ABSTRACT
Monkey orange (Strychnos spp.) is a widely distributed fruit species in Southern Africa commonly consumed by the local population. It has potential to improve the nutritional status of rural populations, being a precious food source in areas with periodic shortages, since it is rich in vitamin C, zinc, and iron. To improve the availability of this food outside its production season, processing and preservation techniques used at household level need upgrading as they are unreliable and their effects on nutritional quality are unknown. Based on this review, we recommend better indigenous fruit production as a sustainable solution to malnutrition in rural areas in transition countries.
Introduction
Monkey orange (Strychnos spp.) is a member of the Loganiaceae family, indigenous to tropical and subtropical Africa.(Citation1) Up to 75 Strychnos species have been recognized in Africa, of which 20 species produce edible fruits in Central and Southern tropical Africa, drought prone areas and semi-arid regions,(Citation2) where the tree remains dormant when water is unavailable.(Citation3) Most commonly consumed species, which are prevalent in woodlands of Southern Africa, are S. innocua (synonym: S. madagascariensis),(Citation3) S. cocculoides, S. pungens and S. spinosa.(Citation1)
Generally, fruits of the four monkey orange species under review are indehiscent, oval shaped, yellow or orange colored, possessing a thick woody shell.(Citation4) The pulp is bright yellow or brown, juicy, sweet and or sour, with few to numerous hard seeds imbedded in the fleshy pulp.(Citation4,Citation5) Whole fruit weighs from 145–383 g(Citation6) and a single tree produces 300–700 fruits, which translates to approximately 40–100 kg fruit weight per tree.(Citation2) Monkey oranges are characterized by their seasonality, being harvested between August and December,(Citation7) the so-called “lean season”– a time of cultivated food shortages in Zimbabwe. Fresh monkey oranges are consumed immediately after cracking and become inedible when left exposed to air due to both microbial spoilage and physico-chemical reactions that cause off flavors and enzymatic browning. The intact fruits have been reported to stay fresh and edible for three months, enabling their trade between countries in Southern Africa.(Citation6,Citation8) During prolonged storage, the fruits are susceptible to deterioration because of high temperature, rainfall, disease, poor handling, and storage conditions that impact physiological and biochemical changes. Reduction of post-harvest losses and increase in shelf life have become urgent needs to promote better use of monkey oranges. To prolong shelf life and maintain quality, the fruit is sometimes domestically processed to jams, juices, wine, beer, fritters, muffins or dried fruit leathers,(Citation9–Citation13) but still most of the product is lost if not consumed fresh.
Monkey oranges are widely consumed by rural communities, particularly women and children in Southern Africa, because of their pleasant taste and flavor.(Citation14) Nutritionally, the fruits possess potential health benefits attributable to high energy, fiber, minerals (iron and zinc) and vitamin C.(Citation15,Citation16) In Zimbabwe, 30% of pregnant and lactating women are assumed to be iron deficient,(Citation17) while maternal anaemia is a common cause of maternal and neonatal mortality.(Citation18) Thirty-three per cent of children 6–59 months old were stunted,(Citation19) and 56% of the same age group suffer some degree of anaemia.(Citation20) Strychnos spp. depending with species have an iron content up to 140 mg/100 g,(Citation22) giving potential to deliver iron when used as a food source by pregnant or lactating women and children. Strychnos spp. have been described to be among the indigenous fruits in many parts of Southern Africa that have commercial potential and contribution to trade.(Citation21) Potential benefits of improved livelihood security through income generation by sale of fruit and fruit products for rural communities also exist.
In Southern Africa, identification of superior genotypes, collection of germplasm,(Citation3) and cultivation of S. spinosa and S. cocculoides for consistent high yields of large, quality fruits are ongoing. On cultivation positive responses from seedlings and grafting were obtained taking three years and four to five years to bear fruit respectively.(Citation53)
Previous reviews of Strychnos spp. by Mwamba(Citation3) and Orwa(Citation10) were directed towards taxonomy, physical properties, ecology, agronomy, reproduction, harvest, selection and genetic resources. The current review provides on overview of nutritional composition, sensory properties, food uses, and impact of processing techniques on nutritional and sensorial quality of the four common Strychnos spp. in Southern Africa. Thereafter, recommendations for future studies pertaining to processing and storage of monkey orange in order to maintain year-round availability, nutrient retention and sensory quality are described.
Sensory properties
Taste and texture
Overall, the ripe monkey orange species has fleshy,(Citation22) sweet,(Citation14) yellow, very aromatic pulp,(Citation5,Citation23) and contains numerous hard brown seeds.(Citation24) There is wide variability in the general description of taste, color, texture and flavour between and within species ().
Table 1. Sensory properties of the four main species of monkey orange fruit.
Fruit sweetness depends highly on sugar composition;(Citation25) Sitrit et al.(Citation24) report accumulation of sugars and organic acids during ripening and sucrose conversion to glucose and fructose at the onset of ripening for S. spinosa. Total sugars were 28.2 g/100 g dw and the most abundant sugar was sucrose (12.9 g/100 g dw), a disaccharide, followed by the monosaccharides glucose (4.6 g/100 g dw) and fructose (1.9 g/100 g dw).(Citation24) It was shown that the degree of monkey orange ripeness had an effect on sugar composition; thus, taste is dependent on the stage of ripening in addition to environmental factors like soil, geographical location and climatic differences.
Organic acids in fruits originate from biochemical processes or from the activity of some microorganisms such as yeasts and bacteria.(Citation25) The presence of organic acids (citric, malic and succinic acids) explains the acidic component that blends with sugars and results in the species characteristic blended acid-sweet taste. Sirit et al.(Citation24) found that S. spinosa contained malic acid 1.9 g/100 g dw, succinic acid 0.5 g/100 g dw and citric acid levels of 2.4 g/100 g dw. Amarteifio and Mosase(Citation14) reported a citric acid content of 0.77 g/100 g dw for S. spinosa, indicating the acidic nature of the species. Citric acid concentrations of 3.8–4.9 g/100 g, found in a sour variety of Ziziphus mauritiana fruit,(Citation26) were higher than that found in S. spinosa. Hence, in comparison with other indigenous sour fruits, S. spinosa contains less citric acid, thus exhibiting a more palatable and less sour taste. However, S. innocua has a bitter taste that can be attributed to the presence of tannins (1.01 ± 0.01 mg/g),(Citation15) though this can vary between and among trees of the same provenance.
Saka et al.(Citation27) reported a mean acidity of 1.13% for S. cocculoides processed juice. The relatively high acidity of Strychnos spp. may contribute to the long shelf life of fresh fruits when compared to other fruits. The pH of S. spinosa was 3.2(Citation14) and 2.8,(Citation24) while that of processed S. cocculoides juice was 3.5.(Citation27)
Partial solubilization of pectin and cellulose during ripening by the endogenous plant enzymes polygalacturonase (PG), pectinmethylesterase (PME), lyase, and rhamnogalacturonase(Citation28) contributes to changes in texture and juiciness of fruits. Though consistency differs among species and level of ripeness, monkey orange fruits exhibit a thick gel or juicy texture. The degree of pectin solubilization determines texture characteristics: the higher the hydrolysis, the juicer the fruit.
Saka et al.(Citation27) compared the sensory properties of sugar plum (Uapaca kirkiana), baobab (Adansonia digitata), mango (Mangifera indica) and S. cocculoides juice, where the monkey orange juice was the most preferred by sensory panelists. These findings concurred with the study of Bille et al.(Citation9) where diluted S. cocculoides juice resulted in high consumer acceptance, especially with improved liquefaction and clarification. Strychnos cocculoides jam was highly scored by consumers, also because of its taste,(Citation29) and in general consumer acceptance of the other monkey orange fruit products was high in literature surveyed.(Citation9,Citation27,Citation29) All in all, the sensory studies indicated the potential for product development and commercialization of the species.
Volatiles
Fresh monkey orange fruit emits a distinct and delicate mixture of complex aroma volatiles, which are perceived by consumers as a mixture of pineapple, apricot, melon, clove, and citrus.(Citation24) According to data of surveys in Zimbabwe, S. cocculoides juice is added to cereal porridge (a thin maize slurry) for sour flavor enhancement and vitamin enrichment.(Citation30) The most abundant volatiles of ripe S. cocculoides pulp of Malawian provenances were acetate and butyrate esters,(Citation31) which exhibit a fruity sweet flavour,(Citation32) this concurs with consumer descriptions of fruit flavor. The main volatile flavor compounds found in the peel of ripe S. spinosa fruits were trans-isoeugenol and eugenol, which have a pungent clove aroma, and p-transanol [4-(1-propenyl)-phenol], while the unripe fruit lacks volatile compounds.(Citation24) Volatile compounds of S. spinosa and S. cocculoides found in literature are shown in , while no data for volatiles of S. pungens and S. innocua were found.
Nutritional composition of fruit flesh
Different units to express nutritional content were used in the literature collected for this review; thus, all data were converted to g/100 g for macronutrients, mg/100 g for micronutrients and dry matter basis where possible to enable data comparison. In some cases, data had to be omitted due to missing water content or dry matter content and when it was not clear whether dry or wet basis was used. In other cases, it was not stated whether the flesh, juice or pulp was used in analysis and therefore data are presented in general terms as the edible portion of the fruit. Where data for a particular species were found from one source, it is assumed to be the mean value.
Macronutrients
The reported minimum and maximum macronutrient composition of monkey orange species varies between and within species (). There was large variation between the minimum and the maximum carbohydrate content within species of monkey orange. S. innocua had the highest total carbohydrate content variation: 15.4 g/100 g dw(Citation15) to 61 g/100 g dw.(Citation16) This appears to result from inaccuracies due to the applied methodology as carbohydrates were determined by an indirect method, namely the difference method. Also, the reported protein range for S. innocua was remarkably wide, namely from 0.3 g/100 g dw(Citation22) to 12.8 g/100 g dw,(Citation33) where outlier values may be due to small sample sizes in the studies. All authors used the Kjeldahl analysis with a conversion factor of 6.25 for protein determination; hence, the methodology cannot be the cause of the observed variation in data. Of the studies under review, no authors analyzed for amino acids.
Table 2. Macronutrient composition1 of the edible portion of monkey orange species S. cocculoides, S. spinosa, S. innocua and S. pungens.
In the literature, there is also a notable variability in fat content for all the species ranging from 0.3 g/100 g dw(Citation22) to 20 g/100 g dw.(Citation34) Saka and Msonthi(Citation16) reported a fat content of 31.2 g/100 g dw for S. spinosa, which was the maximum value for fruits of the same species and other reported species. The high value is an outlier and can be the result of irregularities in the methodology where sample size was n = 1. The large variation in fat content between and within S. spinosa and other species can thus be attributed to sample size and methodology as in some cases authors did not describe the method of analysis.
Reported energy values range from 1315.4 kJ/100 g(Citation33) to 2083.6 kJ/100 g(Citation34) for all the four species. The differences in values between studies and from species to species could result from different coefficients used to compute energy values. In a comparative study, S. spinosa ranked superior to other indigenous fruits; the energy value for S. spinosa was 1923 kJ/100 g, which was higher than for ber (Z. mauritiana), with an energy value of 1588 kJ/100 g, and baobab (A. digitata), with an energy value of 1480 kJ/100 g.(Citation16) Generally, the crude fiber content ranged from 2.5 g/100 g dw(Citation22) to 22.2 g/100 g dw(Citation33) for monkey orange species reviewed. The fiber content of S. spinosa (17.6 g/100 g dw) was similar to that of S. innocua (17.9 g/100 g dw), as reported by Saka and Msonthi.(Citation16) Food high in fiber is often richer in micronutrients,(Citation35) thus this might explain the high micronutrient content of S. innocua and S. spinosa (). The ash content of the four monkey orange species was between 0.5 g/100 g for S. innocua and S. cocculoides(Citation33,Citation34) to 4.7 g/100 g for S. innocua.(Citation15) Arnold et al.(Citation33) reported low ash contents for S. spinosa (1.8 g/100 g dw), S. cocculoides (0.5 g/100 g dw) and S. pungens (1 g/100 g dw); these low contents could be related to soil chemistry and micro climate of sampling locality. The reported variation in moisture content was high for S. innocua: 60%(Citation34) to 91%.(Citation15) The information on sample preparation was not documented and the variation may also be caused by the difficulty to obtain juice and or flesh due to the stickiness of the mesocarp to the endocarp. Thus overall high fiber and fat content can be attributed to and explained by contamination of the edible portion with seed material. Though water content variation was lower within the three species than between the species, the high water quantities can affect the shelf life when fruits are not appropriately stored.
Minerals and vitamins
The reported mean mineral contents of monkey orange show S. innocua as having the highest mean content of Cu (2.4 mg/100 g), Na (99.6 mg/100 g) and Zn (28.7 mg/100 g) (), while S. cocculoides had highest mean content of Fe (70.5 mg/100 g) of the four monkey orange species. However, the large variation in mineral content that exists between species and within species may result from phenotypic variations, climatic differences and soil chemistry, which affection availability and uptake. Some reported mineral contents were obtained from a single literature source such as for S. cocculoides (Mg, Na, Zn, K, and Cu), S. innocua (Zn and Cu) and S. pungens (Mg, Na, Zn, K, and Cu). The data were insufficient for effective comparison of mineral contents between species. When compared to the other indigenous fruits, baobab (A. digitata), marula (Sclerocarya birrea), and the medlar (Vangueria infausta), the highest source of Fe and Zn was from S. spinosa.(Citation14) Though mineral contents show extreme variations in minimum and maximum values, and data are sometimes only from one source without a methodology description, the available data suggest S. innocua and S. cocculoides to be important sources for Zn and Fe, respectively. This warrants further research on validation of mineral contents and bio-accessibility studies of mineral contents of S. innocua and S. cocculoides as they appear to have potential to complement local diets that are mineral deficient. Thereafter, research on bioavailability of Fe and Zn is needed to determine to what extent the species contribute to improving human nutrition.
Table 3. Mineral and vitamin C composition1 of the edible portion of monkey orange species S. cocculoides, S. spinosa, S. innocua and S. pungens.
The vitamin C content of monkey orange fruits ranged from 34.2 mg/100 g dw(Citation33) to 88 mg/100 g dw.(Citation14) In comparison with other fruits, the reported maximum vitamin C content for S. spinosa is comparable to marula (S. birrea) (128.3 mg/100 g), baobab (A. digitata) (141.3 mg/100 g),(Citation14) oranges (50 mg/100 g) and strawberries (59 mg/100 g).(Citation36) Mean vitamin C content of S. spinosa was higher than for the other species.
Other vitamins assayed were thiamine (vitamin B1) and riboflavin (vitamin B2). Wehmeyer(Citation37) found large differences between thiamine and riboflavin contents of S. pungens flesh adjacent to the shell on the one hand, and adjacent to the seed on the other hand. A thiamine content of 2.74 mg/100 g dw and a riboflavin content of 1.85 mg/100 g dw were reported for flesh inside the shell, while a thiamine content of 0.10 mg/100 g dw and a riboflavin content of 0.74 mg/100 g dw were reported for flesh surrounding the seeds.(Citation37) It is not clear why the demarcation was made between flesh around the seed and flesh around the shell for thiamine and riboflavin determinations. However, lower thiamine contents were found for pulp of S. cocculoides: 0.03 mg/100 g dw, S. pungens: 0.05 mg/100 g dw and S. spinosa: 0.23 mg/100 g dw by Arnold et al.(Citation33) No data were found for the riboflavin content of the other three species.
Phytochemicals
Phytochemicals are non-nutritive, biologically active compounds, for example, phenolic acids, flavonoids and carotenoids,(Citation38,Citation39) to which health protective properties are ascribed, such as preventive actions against aging, inflammation and certain cancers.(Citation40) Protective effects of phytochemicals are because of their properties as free radical scavengers, hydrogen–donating compounds, singlet oxygen quenchers and or metal chelators.(Citation41) Nhukarume et al.(Citation42) found S. spinosa had phenolic content radical quenching ability trends and flavonoids, expressed as catechin equivalence comparatively similar to baobab nectar. Proanthocyanidins, expressed as % leucocyanidin equivalence, were comparatively similar to mobola plum (Parinari curatellifolia). A high color intensity is commonly related to a high total antioxidant capacity of a product,(Citation43,Citation44) a relation which warrants further investigation for the monkey orange species as they have bright orange (S. innocua) and orange–brown colors (S. cocculoides, S. spinosa and S. pungens), which promise the fruits to be high in phytochemicals. Quantification and classification of the phenols needs to be carried out in further research.
Anti-nutritional and toxic compounds
Anti-nutritional compounds have a negative effect on the digestibility of protein and carbohydrates, and decrease bioavailability of minerals such as iron, zinc, phosphorous, calcium and or manganese. Anti-nutritional compounds such as tannins (1.01 mg/g), trypsin inhibitors (10.12 mg/g),(Citation15) phytates (1.57 mg/g) and oxalates (0.83 mg/g) were found in S. innocua juice.(Citation34,Citation45) In separate studies of other indigenous fruits, Umaru et al.(Citation46) found for baobab: tannin (2.22 ± 0.32 mg/g), phytates (0.69 ± 1.5 mg/g) and oxalate (9.5 ± 0.42 mg/g) and for marula (S. birrea): tannin (2.04 ± 0.30 mg/g), phytates (3.56 ± 0.54 mg/g) and oxalate (4.9 ± 1.70 mg/g). Tannin, oxalates and phytates values of marula are higher than those reported for S. spinosa. In the study by Bello et al.(Citation15), the reported anti-nutrient values of S. spinosa were too low to be of any nutritional importance as they were not high enough to bind a significant amount of minerals and were below the established toxic level. However, Hassan et al.(Citation34) concluded from their study that the calculated molar ratios of [Phytates]/[Ca] and [Phytates]/[Fe] were above the critical levels that affect bioavailability of Ca and Fe. These results however need to be supported by in vivo bioavailability studies.
A potentially toxic natural secoiridoid, namely kingiside aglucone, was isolated from unripe bitter S. spinosa pulp.(Citation47) Toxic effects of kingiside aglucone in mice were reported for high dosage of unripe pulp of S. spinosa; however, specific searches with extracted ion chromatograms for strychnine, brucine and associated compounds found no terpene indole alkaloids in unripe S. spinosa pulp.(Citation48) The fruit extracts of unripe S. spinosa pulp were also found to be acaricidal on cattle ticks, though the extracts did not show the classical dose dependence that occurs with conventional insecticides(Citation49) and no toxic effects were elicited in guinea pig and mice.(Citation50) For these studies, an extrapolation to human toxicity was not done. Seeds of monkey orange fruits reportedly contain strychnine and are bitter tasting. When swallowed, they pass the body safely but if chewed may cause vomiting and headaches.(Citation53) Thus, according to the studies reviewed, the toxic alkaloids are present in the seeds and unripe pulp of S. spinosa. From the literature, there is no scientific evidence that ripe S. spinosa, S. cocculoides, S. innocua and S. pungens pulp contain alkaloids, neither have the fruits been reported to be toxic in their ripe state. Therefore, the loss of toxic alkaloids after fruit ripening may be caused by alkaloid degradation at maturation that renders the fruit consumable by both animals and human as sensory properties, sugar, color and flavor contents also increase. To the best of our knowledge, there is no research on the toxicity of ripe and unripe pulp and seed of S. pungens and S. cocculoides.
Post-harvest handling and storage
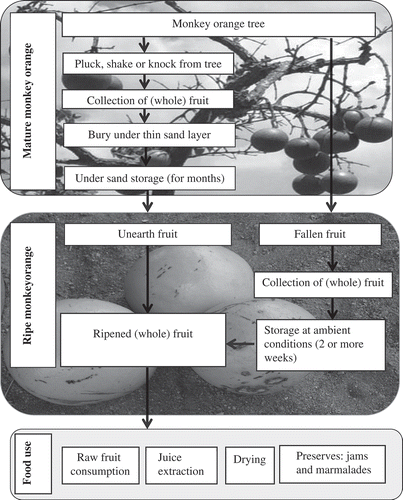
Monkey orange fruits are mainly harvested as mature, fully developed fruit by plucking, knocking, and shaking trees or collecting ripe fallen fruit. When fully developed though unripe fruits are harvested, they are subsequently buried under a thin layer of sand for several months until ripe (or until they liquefy) to protect them from other fruit hunters and animals(Citation2,Citation11,Citation24,Citation51) and to prevent postharvest losses ().(Citation52) After storage, the fruit pulp changes from a dry texture with a pale cream color to a golden color (yellow or brown and juicy depending on species) ready to be consumed.(Citation3)
Burying of the fruits may provide temperatures adequate for increased enzyme activity to degrade pectin, which is catalyzed by pectinases, such as PME and PG, to bring about the ripe texture of the fruits. Like other climacteric fruits during storage and ripening, monkey oranges increase in total soluble solids content (TSS) with accumulation of sucrose, glucose and fructose as well as citric and malic acids as realized for S. spinosa cultivated in Israel.(Citation24)
When picked, ripe monkey orange can be stored for up to two weeks under ambient conditions -mainly in shade-before they spoil.(Citation2,Citation6,Citation8) Slow spoilage can be attributed to the hard shell of the fruit, which also resists fungi and fruit flies.(Citation53) However, generally fresh fruits with a high moisture content like monkey orange undergo direct or indirect nutrient and quality losses upon storage. Limited knowledge of fruit handling and marketing contribute to quality losses.(Citation27) Data for sensory and nutrient composition during storage (from maturity to ripening) for S. cocculoides, S. innocua and S. pungens are not available. Once such data become available, post-harvest handling techniques and storage methods will need to be developed in order to maintain and improve the shelf life of the fruit and fruit products.
Food uses of Strychnos spp.
During prioritization studies of indigenous fruits, individual species were ranked according to their role in food security, commercialization potential, taste, and abundance.(Citation30) From surveys on domestication of the 50 most common indigenous fruit species consumed in Southern African countries (Malawi, Zambia, Zimbabwe, Tanzania, and Mozambique), the monkey orange fruit, namely S. cocculoides, ranked third overall.(Citation6,Citation54) In country-specific results, S. cocculoides was ranked first in Zimbabwe and Tanzania, where interviewed households preferred the fruit tree for domestication as a food source.(Citation55)
Consumption of raw fruits
In households, monkey orange fruit is consumed fresh and unprocessed,(Citation50,Citation56,Citation57) immediately after cracking the shell as it is a traditional belief that unprocessed pulp that is left exposed to atmospheric conditions cannot be consumed later. Depending on the species, monkey orange pulp can be processed to food products and is often cooked with maize meal to a sweet porridge, dissolved in water to create sweet and tasty drinks, dried for later consumption or made into jams and marmalades.(Citation3,Citation12,Citation53)
Processing
Indigenous fruit is processed into value added products to improve digestion, metabolism, and preservation.(Citation8,Citation54,Citation58) Monkey oranges are processed domestically and at small scale to dried products, juices and preserves where generally the preparation methods and conditions vary from place to place. Post-harvest processing such as maceration, drying, juicing, cooking, and storage is expected to have an effect on nutrient content, bioavailability, anti-oxidant activity of bioactive compounds and physical properties.(Citation59,Citation60) For monkey oranges, thermal processing is of utmost importance as all its known products undergo some form of thermal treatment.
Drying
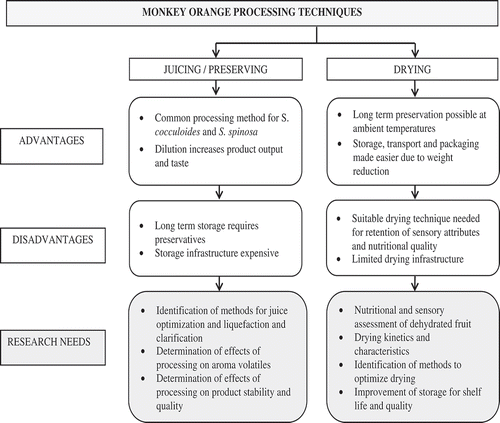
Drying reduces water activity, which diminishes the bacterial activity in the dehydrated food, increases shelf life, reduces the product’s mass and adds value.(Citation58,Citation61) In Southern Africa, monkey orange fruits are commonly dried by fire and direct sun to fruit rolls, leathers, pound into flour (S. cocculoides) used to cook porridge, referred to locally as “bozo” in Mozambique(Citation30) or re-cooked as a sauce.(Citation62) Sun dried monkey orange pulp can be stored from two months to five years,(Citation63) which makes thermal drying an ideal preservation method due to its affordability for rural communities and as a means to secure continuous fruit availability into the next season.
The moisture content of monkey orange ranges from 60%(Citation22) to 91%,(Citation15) of which, depending on the method and degree of heating, a large proportion is removed by the drying process. Sufficiently dried fruit products generally have a residual water content of 18%–24%, and with proper packaging have a long shelf life because of their high-sugar concentration and reduced water activity (0.72–0.75).(Citation64) Due to the characteristics of conventional drying processes, nutrients and compounds sensible to heat, light, and oxygen, such as vitamin C, might be degraded.(Citation65) Other studies have shown that dried fruits are rich and shelf stable sources of dietary polyphenolic and anti-oxidants.(Citation66) The elevated antioxidant activity might be due to the concentration of polyphenolic compounds during drying and the formation of Maillard products,(Citation67) which have an anti-oxidant activity attributable to the high molecular weight of brown pigments formed in the advanced stages of the reaction.(Citation60) Overall the changes in anti-oxidant properties in foods can be attributed to the sum of different or opposite events that retain or decrease anti-oxidant activity in dried products. Thus, further studies measuring anti-oxidant activity and phenol content in dried monkey oranges need to be performed to determine their role in human health.
Moisture content maintains the equilibrium media for the stability of fruit pigments and anti-oxidants such as anthocyanins.(Citation58) Loss of moisture through heat reduces pigment stability and degrades pigments, which results in discoloration of the product.(Citation43) Total color change (∆E) is an important physical property of dehydrated fruit, in which high thermal drying temperatures and time can result in an extremely hard, burnt, off-flavor and tasteless product.(Citation59) Because of their high sugar content, monkey oranges are sticky and difficult to handle in dryers and have a potential for caramelization, which can change fruit to a brown or darker color and has a negative impact on sensory quality attributes. Thus, adequate drying procedures are required to obtain a product of sufficient sensory attributes for the consumer.
The dried products are now mainly produced for home consumption and have not been sold commercially. To the best of our knowledge, there is scanty scientific information about the suitability of drying techniques for monkey orange fruit. Retention of nutrients and sensory appeal in dried products is an important quality aspect of food preservation.(Citation39,Citation59) Thus, studies on the species’ suitability for dried products, the drying kinetics and characteristics of the commonly consumed monkey oranges need to be conducted to avail suitable drying techniques that fit local conditions and offer the possibility of commercialization.
Juice extraction
Juice is extracted at small scale by initially soaking the pulp to soften the tissue around the seed for efficient extraction. Due to their water solubility, vitamin C and other phenolic antioxidants can be leached from the fruit tissue to the processing water, while carotenoids, thiamine, folate and niacin increase during processing as a result of dissociation from plant matrix materials.(Citation44,Citation68) When monkey orange juice is extracted, processing is manually done by mashing, using a hand held whisk or wooden spoon. The pulp is diluted with water, then heated to 92 °C for 3 minutes to initiate precipitation of colloidal substances, which later on can be removed by filtration.(Citation5) The filtrate is obtained by sieving pulp with a muslin cloth and used in processing juice, while the residue remains for jam making.(Citation27) Hence, the juice is expected to retain a significant amount of anti-oxidants after processing.
Clear monkey orange juice was preferred by consumers in comparison to other indigenous fruit juices based on taste, mouth feel, flavor, and sweetness.(Citation9,Citation27,Citation29) During processing, the natural monkey orange color turns dark brown. To enhance the natural juice color, food grade egg yellow or lemon juice as an anti-browning agent is added.(Citation9,Citation27) To increase the shelf life, processed juice of the fruit can be preserved by using benzoic acid and sodium sulphate additives.(Citation3) In orange juice processing, high mechanical extraction pressure application, de-pulping and aeration conditions can reduce the volatile components and alter the flavor of the resultant juice.(Citation69) Since traditional monkey orange juice extraction is mild due to equipment constraints and processing steps are limited to heating and hand extraction, the extent of aroma alteration and odor development by processing is hypothesized to be minimal though there are no studies to this effect. Saka et al.(Citation27) identified a reduction in the mineral elements calcium and magnesium from fresh to processed juice of S. cocculoides. To date, few studies have been done on nutritional and sensorial characterization of fresh and pasteurized monkey orange juice, thus more work is recommended.
Preserves; jams and marmalades
In fruit processing, preserves such as jams, sauces, pickles and chutneys play a significant role in reducing the loss of fresh produce.(Citation70) Traditionally and at small scale, jams and marmalades are processed from monkey orange fruits, whereby the type of preserve depends on species.(Citation3,Citation21) From literature reviewed, jam processes vary from author to author. However, a consensus in the applied methodologies is the use of pulp alone or a mixture of pulp and juice with heating to reach the required soluble solids content and as a pasteurization step before packaging and setting.(Citation9,Citation27) No pectin is added as monkey orange pectin depolymerisation allows jams to set and spread well, a component that contributes to the sensorial quality of monkey orange jam. Contrary to the loss of water soluble anti-oxidants, the removal of water concentrates minerals as shown in the study of Saka et al.(Citation27) where Ca, Mg, K, Na, Zn, and P contents were higher in jam than in the juice (filtrate) from which the residue used for jam processing was obtained. When comparing with other indigenous jams, consumers in the studies of Bille et al.(Citation9) and Saka et al.(Citation27) preferred the monkey orange jam because of its sweet taste and delicate flavor. However, due to thermal processing of fruits, possible undesirable changes in sensorial, functional and nutritional value by destruction of phenolic antioxidants occur.(Citation58,Citation71) As previously alluded, thermal processing can lead to the release of bound phenolic compounds, hence the total phenolic content can be attributed to both free and bound polyphenolic compounds.(Citation25) Thus, the retention or loss of phenolic compounds, nutrients and organoleptic characteristics during jam processing needs to be further investigated.
Overall discussion
Information on the contents of micronutrients of all four species was limited in the literature surveyed, as research emphasis on fruit species was on macronutrients. More research needs to be conducted on micronutrient composition, especially with respect to S. innocua, S. spinosa and S. cocculoides, as their nutritional composition from available literature is exceptional.
In the available literature large variations in nutritional and sensorial attributes were reported, which may have been caused by several factors. Fruit maturation and ripeness at time of analysis was not clarified, and therefore could have promoted the variable data on nutritional composition and sensorial quality. Other factors, such as different agronomic and environmental factors (soil types and climate), contribute to variation as samples spanned from different regions in Southern Africa and as far as Israel. Agro-technical conditions (including irrigation and fertilization) might affect ion uptake, thus resulting in mineral variation within and between species.(Citation24) Methodological differences in conditions and types of assays contributed to variation, where in some cases absence of methodology descriptions made evaluating results and drawing conclusive remarks on nutritional composition difficult. Overall, the data set used was small and thus more work needs to be done by sound sampling plans and reliable food analysis procedures of each monkey orange fruit species.
Processing techniques presented in this article largely require thermal treatments that have negative and or positive consequences on the nutritional and sensorial quality of products and a beneficial role in preservation. However, information on the extent of the effects of processing methods is lacking. The presence, possible release and quantities of toxic alkaloids into the processed products also warrant further investigation for quality control purposes during processing.
Fruit weights for provenances from Tanzania, Zambia and Zimbabwe range from 145–383 g.(Citation6) Pulp contents of large fruits are between 34% and 48%,(Citation6) so the amount of consumed pulp is 130.2–183.4 g, and for small fruits these figures are 50–57%,(Citation6) and 72.5–82.7 g, respectively. Based on these data, an estimate of contribution to the recommended daily intake can be calculated per consumption of 100 g serving of fruit based on Dietary Reference Intakes.(Citation72) On a per serving basis (100 g), S. innocua delivers more than 100% of the RDI for Zn, while S. cocculoides delivers more than 100% of the RDI for Fe and S. spinosa delivers more than 100% of the RDI for vitamin C (). Overall the monkey orange species serve as a rich source of carbohydrates, protein, fiber, Zn, Fe and vitamin C. It is therefore paramount that research clearly assesses the potential beneficial contribution to human health of ingestion of monkey orange as well as the bio- accessibility and bio-availability of its minerals to contribute to micronutrient deficient diets.
Table 4. Monkey orange composition with RDI(Citation72) for children 4–8, pregnant females 19–50, adult males 19–50, and adult females 19–50 years (species: S. spinosa, S. cocculoides, S. innocua and S. pungens).
Conclusions and research priorities
Monkey orange fruits have potential to impart health benefits and improve nutritional status of the rural population thanks to the vitamin C, zinc and iron content. With regard to nutritional and sensorial quality, several recommendations for future research were identified as summarized in . Previous studies concur that monkey orange fruit has desirable sensory properties and its nutritional composition is comparable to and in some cases better than that of its exotic and indigenous counterparts. However, compared to many exotic fruit species, very little research work has been done on processing for value addition of monkey orange fruits in Southern Africa. Minimizing loss of nutrients, anti-oxidants, organoleptic properties and reducing levels of potential toxic alkaloids during processing is important to obtain a nutritious fruit product as processing has an influence on the overall quality of fruit products. The evidence collected in this review shows that the impact of processing is not well documented, and that the assessment of the contribution by monkey orange to nutrient intake of regular consumers is not accurate.
Thus, optimization of the state of the art with respect to processing techniques and assays of the nutritional and sensorial quality of monkey orange products is essential for the implementation of preservation procedures and the consequent promotion of monkey orange consumption. gives an illustration of the advantages, disadvantages and recommendations for monkey orange processing as concluded from this review.
In Africa indigenous fruit trees supplement the diet of many rural families by providing essential micronutrients and health benefits as well as serve as an alternative for cash and income, especially in times of famine.(Citation9,Citation73–Citation75) The wide distribution of the monkey orange trees in drought prone areas and semi-arid regions, coupled with the fruit nutritional quality renders the fruit an important food source for particularly children and pregnant women. Thus, improved manufacturing of processed foods through optimization of preservation techniques as a sustainable solution to malnutrition in rural areas of transition countries needs to be determined.
Funding
We acknowledge The Netherlands Fellowship Programme for financial support [grant number CF9151/2013].
Additional information
Funding
References
- Bisset, N.G. The African species of Strychnos. Part I. The ethnobotany. Lloydia. 1970, 33(2), 201–243.
- Southampton Centre for Underutilised Crops. Monkey orange, strychnos cocculoides, field manual for extension workers and farmers, SCUC: Southampton, UK; 2006.
- Mwamba, C.K. Monkey orange: strychnos cocculoides. Vol. 8: Crops for the future, 2006.
- Coates Palgrave, K.; Drummond, R.B.; Moll, E.J.; Coates Palgrave, M. Trees of southern Africa. Struik Publishers: Cape Town, 2002; pp 923–935.
- Mbiyangandu, K. Composition and proposed use of two wild fruits from Zaire. Food Chem. 1985, 16(2), 175–178.
- Mkonda, A.; Akinnifesi, F.K.; Maghembe, J.A.; Swai, R.; Kadzere, I.; Kwesiga, F.; Saka, J.D.K.; Lungu, S.; Mhango, J. Towards Domestication of ‘Wild Orange’ Strychnos Cocculoides in Southern Africa: A Synthesis of Research and Development Efforts. In Southern African Regional Agroforestry Conference, 2002; Warmbaths: South Africa.
- Akinnifesi, F.; Ajayi, O.; Sileshi, G.; Kadzere, I.; Akinnifesi, A. Domesticating and Commercializing Indigenous Fruit and Nut Tree Crops for Food Security and Income Generation in Sub-Saharan Africa. In Paper presented at the New Crops International Symposium. 2007.
- Ruffo, C.K.; Birnie, A.; Tengnäs, B. Edible wild plants of Tanzania. Vol. 27: Regional Land Management Unit/Sida, 2002.
- Bille, P.; Shikongo-Nambabi, M.; Cheikhyoussef, A. Value addition and processed products of three indigenous fruits in Namibia. Afr. J. Food, Agric. Nutr. 2013, 13(1), 7192–7212.
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: a tree reference and selection guide version 4.0. 2009.
- Rampedi, I.T.; Olivier, J. Traditional beverages derived from wild food plant species in the Vhembe District, Limpopo Province in South Africa. Ecol. Food Nutr. 2013, 52(3), 203–222.
- Zinyama, L.M.; Matiza, T.; Campbell, D.J. The use of wild foods during periods of food shortage in rural Zimbabwe. Ecol. Food Nutr. 1990, 24(4), 251–265.
- McGregor, J. Gathered produce in Zimbabwe’s communal areas changing resource availability and use. Ecol. Food Nutr. 1995, 33(3), 163–193.
- Amarteifio, J.; Mosase, M. The chemical composition of selected indigenous fruits of Botswana. J. Appl. Sci. Environ. Manage. 2006, 10(2).
- Bello, M.; Falade, O.; Adewusi, S.; Olawore, N. Studies on the chemical compositions and anti nutrients of some lesser known Nigeria fruits. Afr. J. Biotech. 2008, 7(21), 3972–3979.
- Saka, J.D.K.; Msonthi, J.D. Nutritional value of edible fruits of indigenous wild trees in Malawi. For. Eco. Manage. 1994, 64, 245–248.
- Banziger, M.; Long, J. The potential for increasing the iron and zinc density of maize through plant-breeding. Food. Nutr. Bull. 2000, 21(4), 397–400.
- Gadaga, T.; Madzima, R.; Nembaware, N. Status of micronutrient nutrition in Zimbabwe: a review. Afr. J. Food, Agric. Nutr. 2009, 9(1), 502–522.
- FNC. Food and Nutrition Council. Zimbabwe National Nutrition Survey: Preliminary Findings. Harare, Zimbabwe, 2010.
- Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International. Zimbabwe Demographic and Health Survey 2010-11. 2012: Calverton, Maryland: ZIMSTAT and ICF International Inc.
- Van Wyk, B.E. The potential of South African plants in the development of new food and beverage products. S. Afr. J. Bot. 2011, 77(4), 857–868.
- Malaisse, F.;Parent, G. Edible wild vegetable products in the Zambezian woodland area: A nutritional and ecological approach. Ecol. Food Nutr. 1985, 18(1), 43–82.
- Tanton, T.; Haq, N.I. Climate change: an exciting challenge for new and underutilised crops. In: New crops and uses: Their role in a rapidly changing world. new crops and uses: their role in a rapidly changing world; Smartt, J., Haq, N. Eds.;Centre for Underutilised Crops: Southampton, UK, 2007; pp. 15–22.
- Sitrit, Y.; Loison, S.; Ninio, R.; Dishon, E.; Bar, E.; Lewinsohn, E.; Mizrahi, Y. Characterization of Monkey orange (Strychnos spinosa Lam.), a potential new crop for arid regions. J. Agric. Food Chem. 2003, 51(21), 6256–6260.
- Lee, P.R.; Tan, R.M.; Yu, B.; Curran, P.; Liu, S.Q. Sugars, organic acids, and phenolic acids of exotic seasonable tropical fruits. Nutr. Food Sci. 2013, 43(3), 267–276.
- Nyanga, L.K.; Nout, M.J.; Gadaga, T.H.; Boekhout, T.; Zwietering, M.H. Traditional processing of masau fruits (Ziziphus mauritiana) in Zimbabwe. Ecol. Food Nutr. 2008, 47(1), 95–107.
- Saka, J.; Rapp, I.; Akinnifesi, F.; Ndolo, V.; Mhango, J. Physicochemical and organoleptic characteristics of Uapaca kirkiana, Strychnos cocculoides, Adansoniadigitata and Mangiferia indica fruit products. Int. J. Food Sci. Tech. 2007, 42(7), 836–841.
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena–An overview. Crit. Rev. Food Sci. Nutr. 2007, 47(1), 1–19.
- Tumeo, T.; Mhango, J.; Munthali, C. Organoleptic attributes and yield of products from Parinari curatellifolia, Strychnos cocculoides, Mangifera indica, Uapaca kirkiana and Vitex doniana in Malawi. In: International Symposium on Underutilized Plants for Food Security, Nutrition, Income and Sustainable Development 806. 2008; pp 513–518.
- Akinnifesi, F.K.; Leakey, R.R.B.; Ajaui, O.C.; Sileshi, G.; Tchoundjeu, Z.; Matakala, P.; Kwesiga, F.R. Indigenous fruit trees in the tropics: domestication, utilization and commercialization. Wallingford: CABI, 2007; pp xiii + 427.
- Shoko, T.; Apostolides, Z.; Monjerezi, M.; Saka, J.D.K. Volatile constituents of fruit pulp of Strychnos cocculoides (Baker) growing in Malawi using solid phase microextraction. S. Afr. J. Bot. 2013, 84, 11–12.
- Bicas, J.L.; Molina, G.; Dionísio, A.P.; Barros, F.F.C.; Wagner, R.; Maróstica Jr, M.R.; Pastore, G.M. Volatile constituents of exotic fruits from Brazil. Food Res. Int. 2011, 44(7), 1843–1855.
- Arnold, T.H.; Wells, M.J.; Wehmeyer, A.S. Khoisan food plants: taxa with potential for future economic exploitation; In Plants for arid lands; Wickens, J.R.; Goodin; D.V. Field, Eds.; Allen and Unwin: London, 1985; pp 69–86.
- Hassan, L.; Abdulmumin, U.; Umar, K.; Ikeh, O.; Aliero, A. Nutritional and anti-nutritional composition of Strychnos innocua Del. (Monkey Orange) fruit pulp grown in Zuru, Nigeria. Nig. J. Bas. Appl. Sci. 2014, 22(1–2), 33–37.
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: a review. J. Food Sci Technol. 2012, 49(3), 255–266.
- Eromosele, I.C.; Eromosele, C.O.; Kuzhkuzha, D.M. Evaluation of mineral elements and ascorbic acid contents in fruits of some wild plants. Plant Foods Hum Nutr (Formerly Qualitas Plantarum). 1991, 41(2), 151–154.
- Wehmeyer, A. Nutrient composition of some edible wild fruits found in the Transvaal. S. Afr. Med. J. 1966, 40(45), 1102–1104.
- Alasalvar, C.; Shahidi, F. Composition, phytochemicals, and beneficial health effects of dried fruits: an overview. Dried Fruits: Phytochem. Health. Eff. 2012, 1–19.
- Fernandes, F.A.; Rodrigues, S.; Law, C.L.; Mujumdar, A.S. Drying of exotic tropical fruits: a comprehensive review. Food Bio Techn. 2011, 4(2), 163–185.
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Pak Dek, M.S.; Hairuddin, M.R. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int. J. Mol. Sci. 2011, 12(7), 4678–4692.
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Nazri, H.S.M.; Diton, N.A.M.; Mokhtar, R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Comp Anal. 2009, 22(5), 388–393.
- Nhukarume, L.; Chikwambi, Z.; Muchuweti, M.; Chipurura, B. Phenolic content and antioxidant capacities of Parinari curatelifolia, Strychnos spinosa and Adansonia digitata. J. Food Biochem. 2010, 34(s1), 207–221.
- Wicklund, T.; Rosenfeld, H.J.; Martinsen, B.K.; Sundfør, M.W.; Lea, P.; Bruun, T.; Blomhoff, R.; Haffner, K. Antioxidant capacity and colour of strawberry jam as influenced by cultivar and storage conditions. LWT – Food Sci. Tech. 2005, 38(4), 387–391.
- Kalt, W. Effects of production and processing factors on major fruit and vegetable antioxidants. J. Food Sci. 2005, 70(1), R11–R19.
- Bello, M.O.; Falade, O.S.; Adewusi, S.R.A.; Olawore, N.O. Studies on the chemical compositions and anti nutrients of some lesser known Nigeria fruits. Afr. J. Biotech. 2008, 7(21), 3972–3979.
- Umaru, H.; Adamu, R.; Dahiru, D.; Nadro, M. Levels of antinutritional factors in some wild edible fruits of Northern Nigeria. Afr. J. Biotech. 2007, 6(16), 1935–1938.
- Msonthi, J.D.; Galeffi, C.; Nicoletti, M.; Messana, I.; Marini-Bettolo, G.B. Kingiside aglucone, a natural secoiridoid from unripe fruits of Strychnos spinosa. Phytochem. 1985, 24(4), 771–772.
- Emmanuel, T.N.; Thokozani, H.; Brighton, M.M.; Humphrey, H.; Steven, R.B.; James, M.; Philip, C.S. Acute mammalian toxicity of four pesticidal plants. J. Med. Plants Res. 2012, 6(13), 2674–2680.
- Madzimure, J.; Nyahangare, E.T.; Hamudikuwanda, H.; Hove, T.; Belmain, S.R.; Stevenson, P.C.; Mvumi, B.M. Efficacy of Strychnos spinosa (Lam.) and Solanum incanum L. aqueous fruit extracts against cattle ticks. Trop. Anim. Health Prod. 2013, 1–7.
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed., Livingstone: London, 1962.
- Motlhanka, D.M.T.; Motlhanka, P.; Selebatso, T. Edible indigenous wild fruit plants of Eastern Botswana. Int. J. Poultry Sci. 2008, 7, 457–460.
- Kalaba, F.; Chirwa, P.; Prozesky, H. The contribution of indigenous fruit trees in sustaining rural livelihoods and conservation of natural resources. J Hortic For. 2009, 1(1), 1–6.
- National Research Council. Lost Crops of Africa. Volume III: Fruits. Washington D.C, USA, 2008.
- Saka, J.K.; Swai, R.; Mkonda, A.; Schomburg, A.; Kwesiga, F.; Akinnifesi, F.K. 44. Processing and utilisation of indigenous fruits of the miombo in southern Africa. In Proceedings of the Regional Agroforesty Conference on Agroforestry Impacts on Livelihoods in Southern Africa: Putting Research into Practise: held at the Aventura Resorts Warmbaths, South Africa, 20-24 May 2002. 2004. World Agroforesty Centre.
- Akinnifesi, F.; Kwesiga, F.; Mhango, J.; Mkonda, A.; Chilanga, T.; Swai, R. Domesticating priority miombo indigenous fruit trees as a promising livelihood option for small-holder farmers in Southern Africa. In: XXVI International Horticultural Congress: Citrus and Other Subtropical and Tropical Fruit Crops: Issues, Advances and 632. 2002.
- Farinu, G.O. Chemical composition of some plant products of the savanna forest zone of Nigeria. Food Chem. 1986, 22(4), 315–320.
- Gomez, M.I. A resource inventory of indigenous and traditional foods in Zimbabwe. Zambezia. 1988, 15(1), 53–73.
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables and grains – A review. Crit. Rev. Food Sci. Nutr. 2015, 55(7), 887–918.
- Chong, C.H.; Law, C.L.; Figiel, A.; Wojdyło, A.; Oziembłowski, M. Colour, phenolic content and antioxidant capacity of some fruits dehydrated by a combination of different methods. Food Chem. 2013, 141(4), 3889–3896.
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10(3), 94–100.
- Chong, C.H.; Law, C.L.; Cloke, M.; Hii, C.L.; Abdullah, L.C.; Daud, W.R.W. Drying kinetics and product quality of dried Chempedak. J. Food Eng. 2008, 88(4), 522–527.
- Mizrahi, Y.; Nerd, A.; Sitrit, Y. New fruits for arid climates. Trends in new crops and new uses. ASHS Press: Alexandria, VA, 2002; pp 378–384.
- Shackleton, C.M.; Dzerefos, C.M.; Shackleton, S.E.; Mathabela, F.R. The use of and trade in indigenous edible fruits in the Bushbuckridge savanna region, South Africa. Ecol. Food Nutr. 2000, 39(3), 225–245.
- Pátkai, G. Fruit and Fruit Products as Ingredients. In Handbook of Fruits and Fruit Processing, Wiley-Blackwell, 2012; pp 263–275.
- Santos, P.H.S.; Silva, M.A. Retention of vitamin C in drying processes of fruits and vegetables – A review. Dry Tech. 2008, 26(12), 1421–1437.
- Bennett, L.E.; Jegasothy, H.; Konczak, I.; Frank, D.; Sudharmarajan, S.; Clingeleffer, P.R. Total polyphenolics and anti-oxidant properties of selected dried fruits and relationships to drying conditions. J. Func. Foods. 2011, 3(2), 115–124.
- Capanoglu, E. Investigating the antioxidant potential of Turkish dried fruits. Int. J. Food Prop. 2013, 17(3), 690–702.
- Hotz, C.; Gibson, R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J. Nutr. 2007, 137(4), 1097–1100.
- Perez-Cacho, P.R.; Rouseff, R. Processing and storage effects on orange juice aroma: a review. J. Agric. Food Chem. 2008, 56(21), 9785–9796.
- Singh, S.; Jain, S.; Singh, S.; Singh, D. Quality changes in fruit jams from combinations of different fruit pulps. J. Food Process. Preserv. 2009, 33(s1), 41–57.
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Physicochemical and sensorial properties of grapefruit jams as affected by processing. Food Bioprocess Techn. 2013, 6(1), 177–185.
- Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification. The National Academies Press: Washington D.C, USA, 2003.
- Legwaila, G.; Mojeremane, W.; Madisa, M.; Mmolotsi, R.; Rampart, M. Potential of traditional food plants in rural household food security in Botswana. J. Hort. For. 2011, 3(6), 171–177.
- Mithöfer, D.; Waibel, H. Income and labour productivity of collection and use of indigenous fruit tree products in Zimbabwe. Agroforestry Systems 2003, 59(3), 295–305.
- Packham, J. The value of indigenous fruit-bearing trees in miombo woodland areas of South-Central Africa. RDFN Paper 15c. 1993.