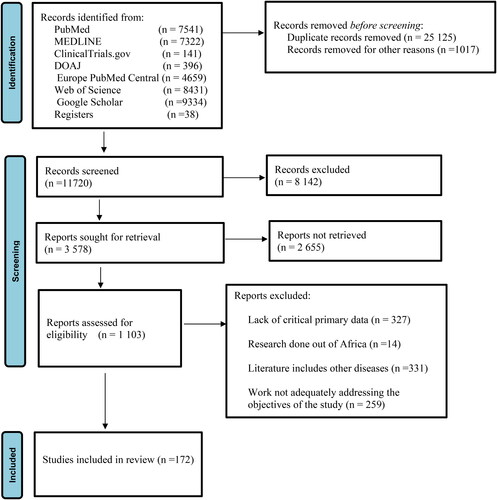
In order to determine relevance to the study, the retrieved articles were assessed by reviewing their abstracts. The articles focusing on ‘Malaria situation AND/OR elimination in Africa AND/OR Zimbabwe were then downloaded and fully reviewed. The results of each article were synthesized and summarized in line with the specific objectives (inclusion). The articles with primary data, accessible full texts and prepared in English language were considered in this case. Articles which were associated with the following traits were excluded (1) lack of critical primary data, research done out of Africa, literature including other diseases and work not adequately addressing the objectives of the study. Focus was mainly on articles with relevant primary data and in order to ensure integrity and quality of this review paper, the PRISMA 2020 Checklist was used and data summarised in a PRISMA flow diagram.
3. Results
3.1. Summary of exclusion and inclusion criteria based PRISMA 2020 checklist
The literature search yielded a total of over thirty six thousand (36,000) articles of which twenty five thousand one hundred and twenty five (25,125) were excluded as duplicates and up to one thousand and seventy (1017) were excluded based on titles and abstracts (). Only one thousand one hundred and three (1103) articles were retrieved and based on the exclusion criteria one hundred and eighty five (185) articles were included. Results for systematic literature search is summarised in .
3.2. The current state of malaria prevention and control, as well as their accessibility
3.2.1. In Africa
Most national malaria control interventions have advocated for universal accessibility of ITNs (WHO, Citation2017) and in period 2011 to 2016, over 800 million ITNs were distributed across sub-Saharan Africa (Olapeju et al., Citation2018). This has translated to an increase in number of individuals sleeping under ITNs from 30 to 54% between 2010 and 2016. Notably, between 67 and 73% of 663 million cases reported between 2000 and 2015 were prevented by ITNs in sub-Saharan Africa (World Health Organisation (WHO, Citation2017). There has been expansion in the provision of ITNs in sub-Saharan Africa between 2000 and 2016. However, a slight decline in coverage ITNs since 2017 was reported in Africa and, 65and 43% of households in Africa owned one or more ITN(s), and children below 5 years slept under ITNs in 2020, respectively (Balakrishnan, Citation2022). Although IRS and LLINs have been adopted in the fight against malaria in Africa, the coverage of these interventions is still below set targets (Allcock et al., Citation2018; Russell et al., Citation2015; Singh et al., Citation2013). It has been reported that accessibilityof IRS and LLINs have been hampered due to prohibitive costs in instances where distribution programmes are not free (Asingizweet al., Citation2019; Ahorlu et al., Citation2019), and lack of availability (Allcock et al., Citation2018; Sangaré et al., Citation2012). However, adoption and uptake of IRS and LLINs has been limited by lack of awareness campaigns on role of LLINs in malaria prevention (Bertheet al., Citation2019; Diemaet al., Citation2017; Allcock et al., Citation2018), and side effects such as skin irritation and residual effects of insecticides (Magaço et al., Citation2019). Alternative uses of LLINs as curtains, fishing nets, and seedlings protectors (Asingizwe et al., Citation2019) have been reported in Africa.
In Africa, ITNs have been attributed to 68% decline in prevalence of Plasmodium falciparum in period 2000–2015 (Slater et al., Citation2020). It should be noted that besides ITNs, seasonal malaria chemoprevention (SMC) and intermittent preventive treatment in pregnancy (IPTp) have been linked to significant reduction in morbidity in African countries (Foy et al., Citation2023). For example, SMC has been applied in West Africa and have effectively reduced malaria morbidity and incidence by about 70% (WHO, Citation2022a). Besides the common malaria control interventions such as IRS and LLINs, other methods involving modification of house (screens, ceilings, and eaves) (Reket al., Citation2018; Getawen et al., Citation2018; Tusting et al., Citation2017; Snetselaar et al., Citation2017; Gimnig and Slutsker, Citation2009), use of repellents(essential oils, spatial, and plants for example Lantana camara; Azadirachta indica and Cymbopogoncitratus) (Masalu et al., Citation2020; Tesfahuneygn & Gebreegziabher, Citation2019) and application of integrated vector (environmental and larval source management) have been applied in Africa (Nalinya et al., Citation2022).
In Southern Africa, countries including Zimbabwe, South Africa and Eswatini, mortality due to malaria was significantly reduced by dichlorodiphenyltrichloroethane (DDT) based indoor residual spraying (IRS) (Mabasoet al., Citation2004). The application of DDT based IRS in Sothern African countries started in the 1940s and was meant to control Anopheles funestus and Anopheles gambiae complex (Nkya et al., Citation2022). Sub-Saharan Africa saw a 50% reduction in malaria cases and a 40% reduction in clinical disease from 2000 to 2015, owing primarily to the extensive implementation of malaria prevention strategies including indoor residual spraying (IRS), and insecticidal treated nets (ITNs) (Oladipo et al., Citation2022). Apart from these vector control interventions, preventive malaria treatment (seasonal malaria chemoprevention (SMC) for under-five children, intermittent preventive treatment of infants (IPTi), and intermittent preventive treatment of pregnant women (IPTp)), treatment of malaria using Artemisinin combination therapy (ACT), and case management have significantly reduced the impact of malaria in the sub Saharan African region (Pryceet al., Citation2018, Kesteman et al., Citation2017).
IRS has reduced number of malarial cases by over 80% in southern Africa countries (Yukichet al., Citation2022). However, the application of IRS in sub Saharan has recently been reduced and this has been reported in Senegal, Tanzania, Madagascar, and Zambia, where IRS application was reduced by 64, 68, 56 and 63%, respectively (Oladipo et al., Citation2022). However, the implementation of prevention chemotherapy in Africa has increased significantly. For instance, 2018, IPTp coverage increased in Madagascar, Nigeria, DR Congo, Madagascar and Nigeria by 23, 23.5, 9.3, 23.1, and 22.5%, respectively (González et al., Citation2023). The implementation and universal accessibility of all these malaria control interventions is linked to investments towards malaria control. For example, up to $2.7 billion was availed for the fight against malaria in 2018 against $8.1 billion which was required for universal access to malaria control interventions (WHO, Citation2019).
3.2.2. In Zimbabwe
The National Malaria Control Programme in Zimbabwe uses vector control (ITNs and IRS) in malaria burdened districts (Gavi et al., Citation2021). In regions with moderate to high transmission, half of the households possess a minimum of one ITN for any two members of the household, and 85 percent of households have at least one ITN and are protected by IRS, indicating a significant degree of protection (Dube et al., Citation2019). For instance, in Mutasa District, Manicaland Province, IRS and ITNs were applied as vector control strategies for 6 and 2 decades, respectively (Mharakurwa et al., Citation2013). In 2014, approximately 92% of the population in Mutasa District was covered by IRS (Sande et al., Citation2016), and during this period, access to ITNs was limited. Between 2012 and 2017, it was noted that 70% of households had least one ITN (Kanyangarara et al., Citation2018). Additionally, with the confirmation of An. arabiensis, An. gambiae complex and An. quadriannulatus as malaria vectors in Zimbabwe, IRS or LLINs have been applied in Malarious distrcts (Sande et al., Citation2015; Masendu et al., Citation2005). Malaria vectors reported in Zimbabwe include Anopheles parensis, Anopheles leesoni, Anopheles confuses, An. funestus, Anopheles aruni (Sande et al., Citation2016). In Chiredzi and Mutasa Districts, An. funestus was identified as one of the problematic vectors (Sande et al., Citation2015; Lukwa et al., Citation2014). However, An. funestus was not widely distributed in Chiredzi district due to IRS application (Masendu et al., Citation2005).
According to Kanyangarara et al., Citation2018, the number of households with one ITN per two people in Zimbabwe was approximately 60.3%. These findings indicate that there is still need to improve accessibility especially malaria burdened districts. Moreover, it was noted that IRS offered protection to more than 80% of malaria vulnerable population in Mutasa and Mutare Districts. Benzene and DDT based IRSs were from 1940s (Pates and Curtis, Citation2005, Mabaso et al., Citation2004), and currently are among the malaria control interventions in Zimbabwe. Notably, IRS implementation in Zimbabwe was in line with recommendation by WHO which targets population coverage of over 80% (Sande et al., Citation2016). However, in Zimbabwe, IRS and ITNs are applied in districts or areas associated with annual parasite index (API) of 5 and 2–4 per 1000 population, respectively U. S. President’s Malaria Initiative, 2020).
3.3. Antimalarial medications, resistance to antimalarial drugs, and the use of medicinal herbs in malaria therapy
3.3.1. In Africa
The treatment of choice for uncomplicated, severe malaria, and expectant mothers are distinct. Chloroquine has been used as first-line antimalarial for uncomplicated malaria caused by P. falciparum in sub-Saharan up to early 2,000 (Flegg et al., Citation2013). African countries such as Zambia, South Africa, Kenya and Tanzania switched to first line malaria treatment based on sulfadoxine/pyrimethamine (SP) in response to chloroquine resistance (Mwendera et al., Citation2016). However, P. falciparum gene mutations have resulted in SP treatment failures (Desai et al., Citation2015). Recently, ACTs have been considered has the primary drug for malaria and in sub-Saharan Africa antimalarial drugs such as artesunate-amodiaquine and artemether-lumefantrine have been used in uncomplicated malaria treatment (Conrad and Rosenthal, Citation2019; WHO, Citation2018).
The antimalarial drugs recommended by the WHO African region include artesunate-pyronaridine, dihydroartemisinin-piperaquine and artesunate mefloquine. Apart from these antimalarial drugs, antimalarials such as artemisinin-piperaquine, artesunate-sulfadoxine-pyrimethamine, arterolane-piperaquine and artemisinin-naphthoquine are in use in Africa (Mhamilawa et al., Citation2020; Conrad and Rosenthal, Citation2019). In cases where intravenous treatment was impossible, rectal artemether and artesunate, and intramuscular artesunate, have been administered (Kremsner et al., Citation2016). However, in African settings, intravenous quinine has always been used in the treatment of severe malaria (Esu et al., Citation2014). Antimalarial preventive drugs including sulfadoxine-pyrimethamine and amodiaquine-sulfadoxinepyrimethamine are used to prevent malaria during pregnancy and in children during rain seasons (Desai et al., Citation2018, Kayentao et al. Citation2013). For travellers visiting high malaria transmission African countries, malaria chemoprophylaxis is mainly based on mefloquine, atovaquone-proguanil and doxycycline (Shellvarajah et al., Citation2017).
Chloroquine resistance was first reported in 1978 and this type of resistance had spread in countries such as Zambia, Sudan, Malawi and Uganda by 1983 (WHO, Citation2013). This has resulted in discontinuation of chloroquine based malaria treatment in most African countries and replacement with sulfadoxine/pyrimethamine (SP) in countries such as Malawi in the early 1990s (Severini and Menegon, Citation2015). It has been reported that parasites resistant to sulfadoxine-pyrimethamine and chloroquine in Africa originated from Southeast Asia. According to Ehrlich et al., (Citation2020), there was a significant decrease in Asn86Tyr, pfmdr1 pfcrtLys76Thr and Asp1246Tyr prevalent in sub-Saharan Africa. This indicates an increase in the prevalence of lumefantrine resistant parasites and resurgence of chloroquine sensitive parasites.
Chloroquine resistance was implicated to mutations in the CQ-resistant transporter (pfcrt) gene and multidrug-resistance gene 1 (pfmdr-1) in P. falciparum (Bin Dajem & Al-Qahtani, Citation2010). The resistance of malaria parasites to CQ was confirmed with CQ treatment in Tanzania and Kenya as early as 1978 and by 1980 it was reported in West Africa (Roux et al., Citation2021). Among other factors, antimalarial drug resistance in Africa has been linked to ineffective use of antimalarial drugs and policy changes. For instance, in Kenya, it was reported that the implementation of IPTp and first-line malaria treatment policy has been followed by the emergence of DNA drug resistance markers (Hemming-Schroeder et al., Citation2018).
Although resistance to chloroquine was noted in African countries, chloroquine has remained as a therapy option for uncomplicated malaria caused by P falciparum (Kgoroebutswe et al., Citation2020). In the early 1990s, P falciparum resistance to chloroquine was reported in southern and eastern part of Africa (Conrad and Rosenthal, Citation2019). However, use of ACTs as alternatives of chloroquine, has resulted in the emergence of malaria parasites with pfmdr1 and pfcrt sequences in the eastern parts of Zimbabwe. The reported shift in polymorphism after the introduction of ACTs has resulted in parasites that are sensitive to chloroquine (Hemming-Schroeder et al., Citation2018; Tumwebaze et al., Citation2017). Up to 90% of countries in sub-Saharan Africa had started using artemisinin-based combination therapy (ACT) for malaria treatment in response to SP and CQ resistance (Frosch et al., Citation2011). Disturbingly, reports of isolated cases of malaria parasites resistant to artemisinin have been noted in sub-Saharan Africa. However, artemisinin resistant parasites are yet to be established in sub Saharan Africa (Lu et al., Citation2017). Although the resistance of malaria parasites to piperaquine is limited in Africa, it was reported in Uganda (Conrad et al., Citation2014).
3.3.2. In Zimbabwe
The cases of chloroquine (CQ) resistance in Zimbabwe were first reported 1984 in Zambezi Valley. Before 1990 chloroquine resistance was confined to Zambezi Valley (Makono and Sibanda, Citation1999) and according to Mharakurwa and Mugochi, (Citation1994), out of the 83% of the chloroquine resistant cases reported in Gokwe, only 3% responded to chloroquine treatment. Although CQ failure was significantly high, the widespread use CQ continued until 2000 (Mlambo et al., Citation2007). Resistance to CQ by parasites has resulted in replacement CQ with artemether-lumefantrine and sulfadoxine-pyrimethamine in 2008 and 2008, respectively. Up to 67% of sampled malaria parasites in 2003 in Mutasa District were resistant to CQ and this was associated with CQ resistance transporter gene (pfcrt) (Ministry of Health and Child Welfare (Zimbabwe), Citation2007). However, in 2013, only 3% of samples had pfcrt mutants, and between 2017 and 2018, 100% of P. falciparum were pfcrt wild type. The presence of P. falciparum with pfcrt wild-type indicated the emergence of CQsusceptible P. falciparum (Mharakurwa et al., Citation2021).
The use plants against malaria was reported in Sub-Saharan African countries. Although in most African countries people have continued to use plants against malaria without ethnobotanical studies, in countries such as Guinea, Kenya, Nigeria and Uganda studies were conducted (Chinsembu, Citation2015). The use of plants including Cassia singueana against malaria in African countries such as Tanzania, Nigeria Burkina Faso and Kenya has been reported (Hiben et al., Citation2016). Apart from Cassia singueana, Azadirachta indica has been used in the management of malaria in Ivory Coast, Kenya, Sudan, Ghana, and Madagascar (Muthaura et al., Citation2011). According to Carraz et al., (Citation2006), Strychnopsis thouarsii is a malaria prophylactic plant in Madagascar. Antimalarial plants have been used in the management of malaria in Zimbabwe. According to Ngarivh et al. (Citation2015), such as Aristolochia albida, Elephantorrhiza goetzei (Harms), Cassia abbreviate, Toddalia asiatica (L.) Lam and Pavetta schumanniana F. Hoffm have been utilized in Manicaland Province, Zimbabwe.
3.4. Malaria vector resistance to currently available control strategies
3.4.1. In Africa
Resistance of malaria vectors to all insecticides recommended by WHO was reported in Africa between 2001 and 2012. In countries such as Zimbabwe, An. funestus and An. gambiaes.l were found to be resistant to carbamates, pyrethroids and organochlorines (Knox et al., Citation2014). The stagnation in the gains made against malaria has been promoted by widespread malaria vector resistance to insecticides (e.g. pyrethroids, organophosphates, carbamates) used in IRS and ITNs. For instance, in Burkina Faso high malaria burden has been linked to vector resistant to pyrethroids (Toé et al., Citation2014). Apart from resistance to commonly used pesticides, behavioral resistance (e.g. resting outdoors, biting outdoors, and changing of biting at times) has been noted in malaria parasites (Foy et al., Citation2023).
Resistance of An. funestus to pyrethroids and carbamates was reported in Africa (Choi et al., Citation2014, Cuamba et al., 2011). In African countries including Mozambique (Casimiro et al., Citation2006), An. arabiensis was found to have reduced resistance to carbamates and An. gambiaes.s. with resistance to pyrethroids and DDT were detected (Chanda et al., Citation2011). Resistance to insecticides was largely linked genetic changes due to metabolic resistance and mutations (Gogue et al., Citation2020; Chanda et al., Citation2020).
The establishment and invasion of Anopheles stephensi in African urban areas (Sinka et al., Citation2020), has resulted in the fight against malaria to be stalled. This malaria parasite (Anopheles stephensi) can proliferate successfully in urban areas and this is linked to increased adaptability of the parasite to breeding in highly polluted aquatic environments (Batra et al., Citation2001) and ability to make use of purpose-built water storage reservoir in urban areas (Thomas et al., Citation2016). Additionally, A. stephensi is insecticide resistant to insecticides used in IRS. For example in a study conducted by Teshome et al., (Citation2023), it was indicated that A. stephensi is resistant to a number of insecticides used in IRS and LLIN.
3.4.2. In Zimbabwe
Malaria vector resistance has significantly hampered malaria control efforts in Zimbabwe. For instance, pyrethroid resistance was reported in Mutasa District, Manicaland Province, Zimbabwe in 2014 (Wesolowski et al., Citation2022). The shift from pyrethroids based IRS to organophosphates spraying in Zimbabwe was prompted by An. funestus resistance to carbamates and pyrethroids (Kanyangarara et al., Citation2016; Choi et al., Citation2014).
In 2014, insecticide An. funestus was reported in Mutasa and Mutare Districts. An. arabiensis samples collected from Chiredzi District, Masvingo Province, Zimbabwe in the early 1980 were resistance to benzene hexachloride (Green, Citation1981). In addition to this, resistance to permethrin and DDT was noted in An. arabiensis from Gokwe District (Masendu et al., Citation2005) and (Munhenga et al., Citation2008). Malaria vectors in Zimbabwe, An. arabiensis and An. funestus, have shown behavioral resistance and continued to spread malaria parasites even if there are efficient vector control methods. For example, An. arabiensis and An. gambiae complex collected from Gokwe South District were exophilic and therefore making malaria vector control difficult. Up to 16% of An. funestus sampled from Mutasa and Mutare Districts had exophilic features and 10% of those found indoors were on unsprayable surfaces (Sande et al., Citation2016). This therefore, presents a new dimension in fight against malaria in Mutare and Mutasa Districts, Manicaland, Zimbabwe.
The use of benzene hexachloride in malaria control programmes in Zimbabwe has resulted in the emergence of malaria vectors (An. arabiensis) which were resistant to benzene hexachloride in Chiredzi district. According to Munhenga et al., (Citation2008), An. arabiensis from Gokwe was found to be resistance to DDT and permethrin. Apart from this, resistance to pyrethroids, carbamates, and organophosphates was noted among An. funestus (Choi et al., Citation2014). Resistance of malaria vectors to insecticides in areas of high agricultural activities (Gwave, Gokwe (cotton production) (Munhenga et al., Citation2008), Burma Valley (tea and banana farming) (PMI Citation2014), Triangle Estates (Sugarcane production), Hippo Valley (Sugarcane production) and Honde Valley (tea and banana farming) (Munhenga et al., Citation2008) indicates that malaria vector resistance in Zimbabwe has been shaped by the use of insecticides in agricultural activities.
3.5. Limitations of vaccines and human immune system in the fight against malaria
Although people in malaria-endemic areas progressively develop a natural immune response against deleterious effects of malaria, this type of immunity is not sterilizing and Plasmodium infections associated with malaria tolerance may still occur throughout the lifetime of immune individuals (Nhama et al., Citation2020). Intriguingly, these immune tolerant individuals may be reservoirs for malaria transmission as they migrate to settings where malaria is less common (Dwomoh et al., Citation2020). Individuals in areas where malaria prevalence is low remain equally naive and susceptible to malaria once they get infected and irrespective of their age. The development of an effective malaria vaccine and protective immunity is limited by the emergence of genetically distinct malaria parasites in natural populations. Genetic variability among Plasmodium species is as result of high rate of genetic recombination that takes place during the sexual stages in female Anopheles mosquitoes. The net effect of this phenomenon is transmission of multiple strains simultaneously and reduction of an antibody–mediated parasite inhibition (Abamecha et al., Citation2020). Even though people from malaria endemic areas are immune to multiple P. falciparum strains due to a series of exposure, genetic diversity of malaria parasite is one of the major contributing factors responsible for the slow acquisition of natural immunity against malaria (Abamecha et al., Citation2020).
3.5.1. In Africa
Malaria vaccine research has evolved in response to a rise in malaria vector and drug resistance. In Africa, twenty-four malaria vaccines are being evaluated at 99 experimental sites (Bhagavathula et al., Citation2016). Mosquirix™ (RTS, S), a vaccine designed against P. falciparum, received approval in the African region in 2015. Though the vaccine (Mosquirix™) is effective against P. falciparum, it was found to be ineffective against infant severe malaria in a Phase III study (RTS. S Clinical Trials Partnership, Citation2014). Based on research findings which indicated that RTS,S/AS01 has significantly low effectiveness protecting infants against malaria, WHO recommended the RTS,S/AS01 for children aged 5 months above. However, WHO approved and recommended the use of Mosquirix among infants in Sub-Saharan Africa in 2021 (Kolawole et al., Citation2023). The approval of malaria vaccine, RTS, S (Mosquirix) in Africa was based on successful pilot studies in subSaharan African countries: Ghana, Kenya and Malawi (Oladipo et al., Citation2022; WHO, Citation2021b).
RTS, S/AS01 was assessed in children and infants when given as a 3-dose vaccine associated with a booster dose. It was noted that the third dose of RTS,S/AS01 was 31.1 and 55.8% effective in protecting infants (6–12 weeks) and children (5–7 months) against the initial and only episode associated with clinical malaria within a period of one year, respectively (RTS. S Clinical Trials Partnership, Citation2015). Aside from the 3-dose vaccine regime, WHO has recommended a 4-dose vaccine regime. Based on findings from a phase 3 investigations, it was concluded that RTS, S/AS01 efficacy among children who were given 4-doses was 36% in 4 years (RTS. S Clinical Trials Partnership, Citation2015). Generally, it was shown that the efficacy of RTS, S/AS01 against clinical malaria decreased with an increase in the follow-up period for both infants and children and dosage systems (3- and 4-dose vaccine regimens). However, the decrease efficacy with an increase in follow-up period was faster in the 3-dose dose vaccine regime than 4-dose group (RTS. S Clinical Trials Partnership, Citation2015). Recently, data from a trial conducted in in west Africa, based on a seven-dose regimen of RTS,S/AS01 indicated an efficacy of 73 and 58% over a follow –up period of 1 and 5 years, respectively (Datoo et al., Citation2024).
Of the vaccinated infants (830,000) in the trials conducted in Ghana, Kenya and Malawi, above 30 and 40% reduction of admitted infants with severe malaria and malaria episode were reported, respectively (Oladipo et al., Citation2022). The reduced efficacy of Mosquirix from its original efficacy which lies between 60 and 70% has serious implications on WHO target of producing malaria vaccine which can protect over 75% of people in malarious regions by 2030. Although there other malaria vaccines such as R21 with efficacy above 70% (Datoo et al., Citation2021), their efficacy in malaria perennial regions is yet to be explored. However, based on data from a 3 phase trial (conducted in Burkina Faso) of Matrix-M (R21), it was shown that R21 has an efficacy of 75% and 67% at seasonal and standard sites among participants of age 5 to 36 months over period of 1 year, respectively (Datoo et al., Citation2024). Generally, it was revealed that when efficacy was followed up after every 3 months over a period 12 months, a decline was noted and finally maintained at above 60% in the last quarter of the year (Datoo et al., Citation2024). The data from this trial was consistent with findings from a phase 2b trial at which was done in Burkina Faso, where the efficacy of R21 was 76% (follow up period of 1 year) (Datoo et al., Citation2021) and 77% over a period of 2 years using a 4-dose R21 regimen for children (age: 5–17 months) (Datoo et al., Citation2022). Following these clinical trials, R21 was approved for use in Nigeria and Ghana (WHO, Citation2024).
High prices, along with compliance and viability issues, may impede the acceptance of such malaria vaccines in Africa (Bhagavathula et al., Citation2016). According to Maxmen, (Citation2021), RTS,S vaccine is characterized by a modest efficacy and requires complex regime of doses and sufficient funding. Scaling up malaria vaccine, however, encounters inherent problems associated with vaccination programs, which include distribution, declining immunity, and financing (Dimala et al., Citation2018). Generally, there is really limited dose supply available for RTS, S in Africa. The capacity of vaccine production in Africa is extremely low as local vaccine production depends on external support (Abiodun et al., Citation2021). Out of the 54 African countries, only 8 have vaccine-manufacturing companies and only Senegal has been approved by WHO to sell vaccines to other countries (Okereke et al., Citation2022). Local production of vaccines in Africa has been linked to poor investment in research, insufficient skills transfer and exodus of highly skilled professionals (Ekström et al., Citation2021). Though this information can apply to on RTS, S and R21 vaccines, it should be noted that this pertains to COVID-19 vaccines. Notably, there are significant differences in GMP production for an mRNA vaccine (COVID-19 vaccines) and malaria (RTS, S and R21) vaccines.
3.6. Evidence of high malaria burden in Africa
In the African region over 67% of deaths due to malaria were among those under age of 5 years (WHO, Citation2021a). In 2020, it was noted that the WHO African region accounted for over 96% and 95% of all malaria related deaths and malaria cases, respectively (WHO, Citation2021a). Up to 50% malaria deaths Worldwide are accounted for in five African countries: Tanzania, Nigeria, DRC and Mozambique (Okokon et al., Citation2010). It was reported that only 15 countries in the WHO African region made significant progress or achieved the target of reducing malaria incidences by a minimum of 40% (African Union, Citation2021). The countries that reduced incidences of malaria by 40% include Ethiopia, Ghana, Cabo Verde, Mauritania, and Gambia. However, the countries that have made significant progress include Eswatini, Togo, Rwanda, Kenya and Equatorial Guinea. Notably, only two African countries recorded zero malaria related deaths since 2018 and these are CaboVerde and Sao Tome & Principe (African Union, Citation2021; WHO, Citation2021a). The WHO African region recorded 405,000 malaria related deaths and up to 28 million cases of malaria casesin 2018 and this translated to 94% and 93% of global malaria deaths and cases, respectively (WHO, Citation2019).
Though there was a significant decrease of malaria incidences (by 17%) and deaths rates (by approximately 26%) in the African region between 2000 and 2013, in some African countries the progress in the fight against malaria has significantly stalled and in some cases reversed since 2014 (Mbacham et al., Citation2019). In 2017 alone, the WHO African region contributed to 92% of global malaria deaths and cases. Generally, a drop in malaria cases and deaths of 38 and 67%, respectively, were reported between 2000 and 2019 (WHO, Citation2020). However, in 2020, malaria case incidence sharply increased due to disruption in the fight against malaria in wake of COVID-19 (WHO, Citation2021a).
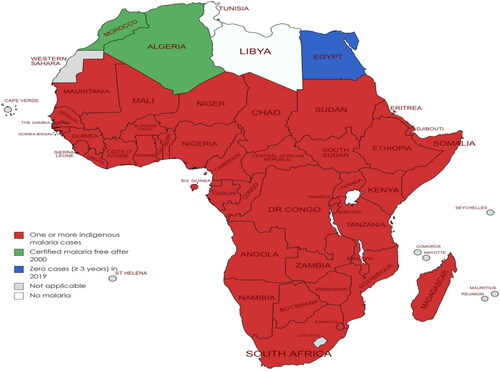
The number of malaria cases increased from 227 to 241 million in the period 2019 and 2020. However, a decrease in the number of malaria related death (by 44%) was reported between 2000 and 2019, and this was associated by significant decrease in death rate by 67% in the same period (WHO, Citation2021a). According to Balakrishnan, (Citation2022), contribution of African countries to global malaria is significantly high. For example, Nigeria, Mozambique, DRC, and Tanzania contribute up to 53% of global malaria deaths. Contrary to this, some African countries have significantly made progress in the fight against malaria. For instance, in 2019, Algeria was certified as malaria free. Generally, malaria cases are reported in many parts of Africa ().
Figure 3. African countries: malaria cases distribution and current status in 2019 (Adapted from WHO database) (WHO, Citation2020). (No Copyright: You can copy, modify, distribute and perform the work, even for commercial purposes, all without asking permission).
Although COVID-19 disrupted malaria control interventions in 2021, significant progress was made in the fight against malaria in the African Region in this period. For instance, in 2021 malaria related deaths dropped slightly to 593,000 from 599,000 reported in 2020 (African Union, Citation2021). In countries such as Cabo Verde malaria cases were reduced to zero since 2018 and in quite a number of African countries (Ghana, Zimbabwe, Mauritania, Togo, South Africa, Ethiopia, and Sierra Leone) significant decline in malaria cases have been noted (WHO, Citation2022a). Notably, the decrease in the number of malaria cases was very high in three countries (Mauritania, Zimbabwe and Ethiopia), with percentage decrease between 68 and 71. Moreover, the number of malaria related deaths decreased significantly in South Africa, Ethiopia and Togo, with highest decrease recorded in Ethiopia (68%) (WHO, Citation2022a). However, in most malaria burdened African countries the decrease in malaria deaths and cases has stalled. In 2021, two countries in the African region (DRC and Nigeria) contributed 47 and 41% of malaria deaths and cases, respectively (WHO, Citation2021a).
In 2018, the ‘high burden to high impact’ (HBHI) initiative was launched in order to reduce malaria burden in most malaria burdened African countries including Niger, DRC, Uganda, Burkina Faso, Nigeria, Mozambique and Ghana (WHO, Citation2019). It has been shown that in the HBHI countries, malaria cases increased in the period 2015–2021. The most impacted countries were DRC, Nigeria and Uganda as indicated by 36, 19 and 25% increase in malaria related deaths between 2015 and 2021, respectively (African Union, Citation2021; WHO, Citation2022a). Aside from the WHO African region missing the GTS set targets (2020), African countries such as Mauritania, Cabo Verde, Gambia, Zimbabwe, Gambia, Algeria, Ghana, Ethiopia, Rwanda, and South Africa were able to reducing malaria incidences by 40% or more (WHO, Citation2022a). Although the HBHI was meant for ten highly malaria burdened countries, small population countries with high malaria incidences such Burundi, Somalia and Guinea implemented HBHI intervention (WHO, Citation2019).
3.7. Malaria in the face of COVID-19 outbreak (infectious disease co-infection)
3.7.1. Africa
COVID-19 seriously disrupted malaria control interventions in African countries and the most impacted were the HBHI countries. Due to COVID-19, African countries were faced with three major hurdles: (1) reducing the instantaneous consequences of COVID-19 on health, (2) minimizing interruptions to vital services, and (3) maintaining a healthy population in the midst of more generalized economic problems. Consequently, the progress achieved before the implementation of HBHI intervention was reversed and this has interrupted the rolling out of the HBHI approach. It was noted that in 2021, countries including Mozambique, Burkina Faso and Cameroon were yet to bounce back to pre-COVID-19 progress (in 2019) (WHO, Citation2022a). According to WHO (Citation2020), the disruptions in the fight against malaria as a result of COVID-19 has contributed significantly to an increase in malaria related deaths of approximately 49,000. As result of this, COVID-19 has negatively impacted on economies of several malaria burdened nations and significantly threatened the delivery of malaria prevention and treatment resources (Gao et al., Citation2023). The measures which were implemented to fight against COVID-19 in African countries impacted negatively on progress made against malaria. For instance, lockdowns reduced availability and accessibility of malaria control interventions (Park et al., Citation2023). In order to prevent the spread of COVID-19, measures including movement restrictions, quarantining, curfews, travel bans, and lockdowns were introduced (Patlolla et al., Citation2022). The implementation of these interventions is believed to have resulted in resurgence of malaria (Mahajan et al., Citation2021). Of the 12 and 5% increase in malaria related deaths and incidence rate reported 2020, respectively, most of them were from the WHO African region and has a result of disruptions associated with COVID-19 (Shah et al., Citation2015).
Apart from the identified factors, malaria vaccine hesitancy is a potential barrier in the fight against malaria. For instance, it was noted that one of the factors which influence malaria vaccination programs includes acceptability of the vaccines such as RTS, S/AS01. Additionally, it was indicated that acceptability is a function of vaccine side effects concerns, quality of healthcare services and community engagement (Mumtaz et al., Citation2023). In a study which was done in Tanzania, Ghana and Nigeria, the acceptance rate of RTS,S vaccine ranged from 92.5 to 96.3%. Although acceptance rate was high, the reasons which were identified for not accepting the malaria vaccines include issues around multiple injections, efficacy profile, safety concerns, and low awareness (Sulaiman et al., Citation2022). RTS, S/AS01 receptivity was identified to be anchored on inadequate awareness, fear of side effects associated with RTS, S/AS01, inefficient vaccination services delivery and poor quality of health services (Dimala et al., Citation2018).
3.7.2. In Zimbabwe
The malaria cases recorded in Zimbabwe (from the beginning of year up to June 2020) were in excess of 30,000 as compared to those recorded in 2019, 2018 and 2017 (in the same period) (Gavi et al., Citation2021). Additionally, malaria related deaths recorded up to June 2020 (350) were significantly higher the yearly totals recorded in the year 2019 and 2018 (311 and 232, respectively). It was concluded that high malaria deaths and cases reported up to June 2020 coincided with the beginning of COVID-19 (Gavi et al., Citation2021). Of all the 62 Zimbabwean districts, 11 had more than expected incidences of malaria up to June 2020 and this indicated possibility of malaria outbreak. Among these 11 districts three are in malaria burdened provinces (Manicaland, Masvingo, Mashonaland East and Mashonaland Central) (Gavi et al., Citation2021). In districts such as Gweru, Centenary, Mazowe, Hwedza, and Makonde the reported mortality due to malaria was more than three times the expected malaria mortality up to June 2020 (Gavi et al., Citation2021).
3.8. Prevalence and contributing factors of malaria in Zimbabwe
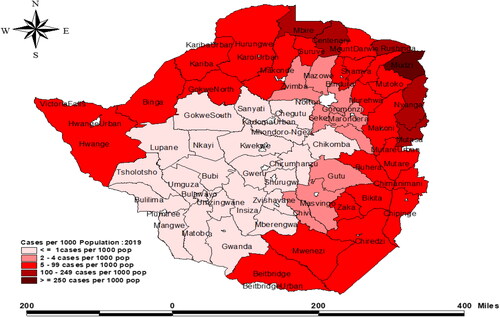
In 2018 alone, WHO reported that out of the 14 million Zimbabweans, 4 million were vulnerable to malaria and up to 1 million malaria cases were confirmed (Gwitira et al., Citation2020). Zimbabwe is subdivided into 10 provinces, which are further subdivided into sixty-five districts, of which 63 are rural. Malaria districts account for approximately 75% of the 65 districts, with 64% classified as high malaria burdened districts (Zimbabwe National Statistical Agency, Citation2012). Malaria prevalence in Zimbabwe is affected by seasonal and geographical variations that are directly linked to rainfall patterns (US President’s Malaria Operational Plan FY, Citation2018; Sande et al., Citation2016). Zimbabwe’s districts are separated into two zones characterized by low and heavy rainfall. Malaria transmission is lower in low rainfall areas such as parts of Matabeleland South Province (less than 700 mm) and higher in high rainfall areas such as Manicaland Province (more than 1,500 mm) (US President’s Malaria Operational Plan FY, Citation2018; Coleman et al., Citation2009). Prevalence of malaria in Zimbabwe is highest among children under the age of five, pregnant women and people with weakened immune system (Chapu & Mgocheki, Citation2017; Mosha et al., Citation2014). Generally, malaria transmission in Zimbabwe is seasonal and directly linked to the geographic variation in rainfall patterns and topology (Manyangadze et al., Citation2017). In line with this transmission pattern, nearly 80% of annual malaria cases are confined to the northern and eastern provinces of Zimbabwe. The most impacted provinces are Mashonaland Central, Mashonaland North, and Manicaland (), these amounts to 30% of the country’s total number of provinces (US President’s Malaria Initiative Plan, Citation2020).
Table 1. Distribution of parasitologically confirmed malaria cases in Zimbabwe.
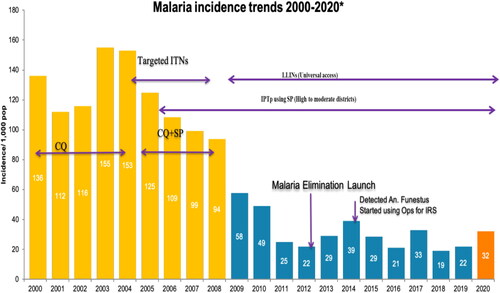
Malaria is a serious disease that affects individuals of all ages in Zimbabwe and according to a 2016 WHO report, 79% of Zimbabweans are susceptible to malaria. Additionally, in the same year, the number of reported malaria cases at the eastern borders of Zimbabwe was significantly high and contributed up to 82% all recorded malaria cases (President’s Malaria Initiative, Citation2018). The malaria incidence was reported to be 139 per 1000 people in 2013. However, between 2012 and 2014, there was a significant increase in malaria-related fatalities (from 6.1 to 13.8%) (US President’s Malaria Operational Plan FY, Citation2018). Malaria-related mortality is reported year round, despite the fact that disease transmission occurs mostly between November and April (US President’s Initiative, Citation2016). The annual number of malaria cases documented changes between 300,000 and 500,000, with a notable continuous decreasing trend recently (). This success prompted a shift in emphasis from malaria control to elimination in a number of provinces including Midlands and Matabeleland, coupled with the launch of pre-elimination of malaria in selected regions (Sande et al., Citation2017). However, there seems to be insufficient and inconclusive evidence that malaria incidences have substantially dropped. In 2019, for example, nearly 310,000 malaria cases (22 per 1000) were recorded across the country (). (President’s Malaria Initiative, Zimbabwe, Citation2020). This was a 19% rise in reported cases of the previous year 2018, and this was associated with an increase in malaria deaths by up to 12.8% from 2018 to 2019 (US Pesident’s Malaria Operational Plan FY, Citation2022) (). In addition, in 2020, malaria occurrences in the country where abnormally high with 135,585 recorded cases and 131 malaria related-fatalities as of April 2020, primarily from four provinces: Matabeleland South, Manicaland, Mashonaland East and Masvingo (US President’s Malaria Operational Plan FY, Citation2022). In light of the resources allocated to malaria control and eradication, as well as the Zimbabwean economy and policies, it is vital to assess if malaria pre-elimination is realistic.
Figure 4. Malaria incidence trends from 2000 to 2020 in Zimbabwe and associated malaria control interventions. Source: Zimbabwe District Health Information System 2 (US President’s Malaria Operational Plan FY, Citation2022). (No Copyright: You can copy, modify, distribute and perform the work).
Malaria is an endemic disease in Zimbabwe and is among the country’s top five causes of illness and death. The Ministry of Health and Child Care (MOHCC) controls malaria through the National Malaria Control Program (NMCP), which employs several interventions such as case management, malaria vector control strategies, and behavioral modification communication (US Pesident’s Malaria Operational Plan FY, Citation2017). The transmission of malaria in Zimbabwe is seasonally and geographically variable and is positively correlated to environmental factors such as altitude and rainfall fluctuations in the country (Mundagowa and Chimberengwa, Citation2020). Districts in the northwestern to the southeastern boundaries of the nation are classified as high malaria transmission zones, whereas districts in the central and southwestern parts of the country are being associated with very low or lack of malaria incidences and transmission () (US President’s Malaria Operational Plan FY, Citation2018). Malaria transmission is most common during the rainy season, when temperatures range between 18 and 30 degrees Celsius. Plasmodium falciparum is the most common malaria pathogen, accounting for 98 percent of all confirmed malaria incidences in Zimbabwe (US President’s Malaria Operational Plan FY, Citation2019, Citation2018). In malaria-burdened areas, transmission occurs all year, with most of incidences occurring between or soon after November to April rainy season.
Figure 5. Malaria cases distribution in Zimbabwe. Source: https://www.cdc.gov/globalhealth/countries/zimbabwe (No Copyright: You can copy, modify, distribute and perform the work).
In the past 13 years, the Zimbabwe NMCP and the Roll Back Malaria Programme (RBMP) have had significant gains in fighting against malaria, leading to a shift in focus from malaria prevention and control to eradication (Sande et al., Citation2016, Citation2017). The two programs, whose administration is centralized in Harare, are guided by provincial and disease control managers who obtain data from district health care teams, whose main aim is to coordinate malaria case management, monitoring, and epidemic response at the local scale (US President’s Malaria Operational Plan FY, Citation2017). The Zimbabwean 2016–2020 National Malaria Strategic Plan directs the deployment of IRS in regions with an annual parasite index (API) of 5 or higher per 1000 inhabitants. LLINs are distributed in locations with an API of 2–4 per 1000 people and the IRS intervention is a routine annual strategy in Zimbabwe, carried out to promote malaria outbreak containment and abatement (US President’s Malaria Operational Plan FY, Citation2022). The NMCP uses a double strategy towards vector control, deploying both ITNs and IRS to malaria endemic regions. In moderate- to - high incidence zones, 51% of homes possess at least one ITN per two household members, and 85% own at least one ITN and are sometimes protected by IRS, indicating excellent protection levels (US President’s Malaria Operational Plan FY, Citation2022).
Despite tremendous progress in achieving malaria eradication, some periodic outbreaks continue to reemerge. For example, Beitbridge District has seen a second malaria epidemic within a year, in 2017. Malaria eradication efforts have been hampered by malaria vector and pathogen insecticide and anti-malarial drug resistance, varied vector behavior, and malaria vector new regions colonization (Gunda et al., Citation2016). Changing climate, inconsistent funding, economic crisis, political disruptions, and growing cross-border human migrations have all hampered the malaria control programs (Gwitira et al., Citation2018; Mundagowa & Chimberengwa, Citation2020). These variables have aided intermittent malaria epidemics across Zimbabwe.
3.9. Steps to malaria elimination in Africa with specific focus on Zimbabwe
After the introduction of the E-8 initiative, malaria incidence status in eight provinces of Zimbabwe was assessed in line with WHO guidelines (WHO, Citation2007). The classification of provinces on malaria status and progress on elimination were based on this criterion: (1) pre-elimination, (2) reintroduction prevention, (3) consolidation and control, and (4) elimination (WHO, Citation2007). In the year 2009, the Elimination 8 (E-8) initiative was launched by Southern African Development Community (SADC) in order to facilitate zero malaria transmission by 2020 in countries such as South Africa, Namibia, Botswana and Eswatini (frontline countries) (SARN, Citation2010).
Following application these cateria, Matabeleland South was the first province implement malaria pre-elimination phase in 2013 (Sande et al., Citation2016). In 2015, Midlands, Mashonaland West Provinces and Matabeleland North were promoted to focus on activities under malaria pre-elimination phase. However, the rest of provinces (Mashonaland Central, Masvingo, Manicaland and Mashonaland East) continue to focus on the consolidation and control phase, due to high malaria incidences (Sande et al., Citation2016).
Under E-8 initiative, countries such as Zambia, Angola, Zimbabwe and Mozambique were classified as second line states which were expect to eliminate malaria by 2030 (SADC, Citation2018). The introduction of E-8 by SADC was in line with the remarkable decline in malaria incidences between 2000 and 2010. This significant decline in malaria cases was due to vector control and better case management (SADC, Citation2018). According to WHO (Citation2021a), Africa is likely to miss its target of eliminating malaria by 2030 as indicated by failure to reduce both malaria mortality and incidences by 40% in 2020.When compared to 2019, the number of malaria deaths in 2020 increased by 68 953 and this has been attributed to the state of health emergence as a result of COVID-19 (WHO, Citation2021a).
4. Conclusion
The major malaria control interventions applied in Sub-Saharan Africa are IRS and ITNs. Apart from IRS and ITNs, chemoprevention (SMC) for under-five children, IPTi, and IPTp, treatment of malaria using Artemisinin combination therapy (ACT), case management, and use of Mosquirix (malaria vaccine) were applied in the sub Saharan African region. The accessibility of these malaria control interventions in most African countries including Zimbabwe is still below the set global and national targets. Additionally, due to vector (to carbamates, pyrethroids and organochlorines) and parasite resistance (to antimalarial drugs such as chloroquine, and sulfadoxine/pyrimethamine) the fight against malaria has stalled and progress made in previously has been reversed in some African countries. The contribution of WHO African region to global malaria incidences is significantly (above 92%). In African countries including Zimbabwe, malaria is most devastating among children under the age of five, pregnant women and people with weakened immune system, especially those living with HIV and AIDS. Generally, malaria transmission in African countries such as Zimbabwe is seasonal and directly linked to the geographic variation in rainfall patterns and topology. Africa is likely to miss its target of eliminating malaria by 2030 as indicated by failure to reduce both malaria mortality and incidences by 40% in 2020. However, the introduction of E-8 by SADC has resulted in a remarkable decline in malaria incidences between 2000 and 2010. Furthermore, the WHO African region introduced the HBHI initiative in order to reduce malaria burden in ten most malaria burdened African countries. The E-8 and HBHI initiatives have been hampered by COVID-19, which disrupted provision and accessibility of malaria control interventions through introduction of measures including movement restrictions, quarantining, curfews, travel bans, and lockdowns. Overall some African countries are moving towards malaria elimination (for example Cabo Verde, Sao Tome & Principe, Zimbabwe, Algeria, Ethiopia, and South Africa), yet some are still far from meeting certain targets (including Niger, DRC, Burkina Faso and Nigeria.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Zakio Makuvara
Zakio Makuvara, the corresponding and first author, is a lecturer and researcher at Great Zimbabwe University. He is currently a PhD student at the University of South Africa, working on antimalarial resistance and the efficacy of plants against malaria.
Solomon R. Magano
Solomon Magano is a Professor of Zoology, currently the Executive Dean in the CAES, University of South Africa.
Grace Mugumbate
Grace Mugumbate is a Professor of Chemistry and drug discovery, currently Pro-Vice Chancellor at the Midlands State University.
References
- Abamecha, A.,Yilma, D.,Addisu, W.,El-Abid, H.,Ibenthal, A.,Noedl, H.,Yewhalaw, D.,Moumni, M., &Abdissa, A. (2020). Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Chewaka District, Ethiopia. Malaria Journal, 19(240), 1–10. 32650784.
- Abiodun, A., Andersen, H., Mamo, L. T., & Sisay, O. B. (2021). Vaccine manufacturing in Africa: what it takes and why it matters. Tony Blair Institute for Global Change.
- African Union. (2021). World malaria report (2021), progress towards the African Union’s goal of eliminating malaria in Africa by 2030. African Union.
- Ahorlu, C. S., Adongo, P., Koenker, H., Zigirumugabe, S., Sika-Bright, S., Koka, E., Tabong, P. T.-N., Piccinini, D., Segbaya, S., Olapeju, B., & Monroe, A. (2019). Understanding the gap between access and use: A qualitative study on barriers and facilitators to insecticide-treated net use in Ghana. Malaria Journal, 18(1), 417. https://doi.org/10.1186/s12936-019-3051-0
- Allcock, S. H., Young, E. H., & Sandhu, M. S. (2018). A cross-sectional analysis of ITN and IRS coverage in Namibia in 2013. Malaria Journal, 17(1), 264. https://doi.org/10.1186/s12936-018-2417-z
- Asingizwe, D., Poortvliet, P. M., Koenraadt, C. J., Van Vliet, A. J., Ingabire, C. M., Mutesa, L., & Leeuwis, C. (2019). Role of individual perceptions in the consistent use of malaria preventive measures: mixed methods evidence from rural Rwanda. Malaria Journal, 18(1), 270. https://doi.org/10.1186/s12936-019-2904-x
- Balakrishnan, V. S. (2022). A new strategy is required for malaria elimination in Africa. The Lancet-Infectious Diseases, 22(2), 170–171. https://doi.org/10.1016/S1473-3099(22)00012-3
- Batra, C. P., Adak, T., Sharma, V. P., & Mittal, P. K. (2001). Impact of urbanization on bionomics of An. culicifacies and An. stephensi in Delhi. Indian Journal of Malariology, 38(3–4), 61–75.
- Beavogui, A. H., Camara, A., Delamou, A., Diallo, M. S., Doumbouya, A., Kourouma, K., Bouedouno, P., Guilavogui, T., Souza, S., Kelley, J., Talundzic, E., Fofana, A., & Plucinski, M. M. (2020). Efficacy and safety of artesunate – amodiaquine and artemether – lumefantrine and prevalence of molecular markers associated with resistance, Guinea: An open‑label two‑arm randomised controlled trial. Malaria Journal, 19(1), 223. https://doi.org/10.1186/s12936-020-03290-w
- Bekono, B. D., Kang, F. N., Onguéné, P. A., Lifongo, L. L., Sippl, W., Fester, K., & Owono, L. C. O. (2020). The potential of anti‑malarial compounds derived from African medicinal plants : A review of pharmacological evaluations from 2013 to 2019. Malaria Journal, 19(1), 183. https://doi.org/10.1186/s12936-020-03231-7
- Berthe, S., Harvey, S. A., Lynch, M., Koenker, H., Jumbe, V., Kaunda-Khangamwa, B., & Mathanga, D. P. (2019). Poverty and food security: Drivers of insecticide-treated mosquito net misuse in Malawi. Malaria Journal, 18(1), 320. https://doi.org/10.1186/s12936-019-2952-2
- Bhagavathula, A. S., Elnour, A. A., & Shehab, A. (2016). Alternatives to currently used antimalarial drugs: in search of a magic bullet. Infectious Diseases of Poverty, 5(1), 103. https://doi.org/10.1186/s40249-016-0196-8
- Bin Dajem, S. M., & Al-Qahtani, A. (2010). Analysis of gene mutations involved in chloroquine resistance in Plasmodium falciparum parasites isolated from patients in the southwest of Saudi Arabia. Annals of Saudi Medicine, 30(3), 187–192. https://doi.org/10.4103/0256-4947.62826
- Carraz, M., Jossang, A., Franetich, J.-F., Siau, A., Ciceron, L., Hannoun, L., Sauerwein, R., Frappier, F., Rasoanaivo, P., Snounou, G., & Mazier, D. (2006). A plant-derived morphinan as a novel lead compound active against malaria liver stages. PLOS Medicine, 3(12), e513. https://doi.org/10.1371/journal.pmed.0030513
- Casimiro, S., Coleman, M., Hemingway, J., & Sharp, B. (2006). Insecticide resistance in Anopheles arabiensis and Anopheles gambiae from Mozambique. Journal of Medical Entomology, 43(2), 276–282. https://doi.org/10.1093/jmedent/43.2.276
- Chanda, E., Hemingway, J., Kleinschmidt, I., Rehman, A. M., Ramdeen, V., Phiri, F. N., Coetzer, S., Mthembu, D., Shinondo, C. J., Chizema-Kawesha, E., Kamuliwo, M., Mukonka, V., Baboo, K. S., & Coleman, M. (2011). Insecticide resistance and the future of malaria control in Zambia. PLOS One, 6(9), e24336. https://doi.org/10.1371/journal.pone.0024336
- Chanda, J., Saili, K., Phiri, F., Stevenson, J. C., Mwenda, M., Chishimba, S., Mulube, C., Mambwe, B., Lungu, C., Earle, D., Bennett, A., Eisele, T. P., Kamuliwo, M., Steketee, R. W., Keating, J., Miller, J. M., & Sikaala, C. H. (2020). Pyrethroid and carbamate resistance in Anopheles funestus Giles along Lake Kariba in southern Zambia. The American Journal of Tropical Medicine and Hygiene, 103(2_Suppl), 90–97. https://doi.org/10.4269/ajtmh.19-0664
- Chapu, G., & Mgocheki, N. (2017). A survey on traditional and modern prophylactic methods of malaria management in a resettlement area in the Southern Lowveld of Zimbabwe. International Journal of tropical disease & Health, 21(1), 1–17. https://doi.org/10.9734/IJTDH/2017/30433
- Chinsembu, K. C. (2015). Plants as antimalarial agents in Sub-Saharan Africa. Acta Tropica, 152, 32–48. https://doi.org/10.1016/j.actatropica.2015.08.009
- Choi, K. S., Christian, R., Nardini, L., Wood, O. R., Agubuzo, E., Muleba, M., Munyati, S., Makuwaza, A., Koekemoer, L. L., Brooke, B. D., Hunt, R. H., & Coetzee, M. (2014). Insecticide resistance and role in malaria transmission of Anopheles funestus populations from Zambia and Zimbabwe. Parasites & Vectors, 7(1), 464. https://doi.org/10.1186/s13071-014-0464-z
- Cohen, M. K. (2024). World malaria day report (pp. 1–2). Centers for Disease Control and Prevention.
- Coleman, M., Coleman, M., Mabuza, A. M., Kok, G., Coetzee, M., & Durrheim, D. N. (2009). Using the SaTScan method to detect local malaria clusters for guiding malaria control programmes. Malaria Journal, 8(1), 68. https://doi.org/10.1186/1475-2875-8-68
- Conrad, M. D., LeClair, N., Arinaitwe, E., Wanzira, H., Kakuru, A., Bigira, V., Muhindo, M., Kamya, M. R., Tappero, J. W., Greenhouse, B., Dorsey, G., & Rosenthal, P. J. (2014). Comparative impacts over 5 years of artemisinin-based combination therapies on Plasmodium falciparum polymorphisms that modulate drug sensitivity in Ugandan children. The Journal of Infectious Diseases, 210(3), 344–353. https://doi.org/10.1093/infdis/jiu141
- Conrad, M. D., & Rosenthal, P. J. (2019). Antimalarial drug resistance in Africa: The calm before the storm? The Lancet. Infectious Diseases, 19(10), e338–e351. https://doi.org/10.1016/S1473-3099(19)30261-0
- Cuamba, N., Morgan, J. C., Irving, H., Steven, A., & Wondji, C. S. (2010). High level of pyrethroid resistance in an Anopheles funestus population of the Chokwe district in Mozambique. PLOS One, 5(6), e11010. https://doi.org/10.1371/journal.pone.0011010
- Datoo, M. S., Dicko, A., Tinto, H., Ouédraogo, J.-B., Hamaluba, M., Olotu, A., Beaumont, E., Ramos Lopez, F., Natama, H. M., Weston, S., Chemba, M., Compaore, Y. D., Issiaka, D., Salou, D., Some, A. M., Omenda, S., Lawrie, A., Bejon, P., Rao, H., … Hill, A. V. S, R21/Matrix-M Phase 3 Trial Group. (2024). Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet, 403(10426), 533–544. https://doi.org/10.1016/S0140-6736(23)02511-4
- Datoo, M. S., Natama, H. M., Somé, A., Bellamy, D., Traoré, O., Rouamba, T., Tahita, M. C., Ido, N. F. A., Yameogo, P., Valia, D., Millogo, A., Ouedraogo, F., Soma, R., Sawadogo, S., Sorgho, F., Derra, K., Rouamba, E., Ramos-Lopez, F., Cairns, M., … Tinto, H. (2022). Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. The Lancet. Infectious Diseases, 22(12), 1728–1736. https://doi.org/10.1016/S1473-3099(22)00442-X
- Datoo, M. S., Natama, M. H., Somé, A., Traoré, O., Rouamba, T., Bellamy, D., Yameogo, P., Valia, D., Tegneri, M., Ouedraogo, F., Soma, R., Sawadogo, S., Sorgho, F., Derra, K., Rouamba, E., Orindi, B., Ramos Lopez, F., Flaxman, A., Cappuccini, F., … Tinto, H. (2021). Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet, 397(10287), 1809–1818. https://doi.org/10.1016/S0140-6736(21)00943-0
- Desai, M., Gutman, J., Taylor, S. M., Wiegand, R. E., Khairallah, C., Kayentao, K., Ouma, P., Coulibaly, S. O., Kalilani, L., Mace, K. E., Arinaitwe, E., Mathanga, D. P., Doumbo, O., Otieno, K., Edgar, D., Chaluluka, E., Kamuliwo, M., Ades, V., Skarbinski, J., … Ter Kuile, F. O. (2015). Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clinical Infectious Diseases, 62(3), 323–333. https://doi.org/10.1093/cid/civ881
- Desai, M., Hill, J., Fernandes, S., Walker, P., Pell, C., Gutman, J., Kayentao, K., Gonzalez, R., Webster, J., Greenwood, B., Cot, M., & Ter Kuile, F. O. (2018). Prevention of malaria in pregnancy. The Lancet-Infectious Diseases, 18(4), e119–e132. https://doi.org/10.1016/S1473-3099(18)30064-1
- Diema, K. K., Dodam, K. K., Aarah-Bapuah, M., & Asibi, A. J. (2017). Barriers to sustained use of the insecticide treated bed net in the upper east region of Ghana. International Journal of Community Medicine and Public Health, 4(2), 500–505. https://doi.org/10.18203/2394-6040.ijcmph20170280
- Dimala, C. A., Kika, B. T., Kadia, B. M., & Blencowe, H. (2018). Current challenges and proposed solutions to the effective implementation of the RTS, S/AS01 malaria vaccine program in sub-Saharan Africa: A systematic review. PLOS One, 13(12), e0209744. https://doi.org/10.1371/journal.pone.0209744
- Dube, B., Mberikunashe, J., Dhliwayo, P., Tangwena, A., Shambira, G., Chimusoro, A., Madinga, M., & Gambinga, B. (2019). How far is the journey before malaria is knocked out in Zimbabwe: Results of the malaria indicator survey 2016. Malaria Journal, 18(1), 196. https://doi.org/10.1186/s12936-019-2823-x
- Dwomoh, D., Adu, B., Dodoo, D., Theisen, M., Iddi, S., & Gerds, T. A. (2020). Evaluating the predictive performance of malaria antibodies and FCGR3B gene polymorphisms on Plasmodium falciparum infection outcome: A prospective cohort study. Malaria Journal, 19(1), 307. https://doi.org/10.1186/s12936-020-03381-8
- Ehrlich, H. Y., Jones, J., & Parikh, S. (2020). Molecular surveillance of antimalarial partner drug resistance in sub-Saharan Africa: A spatial-temporal evidence mapping study. The Lancet-Microbe, 1(5), e209–e217. https://doi.org/10.1016/s2666-5247(20)30094-x
- Ekström, A. M., Tomson, G., Wanyenze, R. K., Bhutta, Z. A., Kyobutungi, C., Binagwaho, A., & Ottersen, O. P. (2021). Addressing production gaps for vaccines in African countries. Bulletin of the World Health Organization, 99(12), 910–912. https://doi.org/10.2471/BLT.21.287381
- Esu, E.,Effa, E. E.,Opie, O. N.,Uwaoma, A., &Meremikwu, M. M. (2014). Artemether for severe malaria. The Cochrane Database of Systematic Reviews, 2014(9), CD010678. https://doi.org/10.1002/14651858.
- Flegg, J. A., Metcalf, C. J. E., Gharbi, M., Venkatesan, M., Shewchuk, T., Hopkins Sibley, C., & Guerin, P. J. (2013). Trends in antimalarial drug use in Africa. The American Journal of Tropical Medicine and Hygiene, 89(5), 857–865. https://doi.org/10.4269/ajtmh.13-0129
- Foy, B. D., Some, A., Magalhaes, T., Gray, L., Rao, S., Sougue, E., Jackson, C. L., Kittelson, J., Slater, H. C., Bousema, T., Da, O., Coulidiaty, A. G. V., Colt, M., Wade, M., Richards, K., Some, A. F., Dabire, R. K., & Parikh, S. (2023). Repeat ivermectin mass drug administrations for malaria control II: Protocol for a double-blind, cluster-randomized, placebo-controlled trial for the integrated control of malaria. JMIR Research Protocols, 12(1), e41197. https://doi.org/10.2196/41197
- Frosch, A. E., Venkatesan, M., & Laufer, M. K. (2011). Patterns of chloroquine use and resistance in sub-Saharan Africa: A systematic review of household survey and molecular data. Malaria Journal, 10(1), 116. https://doi.org/10.1186/1475-2875-10-116
- Gao, L., Shi, Q., Liu, Z., Li, Z., & Dong, X. (2023). Impact of the COVID-19 pandemic on malaria control in Africa: A preliminary analysis. Tropical Medicine and Infectious Disease, 8(1), 67. https://doi.org/10.3390/tropicalmed8010067
- Gavi, S., Tapera, O., Mberikunashe, J., & Kanyangarara, M. (2021). Malaria incidence and mortality in Zimbabwe during the COVID-19 pandemic: Analysis of routine surveillance data. Malaria Journal, 20(1), 233. https://doi.org/10.1186/s12936-021-03770-7
- Getawen, S. K., Ashine, T., Massebo, F., Woldeyes, D., & Lindtjørn, B. (2018). Exploring the impact of house screening intervention on entomological indices and incidence of malaria in Arba Minch town, southwest Ethiopia: A randomized control trial. Acta Tropica, 181, 84–94. https://doi.org/10.1016/j.actatropica.2018.02.009
- Gimnig, J. E., & Slutsker, L. (2009). House screening for malaria control. Lancet, 374(9694), 954–955. https://doi.org/10.1016/S0140-6736(09)61078-3
- Global Technical Strategy for Malaria 2016–2030. (2016). World Health Organisation.
- Gogue, C., Wagman, J., Tynuv, K., Saibu, A., Yihdego, Y., Malm, K., Mohamed, W., Akplu, W., Tagoe, T., Ofosu, A., Williams, I., Asiedu, S., Richardson, J., Fornadel, C., Slutsker, L., & Robertson, M. (2020). An observational analysis of the impact of indoor residual spraying in Northern, Upper East, and Upper West Regions of Ghana : 2014 Through 2017. Malaria Journal, 19(1), 242. https://doi.org/10.1186/s12936-020-03318-1
- González, R., Manun’Ebo, M. F., Meremikwu, M., Rabeza, V. R., Sacoor, C., Figueroa-Romero, A., Arikpo, I., Macete, E., Mbombo Ndombe, D., Ramananjato, R., LIach, M., Pons-Duran, C., Sanz, S., Ramírez, M., Cirera, L., Maly, C., Roman, E., Pagnoni, F., & Menéndez, C. (2023). The impact of community delivery of intermittent preventive treatment of malaria in pregnancy on its coverage in four sub-Saharan African countries (Democratic Republic of the Congo, Madagascar, Mozambique, and Nigeria): A quasi-experimental multicentre evaluation. The Lancet-Global Health, 11(4), e566–e574. https://doi.org/10.1016/S2214-109X(23)00051-7
- Green, C. A. (1981). Malaria epidemiology and anopheline cytogenetics. In R. Pal, J. B. Kitzmiller, & T. Kanda Kodansha (Eds.), Cytogenetics and genetics of vectors: Proceedings of a symposium of the XVIth International Congress of Entomology (p. c1981). Elsevier Biomedical Press.
- Gunda, R., Chimbari, M. J., & Mukaratirwa, S. (2016). Assessment of burden of malaria in gwanda District, Zimbabwe, using the disability adjusted life years. International Journal of Environmental Research and Public Health, 13(2), 244. https://doi.org/10.3390/ijerph13020244
- Gutman, J. R., Stephens, D. K., Tiendrebeogo, J., Badolo, O., Dodo, M., Burke, D., Williamson, J., Vibbert, K., Youll, S. J., Savadogo, Y., & Brieger, W. R. (2020). A cluster randomized trial of delivery of intermittent preventive treatment of malaria in pregnancy at the community level in Burkina Faso. Malaria Journal, 19(1), 282. https://doi.org/10.1186/s12936-020-03356-9
- Gwitira, I., Mukonoweshuro, M., Mapako, G., Shekede, M. D., Chirenda, J., & Mberikunashe, J. (2020). Spatial and spatio-temporal analysis of malaria cases in Zimbabwe. Infectious Diseases of Poverty, 9(1), 146. https://doi.org/10.1186/s40249-020-00764-6
- Gwitira, I., Murwira, A., Mberikunashe, J., & Masocha, M. (2018). Spatial overlaps in the distribution of HIV/AIDS and malaria in Zimbabwe. BMC Infectious Diseases, 18(1), 598. https://doi.org/10.1186/s12879-018-3513-y
- Hemming-Schroeder, E., Umukoro, E., Lo, E., Fung, B., Tomás-Domingo, P., Zhou, G., Zhong, D., Dixit, A., Atieli, H., Githeko, A., Vardo-Zalik, A., & Yan, G. (2018). Impacts of antimalarial drugs on Plasmodium falciparum drug resistance markers, Western Kenya, 2003–2015. The American Journal of Tropical Medicine and Hygiene, 98(3), 692–699. https://doi.org/10.4269/ajtmh.17-0763
- Hiben, M. G., Sibhat, G. G., Fanta, B. S., Gebrezgi, H. D., & Tesema, S. B. (2016). Evaluation of Senna singueana leaf extract as an alternative or adjuvant therapy for malaria. Journal of Traditional and Complementary Medicine, 6(1), 112–117. https://doi.org/10.1016/j.jtcme.2014.11.014
- Ippolito, M. M., Gebhardt, M. E., Ferriss, E., Schue, J. L., Kobayashi, T., Chaponda, M., Kabuya, J.-B., Muleba, M., Mburu, M., Matoba, J., Musonda, M., Katowa, B., Lubinda, M., Hamapumbu, H., Simubali, L., Mudenda, T., Wesolowski, A., Shields, T. M., Hackman, A., … Moss, W. J, Southern and Central Africa International Center of Excellence for Malaria Research. (2022). Scientific findings of the Southern and Central Africa International Center of Excellence for malaria research: ten years of malaria control impact assessments in hypo-, meso-, and holoendemic transmission zones in Zambia and Zimbabwe. The American Journal of Tropical Medicine and Hygiene, 107(4_Suppl), 55–67. https://doi.org/10.4269/ajtmh.21-1287
- Kanyangarara, M., Hamapumbu, H., Mamini, E., Lupiya, J., Stevenson, J. C., Mharakurwa, S., Chaponda, M., Thuma, P. E., Gwanzura, L., Munyati, S., Mulenga, M., Norris, D. E., & Moss, W. J, Southern Africa International Centers of Excellence for Malaria Research. (2018). Malaria knowledge and bed net use in three transmission settings in southern Africa. Malaria Journal, 17(1), 41. https://doi.org/10.1186/s12936-018-2178-8
- Kanyangarara, M., Mamini, E., Mharakurwa, S., Munyati, S., Gwanzura, L., Kobayashi, T., Shields, T., Mullany, L. C., Mutambu, S., Mason, P. R., Curriero, F. C., & Moss, W. J, Southern Africa International Centers of Excellence for Malaria Research. (2016). Individual-and household-level risk factors associated with malaria in Mutasa District, Zimbabwe: A serial cross-sectional study. The American Journal of Tropical Medicine and Hygiene, 95(1), 133–140. https://doi.org/10.4269/ajtmh.15-0847
- Kanyangarara, M., Mamini, E., Mharakurwa, S., Munyati, S., Gwanzura, L., Kobayashi, T., Shields, T., Mullany, L. C., Mutambu, S., Mason, P. R., Curriero, F. C., … & Moss, W. J, Southern Africa International Centers of Excellence for Malaria Research. (2016). Reduction in malaria incidence following indoor residual spraying with Actellic 300 CS in a setting with pyrethroid resistance: Mutasa District, Zimbabwe. PLOS One, 11(3), e0151971. https://doi.org/10.1371/journal.pone.0151971
- Kayentao, K., Garner, P., van Eijk, A. M., Naidoo, I., Roper, C., Mulokozi, A., MacArthur, J. R., Luntamo, M., Ashorn, P., Doumbo, O. K., & ter Kuile, F. O. (2013). Intermittent preventive therapy for malaria during pregnancy using 2 vs. 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: Systematic review and meta-analysis. JAMA, 309(6), 594–604. https://doi.org/10.1001/jama.2012.216231
- Kemibala, E. E., Neto, A. M., Saroli, J., Silva, R., Philbert, A., Ng, K., Foster, W. A., Dekker, T., & Mboera, L. E. G. (2020). Is Anopheles gambiae attraction to floral and human skin–based odours and their combination modulated by previous blood meal experience ? Malaria Journal, 19(1), 318. https://doi.org/10.1186/s12936-020-03395-2
- Kesteman, T., Randrianarivelojosia, M., & Rogier, C. (2017). The protective effectiveness of control interventions for malaria prevention: A systematic review of the literature. F1000Research, 6, 1932. https://doi.org/10.12688/f1000research.12952.1
- Kgoroebutswe, T. K., Makate, N., Fillinger, U., Mpho, M., Segoea, G., Sangoro, P. O., Mutero, C. M., Chanda, E., Ntebela, D., Mogopa, M., Mosweunyane, T., & Nkya, T. E. (2020). Vector control for malaria elimination in Botswana : Progress, gaps and opportunities. Malaria Journal, 19(1), 301. https://doi.org/10.1186/s12936-020-03375-6
- Klooster, V., Jong, D., Ngarivhume, T., & Van, C. I. E. A. (2015). UvA-DARE (Digital Academic Repository) medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. Journal of Ethnopharmacology, 159, 224–237. https://doi.org/10.1016/j.jep.2014.11.011
- Knox, T. B., Juma, E. O., Ochomo, E. O., Pates Jamet, H., Ndungo, L., Chege, P., Bayoh, N. M., N’Guessan, R., Christian, R. N., Hunt, R. H., & Coetzee, M. (2014). An online tool for mapping insecticide resistance in major Anopheles vectors of human malaria parasites and review of resistance status for the Afrotropical region. Parasites & Vectors, 7(1), 76. https://doi.org/10.1186/1756-3305-7-76
- Kolawole, E. O., Ayeni, E. T., Abolade, S. A., Ugwu, S. E., Awoyinka, T. B., Ofeh, A. S., & Okolo, B. O. (2023). Malaria endemicity in Sub-Saharan Africa: Past and present issues in public health. Microbes and Infectious Diseases, 4(1), 242–251.
- Kremsner, P. G., Adegnika, A. A., Hounkpatin, A. B., Zinsou, J. F., Taylor, T. E., Chimalizeni, Y., Liomba, A., Kombila, M., Bouyou-Akotet, M. K., Mawili Mboumba, D. P., Agbenyega, T., Ansong, D., Sylverken, J., Ogutu, B. R., Otieno, G. A., Wangwe, A., Bojang, K. A., Okomo, U., Sanya-Isijola, F., … Krishna, S. (2016). Intramuscular artesunate for severe malaria in African children: A multicenter randomized controlled trial. PLOS Medicine, 13(1), e1001938. https://doi.org/10.1371/journal.pmed.1001938
- Krettli, A. U., Andrade-Neto, V. F., Brandão, G. L., & Ferrari, W. M. S. (2001). The search for new antimalarial drugs from plants used to treat fever and malaria or plants randomly selected: A Review. Mem Inst Oswald Cuz, Rio de Janeiro, 96(November 2001), 1033–1042.
- Lima, R. B. S., Rocha, L. F., Melo, M. R. S., Costa, J. S., Picanço, N. S., Lima, E. S., Vasconcellos, M. C., Boleti, A. P. A., Santos, J. M. P., Amorim, R. C. N., Chaves, F. C. M., Coutinho, J. P., Tadei, W. P., Krettli, A. U., & Pohlit, A. M. (2015). In vitro and in vivo anti‑malarial activity of plants from the Brazilian Amazon. Malaria Journal, 14, 508. https://doi.org/10.1186/s12936-015-0999-2
- Lu, F., Zhang, M., Culleton, R. L., Xu, S., Tang, J., Zhou, H., Zhu, G., Gu, Y., Zhang, C., Liu, Y., Wang, W., Cao, Y., Li, J., He, X., Cao, J., & Gao, Q. (2017). Return of chloroquine sensitivity to Africa? Surveillance of African Plasmodium falciparum chloroquine resistance through malaria imported to China. Parasites & Vectors, 10(1), 355. https://doi.org/10.1186/s13071-017-2298-y
- Lukwa, N., Sande, S., Makuwaza, A., Chiwade, T., Netsa, M., Asamoa, K., Vazquez-Prokopec, G., Reithinger, R., & Williams, J. (2014). Nationwide assessment of insecticide susceptibility in Anopheles gambiae populations from Zimbabwe. Malaria Journal, 13(1)2014, 408. https://doi.org/10.1186/1475-2875-13-408
- Mabaso, M. L. H., Sharp, B., & Lengeler, C. (2004). Historical review of malaria control in southern Africa with emphasis on the use of indoor residual house-spraying. Tropical Medicine & International Health, 9(8), 846–856. https://doi.org/10.1111/j.1365-3156.2004.01263.x
- Magaço, A., Botão, C., Nhassengo, P., Saide, M., Ubisse, A., Chicumbe, S., & Zulliger, R. (2019). Community knowledge and acceptance of indoor residual spraying for malaria prevention in Mozambique: A qualitative study. Malaria Journal, 18(1), 27. https://doi.org/10.1186/s12936-019-2653-x
- Mahajan, N. N., Gajbhiye, R. K., Bahirat, S., Lokhande, P. D., Mathe, A., Rathi, S., Warty, N., Mahajan, K. N., Srivastava, V., Kuppusamy, P., & Mohite, S. C. (2021). Co-infection of malaria and early clearance of SARS-CoV-2 in healthcare workers. Journal of Medical Virology, 93(4), 2431–2438. https://doi.org/10.1002/jmv.26760
- Makono, R., & Sibanda, S. (1999). Review of the prevalence of malaria in Zimbabwe with specific reference to parasite drug resistance (1984–1996). Transactions of the Royal Society of Tropical Medicine and Hygiene, 93(5), 449–452. https://doi.org/10.1016/s0035-9203(99)90331-0
- Manyangadze, T., Chimbari, M. J., Macherera, M., & Mukaratirwa, S. (2017). Micro ‑ spatial distribution of malaria cases and control strategies at ward level in Gwanda district, Matabeleland South, Zimbabwe. Malaria Journal, 16(1), 476. https://doi.org/10.1186/s12936-017-2116-1
- Masalu, J. P., Finda, M., Killeen, G. F., Ngowo, H. S., Pinda, P. G., & Okumu, F. O. (2020). Creating mosquito-free outdoor spaces using transfluthrin-treated chairs and ribbons. Malaria Journal, 19(1), 109. https://doi.org/10.1186/s12936-020-03180-1
- Masendu, H. T., Hunt, R. H., Koekemoer, L. L., Brooke, B. D., Govere, J., & Coetzee, M. (2005). Spatial and temporal distributions and insecticide susceptibility of malaria vectors in Zimbabwe. African Entomology, 13, 25–34.
- Maxmen, A. (2021). Scientists hail historic malaria vaccine approval-but point to challenges ahead. Nature. Available online. https://doi.org/10.1038/d41586-021-02755-5
- Mbacham, W. F., Ayong, L., Guewo-Fokeng, M., & Makoge, V. (2019). Current situation of malaria in Africa. Methods in Molecular Biology, 2013, 29–44. https://doi.org/10.1007/978-1-4939-9550-9_2
- Mhamilawa, L. E., Ngasala, B., Morris, U., Kitabi, E. N., Barnes, R., Soe, A. P., Mmbando, B. P., Björkman, A., & Mårtensson, A. (2020). Parasite clearance, cure rate, post‑treatment prophylaxis and safety of standard 3-day versus an extended 6-day treatment of artemether – lumefantrine and a single low-dose primaquine for uncomplicated Plasmodium falciparum malaria in Bagamoyo district, Tanzania : A randomized controlled trial. Malaria Journal, 19(1), 216. https://doi.org/10.1186/s12936-020-03287-5
- Mharakurwa, S., Matsena-Zingoni, Z., Mudare, N., Matimba, C., Gara, T. X., Makuwaza, A., Maponga, G., Munyati, S., Gwanzura, L., Mutambu, S. L., Mason, P., Kobayashi, T., Midzi, N., Moss, W. J., & Ippolito, M. M. (2021). Steep rebound of chloroquine-sensitive Plasmodium falciparum in Zimbabwe. The Journal of Infectious Diseases, 223(2), 306–309. https://doi.org/10.1093/infdis/jiaa368
- Mharakurwa, S., & Mugochi, T. (1994). Chloroquine-resistant falciparum malaria in an area of rising endemicity in Zimbabwe. The Journal of Tropical Medicine and Hygiene, 97(1), 39–45.
- Mharakurwa, S., Mutambu, S. L., Mberikunashe, J., Thuma, P. E., Moss, W. J., & Mason, P. R., Southern Africa ICEMR Team. (2013). Changes in the burden of malaria following scale up of malaria control interventions in Mutasa District, Zimbabwe. Malaria Journal, 12(1), 223. https://doi.org/10.1186/1475-2875-12-223
- Ministry of Health and Child Welfare. (2007). National malaria control programme strategy. Zimbabwe: Ministry of Health and Child Welfare.
- Mlambo, G., Sullivan, D., Mutambu, S. L., Soko, W., Mbedzi, J., Chivenga, J., Gemperli, A., & Kumar, N. (2007). High prevalence of molecular markers for resistance to chloroquine and pyrimethamine in Plasmodium falciparum from Zimbabwe. Parasitology Research, 101(4), 1147–1151. https://doi.org/10.1007/s00436-007-0597-5
- Moon, T. D., Hayes, C. B., Blevins, M., Lopez, M. L., Green, A. F., González-Calvo, L., …& Olupona, O, Ogumaniha-SCIP Zambézia Consortium. (2016). Factors associated with the use of mosquito bed nets: Results from two cross-sectional household surveys in Zambézia Province, Mozambique. Malaria Journal, 15(1), 196. https://doi.org/10.1186/s12936-016-1250-5
- Mosha, J. F., Sturrock, H. J. W., Greenwood, B., Sutherland, C. J., Gadalla, N. B., Atwal, S., Hemelaar, S., Brown, J. M., Drakeley, C., Kibiki, G., Bousema, T., Chandramohan, D., & Gosling, R. D. (2014). Hot spot or not: A comparison of spatial statistical methods to predict prospective malaria infections. Malaria Journal, 13(1), 53. https://doi.org/10.1186/1475-2875-13-53
- Mugwagwa, N., Mberikunashe, J., Gombe, N. T., Tshimanga, M., Bangure, D., & Mungati, M. (2015). Honde valley, Mutasa district, Zimbabwe, 2014 : Factors associated with malaria infection in Honde valley, Mutasa district, Zimbabwe, 2014 : A case control study. BMC Research Notes, 8. (1), 829. https://doi.org/10.1186/s13104-015-1831-3
- Mulaw, T., Wubetu, M., Dessie, B., Demeke, G., & Molla, Y. (2019). Evaluation of antimalarial activity of the 80% methanolic stem bark extract of Combretum molle against Plasmodium berghei in MICE. Journal of Evidence-Based Integrative Medicine, 24, 2515690X19890866. https://doi.org/10.1177/2515690X19890866
- Mumtaz, H., Nadeem, A., Bilal, W., Ansar, F., Saleem, S., Khan, Q. A., Tango, T., Farkouh, C., Belay, N. F., Verma, R., Farkouh, M., & Saqib, M. (2023). Acceptance, availability, and feasibility of RTS, S/AS01 malaria vaccine: A review. Immunity, Inflammation and Disease, 11(6), e899. https://doi.org/10.1002/iid3.899
- Mundagowa, P. T., & Chimberengwa, P. T. (2020). Malaria outbreak investigation in a rural area south of Zimbabwe : A case–control study. Malaria Journal, 19(1), 197. 10. https://doi.org/10.1186/s12936-020-03270-0
- Munhenga, G., Masendu, H. T., Brooke, B. D., Hunt, R. H., & Koekemoer, L. K. (2008). Pyrethroid resistance in the major malaria vector Anopheles arabiensis from Gwave, a malaria-endemic area in Zimbabwe. Malaria Journal, 7(1), 247. https://doi.org/10.1186/1475-2875-7-247
- Muthaura, C. N., Keriko, J. M., Derese, S., Yenesew, A., & Rukunga, G. M. (2011). Investigation of some medicinal plants traditionally used for treatment of malaria in Kenya as potential sources of antimalarial drugs. Experimental Parasitology, 127(3), 609–626. https://doi.org/10.1016/j.exppara.2010.11.004
- Mwendera, C., de Jager, C., Longwe, H., Phiri, K., Hongoro, C., & Mutero, C. M. (2016). Malaria research and its influence on anti-malarial drug policy in Malawi: A case study. Health Research Policy and Systems, 14(1), 41. https://doi.org/10.1186/s12961-016-0108-1
- Nalinya, S., Musoke, D., & Deane, K. (2022). Malaria prevention interventions beyond long-lasting insecticidal nets and indoor residual spraying in low-and middle-income countries: A scoping review. Malaria Journal, 21(1), 31. https://doi.org/10.1186/s12936-022-04052-6
- Ngarivhume, T.,Van’t Klooster, C. I. E. A.,De Jong, J. T. V. M., &Van Der Westhuizen, J. H. (2015). Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. Journal of Ethnopharmacology, 159, 224–237. https://doi.org/10.1016/j.jep.2014.11.011 PMID: 25449454
- Nhama, A., Varo, R., & Bassat, Q. (2020). Highlighting the burden of malarial infection and disease in the neonatal period: Making sense of different concepts. Malaria Journal, 19(1), 311. https://doi.org/10.1186/s12936-020-03394-3
- Nkya, T. E., Fillinger, U., Sangoro, O. P., Marubu, R., Chanda, E., & Mutero, C. M. (2022). Six decades of malaria vector control in southern Africa: A review of the entomological evidence-base. Malaria Journal, 21(1), 279. https://doi.org/10.1186/s12936-022-04292-6
- Odufuwa, O. G., Ross, A., Mlacha, Y. P., Juma, O., Mmbaga, S., Msellemu, D., & Moore, S. (2020). Household factors associated with access to insecticide‑treated nets and house modification in Bagamoyo and Ulanga districts, Tanzania. Malaria Journal, 19(1), 220. https://doi.org/10.1186/s12936-020-03303-8
- Okereke, M., Mashavakure, H. M., & Abdulwasiu, M. A. (2022). An evaluation of local pharmaceutical manufacturing in Zimbabwe: How prepared is Zimbabwe to produce COVID-19 vaccines? Innovations in Pharmacy, 13(1), 10. https://doi.org/10.24926/iip.v13i1.4656
- Okokon, J. E., Okokon, P. J., & Sahal, D. (2010). In vitro antiplasmodial activity of some medicinal plants from Nigeria. International Journal of Herbal Medicine, 5, 102–109.
- Oladipo, H. J., Tajudeen, Y. A., Oladunjoye, I. O., Yusuff, S. I., Yusuf, R. O., Oluwaseyi, E. M., AbdulBasit, M. O., Adebisi, Y. A., & El-Sherbini, M. S. (2022). Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Annals of Medicine and Surgery (2012), 81, 104366. https://doi.org/10.1016/j.amsu.2022.104366
- Olapeju, B., Choiriyyah, I., Lynch, M., Acosta, A., Blaufuss, S., Filemyr, E., Harig, H., Monroe, A., Selby, R. A., Kilian, A., & Koenker, H. (2018). Age and gender trends in insecticide-treated net use in sub-Saharan Africa: A multi-country analysis. Malaria Journal, 17(1), 423. https://doi.org/10.1186/s12936-018-2575-z
- Park, J., Kang, S., Seok, D., Baek, Y. J., An, S. Y., Lee, J., Jun, A., & Kim, S.-Y. (2023). Barriers against and strategies for malaria control during the COVID-19 pandemic in low-and middle-income countries: A systematic review. Malaria Journal, 22(1), 41. https://doi.org/10.1186/s12936-023-04452-2
- Pates, H., & Curtis, C. (2005). Mosquito behaviour and vector control. Annual Review of Entomology, 50(1), 53–70. https://doi.org/10.1146/annurev.ento.50.071803.130439
- Patlolla, A. K., Smith, Z., & Tchounwou, P. (2022). Indirect impacts of COVID-19 on the environment: A Global Review. International Journal of Biomedical and Clinical Analysis, 2(1), 9. https://doi.org/10.61797/ijbca.v2i1.160
- PMI. (2014). Africa IRS (AIRS). Project indoor residual spraying (IRS 2) task order four. In USAID (Eds.). 2013 Zimbabwe end-of-spray report 2014. PMI|Africa IRS (AIRS) Project Indoor Residual Spraying (IRS 2) Task Order Four, ABT Associates Inc.
- Pornputtapong, N., Suriyapakorn, B., Satayamapakorn, A., Larpadisorn, K., Janviriyakul, P., & Khemawoot, P. (2020). In silico analysis for factors affecting anti‑malarial penetration into red blood cells. Malaria Journal, 19(1), 215. https://doi.org/10.1186/s12936-020-03280-y
- Premaratne, R., Wickremasinghe, R., Ranaweera, D., Gunasekera, W. M. K. T. d A. W., Hevawitharana, M., Pieris, L., Fernando, D., & Mendis, K. (2019). Technical and operational underpinnings of malaria elimination from Sri Lanka. Malaria Journal, 18(1), 256. https://doi.org/10.1186/s12936-019-2886-8
- Pryce, J., Richardson, M., & Lengeler, C, Cochrane Infectious Diseases Group. (2018). Insecticide-treated nets for preventing malaria. Cochrane Database of Systematic Reviews, 11, 1–68. https://doi.org/10.1002/14651858.CD000363.pub3
- Quanquin, N. M., Barres, L. G., Aliyari, S. R., Day, N. T., Gerami, H., Fisher, S. J., Kakuru, A., Kamya, M. R., Havlir, D. V., Feeney, M., Dorsey, G., Cheng, G., & Gaw, S. L. (2020). Gravidity – dependent associations between interferon response and birth weight in placental malaria. Malaria Journal, 19(1), 280. https://doi.org/10.1186/s12936-020-03351-0
- Rek, J. C., Alegana, V., Arinaitwe, E., Cameron, E., Kamya, M. R., Katureebe, A., Lindsay, S. W., Kilama, M., Staedke, S. G., Todd, J., Dorsey, G., & Tusting, L. S. (2018). Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: A cohort study. The Lancet. Planetary Health, 2(2), e83–e94. https://doi.org/10.1016/S2542-5196(18)30010-X
- Roux, A. T., Maharaj, L., Oyegoke, O., Akoniyon, O. P., Adeleke, M. A., Maharaj, R., & Okpeku, M. (2021). Chloroquine and sulfadoxine–pyrimethamine resistance in Sub-Saharan Africa—a review. Frontiers in Genetics, 12, 668574. https://doi.org/10.3389/fgene.2021.668574
- RTS. S Clinical Trials Partnership. (2015). Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet, 386(9988), 31–45. https://doi.org/10.1016/S0140-6736(15)60721-8
- RTS. S Clinical Trials Partnership. (2014). Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: A phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLOS Medicine, 11(7), e1001685. https://doi.org/10.1371/journal.pmed.1001685
- Russell, C. L., Sallau, A., Emukah, E., Graves, P. M., Noland, G. S., Ngondi, J. M., Ozaki, M., Nwankwo, L., Miri, E., McFarland, D. A., Richards, F. O., & Patterson, A. E. (2015). Determinants of bed net use in Southeast Nigeria following mass distribution of LLINs: Implications for social behavior change interventions. PLOS One, 10(10), e0139447. https://doi.org/10.1371/journal.pone.0139447
- SADC. (2018). Windhoek declaration on eliminating malaria in the SADC region—2018. Windhoek.
- Sande, S., Zimba, M., Chinwada, P., Masendu, H. T., & Makuwaza, A. (2015). Malaria vector species composition and relative abundance in Mutare and Mutasa Districts, Zimbabwe. Journal of Entomological and Acarological Research, 47(3), 79. 2015https://doi.org/10.4081/jear.2015.4955
- Sande, S., Zimba, M., Chinwada, P., Masendu, H. T., & Makuwaza, A. (2016). Insights into resting behavior of malaria vector mosquitoes in Mutare and Mutasa Districts of Manicaland Province, Zimbabwe. Journal of Medical Entomology, 53(4), 866–872. https://doi.org/10.1093/jme/tjw044
- Sande, S., Zimba, M., Mberikunashe, J., Tangwena, A., & Chimusoro, A. (2017). Progress towards malaria elimination in Zimbabwe with special reference to the period 2003–2015. Malaria Journal, 16(1), 295. https://doi.org/10.1186/s12936-017-1939-0
- Sangaré, L. R., Weiss, N. S., Brentlinger, P. E., Richardson, B. A., Staedke, S. G., Kiwuwa, M. S., & Stergachis, A. (2012). Determinants of use of insecticide treated nets for the prevention of malaria in pregnancy: Jinja, Uganda. PLOS One, 7(6), e39712. https://doi.org/10.1371/journal.pone.0039712
- SARN. (2010). Malaria elimination technical meeting November 2–4, 2010: Maputo. Southern Africa Roll Back Malaria Network. http://tis.sadc.int/english/sarn/elimination-eight-e8/.
- Severini, C., & Menegon, M. (2015). Resistance to antimalarial drugs: An endless world war against Plasmodium that we risk losing. Journal of Global Antimicrobial Resistance, 3(2), 58–63. https://doi.org/10.1016/j.jgar.2015.02.002
- Shah, J. A., Emina, J. B., Eckert, E., & Ye, Y. (2015). Prompt access to effective malaria treatment among children under five in sub-Saharan Africa: A multi-country analysis of national household survey data. Malaria Journal, 14(1), 329. https://doi.org/10.1186/s12936-015-0844-7
- Shellvarajah, M., Hatz, C., & Schlagenhauf, P. (2017). Malaria prevention recommendations for risk groups visiting sub-Saharan Africa: A survey of European expert opinion and international recommendations. Travel Medicine and Infectious Disease, 19, 49–55. https://doi.org/10.1016/j.tmaid.2017.09.002
- Singh, M., Brown, G., & Rogerson, S. J. (2013). Ownership and use of insecticide-treated nets during pregnancy in sub-Saharan Africa: A review. Malaria Journal, 12(1), 268. https://doi.org/10.1186/1475-2875-12-268
- Sinka, M. E., Pironon, S., Massey, N. C., Longbottom, J., Hemingway, J., Moyes, C. L., & Willis, K. J. (2020). A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proceedings of the National Academy of Sciences of the United States of America, 117(40), 24900–24908. https://doi.org/10.1073/pnas.2003976117
- Slater, H. C., Foy, B. D., Kobylinski, K., Chaccour, C., Watson, O. J., Hellewell, J., Aljayyoussi, G., Bousema, T., Burrows, J., D’Alessandro, U., Alout, H., Ter Kuile, F. O., Walker, P. G. T., Ghani, A. C., & Smit, M. R. (2020). Ivermectin as a novel complementary malaria control tool to reduce incidence and prevalence: A modelling study. The Lancet. Infectious Diseases, 20(4), 498–508. https://doi.org/10.1016/S1473-3099(19)30633-4
- Snetselaar, J., Njiru, B. N., Gachie, B., Owigo, P., Andriessen, R., Glunt, K., Osinga, A. J., Mutunga, J., Farenhorst, M., & Knols, B. G. J. (2017). Eave tubes for malaria control in Africa: Prototyping and evaluation against Anopheles gambiaess and Anopheles arabiensis under semi-field conditions in western Kenya. Malaria Journal, 16(1), 276. https://doi.org/10.1186/s12936-017-1926-5
- Somé, F. A., Bazié, T., Ehrlich, H. Y., Goodwin, J., Lehane, A., Neya, C., Zachari, K., Wade, M., Ouattara, J. M., Foy, B. D., Dabiré, R. K., Parikh, S., & Ouédraogo, J. B. (2020). Investigating selected host and parasite factors potentially impacting upon seasonal malaria chemoprevention in Bama, Burkina Faso. Malaria Journal, 19(1), 238. https://doi.org/10.1186/s12936-020-03311-8
- Sulaiman, S. K., Musa, M. S., Tsiga-Ahmed, F. I., Dayyab, F. M., Sulaiman, A. K., & Bako, A. T. (2022). A systematic review and meta-analysis of the prevalence of caregiver acceptance of malaria vaccine for under-five children in low-income and middle-income countries (LMICs). PLOS One, 17(12), e0278224. https://doi.org/10.1371/journal.pone.0278224
- Tahghighi, A., Mohamadi-Zarch, S.-M., Rahimi, H., Marashiyan, M., Maleki-Ravasan, N., & Eslamifar, A. (2020). In silico and in vivo anti‑malarial investigation methylene) hydrazine derivatives. Malaria Journal, 19(1), 231. https://doi.org/10.1186/s12936-020-03269-7
- Takarinda, K. P., Nyadundu, S., Govha, E., Gombe, N. T., Chadambuka, A., Juru, T., & Tshimanga, M. (2022). Factors associated with a malaria outbreak at Tongogara refugee camp in Chipinge District, Zimbabwe, 2021: A case–control study. Malaria Journal, 21(1), 94. https://doi.org/10.1186/s12936-022-04106-9
- Tesfahuneygn, G., & Gebreegziabher, G. (2019). Medicinal plants used in traditional medicine by Ethiopians: A review article. Journal of Respiratory Medicine for Lung Disease, 4(1), 1–3.
- Teshome, A., Erko, B., Golassa, L., Yohannes, G., Irish, S. R., Zohdy, S., Yoshimizu, M., & Dugassa, S. (2023). Resistance of Anopheles stephensi to selected insecticides used for indoor residual spraying and long-lasting insecticidal nets in Ethiopia. Malaria Journal, 22(1), 218. https://doi.org/10.1186/s12936-023-04649-5
- Thomas, S., Ravishankaran, S., Justin, J. A., Asokan, A., Mathai, M. T., Valecha, N., Thomas, M. B., & Eapen, A. (2016). Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai, India. Malaria Journal, 15(1), 274. https://doi.org/10.1186/s12936-016-1321-7
- Toé, K. H., Jones, C. M., N’Fale, S., Ismail, H. M., Dabiré, R. K., & Ranson, H. (2014). Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerging Infectious Diseases, 20(10), 1691–1696. https://doi.org/10.3201/eid2010.140619
- Tumwebaze, P., Tukwasibwe, S., Taylor, A., Conrad, M., Ruhamyankaka, E., Asua, V., Walakira, A., Nankabirwa, J., Yeka, A., Staedke, S. G., Greenhouse, B., Nsobya, S. L., Kamya, M. R., Dorsey, G., & Rosenthal, P. J. (2017). Changing antimalarial drug resistance patterns identified by surveillance at three sites in Uganda. The Journal of Infectious Diseases, 215(4), 631–635. https://doi.org/10.1093/infdis/jiw614
- Tusting, L. S., Bottomley, C., Gibson, H., Kleinschmidt, I., Tatem, A. J., Lindsay, S. W., & Gething, P. W. (2017). Housing improvements and malaria risk in sub-Saharan Africa: A multi-country analysis of survey data. PLOS Medicine, 14(2), e1002234. https://doi.org/10.1371/journal.pmed.1002234
- US President’s Malaria Initiative, Zimbabwe. (2020). CDC global health [Internet]. https://www.cdc.gov/globalhealth/countries/zimbabwe/annual-report/pmi.html.
- US President’s Malaria Initiative. (2018). Zimbabwe malaria operational plan FY 2018.
- US President’s Malaria Operational Plan FY, (2017). President’ malaria initiative Zimbabwe, malaria operational plan FY 2017.
- US President’s Malaria Operational Plan FY, (2019). President’ malaria initiative Zimbabwe, malaria operational plan FY 2019.
- US President’s Malaria Operational Plan FY, (2022). President’ malaria initiative Zimbabwe, malaria operational plan FY 2022.
- US President’s Initiative. (2016). Fighting malaria and saving lives. Washington DC. Retrieved from https://www.pmi.gov
- U. S. President’s malaria initiative Zimbabwe. (2018). Malaria operational plan FY 2018. Retrieved from https://www.pmi.gov
- U. S. President’s malaria initiative Zimbabwe. (2020). Malaria operational plan FY 2020. Retrieved from https://www.pmi.gov
- Verde, C., Tomé, S., Silva, J. R. D. A., Ramos, A. D. S., Machado, M., De Moura, D. F., Neto, Z., Canto-Cavalheiro, M. M., Figueiredo, P., Rosário, V. E., Amaral, A. C. F., & Lopes, D. (2011). A review of antimalarial plants used in traditional medicine in communities in Portuguese-Speaking countries: Brazil, Mozambique. Memorias do Instituto Oswald Cruz, 106, 142–158.
- Wesolowski, A., Ippolito, M. M., Gebhardt, M. E., Ferriss, E., Schue, J. L., Kobayashi, T., Chaponda, M., Kabuya, J.-B., Muleba, M., Mburu, M., Matoba, J., Musonda, M., Katowa, B., Lubinda, M., Hamapumbu, H., Simubali, L., Mudenda, T., Shields, T. M., Hackman, A., … Moss, W. J, Southern and Central Africa International Center of Excellence for Malaria Research. (2022). Policy implications of the Southern and Central Africa International Center of Excellence for Malaria Research: Ten years of malaria control impact assessments in hypo-, meso-, and holoendemic transmission zones in Zambia and Zimbabwe. The American Journal of Tropical Medicine and Hygiene, 107(4_Suppl), 68–74. https://doi.org/10.4269/ajtmh.21-1288
- WHO. (2007). Malaria elimination: A field manual for low and moderate endemic countries. World Health Organization.
- WHO. (2013). World malaria report 2013. World Health Organization.
- WHO. (2017). World malaria report 2016. World Health Organization.
- WHO. (2018). World malaria report 2018. World Health Organization.
- WHO. (2019). HBHI approach in world malaria report 2019 [Online]. Accessed 5 July 2022. https://www.who.int/publications/i/item/9789240015791.
- WHO. (2019). World malaria report. World Health Organisation.
- WHO. (2020). Global trends in the burden of malaria, world malaria report. World Health Organisation.
- WHO. (2020). Global malaria programme, the potential impact of health service disruptions on the burden of malaria: A modelling analysis for countries in Sub-Saharan Africa. World Health Organisation.
- World Health Organization. (2020). The E-2020 eliminating 2019 progress report. World Health Organisation.
- WHO. (2021a). World Malaria Report 2021. World Health Organization. License: CC BY-NC-SA 3.0
- WHO. (2021b). WHO recommends groundbreaking malaria vaccine for children at risk, historic RTS,S/AS01 recommendation can reinvigorate the fight against malaria. https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk
- WHO. (2022a). The transformation agenda of the World Health Organization Secretariat in the African region 2015–2020 [Online]. Accessed 3 June 2022. https://www.afro.who.int/regional-director/transformation-agenda
- WHO. (2022b). Tackling emerging antimalarial drug resistance in Africa. https://www.who.int/news/item/18-11-2022-tackling-emerging-antimalarial-drug-resistance-in-africa.
- WHO. (2024). Highlights from the meeting of the strategic advisory group of experts (SAGE) on Immunization 11–13 March 2024. World Health Organization.
- WHO. (2022c). World malaria report, Progress on malaria control in countries, WHO in an era of transformation, African region. https://www.afro.who.int.
- Winskill, P., Walker, P. G., Cibulskis, R. E., & Ghani, A. C. (2020). Prioritizing the scale up of interventions for malaria control and elimination. Malaria Journal, 18(1), 122. https://doi.org/10.1186/s12936-019-2755-5
- Wu, Y., Soe, M. T., Aung, P. L., Zhao, L., Zeng, W., Menezes, L., Yang, Z., Kyaw, M. P., & Cui, L. (2020). Efficacy of artemether ‑ lumefantrine for treating uncomplicated Plasmodium falciparum cases and molecular surveillance of drug resistance genes in Western Myanmar. Malaria Journal, 19(1), 304. https://doi.org/10.1186/s12936-020-03376-5
- Yukich, J., Digre, P., Scates, S., Boydens, L., Obi, E., Moran, N., Belemvire, A., Chamorro, M., Johns, B., Malm, K. L., Kolyada, L., Williams, I., Asiedu, S., Fomba, S., Mihigo, J., Boko, D., Candrinho, B., Muthoni, R., Opigo, J., … Robertson, M. (2022). Incremental cost and cost-effectiveness of the addition of indoor residual spraying with pirimiphos-methyl in sub-Saharan Africa versus standard malaria control: Results of data collection and analysis in the next generation indoor residual sprays (NgenIRS) project, an economic-evaluation. Malaria Journal, 21(1), 185. https://doi.org/10.1186/s12936-022-04160-3
- Zimbabwe National Statistical Agency. (2012). Census 2012: Preliminary report. Harare, Zimbabwe: Zimbabwe National Statistics Agency.