ABSTRACT
The gut microbiome (GM) influences multiple processes during host development and maintenance. To study these events, fecal microbiota transfer (FMT) to germ-free (GF) recipients is often performed. Mouse models of disease are also susceptible to GM-dependent effects, and cryo-repositories often store feces from donated mouse strains. Shipping live mice may affect the GM and result in an inaccurate representation of the baseline GM. We hypothesize that the use of such fecal samples for FMT would transfer shipping-induced changes in the donor GM to GF recipients. To test this, donor mice originating from two suppliers were shipped to the University of Missouri. Fecal samples collected pre- and post-shipping were used to inoculate GF mice. Pre- and post-shipping fecal samples from donors and fecal and/or cecal contents were collected from recipients at 1 and 2 weeks post-FMT. 16S rRNA sequencing revealed supplier-dependent effects of shipping on the donor microbiome. FMT efficiency was independent of shipping timepoint or supplier, resulting in the transmission of shipping-induced changes to recipient mice; however, the effect of supplier-origin (SO) microbiome remained evident. While shipping may cause subtle changes in fecal samples collected for FMT, such effects are inconsistent among SO GMs and minor in comparison to other biological variables.
Introduction
The gut microbiome (GM) affects numerous host developmental processes and is essential for long-term host health. While a wide range of different community compositions can perform comparable function during health,Citation1 sufficient change (i.e., dysbiosis) is often associated with the disease.Citation2 Mice used in biomedical research are traditionally colonized with a specific pathogen-free (SPF) microbiome, and mice purchased from different suppliers harbor distinct supplier-origin (SO) GMs.Citation3–6 Following purchase from one of the suppliers of SPF mice, myriad husbandry and institution-specific factors,Citation7–9 as well as shipping itself,Citation10 can influence the composition of the microbiome at an individual and colony level. As in human health, the phenotype of many mouse models are influenced by specific features within the GM. Indeed, there are numerous reports of changes or even complete loss of phenotype in mouse models in association with a change in the microbiome due to institution-specific practices.Citation11–14
Periodic collection and preservation of fecal samples from a research colony allows for benchmarks against which samples can be compared in the future. In the event of changes in model phenotypes, the microbiome may be compared to banked samples to determine if changes in community composition have occurred potentially contributing to the changes in model phenotype. Ostensibly, banked samples could also be used to re-inoculate mice via fecal microbiota transfer (FMT) in efforts to restore microbiome-dependent phenotypes. Related to this, the University of Missouri (MU) National Institutes of Health (NIH)-funded Mutant Mouse Resource and Research Center (MMRRC) at the MU and other consortium members routinely collect feces from mouse strains donated for cryopreservation, in an effort to preserve the GM at the time of strain curation. While some donating investigators collect and submit fecal samples alongside mice submitted for cryopreservation, the MU MMRRC collects samples from donated mouse strains after arrival at our facility and prior to cryopreservation. Considering the potential effect of shipping mice for cryopreservation on the composition of fecal samples collected for posterity, we wanted to investigate whether shipping mice to be used as donors significantly affects the composition of their GM, and if so, whether any shipping-induced changes in the GM of donors are maintained following the reconstitution of germ-free (GF) mice.
To do so, fecal samples were collected from donor mice pre- and post-shipping and used to reconstitute recipient mice via FMT. FMT can be performed using GF or antibiotic-treated recipient mice. To eliminate potential effects of variability among antibiotic-treated recipient mice, GF recipients were used. Lastly, to determine whether any observed effects of shipping are consistent across different SPF microbiomes, donor mice from two suppliers with distinct SO GMs were used. Fecal samples collected from C57BL6/J and C57BL/6NHsd donors pre- and post-shipping and from recipient GF Swiss Webster mice at 1 and 2 weeks post-FMT were subjected to DNA extraction, 16S rRNA library preparation and sequencing, and testing for the effects of shipping on the GM of mice to be used as FMT donors.
Methods
Mice
Donor mice used in the current study were female, adult (5–6 weeks old) C57BL/6J (Jackson Laboratory, n = 12) or C57BL/6NHsd (Envigo, n = 12) mice. Only female donor mice were used so as to avoid sex as a variable between donors and recipients. Prior to the study (during the acclimation period), mice were housed in individually ventilated microisolator cages (Allentown) on a 14:10-h light:dark cycle. Recipient mice were GF, female, adult (7–8 weeks old) Swiss Webster mice (Taconic, n = 24), shipped from the supplier in microisolator cages within a flexible film isolator to maintain GF status. Following the inoculation of GF recipient mice via FMT, mice were housed in individually ventilated microisolator cages (Thoren, Hazleton, PA) on a 14:10-h light:dark cycle. Temperature was maintained at 22 ± 2°C, with a relative humidity of 30% to 70%. Mice were individually housed on PAPERCHIP® bedding (Watertown, TN) with ad libitum access to commercial rodent diet (Formulab Diet 5008, Purina) and autoclaved, acidified water (acidified to pH 2.5–3.5 with HCl). Autoclaved nestlets were supplied for each cage for additional enrichment. All sentinel mice monitoring these colonies were consistently seronegative for Mouse Hepatitus Virus (MHV), Minute Virus of Mice (MVM), Mouse Parvovirus (MPV), Parvovirus NS-1, Theiler’s Murine Encephalomyelitis Virus (TMEV), Murine rotavirus, Mycoplasma pulmonis, and Sendai virus. Polymerase chain reaction (PCR) testing was negative for fur mites and pinworms.
Experimental design
Donor mice were shipped from their respective suppliers directly to UT Southwestern Medical Center (UTSMC). Mice were allowed to acclimate for 2 weeks, to mitigate any effects of that initial shipment on the GM. Following that, fecal samples were collected from all donor mice the morning of the day they were shipped overnight from UTSMC to MU. Fecal samples collected pre-shipping were also shipped overnight to MU on dry ice. Upon arrival at MU, fecal samples were again collected from all donor mice as they were unpacked. To control for sample preservation, these post-shipping fecal samples were also frozen immediately after collection and kept at −80°C until ready for use.
GF recipient mice were shipped directly to MU. Upon arrival, mice were randomly assigned (using a random number generator) to the pre- or post-shipping group. As they were unpacked from the flexible film isolator, mice then received their assigned FMT using donor material collected pre- or post-shipping from UTSMC to MU. Recipient mice were then individually housed for 2 weeks, with fecal sample collection occurring at 7 and 14 d post-FMT. Terminal cecal samples were collected at 14 d post-FMT.
Fecal microbiota transfer
Working in a biosafety cabinet, fecal pellets were removed from the freezer. To prepare donor fecal material for FMT, one fecal pellet per procedure was placed in a 2 mL round-bottom tube containing a 0.5 cm diameter stainless steel bead and 500 µL of sterile phosphate-buffered saline and agitated at 1/30 s using a TissueLyser II (Qiagen) for 60 s. Following a pulse centrifugation, the supernatant was carefully collected using a sterile 1 mL pipette and transferred to a nylon mesh filter with a 40 µm pore size and allowed to flow into a collection tube. Collection tubes containing filtered FMT material were then sealed and promptly transported to animal rooms for FMT procedures.
DNA extraction
Fecal DNA was extracted using PowerFecal kits (Qiagen) according to the manufacturer instructions, except that samples were homogenized in bead tubes using a TissueLyser II (Qiagen) for 10 min at 30/s, instead of using the vortex adapter described in the protocol, before proceeding according to the protocol and eluting in 100 µL of elution buffer (Qiagen). DNA yields were quantified via fluorometry (Qubit 2.0, Invitrogen, Carlsbad, CA) using quant-iT BR dsDNA reagent kits (Invitrogen) and normalized to a uniform concentration and volume.
16S rRNA library preparation and sequencing
Library preparation and sequencing were performed at the MU Genomics Technology Core. Bacterial 16S rRNA amplicons were constructed via amplification of the V4 region of the 16S rRNA gene with universal primers (U515F/806 R) previously developed against the V4 region, flanked by Illumina standard adapter sequences.Citation15,Citation16 Dual-indexed forward and reverse primers were used in all reactions. PCR was performed in 50 µL reactions containing 100 ng metagenomic DNA, primers (0.2 µM each), dNTPs (200 µM each), and Phusion high-fidelity DNA polymerase (1 U, Thermo Fisher). Amplification parameters were 98°C(3 min) + [98°C(15 sec) +50°C(30 sec) +72°C(30 sec)] × 25 cycles + 72°C(7 min). Amplicon pools (5 µL/reaction) were combined, thoroughly mixed, and then purified by the addition of Axygen Axyprep MagPCR clean-up beads to an equal volume of 50 µL of amplicons and incubated for 15 min at room temperature. Products were then washed multiple times with 80% ethanol, and the dried pellet was resuspended in 32.5 µL EB buffer (Qiagen), incubated for 2 min at room temperature, and then placed on the magnetic stand for 5 min. The final amplicon pool was evaluated using the Advanced Analytical Fragment Analyzer automated electrophoresis system, quantified using quant-iT HS dsDNA reagent kits, and diluted according to Illumina’s standard protocol for sequencing as 2 × 250 bp paired-end reads on the MiSeq instrument.
Informatics analysis
Sequences were processed using the Quantitative Insights into Molecular Ecology 2 v2021.8Citation17 framework. Paired-end reads were trimmed of the universal primers and Illumina adapters using cutadapt.Citation18 Reads were then denoised into unique amplicon sequence variants using DADA2Citation19 with the following parameters: (1) reads were truncated to 150 bp in length, (2) reads with greater than two expected errors were discarded, (3) reads were merged with a minimum overlap of 12 bp, and (4) chimeras were removed using the “consensus” method. Unique sequences were filtered between 249 and 257 bp in length. The resulting feature table was rarefied to an even depth of 43,675 features per sample. PASTCitation20 v.4.03 was then used to evaluate alpha (within-sample) and beta (between-sample) diversity.
Statistical analysis
Differences in univariate data (i.e., Chao1 and Shannon indices) were assessed using one- or two-way analysis of variance (ANOVA). Univariate data were fitted to linear mixed models with mouse treated as a random effect when appropriate. Tukey post hoc testing was performed to identify differences between individual groups. Differences in multivariate data (i.e., beta diversity) were assessed using permutational multivariate analysis of variance (PERMANOVA). Differences in beta diversity were visualized using a principal coordinate analysis (PCoA) of a quarter-root transformed feature table. Beta diversity was compared using Bray–Curtis (weighted) and Jaccard (unweighted) distances.
Results
Shipping subtly affects the donor microbiome but SO differences remain dominant
Comparison of the richness of C57BL/6J (B6J) and C57BL/6NHsd (B6N) donors pre- and post-shipping using a linear mixed model revealed the expected SO effect (p < 0.001, F = 297) and a significant, albeit much smaller, effect of shipping (p = 0.003, F = 10.9) with no interaction between effects (). There were similarly weighted effects of supplier (p < 0.001, F = 783) and shipping (p = 0.017, F = 6.7) on Shannon diversity and no interaction ().
Figure 1. Box plots showing the pre- and post-shipping Chao1 index (a) and Shannon diversity index (b) in C57BL/6J (B6J) and C57BL/6N (B6N) donor mice originally purchased from Jackson Laboratory and Envigo, respectively; bars denote p < 0.001, two-way ANOVA. PCoA plot (c) based on Jaccard distances, showing separation of B6J and B6N donors, and overlap within each substrain of samples collected pre- and post-shipping; legend at right. Ellipses indicate 95% confidence intervals. Results of two-way PERMANOVA using Jaccard distances are shown.
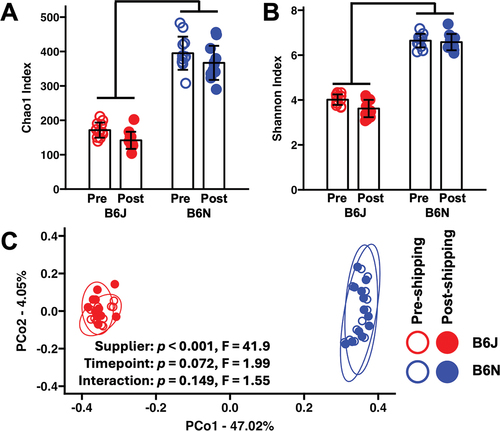
Comparison of beta-diversity via PCoA using Jaccard distances revealed clear separation of SO GMs but negligible separation of pre- and post-shipping samples (). Two-way PERMANOVA revealed a significant effect of SO GM (p < 0.0001, F = 34.7, R2 = 0.469) and no significant effect of shipping (p = 0.13, F = 1.7, R2 = 0.022) or interaction. Using Bray–Curtis distances, PCoA showed a similar pattern, but PERMANOVA now detected a subtle effect of shipping on the donor microbiome (p = 0.04, F = 2.7, R2 = 0.020) but no interaction between supplier and timepoint (p = 0.167, F = 1.4, Supplemental Figure S1).
Comparison of the taxonomic abundance at the phylum level revealed the expected SO effects on multiple phyla including Verrucomicrobiota, Desulfobacterota, and Patescibacteria (Supplemental Figure S2). Shipping-dependent decreases in the abundance of Actinobacteriota, Pseudomonadota, and Campilobacterota were also observed. Bacteroidota (72.2%), Bacillota (19.9%), Verrucomicrobiota (7.23%), Pseudomonadota (0.43%), and Actinobacteriota (0.28%) were the dominant phyla in B6J donors. Bacteroidota (61.5%), Bacillota (36.5%), Desulfobacterota (0.97%), Deferribacterota (0.44%), and Patescibacteria (0.22%) were the most abundant phyla in B6N donors. In the low-richness, Jackson-origin GM, 44% of detected genera were shared between B6J pre- and post-shipping donors and recipients, whereas 53% of genera detected in the high-richness Envigo-origin GM were shared between B6J pre- and post-shipping donors and recipients (Supplementary Figure S3).
Reconstitution of GF recipients results in reduced richness but equivalent alpha-diversity
Following the inoculation of GF recipients with donor fecal material collected pre- or post-shipping, recipient mice were maintained under barrier conditions and fecal samples were collected at 1 and 2 weeks post-FMT. Cecal contents were also collected at 2 weeks post-FMT. In GF mice receiving FMT from pre- and post-shipping B6J mice, microbial richness reached between 50% and 60% of donor richness by 1 week post-FMT and failed to increase beyond that in feces or cecum 2 weeks later (). One recipient of FMT with material from a post-shipping B6J failed to develop richness on par with others in the group, and two-way ANOVA detected significant effects of shipping (p = 0.009, F = 7.8) and donor versus recipient (p < 0.001, F = 22.1), with no interaction. Pairwise post hoc comparisons found significantly lower richness in recipients at all timepoints compared to their respective donor group. In contrast, the richness of mice receiving FMT from pre- and post-shipping B6N donors reached 50–60% of donor richness by 1 week post-FMT but continued to develop before reaching 70–80% of donor richness by 2 weeks, despite the B6N microbiome being over twice as rich as the B6J microbiome. Here, two-way ANOVA failed to detect an effect of shipping (p = 0.965, F = 0) but did detect an overall difference between donors and recipients (p < 0.001, F = 24.1) and no interaction (). Pairwise comparisons found reduced richness in recipients of pre-shipping material relative to donors at all timepoints. In recipients of post-shipping B6N material, fecal richness failed to achieve donor richness, but cecal contents of recipients were not different from donor material.
Figure 2. Box plots showing the Chao1 index in feces of ex-GF FMT recipients of material from B6J donors (a) or B6N donors (b) 1 week (1w) or 2 weeks (2w) post-FMT, or cecal contents (c) 2 weeks post-FMT, in relation to the relevant donor (d) group. (c,d) Box plots showing Shannon diversity in the same groups. Bars represent significantly differing groups. p < 0.05 Tukey post hoc testing.
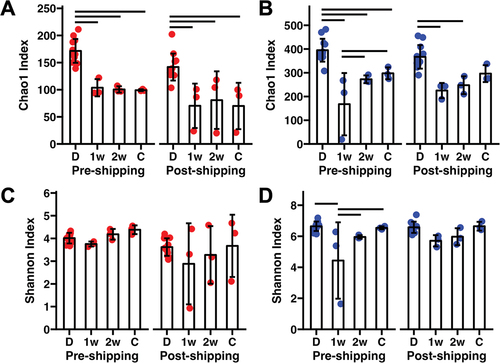
Similar comparison of the Shannon diversity revealed a significant effect of shipping on B6J donors and their recipients (p = 0.007, F = 8.3) but no overall (p = 0.322, F = 1.2) or pairwise differences between donors and recipients and no interactions (). In mice receiving B6N material, however, there was no effect of shipping on Shannon diversity (p = 0.192, F = 1.8), and a significant effect of donor versus recipient (p < 0.001, F = 9.1) with no interaction (). Pairwise comparisons found that while fecal samples from mice receiving pre-shipping material from B6N had not reached full diversity at 1 week post-FMT, no differences were detected between either recipient group and their B6N donors in fecal or cecal diversity at 2 weeks post-FMT. Collectively, these data suggest that while the GM of B6N mice is more efficient at comprehensive colonization of GF mice than the GM of B6J mice, both GMs mature within recipients and develop alpha-diversity comparable to their donors. These data also suggest that the B6J GM is preferentially susceptible to the effects of shipping.
Beta-diversity in recipients replicates effects of shipping on donor microbiome
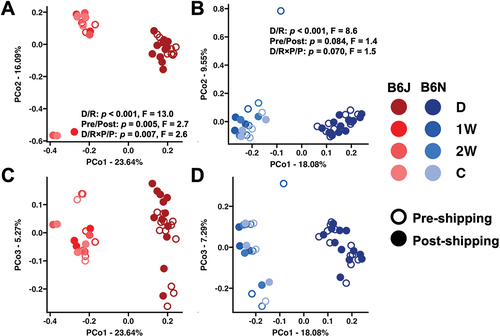
Beta-diversity among the entire dataset was dominated by the differences between B6J and B6N donors and their respective recipients (Supplemental Figure S4). To better resolve the effects of shipping, data were stratified by donor substrain and analyzed separately. Within both donor substrain and their respective recipients, samples separated along PCo1 according to their status as a donor or recipient (). Notably, within B6J and their recipients, examination of PCo3 revealed complete separation of recipient samples from pre- or post-shipping samples from the same mice at both timepoints and in cecal contents (). No such separation was observed among recipients of FMT from B6N donors () on PCo3 or subsequent coordinates. Two-way PERMANOVA confirmed significant differences between donors and recipients in both donor substrains, but significant effects or interactions of donor shipping were only detected in B6J mice, again suggesting selective effects of shipping on the B6J donors and recipients. Lastly, rather than comparing groups, we determined the specific Jaccard distance between each recipient and their specific donor sample and then assessed the effect of substrain, shipping, and timepoint post-FMT on mean donor–recipient distance using a three-way ANOVA. No significant effect of any factor was detected (substrain: p = 0.28, F = 1.2; shipping: p = 0.291, F = 1.2; timepoint: p = 0.661, F = 0.4).
Figure 3. PCoA plots showing similarity between pre- and post-shipping C57BL/6J (a) or C57BL/6N (b) donor material and their respective recipient feces at 1 week (1w) or 2 weeks (2w) post-FMT or cecal contents. (c, d) The same data ordinated using PCo1 and PCo3, showing the separation of B6J recipients based on the timing of donor sample collection.
Discussion
The current study was designed to determine whether the effects of shipping on the microbiome of donor mice resulted in similar changes in the microbiome of recipient mice receiving donor feces via FMT. A secondary question was whether the potential effects of shipping on donor and recipient microbiomes were consistent across different SPF mouse microbiomes. Using C57BL/6 substrains purchased from the Jackson Laboratory or Envigo as donors, we used GF Swiss Webster mice as recipients, rather than antibiotic-treated pseudo-GF mice to eliminate any effect of differences in the recipient microbiome pre-FMT. It is important to note that subtle strain-dependent effects on the gut microbiome have been observed in inbred and outbred strains from Jackson and Envigo; however, this effect is minimal relative to vendor-specific differences.Citation5 As previously reported,Citation10 shipping resulted in subtle changes in the GM of donor mice. However, the current data also suggest that the effect of shipping on donor and recipient microbiomes is not consistent in both SPF microbiomes. A greater proportion of the Envigo GM successfully colonized the recipient gut compared to the Jackson GM, and the effect of shipping donor mice was less apparent in recipients of FMT from Envigo donors. These outcomes are counterintuitive given the substantially greater richness of the Envigo GM and presumably more opportunities for rare taxa not to colonize the recipients.
Despite those findings, the mean Jaccard distance between donors and recipients did not differ between substrains or use of pre- versus post-shipping samples. Thus, the differences observed between recipients of B6J fecal material collected from pre- and post-shipping donors likely reflect the effects of shipping on the donor microbiome, as opposed to true differences in transfer efficiency.
The major limitation of this work is the limited sample size, which was directly related to the cost of GF recipient mice. Attempts were made to maximize the value of GF recipients through sampling at multiple timepoints and use of two SPF microbiomes. The low sample size is of greatest concern in the interpretation of negative findings, and the significant differences detected between groups are compelling based on the similarity of outcomes in B6J and B6N or across multiple recipient timepoints. Related to this, an additional limitation is the use of only female mice. A larger study using female and male donors and GF recipients was cost-prohibitive, resulting in the use of only female donors and recipients to avoid sex as an experimental variable.
In a real-world scenario wherein banked fecal samples are needed in order to characterize the historical microbiome of a mouse colony or colonize a different group of mice, these data indicate that donor samples may be subtly influenced by shipping in some mice, but that the effect of shipping is minor compared to other biological differences such as SO GMs. As mentioned earlier, only a handful of genetic backgrounds are readily available as GF mice, and scenarios in which the GM needs to be restored in a live colony would likely require antibiotic-mediated depletion of the endogenous GM prior to FMT. Previous work has demonstrated the hierarchical nature of SO GMs, exemplified by an inability to deplete and supplant the Envigo GM with the less rich Jackson GM, and faithful, complete transfer in the reciprocal direction.Citation21 Thus, effective reconstitution of live colonies may require rederivation or cross-foster approaches in some cases.
Supplemental Material
Download Zip (1.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All sequencing data supporting the current research project are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive as BioProject PRJNA1083537.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/29933935.2024.2363858.
Additional information
Funding
References
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature [Internet]. 2007;449(7164):804–810. http://www.ncbi.nlm.nih.gov/pubmed/17943116. 10.1038/nature06244.
- de Vos WM, de Vos EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev [Internet]. 2012;70(Suppl 1):S45–56. http://www.ncbi.nlm.nih.gov/pubmed/22861807.
- Hirayama K, Endo K, Kawamura S, Mitsuoka T. Comparison of the intestinal bacteria in specific pathogen free mice from different breeders. Exp Anim [Internet]. 1990;39(2):263–267. https://www.ncbi.nlm.nih.gov/pubmed/2141820.
- Hufeldt MR, Nielsen DS, Vogensen FK, Midtvedt T, Hansen AK. Variation in the gut microbiota of laboratory mice is related to both genetic and environmental factors. Comp Med [Internet]. 2010;60:336–347. http://www.ncbi.nlm.nih.gov/pubmed/21262117.
- Ericsson AC, Davis JW, Spollen W, Bivens N, Givan S, Hagan CE, McIntosh M, Franklin CL, Heimesaat MM. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS One [Internet]. 2015;10(2):e0116704. http://www.ncbi.nlm.nih.gov/pubmed/25675094. 10.1371/journal.pone.0116704.
- Rasmussen TS, de VL, Kot W, Hansen LH, Castro-Mejia JL, Vogensen FK, Hansen AK, Nielsen DS. Mouse vendor influence on the bacterial and viral gut composition exceeds the effect of diet. Viruses [Internet]. 2019;11(5):435. https://www.ncbi.nlm.nih.gov/pubmed/31086117. 10.3390/v11050435.
- Long LL, Svenson KL, Mourino AJ, Michaud M, Fahey JR, Waterman L, Vandegrift KL, Adams MD. Shared and distinctive features of the gut microbiome of C57BL/6 mice from different vendors and production sites, and in response to a new vivarium. Lab Anim [Internet]. 2021;50(7):185–195. https://www.ncbi.nlm.nih.gov/pubmed/34127866. 10.1038/s41684-021-00777-0.
- Unger AL, Eckstrom K, Jetton TL, Kraft J. Facility-dependent metabolic phenotype and gut bacterial composition in CD-1 mice from a single vendor: a brief report. PLOS ONE [Internet]. 2020;15(9):e0238893. https://www.ncbi.nlm.nih.gov/pubmed/32956361.
- Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B, Wilson ID, McBain AJ, Ahmed N. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLOS ONE [Internet]. 2010;5(1):e8584. http://www.ncbi.nlm.nih.gov/pubmed/20052418. 10.1371/journal.pone.0008584.
- Montonye DR, Ericsson AC, Busi SB, Lutz C, Wardwell K, Franklin CL. Acclimation and institutionalization of the mouse microbiota following transportation. Front Microbiol [Internet]. 2018;9:1085. https://www.ncbi.nlm.nih.gov/pubmed/29892276.
- Wolf KJ, Daft JG, Tanner SM, Hartmann R, Khafipour E, Lorenz RG. Consumption of acidic water alters the gut microbiome and decreases the risk of diabetes in NOD mice. J Histochem Cytochem [Internet]. 2014;62(4):237–250. https://www.ncbi.nlm.nih.gov/pubmed/24453191.
- Sofi MH, Gudi R, Karumuthil-Melethil S, Perez N, Johnson BM, Vasu C. pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes [Internet]. 2014;63(2):632–644. https://www.ncbi.nlm.nih.gov/pubmed/24194504. 10.2337/db13-0981.
- Zhao Y, Tarbell KV. Comment on Sofi et al. pH of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes. 2014;63(2):632–644. Diabetes [Internet] 2015; 64:e19. https://www.ncbi.nlm.nih.gov/pubmed/26207042.
- Yang I, Eibach D, Kops F, Brenneke B, Woltemate S, Schulze J, Bleich A, Gruber AD, Muthupalani S, Fox JG. et al. Intestinal microbiota composition of interleukin-10 deficient C57BL/6J mice and susceptibility to Helicobacter hepaticus-induced colitis. PLoS One [Internet]. 2013;8(8):e70783. http://www.ncbi.nlm.nih.gov/pubmed/23951007 10.1371/journal.pone.0070783.
- Walters WA, Caporaso JG, Lauber CL, Berg-Lyons D, Fierer N, Knight R. PrimerProspector: de novo design and taxonomic analysis of barcoded polymerase chain reaction primers. Bioinformatics [Internet]. 2011;27(8):1159–1161. http://www.ncbi.nlm.nih.gov/pubmed/21349862. 10.1093/bioinformatics/btr087.
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci [Internet]. 2011;108(supplement_1):4516–4522. http://www.ncbi.nlm.nih.gov/pubmed/20534432.
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. https://www.ncbi.nlm.nih.gov/pubmed/31341288 10.1038/s41587-019-0209-9.
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetJ [Internet]. 2011;17(1):10–12. https://journal.embnet.org/index.php/embnetjournal/article/view/200.
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Natu Methods [Internet]. 2016;13(7):581–583. https://www.ncbi.nlm.nih.gov/pubmed/27214047.
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4:9.
- Ericsson AC, Personett AR, Turner G, Dorfmeyer RA, Franklin CL. Variable colonization after reciprocal fecal microbiota transfer between mice with low and high richness microbiota. Front Microbiol [Internet]. 2017;8:196. https://www.ncbi.nlm.nih.gov/pubmed/28280484.
