?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To create pH-responsive microcapsules, the research involved microencapsulating n-eicosane phase change material (PCM) with poly(MAA-co-ACR-co-ACN) for application on sanitary napkins. The in-situ polymerization technique was employed for polymer synthesis. SEM, FTIR, and TGA were used to verify the chemical structure and composition of pH-responsive microcapsules through morphology and core-shell structure analysis. The free swell capacity and absorption under load in tap water and saline solution were used to evaluate the absorbency of pH-responsive coated sanitary napkins. Furthermore, strike through and the pH level of every coated sample was investigated. Microcapsule coated napkins have an acidic pH value, and they are better suited for maintaining the acidic pH of the vagina during menstruation, as at the time of menstruation, pH of the blood is alkaline. So, the present work suggests the pH-responsive microcapsules for coating the top layer of feminine napkin and the comparative result suggested by SEM, FTIR, TGA, absorbency capacity, strike through and pH, clearly supports the process.
1. Introduction
The significance of living in a world of hygiene and freshness cannot be underestimated by humans. Women’s menstrual phase begins with menarche, which is a biological milestone, and an average of 1800 days in lifetime, women use the sanitary pad[Citation1,Citation2] Routine menstrual period 35 ml blood has been lost generally. The situation for girls is being worsened by a lack of knowledge on menstruation preparedness and management in many developing countries. Indian society still considers menstruation to be a taboo, even though it is a natural process. The smell of menstrual fluid can be unpleasant, especially when exposed to air. The strategies of women are influenced by their personal preferences, availability of resources, economic/cultural status, and knowledge of education and menstruation [Citation3,Citation4] The medical literature indicates that a large proportion of girls are not aware and unprepared for menarche or other diseases due to their inexperience with menstrual management. The environment is significantly affected by menstrual waste worldwide, India being an exception. Their daily routine involves using menstrual pads and diapers for menstrual conditions. Health is negatively affected by poor genital hygiene in either situation. When choosing sanitary pads/diapers, chemicals and microbes are also a concern. Even though synthetic plastic materials are well known for releasing volatile organic compounds and hormone-disrupting toxins, they have been utilized as liquid absorbents to enhance efficiency[Citation5–7] The development of future products was focused on the thorough characterization of the fabrics used in daily sanitary pads. Polypropylene was utilized as the material for the samples in the FTIR (fourier transform infrared)/ATR (attenuated total reflectance) spectrum spectrum. Despite the Indian Government’s efforts to ensure that all women are provided with sanitary pads to manage menstruation, managing menstrual waste is also a major concern. Biomaterials have been integrated into the healthcare industry to improve the quality of life for both normal and critically ill hospitalized patients, as a result of current advances. The accumulation of waste, which in turn leads to the spread of diseases, can be caused by non-biodegradable materials. The issue of neglect in managing sanitary pads, adult diapers, and similar materials is widely reported. It is imperative that we have a pad/diaper that is antimicrobial, biodegradable, and has a high holding capacity.[Citation8–10]
Consumption of sanitary napkins has been increased day by day, and to fulfill the demand and comfort improvement, textile industries need versatile and smart solution. So, recent scenario is concentrating on smart textile for anti-bacterial, water repellent, UV resistant, and such other properties related to health hygiene[Citation11–13] Sanitary napkin with specific properties like anti-bacterial, water absorbency in free and under load condition, have been studied and the products are available in market also. Moreover, the menstrual blood has typical odor and the pH of the same is 7.4, and the pH of vagina is generally between 3.8 to 5.0 [Citation14] As a result, there is increment in pH wearer feel discomfort. There are many inventions and researches have been performed for super hydrophobicity by imparting hydrogel as a smart material in sanitary napkin, and they attracted consumer market also [Citation4,Citation15,Citation16] For pH controlling at the vagina pH-responsive polymer coated phase change material (PCM) can be used which may be microencapsulated. PCMs has the property of transforming from one state to another for stopping running off.[Citation17,Citation18,] Microcapsule is the product in which the core portion is encapsulated with hard shell for generating a very small sphere, and the process is acknowledge as microencapsulation.[Citation19] Physical as well chemical process can be used for microencapsulating the PCMs, and the chemical process is the mostly used and reliable as well. Various materials are studied for hard shell application and those are polystyrene, poly(methyl methacrylate), polyurethane, silica, titanium dioxide, aluminum hydroxide, zinc oxide, copper oxide, and metal oxide’s hybrids also.[Citation17] pH-responsive polymers are those who reacts with the very small change in pH of the solution due to structural change, solubility, surface activity, and chain configuration. Commonly, pH-responsive polymers possess weak acidic or basic groups, and such groups can absorb or release hydrogen with the change in the surrounding pH.[Citation20,Citation21Citation22]
So, in presented work, n-eicosane has been microencapsulated with poly (methacrylic acid-co-acrylamide-co-acrylonitrile) [poly(MAA-co-ACR-co-ACN)] a pH-responsive polymeric coat. n-eicosane is an organic PCM suitable for the human body by ranging with phase transition temperatures. Due to ionization of the -COOH functional group present in poly(MAA-co-ACR-co-ACN), structure is changed. This microencapsulated structure of PCM and pH-responsive polymer is used for coating the feminine hygiene napkin by showing pH-stimuli responsive controlled-release performance. As a result, pH-responsive polymer poly(MAA-co-ACR-co-ACN) helps in preventing the acidic pH during menstrual period, and PCM core material helps in maintaining the temperature at optimum level. n-eicosane was microencapsulated with pH-responsive polymer by in-situ polymerization and then coated on hygiene napkin, and the performance characteristics had been studied for analyzing the aim of the work. The goal of this study is to open up a new zone for developing dual functional microencapsulated PCMs and applying the same on sanitary napkin for controlling the pH.
2. Material and method
2.1. Materials
Methacrylic acid (MAA), diallyl maleate (DAM), potassium persulfate (K2S2O8) and benzoyl peroxide (BP) were obtained from A. B. Enterprise, Mumbai. n-Eicosane supplied by Shanghai Macklin Biochemical Co. Ltd. China. Acrylonitrile (ACN) and toluene were purchased from Solvochem, Ahmedabad. While acrylamide (ACR), ammonium dodecyl sulfate (ADS), nitrogen gas and liquor ammonia were obtained from Madhu Chemicals-Mumbai, Pawan Chemicals-Mumbai, Yash Organic-Mumbai, Arihant Chemicals-Vapi, Triveni Chemicals-Vapi, Sunshine Industrial Gase-Ahmedabad, Suvidhi Industry-Vapi, respectively. All the reagents used in the work are analytical grade and so used directly as received without supplementary refinement.
2.2. Preparation of pH-stimuli microcapsules
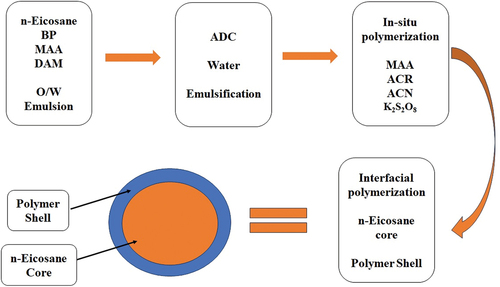
n-eicosane as a core and poly(MAA-co-ACR-co-ACN) as a shell was formulated by in-situ polymerization technique for preparing microcapsule and the route for preparing the microcapsule is shown in . The preparation involves three stages. In the first stage, oil phase consisting MAA, DAM, and n-eicosane has been dispersed in the water by formulating the oil-in-water (O/W) emulsion with the help of ADS, an anionic detergent. In order to start the in-situ copolymerization of MAA and DAM, BP was utilized as an initiator that dissolves in oil, and the O/W emulsion was warmed to 75°C. The second stage involves the addition of MAA, ACR, and ACN co-monomers for performing copolymerization with the addition of K2S2O8, another initiator. Finally, the shell of poly(MAA-co-ACR-co-ACN) has been developed on n-eicosane core.
The synthetic route consisting the addition of 400 ml deionized water taken in 1000 ml five neck round bottom flask ()[Citation23] 18.0 g of MAA, 1.0 g DAM, 0.1 g BP, and 20.0 g n-eicosane was added in the flask by maintaining the temperature 55°C with constant stirring. 2.5 g ADS was added in above mixture after 15 minutes, and then the mixture is allowed to stand at 55°C for 2.5 hours with the purge of nitrogen. As the stable O/W emulsion is produced the temperature gradually increased to 75°C, so free radical polymerization took place under the presence of nitrogen gas, and the mixture is allowed to stand at the same temperature for 3 hours with constant agitation. After 3 h, mixture of 8.0 g MAA, 4.0 g ACR, 0.2 g ACN, and 0.03 g K2S2O8 introduced in above system. After mixing the flask was heated at 82°C for 2.5 h with constant stirring to accomplish interfacial polymerization. At the end of reaction, yield was washed with deionized water and white powders collected by filtration, which are pH-responsive polymer microcapsules. They are dried at room temperature.
2.3. Characterization
JSM 7001 F scanning electron microscope (SEM) was used for study the morphology of pH-stimuli microcapsules. Nano MeasureTM software was utilized for measurement for particle size distribution and mean particle size of pH-responsive microcapsule. For FTIR (Fourier-transform infrared spectroscopy), PerkinElmer Spectrum™ 3 FT-IR spectrometer was used, which can analyze the chemical structure and composition of pH-responsive microcapsules. TGA (thermogravimetric analysis) investigations of pH-responsive microcapsules were carried out with 1.0 g of initial sample material at the heating rate of 20 K/min using Parkin Elmer TGA model 4000.
2.4. Application on Sanitary Napkin
Five layers have been formulated and fabricated for preparing the pad, including a self-adhesive tape which attached to the under garment. Tape is covered using cotton coated with paraffin wax for inhibiting leakage. Third layer is made with developed super adsorbent polymer. Again, the thin cotton has been pressed and attached to the third layer. Top layer of the pad has been made with cotton coated with formulated microencapsulated SAP.
For preparing top layer of sanitary napkin, it immersed in 30 g of pH-responsive microcapsules dispersion for 30 minutes. Then, the layer was rolled in glass rod and baked at 82°C for 3 minutes. The said process was repeated three times. Three samples were prepared: S-1 for n-eicosane, S-2 for poly(MAA-co-ACR-co-ACN) and S-3 for pH-responsive microencapsulated polymers.
2.5. Free swell capacity (FSC)
The amount of distilled water that is absorbed by the sample during its freely swollen condition is measured by this test result. In terms of convenience and speed, the tea-bag method is the best option for samples with limited quantities. Citation25,Citation26 The sample was put into a tea bag and immersed in water or saline solution for an hour after being prepared. Once the tea bag had fully swollen, it was once more weighed and its swelling capacity was calculated.
Where, T1 and T2 are the tea bag absorption factor, W0 is the weight of prepared sample of sanitary napkin, W1 is the weight of dry empty tea bag, W2 is the weight of swollen sample.
2.6. Absorption under load (AUL)
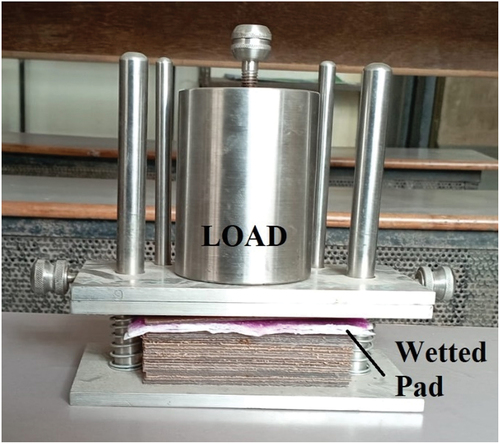
AUL determines the capacity of SAP samples to absorb fluid at certain pressures. The filter screen was filled with an SAP sample of 0.9 grams to create an even bed. On the bed, a piston made of aluminum was placed. The entire assembly weighted W1. Tap water and saline solution were added after placing a porous disk in a tray (). The whole assembly was placed on the filter paper with load (0.3 psi).[Citation22,Citation24,Citation27] The assembly was permitted to remain in its current state for an hour. After the set period, the assembly was lifted, the load was taken out, and the cylinder was reweighted as W2. The AUL was calculated with the following equation:
2.7. Determination of pH of top layer
pH of the saline solution was measured using pH-meter. 0.5 g of sample was added gradually to 100 ml of a saline solution with stirring. After settling of the solution, pH was measured.[Citation22,Citation28,Citation29]
2.8. Strike through
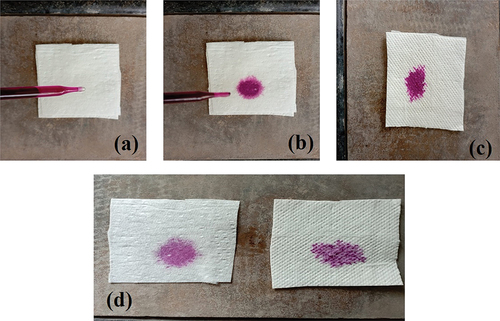
The transportation speed of fluid traveling from the surface on which it absorbs to the inner zone of pad is measured with strike through test. Higher value of strike through indicates the top layer of pad is quickly absorbing the fluid, and so the skin feels dry. A 5 ml drop of 0.9% saline solution is used to conduct the test, and the penetration time is recorded (). On the microencapsulated SAP-coated napkin, a drop of test solution is allowed to fall freely, and the amount of time it takes for the solution to absorb from the top layer to the inner layer is recorded. Citation30
3. Result and discussion
3.1. Characterization
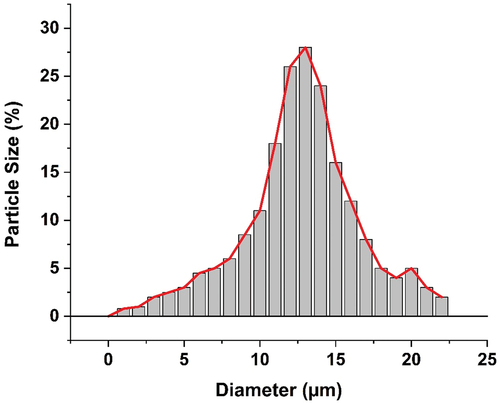
SEM analysis of pH-responsive microcapsules was performed to study the microstructure and morphology of prepared microcapsule, and the obtained result is shown in . The figure clearly indicates that the geometry of prepared pH-responsive microcapsules is uniform and spherical in shape. Final microcapsules do not contain or shown any aggregation. Also, SEM micrographs suggests the particle size distribution of prepared microcapsule is between 1 to 20 µm, and the all-present microcapsules are showing diameter between 8 µm to 15 µm (). SEM images in also support the crosslinking process, which can improve the tightness of poly(MAA-co-ACR-co-ACN) shell, as the pH-responsive microcapsules has little rough surface, and surface if crack or wrinkle free. The result of SEM give the affirmative support that the n-eicosane core has been successfully capsulated with poly(MAA-co-ACR-co-ACN) shell by in-situ polymerization technique. FTIR of the synthesized microcapsules analyzed as suggest in . indicates the FTIR spectra for pure n-eicosane, poly(MAA-co-ACR-co-ACN) and microcapsules. Also from the figure, it is clear that particular picks of pH-responsive microcapsules are supporting the picks of pure n-eicosane and poly(MAA-co-ACR-co-ACN) which support the coexistence of n-eicosane as a core and poly(MAA-co-ACR-co-ACN) as a shell.
Figure 5. SEM Micrographs: (A, B) pH-responsive microcapsules, (C) close-up pH-responsive microcapsules, and (D) damaged microcapsules.
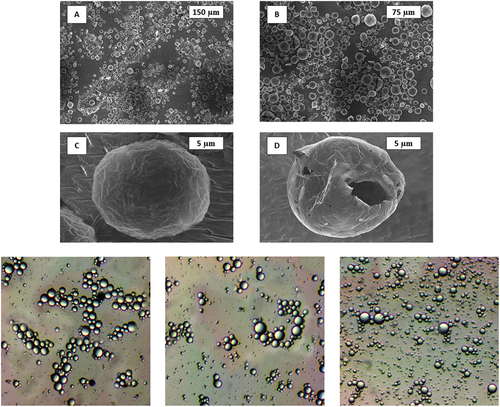
Figure 7. FTIR spectra of pure n-eicosane, poly(MAA-co-ACR-co-ACN), and pH-responsive microcapsules.
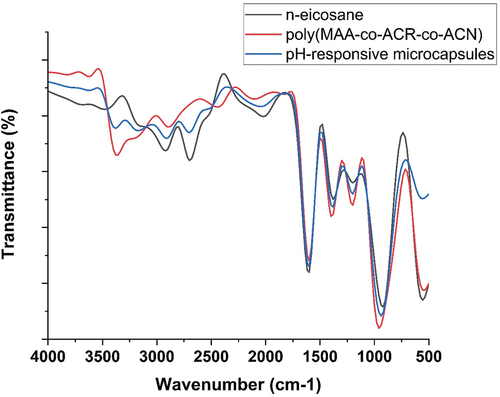
Table 1. The characteristic IR bands of pH-responsive microcapsules.
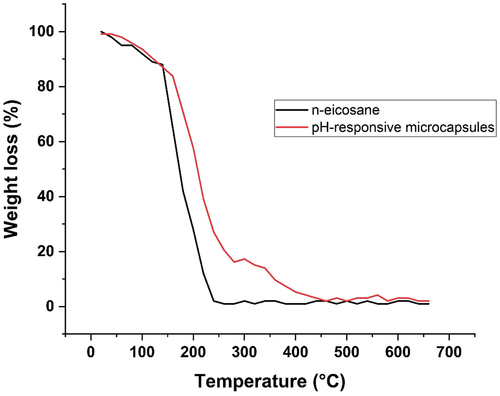
The inspection of TGA curve () for pH-responsive microcapsules reveals that by heating the microcapsules a slight initial weight loss was observed due to loss of adsorbed water. The steep fall in weight was recorded over the temperature range of 152-247°C due to vaporization of n-eicosane. It clearly indicates the thermal degradation of poly(MAA-co-ACR-co-ACN) shell also. This second stage of significant weight loss belongs to fast degradation of poly(MAA-co-ACR-co-ACN). For microcapsules, Tmax of n-eicosane is increases as it is capsulated with poly(MAA-co-ACR-co-ACN) shell. So, the shell of polymer decreased the thermal degradation of core by acting as a barrier.
3.2. Absorption capacity
Free swell capacity (FSC) and absorption under load (AUL) of all the samples treated with pure n-eicosane, poly(MAA-co-ACR-co-ACN) and pH-responsive microcapsules in tap water and saline solution are shown . The highest FSC value 94.23 g/g is attributed to sample prepared with S-3, While in saline water, the FSC value is comparatively lower than the value obtained with tap water. 55.87 g/g attributed to sample SAP3, while other samples are between 32.42 to 41.78 g/g.
Table 2. Absorption capacity of napkin.
also suggests that values for AUL are lower than FSC for each sample. The values obtained in tap water are between 20.18 to 52, while in saline solution those are between 12.23 to 28.92. As per the market scenario, demand of the thinner hygiene with less fluff and more hydrophobicity is comparatively higher. AUL limits the amount of S-3 present in hygienic material. Currently, the value accepted by the hygiene industries is between 18 to 30 g/g, but values can be increased to 50 g/g. according to present work, in saline solution absorption capacity is decreased as compared to the tap water.[Citation22,Citation27,Citation29 Citation31] So, the osmotic pressure is reduced between solution and hydrogel, which reduced the electrostatic force resulting in reduction of water absorption.
S-1 sample has the higher value absorption due to the presence of super absorbent polymer available in it with cotton pulp. The same phenomenon happened in the prepared S-2 and S-3 used in research work, and coated on needle punched cotton adjusted to the top layer. So, the absorption of prepared napkin has also higher absorption and it can replace control one. Also, it provides necessary suppleness, liquid absorptivity, and required breathability
3.3. pH
pH of menstrual blood is nearly 7.4, while the vagina has the pH value ranges between 3.8 to 5.0. Vaginal mucosa can be protected from pathogenic organisms by having a lower pH value (more acidic) compared to blood or interstitial fluids. So, the sanitary napkin must maintain the pH at acidic level for wearer comfort. pH of each sample has been analyzed and tabulated in . S-3 has the pH (3.02) which is acidic; so, when the menstrual blood with the pH of 7.4 will come on contact at the top layer of prepared pad, due to the reaction between fluid and S-3, the pH will lower down, and wearer will feel comfort. It also reduces the itchiness.
Table 3. pH of coated napkin.
3.4. Strike through
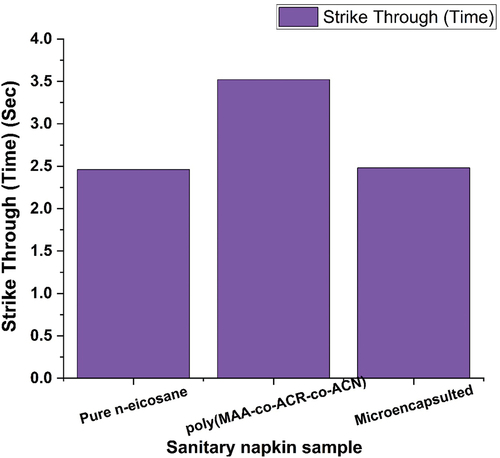
suggests that the coated sample with microencapsulated product S-3 has the higher speed of transportation, and so, the time required for passing the fluid from surface to the inner part is less which 2.48 seconds only. Slightly lower seconds (2.46) had been required for the fluid transportation in the napkin where the top layer was coated with pure n-eicosane (S-1). Whereas, poly(MAA-co-ACR-co-ACN) S-2 coated layer has the time very near for striking. The higher strike through is received due to the presence of SAP. As the presence of cotton and needle punched cotton at top layer in prepared samples, due to hydrophilicity of cotton and absorbency capacity of SAPs, the prepared napkins also suggest the comparable strike through property with the control sample.
4. Conclusion
In the work presented here, encapsulation of n-eicosane into the poly(MAA-co-ACR-co-ACN) has been achieved successfully, which is denoted as pH-responsive microcapsules. Microcapsules contain PCM as core and pH-responsive polymer as shell. The emulsion of the microcapsules has been performed by in-situ polymerization technique. SEM and FTIR analysis supports the formation of pH-responsive microcapsules by analyzing their chemical structure and compositions. Total absorption capacity, in terms of FSC and AUL of the cotton carded web coated with S-1 to S-3 was measured. The results have discussed in tap water and saline solution by suggesting that microcapsule coated sample shows excellent absorbency which is required by sanitary napkin. Strike through property has also shown the excellent performance in case of S-3, which is prepared during this study. Subsequently, pH measurement in saline solution performed, and the results were achieved between 2.94 to 3.08. Finally, the pH-responsible microcapsules can be utilized for coating the top layer of sanitary napkin, which controls the pH in acidic region required for vagina, and also shows the higher absorbency in free swell as well as under load.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Anand, E.; Singh, J.; Unisa, S. Menstrual Hygiene Practices and Its Association with Reproductive Tract Infections and Abnormal Vaginal Discharge among Women in India. Sex Reprod Health. 2015, 64, 249–254. DOI:10.1016/j.srhc.2015.06.001.
- Park, C. J.; Barakat, R.; Ulanov, A.; Li, Z.; Lin, P.-C.; Chiu, K.; Zhou, S.; Perez, P.; Lee, J.; Flaws, J., et al. Sanitary Pads and Diapers Contain Higher Phthalate Contents than Those in Common Commercial Plastic Products. Reprod. Toxicol. 2019, 84, 114–121. DOI: 10.1016/j.reprotox.2019.01.005.
- Kaur, R.; Kaur, K.; Kaur, R. Menstrual Hygiene, Management, and Waste Disposal: Practices and Challenges Faced by Girls/Women of Developing Countries. J. Environ. Public Health. 2018, 2018, 1–9. DOI: 10.1155/2018/1730964.
- Shibly, M. M. H.; Hossain, M. A.; Hossain, M. F.; Nur, M. G.; Hossain, M. B. Development of biopolymer-based Menstrual Pad and Quality Analysis against Commercial Merchandise. Bull Natl Res Cent. 2021, 451, 50. DOI: 10.1186/s42269-021-00504-2.
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R. C.; Faisal, P. A. Phthalates Impact Human Health: Epidemiological Evidences and Plausible Mechanism of Action. J. Hazard. Mater. 2017, 340, 360–383. DOI: 10.1016/j.jhazmat.2017.06.036.
- Bolden, A. L.; Rochester, J. R.; Schultz, K.; Kwiatkowski, C. F. Polycyclic Aromatic Hydrocarbons and Female Reproductive Health: A Scoping Review. Reprod. Toxicol. 2017, 73, 61–74. DOI: 10.1016/j.reprotox.2017.07.012.
- Sivakami, M.; Maria van Eijk, A.; Thakur, H.; Kakade, N.; Patil, C.; Shinde, S.; Surani, N.; Bauman, A.; Zulaika, G.; Kabir, Y., et al. Effect of Menstruation on Girls and Their Schooling, and Facilitators of Menstrual Hygiene Management in Schools: Surveys in Government Schools in Three States in India, 2015. J Global Health. 2019, 9. DOI: 10.7189/jogh.09.010408
- Loo, S.; Vásquez, L.; Athanassiou, A.; Fragouli, D. Polymeric Hydrogels—A Promising Platform in Enhancing Water Security for a Sustainable Future. Adv. Mater. Interfaces. 2021, 8. DOI: 10.1002/admi.202100580.
- Sommer, M.; Chandraratna, S.; Cavill, S.; Mahon, T.; Phillips-Howard, P. Managing Menstruation in the Workplace: An Overlooked Issue in Low- and middle-income Countries. Int J Equity Health. 2016, 151, 86. DOI: 10.1186/s12939-016-0379-8.
- White, H. L.; Mwapasa, T.; Mphasa, M.; Kalonde, P. K.; Feasey, N.; Oliver, D. M.; Ormsby, M. J.; Morse, T.; Chidziwisano, K.; Quilliam, R. S. Open Defaecation by Proxy: Tackling the Increase of Disposable Diapers in Waste Piles in Informal Settlements. Int. J. Hyg. Environ. Health. 2023, 250, 114171. DOI: 10.1016/j.ijheh.2023.114171.
- Chen, K.; Zhou, J.; Hu, J.; Zhang, J.; Heng, T.; Xu, C.; Wang, X.; Liu, J.; Yu, K. Preparation of pH-Responsive Dual-Compartmental Microcapsules via Pickering Emulsion and Their Application in Multifunctional Textiles. ACS Appl. Mater. Interfaces. 2021, 13(1), 1234–1244. DOI: 10.1021/acsami.0c18043.
- Lu, L.; Hu, C.; Zhu, Y.; Zhang, H.; Li, R.; Xing, Y. Multi-functional Finishing of Cotton Fabrics by water-based layer-by-layer Assembly of metal–organic Framework. Cellulose. 2018, 25(7), 4223–4238. DOI: 10.1007/s10570-018-1838-8.
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Wei, G.; Zhou, H. Multifunctional Janus Fibrous Hybrid Membranes with Sandwich Structure for on-demand Personal Thermal Management. Nano Energy. 2019, 63, 103808. DOI: 10.1016/j.nanoen.2019.06.004.
- Lin, Y.-P.; Chen, W.-C.; Cheng, C.-M.; Shen, C.-J. Vaginal pH Value for Clinical Diagnosis and 460Treatment of Common Vaginitis. Diagnostics. 2021,11(11), 1996. DOI: 10.3390/diagnostics11111996.
- Enawgaw, H.; Tesfaye, T.; Yilma, K. T.; Limeneh, D. Y. Synthesis of a Cellulose-Co-AMPS Hydrogel for Personal Hygiene Applications Using Cellulose Extracted from Corncobs. Gels. 2021, 7(4), 236. DOI: 10.3390/gels7040236.
- Peng, M. C.; Sethu, V.; Selvarajoo, A. Performance Study of Chia Seeds, Chia Flour and Mimosa Pudica Hydrogel as polysaccharide-based Superabsorbent Polymers for Sanitary Napkins. Mater. Today Commun. 2021, 26, 101712. DOI: 10.1016/j.mtcomm.2020.101712.
- Guo, Z.; Liu, H.; Wu, Y.; Wang, X.; Wu, D. Design and Fabrication of pH-responsive Microencapsulated Phase Change Materials for Multipurpose Applications. React. Funct. Polym. 2019, 140, 111–123. DOI: 10.1016/j.reactfunctpolym.2019.04.015.
- Liu, G.; Lin, G.; Lin, X.; Zhou, H.; Chen, H.; Hao, L.; Zhou, X. Enzyme and pH dual-responsive Avermectin nano-microcapsules for Improving Its Efficacy. Environ. Sci. Pollut. Res. 2019, 26(24), 25107–25116. DOI: 10.1007/s11356-019-05804-9.
- Zhao, C. Y.; Zhang, G. H. Review on Microencapsulated Phase Change Materials (Mepcms): Fabrication, Characterization and Applications. Renew Sustain Energy Rev. 2011, 158, 3813–3832. DOI: 10.1016/j.rser.2011.07.019.
- Liu, H.; Wang, X.; Wu, D. Innovative Design of Microencapsulated Phase Change Materials for Thermal Energy Storage and Versatile Applications: A Review. Sustain. Energy Fuels. 2019, 3(5), 1091–1149. DOI: 10.1039/C9SE00019D.
- Seitz, S.; Ajiro, H. Self-assembling Weak Polyelectrolytes for the layer-by-layer Encapsulation of paraffin-type Phase Change Material Icosane. Solar Energy Mater. Solar Cells. 2019, 190, 57–64. DOI: 10.1016/j.solmat.2018.10.012.
- Bachra, Y.; Grouli, A.; Damiri, F.; Bennamara, A.; Berrada, M. A New Approach for Assessing the Absorption of Disposable Baby Diapers and Superabsorbent Polymers: A Comparative Study. Results Mater. 2020, 8, 100156. DOI: 10.1016/j.rinma.2020.100156.
- Madhu, C. R. AA, ACR, and ACN Polymer (Synthetic Thickener) Formulation: Utilisation of Reactive Printing of Cotton. Curr. Physical Chem. 2022, 122, 159–170. DOI:10.2174/1877946812666220301125133.
- EDANA. Absorbency against Pressure, No NWSP 242.0.R2 (19), Harmonized Nonwovens Standard Procedure: Polyacrylate Superabsorbent Powders – Gravimetric Determination of Absorption against Pressure, 2019a.
- EDANA, 2019 Free swell capacity, No NWSP 080.9.R1 (19) Harmonized Nonwovens Standard Procedure: Polyacrylate Superabsorbent Powders – Determination of the Free Swell Capacity in Saline by Gravimetric Measurement
- Yimin, Qin 2016 Superabsorbent polymers and their medical applications Medical Textile Materials (Elsevier)
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of Chitosan Derivative Graft Acrylic Acid Superabsorbent Polymers and Its Application as Water Retaining Agent. Int. J. Biol. Macromol. 2018, 115, 754–761. DOI: 10.1016/j.ijbiomac.2018.04.072.
- EDANA. Determination of pH, No NWSP 200 0. R2 (19), Harmonized Nonwovens Standard Procedure: Polyacrylate Superabsorbent Powders – Determination of pH, 2019b.
- Klinpituksa, P.; Kosaiyakanon, P. Superabsorbent Polymer Based on Sodium Carboxymethyl Cellulose Grafted Polyacrylic Acid by Inverse Suspension Polymerization. Int. J. Polym. Sci. 2017, 2017, 1–6. DOI: 10.1155/2017/3476921.
- EDANA, 2018 Guidelines for the Testing of Feminine Hygiene Products – version 13th December 2018 European Disposables and Nonwovens Association
- Likhitha, M.; Sailaja, R. R. N.; Priyambika, V. S.; Ravibabu, M. V. Microwave Assisted Synthesis of Guar Gum Grafted Sodium acrylate/cloisite Superabsorbent Nanocomposites: Reaction Parameters and Swelling Characteristics. Int. J. Biol. Macromol. 2014, 65, 500–508. DOI: 10.1016/j.ijbiomac.2014.02.008.