ABSTRACT
There are many studies regarding stop the progression to type 2 diabetes (T2D) through dietary control but there is no global recommendation for a specific diet. The strongest evidence is weight control through nutritional education but it does not include elderly people because of the risk of malnutrition that would entail. Therefore, the search for specific foods that can help slow down the progress towards T2D are of great interest. Although the controversy between fish consumption and the risk of developing T2D, it has been observed that oily fish could play a protective role. This type of fish may contain large amounts of persistent organic pollutants (POPs) but, on the contrary, contain omega-3 with effects on the cardiovascular system. In addition, the presence of taurine, a semi-essential amino acid very present in oily fish and which has been studied its antidiabetogenic effect, could play an essential role in the protective effect of this type of fish against T2D. Among them, the one with the highest concentrations of omega-3 and taurine as well as low concentration of POPs is sardine. An integrative review of observational studies and clinical trials was performed to investigate the association between sardine consumption and T2D prevention.
T2D risk
Diabetes mellitus (DM) has become a worldwide health problem and its prevalence is continuously increasing. Type 2 diabetes (T2D) is the most common form of DM, covering between 90 and 95% of the total cases, and it is, together with its complications, a major cause of early death particularly in low and middle-income countries .[Citation1]
T2D is led by a first state named prediabetes (preDM) which is characterized by high circulating glucose levels above normal but without reaching diabetes levels. According to the American Diabetes Association (ADA), preDM may be diagnosed based on plasma glucose criteria, by the presence of impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) and/or high values of glycated hemoglobin (HbA1c) .[Citation2]
The natural history of preDM predicts that up to 70% of individuals with this condition will develop T2D and the annual conversion ratio in general population is around 5–10% .[Citation3,Citation4] But, both prevalence of T2D and preDM increase significantly with age[Citation5] and it is considered that the number of new-onset T2D in ≥65 years old is increasing 4.5-fold compared to 3-fold in total population .[Citation6] Precisely, in the majority of European countries the prevalence of T2D is between 10–20% in people aged 60–80 years and the prevalence of preDM is between 15 and 20% for people older than 60 years of age .[Citation7]
T2D prevention
As preDM is a reversible condition, many therapies have been developed to reduce the conversion ratio to T2D. Most of them aim to reduce body weight due to studies in which it was observed that intentional weight loss in overweight participants were associated with a lower rate of T2D development .[Citation8] This is due to increasing adiposity could be responsible for insulin resistance (IR) that drives in T2D .[Citation9]
Specifically, with a weight loss goal intervention, the incidence of converting from preDM to T2D reduced. In particular, the percentage conversion to T2D was approximately 9% for subjects who lost at least 5% of their body weight .[Citation10] Also, it has been reported by losing about a 7% of body weight, a reduction of 58% to the risk of manifesting T2D can be achieved .[Citation11,Citation12] According to previous studies, nutrition interventions have significant effects on weight loss .[Citation13–15] Weight loss by regulating lifestyle interventions, including nutrition interventions, has been associated with a lower incidence of diabetes .[Citation16] Precisely, studies reveal that intensive lifestyle modification has a higher effect on T2D prevention than common used hypoglycemic drugs, [Citation17] which together with gastric surgery are only recommended to those with high risk of developing T2D and a BMI ≥ 25 kg/m2 or BMI ≥ 35 kg/m2, respectively .[Citation18]
Despite that, overweight and obesity account for 44% of the T2D cases[Citation19] and the connotation “diabesity” was created as the modern epidemic, [Citation20] there are non-obese people that develop T2D in which weight loss should not be a proposed intervention. This becomes especially sensitive in the highest prevalence age group, the elderly where weight loss in ≥65 year old individuals has been reported to cause loss of lean body mass, bone mineral density, and fat mass, leading to different health problems, [Citation21] despite its usual beneficial effects in physical function and metabolic parameters .[Citation22] Even so, some epidemiological studies have shown that it leads to increased mortality, [Citation23,Citation24] concretely, the crude mortality rate for ≥65 years old in those who lost weight was 6%, compared with 2.6% in the stable weight and 2.4% in the weight gain group .[Citation25]
Generally, nutritional interventions used for weight loss are based on hypocaloric diets that and, even in the presence of obesity, caloric restriction in this group is not always advised because can lead to malnutrition .[Citation26] A more interesting intervention, for this age group but also for all the people without weight problems but with T2D risk, would be one based on changing dietary patterns instead of focused on restricting caloric intake .[Citation27]
According to ADA, there is not a recommended diet to treat T2D or to prevent it, although it advises following a diet rich in whole grain, fruits, vegetables, seafood omega-3 fats and poultry. A dietary pattern similar to the Mediterranean diet has been seen to reduce mortality due to diabetes condition or cardiovascular disease (CVD) in a 50% in an aging population with normal weight but also with a BMI above 25 .[Citation28] Many dietary treatments have been tested in which they demonstrate that caloric restriction is not the only way to achieve preDM control.
On one hand, studies based on the association of micronutrients intake and T2D risk have been demonstrated protective associations especially for calcium and vitamin D with odds ratio (OR) 0.82, [Citation29] vitamin E[Citation30] with OR 0.80, and magnesium with OR 0.66 .[Citation31] Moreover, recent review has been published where dietary polyphenols showed antidiabetic activity in human study promoting the prevention and management of T2D. Especially resveratrol, curcumin, and anthocyanins, despite the structure–activity relationship are still not clear .[Citation32]
On the other hand, the largest number of investigations focuses especially on the study of macronutrients. Because of its direct involvement with glycemia, carbohydrates have been among the most studied and, in general, have shown: low carbohydrates diet has demonstrated a reduction of HbA1c and an improvement of glycemic control[Citation33]; a positive association with a diet based on low dietary glycemic index and/or glycemic load and protective effects against T2D[Citation34]; a high-fiber diet, translates into ≥30gr/day, showed no weight loss but an improvement in blood glucose levels .[Citation35]
Related to fats, different studies have shown clear associations. In an observational study, recently-diagnosed diabetics had both higher relative intake of total fat and concretely saturated fatty acids from animal fat sources compared with healthy controls .[Citation36] Also, it has been demonstrated, with a clear dose–response connection, an inverse relation between polyunsaturated fatty acids (PUFA) consumption and risk to develop T2D with the highest quintile of intake 0.87 of relative risk .[Citation37] Especially, this opposite relation is observed in omega-6 fatty acid (FA) consumption[Citation38,Citation39] and an inverse association between the amount of omega-3 FA and insulin resistance was studied .[Citation40]
Regarding proteins, a study made in 2004 demonstrated that a high-protein but also low-carbohydrate diet improves HbA1c in an obese population but the protein role was not clear .[Citation41] On the contrary, some years after, it was observed that a high-protein intake may be associated with increased risk[Citation42] . The source of the protein consumed could be responsible for the difference in both results. Therefore, the risk of developing T2D was observed according to the consumption of food groups. The association between high meat intake, especially processed meat, and an increased risk of T2D, was especially clear. But, for fish consumption, observational studies showed confusing results .[Citation43]
Fish consumption
The observation that communities consuming a diet rich in fish present a low grade of chronic diseases[Citation44–47] promotes the investigation to understand what is the role of this food on health. Fish has been seen to have many potential beneficial effects on metabolism although its physiological mechanisms are not well known yet. This assumption comes from different epidemiological studies in which an inverse relation between fish consumption and the incidence of CVD, T2D, and cognitive decline have been observed .[Citation44,Citation48,Citation49] Fish consumption could also play a role in preventing metabolic syndrome and it has been seen that the effect might be stronger in men than women .[Citation50]
Particularly, the first population-based prospective study to investigate the effect of fish intake in the development of T2D reported that people who weekly consume fish were correlated with a 25% decrease of T2D risk in comparison with who consume less than one portion per week .[Citation44] A study on the Japanese population revealed that only small and medium-sized fish (horse mackerel, sardine, saury, mackerel, and eel) are associated with the decrease of T2D incidence with 0,68 OR whereas big-sized fish (salmon, skipjack tuna, cod, flatfish, and sea bream) have no relation with it .[Citation46]
Evidence from other studies show that a higher fish or fish oil consumption lowers the risk of CVD and sudden death .[Citation50] Therefore, it makes sense that the Japanese population present lower rates of CVD death compared to Western population, as their fish intake is much higher .[Citation51,Citation52] Similar results were found in an epidemiological study among the Dutch population, in which those who ate fish once or twice per week had a 50% less CVD mortality risk compared to those who ate fewer .[Citation53] More specifically, in different meta-analyzes and reviews, the intake of between 2 and 4 servings of fish per week was associated with a 4% reduction in the risk of suffering cerebrovascular disease[Citation54] and 18% of having a stroke .[Citation55] In the same line, a consumption of 4 servings of fish per week reduced acute coronary syndrome by 21% .[Citation56] On the other hand, with a minimum consumption of 1 time per week of fish, a reduction of around 50% due to sudden death has been observed[Citation57] and with the consumption of small amounts of fish a 17% reduction in death due to coronary illness .[Citation58] Finally, fish consumption has been associated with a lower prevalence of atherosclerosis .[Citation59–61]
However, some cohort studies reveal a positive association between fish consumption and T2D risk .[Citation62,Citation63] It is thought that this positive association could be due to environmental contaminants in fish such as persistent organic pollutants (POPs), dioxins or other contaminants like methyl-mercury. It is believed that some POPs could induce abdominal obesity, impair insulin sensitivity, and reduce glucose intake[Citation64,Citation65] and it has been observed a positive association of some POPs with T2D, [Citation66] concretely, a dose-response relation between serum concentrations and T2D prevalence with 25th, 50th, 75th, and 90th percentiles of POPS and OR 1.0, 14.0, 14.7, 38.3, and 37.7 .[Citation67]
Despite that, data were not sufficient to establish causality and, especially with fish consumption, there are no studies that show a higher incidence of T2D due to POPs intake through fish in diet. Therefore, there are gaps on experimental data to confirm the chemical exposure in the pathogenesis of T2D[Citation66] and there is much work in limiting contaminants level in fish so this should not take relevance to the beneficial effect of its intake .[Citation45]
The way that fish reduces T2D risk is controversial, but it has been discussed that insulin sensitivity is positively associated with the content of omega-3 FA in cell membranes as PUFA are capable of increasing membrane fluidity, the amount of insulin receptors, and the action of insulin .[Citation68] Moreover, the beneficial action of fish could be also led by its huge content of proteins and amino acids, as fish proteins play a role in increasing satiety which explains its involvement in weight loss, [Citation69] and also have many essential amino acids. Therefore, the anti-inflammatory effect of fish consumption, which is generally attributed to omega-3 FA intake, could also be due to the consumption of fish proteins .[Citation70]
Nevertheless, higher fish intake is usually linked to healthier diets and physical activity which could mean the protective effect against T2D and other diseases may be due to the synergistic action of all these parameters. According to that, a review about the effect of the Mediterranean diet on prevention of CVD suggests that this kind of diet which is rich in fish, has a protective effect on some risk factors considering that it is capable of reducing total cholesterol and blood pressure .[Citation71] Because of all these beneficial effects of fish, dietary guidelines recommend the intake of this food, preferentially oily fish, at least twice a week .[Citation2]
Oily fish
Due to the controversial results obtained in different observational but also interventional studies between fish consumption and T2D risk, a meta-analyses was realized dividing lean and oily fish due to its composition .[Citation72] Lean fish store lipid in the liver while oily fish store lipid in the flesh, which leads to a higher fat intake when consuming oily fish. In this meta-analyses, a significant effect of oily fish intake on risk of T2D was shown. Concretely, dose-response analysis suggested that 80 g intake of oily fish per day may reduce the risk of T2D by 20% .[Citation72]
Changes in lipid profile have been observed when a diet rich in oily fish is followed. Exactly, oily fish is rich in PUFA omega-3 FA, both of α-linolenic acid (ALA) as well as eicosanoid acid (EPA) and docosahexaenoic acid (DHA) which have been the primary seafood components with proven health benefits. Therefore, nutritional recommendations are made to ensure their consumption in the prevention of CVD including 400–500 mg/day of EPA and DHA or at least 2 servings/week of oily fish .[Citation73] But, EPA and DHA are not the only ones, most lipids present in oily fish are found to be involved in insulin signaling and inflammation, [Citation74] especially ceramides, sphingolipids which are thought to be the junction between excess of nutrients, proinflammatory cytokines, and insulin resistance .[Citation75] An oily fish intake 100–150 gr/meal at least four time a week for 8 weeks produced a decrease of plasma lipids (ceramides, lysophosphatidylcholines, diacyglycerols, lysophosphatidylethanolamines and phosphatidylcolines) with an average reduction of −0.5 fold change .[Citation74] Moreover, oily fish consumption in 150gr/meal for 5 meals/week during 4 weeks demonstrated a decreased in serum triglycerides (TAG) whereas it increases HDL cholesterol .[Citation76] Both parameters were widely observed in various studies and a meta-analysis concluded that oily fish consumption (ranging 20–150g/day for 4–24 weeks) in healthy or with CV risk (hyperlipidemia, hypertriacylglycerolaemia or obese) adults, leads to a moderately significant reduction in plasma TAG levels (−9.73 mg/dL) and an increase in HDL cholesterol levels (2.32 mg/dL)[Citation77] which is associated with reducing CVD risk factors[Citation78] strongly associated with T2D pathogenesis .[Citation79]
Despite the fact that PUFA blood profile has been shown to be modified in patients with T2D as a diminished (EPA+DHA)/arachidonic acid ratio has been seen, [Citation80] it has been observed that people with higher plasma phospholipid omega-3 FA have lower risk of T2D progression .[Citation81] It has been described that higher protective effect of oily fish compared to lean fish against T2D is due to its huge content in omega-3 FA, which improves insulin-stimulated oxidative and non-oxidative glucose disposal through the reduction in the inhibitory effect of excessive β-oxidation in humans[Citation82] and prevent diet-induced insulin resistance through the insulin-sensitizing action of omega-3 FA in a nonhuman primate model .[Citation83] Interventions with omega-3 mix supplementation did not show the same effect (). Even the omega-3s called EPA and DHA, proposed as responsible for those benefits, [Citation44,Citation45,Citation51] could not demonstrate their benefits in isolated supplementation .[Citation84] All of this, leads us to think about the whole food as the developer of this beneficial effect against T2D progression. Therefore, the type of fish, cooking methods or environmental contaminants could have an impact on results .[Citation72]
Figure 1. Justification for the choice of sardines in relation to possible preventive effects in humans against the risk of developing T2D.
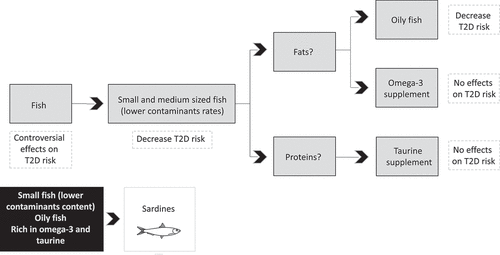
Although the effect of omega-3 FA, this action may also be assisted by fish proteins and vitamins, which are thought to improve insulin sensitivity and have antihypertensive, antioxidant, antiproliferative and anticoagulant capacities .[Citation76,Citation85–88] Different bioactive motifs have been found which are thought to have antidiabetic and hypocholesterolemic properties .[Citation88] Moreover, in a model of high-fat-fed rats supplemented with different fish proteins a reduction of proinflammatory cytokines was seen, [Citation87] although only salmon proteins promotes an improvement in adipose tissue composition .[Citation86]
So, according to the previous statements, oily fish would be capable of improving endothelial dysfunction, and arrhythmia, reducing microvascular complications, and improving other mechanisms present in both T2D and CVD .[Citation89]
On the differences between lean and oily fish, apart from fat content in the flesh, increased presence of fat-soluble vitamins such as vitamin D in oily fish .[Citation90] There are no major differences in overall protein content between white and blue fish, but there are specific differences with some amino acids .[Citation91]
Taurine
One of the amino acids highly present in seafood and specially in oily fish is taurine (2-aminoethanesulfonic acid), a semi-essential amino acid which is normally found in high concentrations in β pancreatic islets .[Citation92] Taurine is normally assimilated through diet but it can also be synthesized in small proportions in the pancreas with the presence of cysteine. Its main functions involve oxidative stress, Ca+2 transport regulation, osmoregulation, and anti-inflammation .[Citation93,Citation94]
Taurine levels have been found decreased in subjects with preDM or T2D, which is thought to be due to the overactivity of the polyol pathway coming from the increased glucose levels .[Citation95,Citation96] This fact suggests a lack of taurine as a role in T2D development and further complications which have been demonstrated in T2D animal models where taurine supplementation among T2D rats has been observed to decrease glucose levels and increase plasma insulin, [Citation97] but clinical trials have not been able to demonstrate this fact on human[Citation98,Citation99] ().
Table 1. Studies with taurine supplementation.
Taurine has been described to have hypoglycemic effects (improving hepatic insulin sensitivity, stimulating glycogen synthesis in the liver and glucose uptake in peripheral tissues such as the liver and skeletal muscle) and is also capable of decreasing serum cholesterol in different ways .[Citation93] It plays a role in bile acids and its transporters, [Citation104] and also enhances the expression of 7α-hydroxylase, which is involved in biliary acid synthesis .[Citation105] At the glycemic level, taurine has been seen to promote nuclear expression of PDX-1 factor, which is required for the expression of insulin, and various genes involved in the same pathways such as Glut-2, GK, Sur-1, Pcsk-1 .[Citation92] Moreover, a function as an antioxidant has been reported as well as promoting autophagy by interacting with taurine-induced nuclear translocation of transcription factor EB (TFEB) .[Citation92]
In other way, taurine has been shown to participate in the modulation of circadian rhythm. The circadian clock maintains alignment of peripheral tissue clocks present in nearly all cells and circadian rhythms are controlled by oscillators, which depend on specific clock genes .[Citation106] Peripheral clocks regulating local metabolic rhythms are determined by feeding and fasting cycles. Moreover, nutrients reset peripheral circadian clocks and the local clock genes control downstream metabolic processes[Citation107] and, specially, the circadian system which has been shown to regulate glucose metabolism .[Citation108] Concretely, taurine supplementation in mice model demonstrated the modulation of clock genes expression in β cells in those consuming a diet rich in high fat diet[Citation102] which is linked to an improvement with sleep quality[Citation109] and negatively associated with obesity and T2D development .[Citation110]
Furthermore, the supplementation through the combination between omega-3 + taurine in humans has shown a cardioprotective and anti-inflammatory effect greater than both elements separately .[Citation111] Also, in mice with T2D the combination of fish oil + taurine showed a decrease in glucose and insulin levels and improved the leptin resistance contributing to the suppression of weight gain[Citation112] ().
Table 2. Studies with taurine and omega-3/oily fish supplementation.
Sardines
Among the oily fish (Supplementary Table 1), sardines are the richest in lipids. Specifically, it is the oily fish with the most PUFA content. Moreover, as one of the oily fishes richest in omega-3 FA and due to its whole nutritional composition, sardines are believed to have many potential beneficial effects on health (). As already mentioned, its high omega-3 FA content contributes in reducing CVD and T2D risk. Moreover, sardines are the richest oily fish in proteins and most of amino acids (Supplementary Table 1), of which, taurine described is one of the highest among oily fish[Citation114] and contains between 122 mg/100 gr of product[Citation115] and 147 mg/100 gr of product[Citation116] in fresh weight, depending on the specie. Its take on relevance in the prevention of CVD and T2D as it is thought to have hypoglycemic, antioxidant, and anti-inflammatory actions. Sardines are also a source of calcium and vitamin D (Supplementary Table 1), which is already known to play a role in reducing T2D risk .[Citation29]
Table 3. Sardines composition by United States Department of Agriculture (USDA) .[Citation113].
Apart from its favorable nutritional composition, sardines are also one of the fish with lower contaminants content and the results indicated that sardines are safe for consumption based on the toxicologically relevant parameters regulated by European Commission .[Citation117] This can be explained because the concentrations of POPs increase their concentration through the trophic chain. This is because large predatory fish accumulate more pollutants because they have a longer life since they cannot be caught by other medium and small fish .[Citation118,Citation119]
Sardines can also be caught year round and are economically affordable by the public. With these reasons, they are proposed to be the best option to prevent T2D progression and diminish CVD risk ().
There have not been many studies where sardine was given as an intervention (). Human studies in which this fish has been supplemented, ensuring at least double weekly fish consumption from sardine-based, reveal a positive effect in overweight or obese non-treated T2D patients with HbA1c between 6.0 and 8.0% and aged between 40 and 70 years old. Participants increased of adiponectin from 2.1 ± 0.3 μg/mL at baseline to 3.0 ± 0.3 μg/mL after 6 months intervention, a biomarker related to an improvement of insulin sensitivity and the decreased of CVD risk index .[Citation122]
Table 4. Studies with sardine supplementation.
Moreover, in diabetic humans receiving hemodialysis, sardines consumption 3 times/week during 8 weeks have promoted a significant reduction −1.01 ± 1.11 mg/L of C-reactive protein which is known for its role in inflammation and considered as a T2D and CVD-progression marker .[Citation123]
Also, animal model studies have reported the beneficial actions of this fish. Reduction of systolic and diastolic pressure, reduction of glycemia and HbA1c, reduction of 137 and total cholesterol and increases of adiponectin, HDL cholesterol, and EPA+DHA have been observed after administering sardines .[Citation120,Citation121] Furthermore, studies report an improvement of insulin resistance, increased activity of PI3K/Akt pathway, and translocation of GLUT4 transporters which all together contribute to the improvement of insulin action and glucose homeostasis .[Citation124]
Although studies with sardine supplementation are not very common, research with its main components has been performed more regularly (). In the case of sardine oil supplementation, it has been seen to reduce TAG as well as total and LDL cholesterol. However, the effects of sardine on glycemia have not been as clearly described as different controversial results have been published.
Table 5. Studies with sardine oil supplementation.
On the other hand, studies with animal models in which sardine protein was supplemented have reported a reduction of white adipose tissue, improvements on insulin sensitivity and glycemia[Citation124,Citation129] ().
Table 6. Studies of sardine protein supplementation.
Conclusions
Non-communicable diseases, especially T2D, are becoming widespread. Researchers must investigate the best strategies to prevent its development and weight loss is one of the most studied areas. However, in elderly people, who are at greater risk of developing T2D, we know that it may be more interesting to focus on the quality of the food they eat.
Fish consumption has been studied on various occasions, with some contradictory results due to the differences between the species consumed. The fish with the least amount of toxic compounds as well as the highest amount of PUFA, especially omega-3 FA, could be the most interesting due to the subject’s improvement in insulin sensitivity.
Fish also contain other interesting nutrients that can stop or delay progression to T2D. Among them, protein content and more specifically some amino acids. Taurine is one of the most present, especially in oily fish, and has been related to a hypoglycemic effect but without being able to demonstrate it in the supplementing trials in isolation.
The fish with the lowest concentration of POPs and the highest content of PUFA as well as taurine is sardine. An in vivo study in rats concluded that sardine consumption reduced glycemia and HbA1c, but the few clinical trials investigating the effect of sardine consumption with diabetes were unable to link it. Instead, they observed a decrease in CRP and an increase in circulating adiponectin.
On the other hand, some in vitro studies in rats, not carried out in humans to reproduce effects, with sardine-derived products such as oil or proteins, produced an improvement in glycemia as well as an improvement in sensitivity to insulin or in the absorption of glucose.
More interventional studies need to be carried out to know discover the impact of sardine consumption in metabolic diseases is. Specially, it could be interesting to promote clinical trials in order to evaluate the preventive effects that a sardine-enriched diet could have against T2D. This would be especially interesting in a high-risk population with preDM but also with a high level of fragility for example an old-age population.
We consider that is very important to know the mechanisms by which sardine consumption, through its nutrients acts on glucose homeostasis as well as insulin secretion or sensibility. Therefore, mechanistic studies involving the composition of the gut microbiota, transcriptomic, or metabolomics could be of great interest in the scientific community.
To summaries, it may be both timely and appropriate to suggest that the elderly tailor their dietary intake to increase the consumption of sardine as all studies carried out to date prove that its consumption is safe. Moreover, it could lead to a health benefit by promoting the consumption of healthy fats, as well as vitamins and minerals potentially at risk in this population group such as calcium or vitamin D and reducing the risk of protein-energy malnutrition. In conclusion, after all the scientific evidence presented in this review, sardine consumption could have a protective effect against the development of metabolic diseases such as T2D with such a high prevalence and incidence in this group.
Supplemental Material
Download MS Word (24.7 KB)Supplementary data
Supplemental data for this article can be accessed on the publisher’s website.
References
- World Health Organization. International Encyclopedia of Public Health. http://www.who.int/diabetes/en/(accessed Aug 11, 2019).
- American Diabetes Association. Standards of Medical Care in Diabetes 2018. Diabetes Care. 2018, 41(Supplement 1), S1. DOI: 10.2337/dc18-Sint01.
- Forouhi, N. G.; Luan, J.; Hennings, S.; Wareham, N. J. Incidence of Type 2 Diabetes in England and Its Association with Baseline Impaired Fasting Glucose: The Ely Study 1990–2000. Diabet. Med. 2007, 24(2), 200–207. DOI: 10.1111/j.1464-5491.2007.02068.x.
- Nathan, D. M.; Davidson, M. B.; DeFronzo, R. A.; Heine, R. J.; Henry, R. R.; Pratley, R.; Zinman, B. Impaired Fasting Glucose and Impaired Glucose Tolerance: Implications for Care. Diabetes Care. 2007, 30(3), 753–759. DOI: 10.2337/dc07-9920.
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of Diabetes Mellitus and Impaired Glucose Regulation in Spain: The [email protected] Study. Diabetologia. 2012, 55(1), 88–93.
- Narayan, K. M. V.; Boyle, J. P.; Geiss, L. S.; Saaddine, J. B.; Thompson, T. Impact of Recent Increase in Incidence on Future Diabetes Burden: U.S., 2005–2050. Diabetes Care. 2006, 29(9), 2114–2116. DOI: 10.2337/dc06-1136.
- DECODE study group. Diabetes and Impaired Glucose Regulation in 13 European Cohorts. Diabetes Care. 2003, 26(1), 61–69. DOI: 10.2337/diacare.26.1.61.
- Will, J. C.; Williamson, D. F.; Ford, E. S.; Calle, E. E.; Thun, M. J. Intentional Weight Loss and 13-year Diabetes Incidence in Overweight Adults. Am. J. Public Health. 2002, 92(8), 1245–1248. DOI: 10.2105/AJPH.92.8.1245.
- DeFronzo, R. A.; Ferrannini, E.; Groop, L.; Henry, R. R.; Herman, W. H.; Holst, J. J.; Hu, F. B.; Kahn, C. R.; Raz, I.; Shulman, G. I.; et al. Type 2 Diabetes Mellitus. Nat Rev Dis Primers. 2015, 1, 15019.
- Lindström, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J. Finnish Diabetes Study. Diabetes Care. 2003, 26(12), 3230–3236.
- Schellenberg, E. S.; Dryden, D. M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients with and at Risk for Type 2 Diabetes. Ann. Intern. Med. 2013, 159(8), 543–551. DOI: 10.7326/0003-4819-159-8-201310150-00007.
- Fox, C. S.; Golden, S. H.; Anderson, C.; Bray, G. A.; Burke, L. E.; de Boer, I. H.; Deedwania, P.; Eckel, R. H.; Ershow, A. G.; Fradkin, J.; et al. Update on Prevention of Cardiovascular Disease in Adults with Type 2 Diabetes Mellitus in Light of Recent Evidence. Circulation. 2015, 132(8), 691–718.
- Curioni, C. C.; Lourenço, P. M. Long-term Weight Loss after Diet and Exercise: A Systematic Review. Int. J. Obes. 2005, 29(10), 1168–1174. DOI: 10.1038/sj.ijo.0803015.
- Ditschuneit, H. H.; Flechtner-mors, M.; Johnson, T. D.; Adler, G. Metabolic and Weight-loss Effects of a Long-term Dietary Intervention in Obese Patients. Am. J. Clin. Nutr. 1999, 69(2), 198–204. DOI: 10.1093/ajcn/69.2.198.
- Goodpaster, B. H.; Delany, J. P.; Otto, A. D.; Kuller, L.; Vockley, J.; South-Paul, J. E.; Thomas, S. B.; Brown, J.; McTigue, K.; Hames, K. C.; et al. Effects of Diet and Physical Activity Interventions on Weight Loss and Cardiometabolic Risk Factors in Severely Obese Adults: A Randomized Trial. JAMA. 2010, 304(16), 1795–1802.
- Hamman, R. F.; Wing, R. R.; Edelstein, S. L.; Lachin, J. M.; Bray, G. A.; Delahanty, L.; Hoskin, M.; Kriska, A. M.; Mayer-David, E. J.; Pi-Sunyer, X.; et al. Effect of Weight Loss with Lifestyle Intervention on Risk of Diabetes. Diabetes Care. 2006, 29(9), 2102–2107.
- The Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346(6), 393–403. DOI: 10.1056/NEJMoa012512.
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C. D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric Surgery and Long-term Cardiovascular Events. JAMA. 2012, 307(1), 56–65.
- Fried, M.; Yumuk, V.; Oppert, J. M.; Scopinaro, N.; Torres, A. J.; Weiner, R.; Yashkov, Y.; Frühbeck, G. European Association for the Study of Obesity, International Federation for the Surgery of Obesity - European Chapter: Interdisciplinary European Guidelines on Metabolic and Bariatric Surgery. Obes. Facts. 2013, 6(5), 449–468. DOI: 10.1159/000355480.
- Farag, Y. M. K.; Gaballa, M. R. Diabesity: An Overview of a Rising Epidemic. Neprol. Dial. Transplant. 2011, 26(1), 28–35. DOI: 10.1093/ndt/gfq576.
- Waters, D. L.; Ward, A. L.; Villareal, D. T. Weight Loss in Obese Adults 65 Years and Older: A Review of the Controversy. Exp. Gerontol. 2013, 48(10), 1054–1061. DOI: 10.1016/j.exger.2013.02.005.
- Forsythe, L. K.; Wallace, J. M.; Livingstone, M. B. Obesity and Inflammation: The Effects of Weight Loss. Nutr. Res. Rev. 2008, 21(2), 117–133. DOI: 10.1017/S0954422408138732.
- Ler, L.; Odell, P. M.; DAgostino, R. B.; Stokes, J., 3rd; Kreger, B. E.; Belanger, A. J.; Brownell, K. D. Variability of Body Weight and Health Outcomes in the Framingham Population. N. Engl. J. Med. 1991, 324(26), 1839–1844. DOI: 10.1056/NEJM199106273242602.
- Blair, S. N.;. Evidence for Success of Exercise in Weight Loss and Control. Ann. Intern. Med. 1993, 119(7), 702–706. Pt 2. DOI: 10.7326/0003-4819-119-7_Part_2-199310011-00015.
- Newman, A. B.; Yanez, D.; Harris, T.; Duxbury, A.; Enright, P. L.; Fried, L. P. Weight Change in Old Age and Its Association with Mortality. J. Am. Geriatr. Soc. 2001, 49(10), 1309–1318. DOI: 10.1046/j.1532-5415.2001.49258.x.
- Darmon, P.; Kaiser, M. J.; Bauer, J. M.; Sieber, C. C.; Pichard, C. Restrictive Diets in the Elderly: Never Say Never Again? Clin. Nutr. 2010, 29(2), 170–174. DOI: 10.1016/j.clnu.2009.11.002.
- Díaz-Rizzolo, D. A.; Kostov, B.; López-Siles, M.; Serra, A.; Colungo, C.; González-de-Paz, L.; Martínez-Medina, M.; Sisó-Almirall, A.; Gomis, R. Healthy Dietary Pattern and Their Corresponding Gut Micrbiota Profile are Linked to a Lower Risk of Type 2 Diabetes, Independent of the Presence of Obesity. Clin. Nutr. 2020, 39(2), 524–532. DOI: 10.1016/j.clnu.2019.02.035.
- Knoops, K. T.; de Groot, L. C.; Kromhout, D.; Perrin, A. E.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W. A. Mediterranean Diet, Lifestyle Factors, and 10-Year Mortality in Elderly the HALE Project. JAMA. 2004, 292(12), 1433–1439. DOI: 10.1001/jama.292.12.1433.
- Pittas, A. G.; Lau, J.; Hu, F.; Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2007, 92(6), 2017–2029. DOI: 10.1210/jc.2007-0298.
- Mayer-Davis, E. J.; Costacou, T.; King, I.; Zaccaro, D. J.; Bell, R. A. Plasma and Dietary Vitamin E in Relation to Incidence of Type 2 Diabetes. Diabetes Care. 2002, 25(12), 2172–2177. DOI: 10.2337/diacare.25.12.2172.
- Lopez-Riadura, R.; Willett, W. C.; Rimm, E. B.; Liu, S.; Stampfer, M. J.; Manson, J. E.; Hu, F. B. Magnesium Intake and Risk of Type 2 Diabetes in Men and Women. Diabetes Care. 2004, 27(1), 134–140. DOI: 10.2337/diacare.27.1.134.
- Sun, C.; Zhao, C.; Guven, E. C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K. M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabtic Agents: Advances and Opporunities. Food Frontiers. 2020, 1(1), 18–44. DOI: 10.1002/fft2.15.
- Saslow, L. R.; Kim, S.; Daubenmier, J. J.; Moskowitz, J. T.; Phinney, S. D.; Goldman, V.; Murphy, E.; Cox, R. M.; Moran, P.; Hecht, F. M. A Randomized Pilot Trial of A Moderate Carbohydrate Diet Compared to A Very Low Carbohydrate Diet in Overweight or Obese Individuals with Type 2 Diabetes Mellitus or Prediabetes. PLoS One. 2014, 9(4), e91027. DOI: 10.1371/journal.pone.0091027.
- Greenwood, D. C.; Threapleton, D. E.; Evans, C. E.; Cleghorn, C. L.; Nykjaer, C.; Woodhead, C.; Burley, V. J. Glycemic Index, Glycemic Load, Carbohydrates, and Type 2 Diabetes: Systematic Review and Dose-response Meta-analysis of Prospective Studies. Diabetes Care. 2013, 36(12), 4166–4171. DOI: 10.2337/dc13-0325.
- Rendell, M.;. Dietary Treatment of Diabetes Mellitus. New Engl. J. Med. Ed. 2000, 342(19), 1440–1441. DOI: 10.1056/NEJM200005113421910.
- Thanopoulou, A. C.; Karamanos, B. G.; Angelico, F. V.; Assaad-Khalil, S. H.; Barbato, A. F.; Del Ben, M. P.; Djordjevic, P. B.; Dimitrijevic-Sreckovic, V. S.; Galloti, C. A.; Katsilambros, N. L.; et al. Dietary Fat Intake as Risk Factor for the Development of Diabetes: Multinational, Multicenter Study of the Mediterranean Group for the Study of Diabetes (MGSD). Diabetes Care. 2003, 26(2), 302–307.
- Meyer, K. A.; Kushi, L. H.; Jacobs, D. R.; Folsom, A. R. Dietary Fat and Incidence of Type 2. Diabetes Care. 2001, 24(9), 1528–1535. June 2000. DOI: 10.2337/diacare.24.9.1528.
- Hu, F. B.; van Dam, R. M.; Liu, S. Diet and Risk of Type II Diabetes: The Role of Types of Fats and Carbohydrates. Diabetologia. 2001, 44(7), 805–817. DOI: 10.1007/s001250100547.
- Lichtenstein, A. H.; Schwab, U. S. Relationship of Dietary Fat to Glucose Metabolism. Atherosclerosis. 2000, 150(2), 227–243. DOI: 10.1016/S0021-9150(99)00504-3.
- Pan, D. A.; Lillioja, S.; Milner, M. R.; Kriketos, A. D.; Baur, L. A.; Bogardus, C.; Storlien, L. H. Skeletal Muscle Membrane Lipid Composition Is Related to Adiposity and Insulin Action. J. Clin. Invest. 1995, 96(6), 2802–2808. DOI: 10.1172/JCI118350.
- Brinkworth, G. D.; Noakes, M.; Parker, B.; Foster, P.; Clifton, P. M. Long-term Effects of Advice to Consume a High-protein, Low-fat Diet, Rather than a Conventional Weight-loss Diet, in Obese Adults with Type 2 Diabetes: One-year Follow-up of a Randomised Trial. Diabetologia. 2004, 47(10), 1677–1686. DOI: 10.1007/s00125-004-1511-7.
- van Nielen, M.; Feskens, E. J. M.; Mensink, M.; Sluijs, I.; Molina, E.; Amiano, P.; Ardanaz, E.; Balkau, B.; Beulens, J. W. L.; Boeing, H.; et al. Dietary Protein Intake and Incidence of Type 2 Diabetes in Europe: The EPIC-InterAct Case-Cohort Study. Diabetes Care. 2014, 37(7), 1854–1862.
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis of Cohort Studies. Nutrients. 2017, 9(9), 1–17. DOI: 10.3390/nu9090982.
- Patel, P. S.; Sharp, S. J.; Luben, R. N.; Khaw, K. T.; Bingham, S. A.; Wareham, N. J.; Forouhi, N. G. Association between Type of Dietary Fish and Seafood Intake and the Risk of Incident Type 2 Diabetes: The European Prospective Investigation of Cancer (Epic)-norfolk Cohort Study. Diabetes Care. 2009, 32(10), 1857–1863. DOI: 10.2337/dc09-0116.
- Mozaffarian, D.; Rimm, E. B.; Intake, F. Contaminants, and Human Health. JAMA. 2006, 296(15), 1885. DOI: 10.1001/jama.296.15.1885.
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Matsushita, Y.; Poudel-Tandukar, K.; Kato, M.; Oba, S.; Inoue, M.; Tsugane, S.; et al. Fish Intake and Type 2 Diabetes in Japanese Men and Women: The Japan Public Health Center-based Prospective Study. Am. J. Clin. Nutr. 2011, 94(3), 884–891.
- Kromann, N.; Green, A. Epidemiological Studies in the Upernavik District, Greenland. Incidence of Some Chronic Diseases 1950–1974. Acta. Med. Scand. 1980, 208(401), 401–406.
- Zheng, J.; Huang, T.; Yu, Y.; Hu, X.; Yang, B.; Li, D. Fish Consumption and CHD Mortality: An Updated Meta-analysis of Seventeen Cohort Studies. Public Health Nutr. 2012, 15(4), 725–737. DOI: 10.1017/S1368980011002254.
- Qin, B.; Plassman, B. L.; Edwards, L. J.; Popkin, B. M.; Adair, L. S.; Mendez, M. A. Fish Intake Is Associated with Slower Cognitive Decline in Chinese Older Adults1–3. J. Nutr. 2014, 144(10), 1579–1585. DOI: 10.3945/jn.114.193854.
- Esmailzadehha, N.; Ziaee, A.; Kazemifar, A. M.; Ghorbani, A.; Oveisi, S. Prevalence of Metabolic Syndrome in Qazvin Metabolic Diseases Study (QMDS), Iran: A Comparative Analysis of Six Definitions. Endocr. Regul. 2013, 47(3), 111–120. DOI: 10.4149/endo_2013_03_111.
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of Eicosapentaenoic Acid on Major Coronary Events in Hypercholesterolaemic Patients (JELIS): A Randomised Open-label, Blinded Endpoint Analysis. Lancet. 2007, 369(9567), 1090–1098.
- Iso, H.; Kobayashi, M.; Ishihara, J.; Sasaki, S.; Okada, K.; Kita, Y.; Kokubo, I.; Tsugane, S. Intake of Fish and N3 Fatty Acids and Risk of Coronary Heart Disease among Japanese: The Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation. 2006, 113(2), 195–202. DOI: 10.1161/CIRCULATIONAHA.105.581355.
- de Goede, J.; Geleijnse, J. M.; Boer, J. M. A.; Kromhout, D.; Verschuren, W. M. M. Marine (N-3) Fatty Acids, Fish Consumption, and the 10-Year Risk of Fatal and Nonfatal Coronary Heart Disease in a Large Population of Dutch Adults with Low Fish Intake. J. Nutr. 2010, 140(5), 1023–1028. DOI: 10.3945/jn.109.119271.
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F. B.; et al. Association between Fish Consumption, Long Chain Omega 3 Fatty Acids, and Risk of Cerebrovascular Disease: Systematic Review and Meta-analysis. BMJ. 2012, 345(7881), 1–9. DOI: 10.1136/bmj.e6698.
- He, K.; Song, Y.; Daviglus, M. L.; Liu, K.; Van Horn, L.; Dyer, A. R.; Goldbourt, U.; Greenland, P. Fish Consumption and Incidence of Stroke: A Meta-analysis of Cohort Studies. Stroke. 2004, 35(7), 1538–1542. DOI: 10.1161/01.STR.0000130856.31468.47.
- Leung Yinko, S. L. L.; Stark, K. D.; Thanassoulis, G.; Pilote, L. Fish Consumption and Acute Coronary Syndrome: A Meta-analysis. Am. J. Med. 2014, 127(9), 848–857. DOI: 10.1016/j.amjmed.2014.04.016.
- Mozaffarian, D.; Prineas, R. J.; Stein, P. K.; Siscovick, D. S. Dietary Fish and N-3 Fatty Acid Intake and Cardiac Electrocardiographic Parameters in Humans. J. Am. Coll. Cardiol. 2006, 48(3), 478–484. DOI: 10.1016/j.jacc.2006.03.048.
- König, A.; Bouzan, C.; Cohen, J. T.; Connor, W. E.; Kris-Etherton, P. M.; Gray, G. M.; Lawrence, R. S.; Savitz, D. A.; Teutsche, S. M. A Quantitative Analysis of Fish Consumption and Coronary Heart Disease Mortality. Am. J. Prev. Med. 2005, 29(4), 335–346. DOI: 10.1016/j.amepre.2005.07.001.
- Buscemi, S.; Nicolucci, A.; Lucsiano, G.; Galvano, F.; Grosso, G.; Belmonte, S.; Sprini, D.; Migliaccio, S.; Cianferotti, L.; Brandi, M. L.; et al. Habitual Fish Intake and Clinically Silent Carotid Atherosclerosis. Nutr. J. 2014, 13(1). DOI: 10.1186/1475-2891-13-2.
- He, K.; Liu, K.; Daviglus, M. L.; Mayer-Davis, E.; Jenny, N. S.; Jiang, R.; Ouyang, P.; Steffen, L. M.; Siscovick, D.; Wu, C.; et al. Intakes of Long-chain N-3 Polyunsaturated Fatty Acids and Fish in Relation to Measurements of Subclinical Atherosclerosis. Am. J. Clin. Nutr. 2008, 88(4), 1111–1118.
- Erkkila, A. T.; Lichtenstein, A. H.; Mozaffarian, D.; Herrington, D. M. Fish Intake Is Associated with a Reduced Progression of Coronary Artery Atherosclerosis in Postmenopausal Women with Coronary Artery Disease. Am. J. Clin. Nutr. 2004, 80(3), 626–632. DOI: 10.1093/ajcn/80.3.626.
- Lee, C.; Liese, A.; Wagenknecht, L.; Lorenzo, C.; Haffner, S.; Hanley, A. Fish Consumption, Insulin Sensitivity and Beta-cell Function in the Insulin Resistance Atherosclerosis Study (IRAS). Nutr. Metab. Cardiovasc Dis. 2013, 23(9), 829–835. DOI: 10.1016/j.numecd.2012.06.001.
- Kaushik, M.; Mozaffarian, D.; Spiegelman, D.; Manson, J. E.; Willett, W. C.; Hu, F. B. Long-chain Omega-3 Fatty Acids, Fish Intake, and the Risk of Type 2 Diabetes Mellitus. Am. J. Clin. Nutr. 2009, 90(3), 613–620. DOI: 10.3945/ajcn.2008.27424.
- Wu, H.; Bertrand, K. A.; Choi, A. L.; Hu, F. B.; Laden, F.; Grandjean, P.; Sun, Q. Persistent Organic Pollutants and Type 2 Diabetes: A Prospective Analysis in the Nurses Health Study and Meta-analysis. Environ. Health Perspect. 2013, 121(2), 153–161. DOI: 10.1289/ehp.1205248.
- Ruzzin, J.; Petersen, R.; Meugnier, E.; Madsen, L.; Lock, E. J.; Lillefosse, H.; Ma, T.; Pesenti, S.; Sonne, S. B.; Marstrand, T. T.; et al. Persistent Organic Pollutant Exposure Leads to Insulin Resistance Syndrome. Environ. Health Perspect. 2010, 118(4), 465–471.
- Taylor, K. W.; Novak, R. F.; Anderson, H. A.; Birnbaum, L. S.; Blystone, C.; DeVito, M.; Jacobs, D.; Kohrle, J.; Lee, D.-H.; Rylander, L.; et al. Evaluation of the Association between Persistent Organic Pollutants (Pops) and Diabetes in Epidemiological Studies: A National Toxicology Program Workshop Review. Environ. Health Perspect. 2013, 121(7), 774–783.
- Lee, D. H.; Lee, I. K.; Song, K.; Steffes, M.; Toscano, W.; Baker, B. A.; Jacobs, D. R., Jr. A Strong Dose-response Relation between Serum Concentrations of Persistent Organic Pollutants and Diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006, 29(7), 1638–1644.
- Borkman, M.; Storlien, L. H.; Pan, D. A.; Jenkins, A. B.; Chisholm, D. J.; Campbell, L. V. The Relation between Insulin Sensitivity and the Fatty-Acid Composition of Skeletal-Muscle Phospholipids. N. Engl. J. Med. 1993, 328(4), 238–244. DOI: 10.1056/NEJM199301283280404.
- Uhe, A. M.; Collier, G. R.; ODea, K. A Comparison of the Effects of Beef, Chicken and Fish Protein on Satiety and Amino Acid Profiles in Lean Male Subjects. J. Nutr. 1992, 122(3), 467–472. DOI: 10.1093/jn/122.3.467.
- Pilon, G.; Ruzzin, J.; Rioux, L. E.; Lavigne, C.; White, P. J.; Frøyland, L.; Jacqes, H.; Bryl, P.; Beaulieu, L.; Marette, A. Differential Effects of Various Fish Proteins in Altering Body Weight, Adiposity, Inflammatory Status, and Insulin Sensitivity in High-fat-fed Rats. Metabolism. 2011, 60(8), 1122–1130.
- Rees, K.; Hartley, L.; Flowers, N.; Clarke, A.; Hooper, L.; Thorogood, M.; Stranges, S. Mediterranean Dietary Pattern for the Primary Prevention of Cardiovascular Disease (Review). Cochrane Database Syst. Rev. 2016, 12(8), CD009825.
- Zhang, M.; Picard-deland, E.; Marette, A. Fish and Marine Omega-3 Polyunsatured Fatty Acid Consumption and Incidence of Type 2 Diabetes : A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2013, 2013, 501015. DOI: 10.1155/2013/501015.
- Lichtenstein, A. H.; Appel, L. J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H. A.; Franklin, B.; Kris-Etherton, P.; Harris, W. S.; Howard, B.; et al. Diet and Lifestyle Recommendations Revision 2006 A Scientific Statement from the American Heart Association Nutrition Committee. Ciruculation. 2006, 114(1), 82–96.
- Lankinen, M.; Schwab, U.; Erkkilä, A.; Seppänen-Laakso, T.; Hannila, M. L.; Mussalo, H.; Lehto, S.; Uusituupa, M.; Gylling, H.; Oresic, M. Fatty Fish Intake Decreases Lipids Related to Inflammation and Insulin Signaling — A Lipidomics Approach. Plos One. 2009, 4(4), 1–9. DOI: 10.1371/journal.pone.0005258.
- Summers, S. A.;. Progress in Lipid Research Ceramides in Insulin Resistance and Lipotoxicity. Prog. Lipid Res. 2006, 45(1), 42–72. DOI: 10.1016/j.plipres.2005.11.002.
- Hagen, I. V.; Helland, A.; Bratlie, M.; Brokstad, K. A.; Rosenlund, G.; Sveier, H.; Mellgren, G.; Gudbrandsen, O. A. High Intake of Fatty Fish, but Not of Lean Fish, Affects Serum Concentrations of TAG and HDL-cholesterol in Healthy, Normal-weight Adults : A Randomised Trial. Br. J. Nutr. 2016, 116(4), 648–657. DOI: 10.1017/S0007114516002555.
- Alhassan, A.; Young, J.; Lean, M. E. J.; Lara, J. Consumption of Fi Sh and Vascular Risk Factors : A Systematic Review and Meta-analysis of Intervention Studies. Atherosclerosis. 2017, 266, 87–94. DOI: 10.1016/j.atherosclerosis.2017.09.028.
- Manninen, V.; Tenkanen, L.; Koskinen, P.; Huttunen, J. K.; Manttari, M.; Heinonen, O. P.; Frick, M. H. Joint Effects of Serum Triglyceride and LDL Cholesterol and HDL Cholesterol Concentrations on Coronary Heart Disease Risk in the Helsinki Heart Study. Implications for Treatment. Circulation. 1992, 85(1), 37–45. DOI: 10.1161/01.CIR.85.1.37.
- Kim, J.; Koh, K. K.; Quon, M. J. The Union of Vascular and Metabolic Actions of Insulin in Sickness and in Health. Arterioscler Thromb. Vasc. Biol. 2005, 46, 1978–1985.
- Imamura, S.; Morioka, T.; Yamazaki, Y.; Numaguchi, R.; Urata, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shoji, T.; Emoto, M.; et al. Plasma Polyunsaturated Fatty Acid Profile and Delta-5 Desaturase Activity are Altered in Patients with Type 2 Diabetes. Metabolism. 2014, 63(11), 1432–1438.
- Djoussé, L.; Biggs, M. L.; Lemaitre, R. N.; King, I. B.; Song, X.; Ix, J. H.; Mukamal, K. J.; Siscovick, D. S.; Mozaffarian, D. Plasma Omega-3 Fatty Acids and Incident Diabetes in Older Adults. Am. J. Clin. Nutr. 2011, 94(2), 527–533.
- Stephens, F. B.; Mendis, B.; Shannon, C. E.; Cooper, S.; Ortori, C. A.; Barrett, D. A.; Mansell, P.; Tsintzas, K. Fish Oil Omega-3 Fatty Acids Partially Prevent Lipid Induced Insulin Resistance in Human Skeletal Muscle without Limiting Acylcarnitine Accumulation. Clin. Sci. (Lond). 2014, 127(5), 315–322. DOI: 10.1042/CS20140031.
- Bremer, A. A.; Stanhope, K. L.; Graham, J. L.; Cummings, B. P.; Ampah, S. B.; Saville, B. R.; Havel, P. J. Fish Oil Supplementation Ameliorates Fructose- Induced Hypertriglyceridemia and Insulin Resistance in Adult Male Rhesus Macaques. J. Nutr. 2014, 144(1), 5–11. DOI: 10.3945/jn.113.178061.
- Chen, C.; Yu, X.; Shao, S. Effects of Omega-3 Fatty Acid Supplementation on Glucose Control and Lipid Levels in Type 2 Diabetes: A Meta-analysis. PLoS One. 2015, 10(10), 1–14.
- Ouellet, V.; Marois, J.; Weisnagel, S. J.; Jacques, H. Dietary Cod Protein Improves Insulin Sensitivity in Insulin-Resistant Men and Women. Diabetes Care. 2007, 30(11), 2816–2821. DOI: 10.2337/dc07-0273.
- Drotningsvik, A.; Mjøs, S. A.; Pampanin, D. M.; Slizyte, R.; Carvajal, A.; Remman, T.; Høgøy, I.; Gudbrandsen, O. A. Dietary Fish Protein Hydrolysates Containing Bioactive Motifs Affect Serum and Adipose Tissue Fatty Acid Compositions, Serum Lipids, Postprandial Glucose Regulation and Growth in Obese Zucker Fa/fa Rats. Br. J. Nutr. 2016, 116(8), 1336–1345. DOI: 10.1017/S0007114516003548.
- Ryan, J. T.; Ross, R. P.; Bolton, D.; Fitzgerald, G. F.; Stanton, C. Bioactive Peptides from Muscle Sources : Meat and Fish. Nutrients. 2011, 3(9), 765–791. DOI: 10.3390/nu3090765.
- Kim, S. K.; Wijesekara, I. Development and Biological Activities of Marine-derived Bioactive Peptides : A Review. J. Funct. Foods. 2010, 2(1), 1–9. DOI: 10.1016/j.jff.2010.01.003.
- Boukhari, N.; Taleb-Senouci, D.; Chabane, F. Z.; Besbes, M.; Lamri-Senhadji, M. Y. Fish By-products Oil Corrects Dyslipidemia, Improves Reverse Cholesterol Transport and Stimulates Paraoxonase-1 Activity in Obese Rat. Ann. Cardiol. Angeiol. (Paris). 2013, 62(3), 149–154. DOI: 10.1016/j.ancard.2013.04.007.
- Lu, Z.; Chen, T. C.; Zhang, A.; Persons, K. S.; Kohn, N.; Berkowitz, R.; Martinello, S.; Holick, M. F. An Evaluation of the Vitamin D3 Content in Fish: Is the Vitamin D Content Adequate to Satisfy the Dietary Requirement for Vitamin D? J. Steroid. Biochem. Mol. Biol. 2007, 103,(3–5), 642–644.
- Tratado de nutrición; Hernández M., Sastre, A. Ediciones Días de Santos S.A.: Madrid, Spain, 1999.
- Carneiro, E. M.; Latorraca, M. Q.; Araujo, E.; Beltrá, M.; Oliveras, M. J.; Navarro, M.; Berná, G.; Bedoya, F. J.; Velloso, A.; Soria, B.; et al. Taurine Supplementation Modulates Glucose Homeostasis and Islet Function. J. Nutr. Biochem. 2009, 20(7), 503–511.
- Imae, M.; Asano, T.; Murakami, S. Potential Role of Taurine in the Prevention of Diabetes and Metabolic Syndrome. Amino Acids. 2014, 46(1), 81–88. DOI: 10.1007/s00726-012-1434-4.
- Schaffer, S. W.; Jong, C. J.; Ramila, K. C.; Azuma, J. Physiological Roles of Taurine in Heart and Muscle. J. Biomed. Sci. 2010, 17(Suppl 1), 1–8. DOI: 10.1186/1423-0127-17-S1-S2.
- Ribeiro, R. A.; Santos-Silv, J. C.; Vettorazzi, J. F.; Cotrim, B. B.; Mobiolli, D. D. M.; Boschero, A. C.; CArneiro, E. M. Taurine Supplementation Prevents Morpho-physiological Alterations in High- Fat Diet Mice Pancreatic B-cells. Amino Acids. 2012, 43(4), 1791–1801. DOI: 10.1007/s00726-012-1263-5.
- Hansen, S. H.;. The Role of Taurine in Diabetes and the Development of Diabetic Complications. Diabetes Metab. Res. Rev. 2001, 17(5), 330–346. DOI: 10.1002/dmrr.229.
- Gavrovskaya, L. K.; Ryzhova, O. V.; Safonova, A. F.; Matveev, A. K.; Sapronov, N. S. Protective Effect of Taurine on Rats with Experimental Insulin-dependent Diabetes Mellitus. Bull. Exp. Biol. Med. 2008, 146(2), 226–228. DOI: 10.1007/s10517-008-0258-4.
- Brøns, C.; Spohr, C.; Storgaard, H.; Dyerberg, J.; Vaag, A. Effect of Taurine Treatment on Insulin Secretion and Action, and on Serum Lipid Levels in Overweight Men with a Genetic Predisposition for Type II Diabetes Mellitus. Eur. J. Clin. Nutr. 2004, 58(9), 1239–1247. DOI: 10.1038/sj.ejcn.1601955.
- Chauncey, K. B.; Tenner, T. E.; Lombardini, J. B.; Jones, B. G.; Brooks, M. L.; Warner, R. D.; David, R. L.; Ragain, R. M. The Effect of Taurine Supplementation on Patients with Type 2 Diabetes Mellitus. Taur. 5. Adv. Exp. Med. Biol. 2003, 526, 91–96.
- Kaneko, H.; Kobayashi, M.; Mizunoe, Y.; Yoshida, M.; Yasukawa, H.; Hoshino, S.; Itagawa, R.; Furuichi, T.; Okita, N.; Sudo, Y.; et al. Taurine Is an Amino Acid with the Ability to Activate Autophagy in Adipocytes. Amino Acids. 2018, 50(5), 527–535.
- Das, J.; Vasan, V.; Sil, P. C. Taurine Exerts Hypoglycemic Effect in Alloxan-induced Diabetic Rats, Improves Insulin-mediated Glucose Transport Signaling Pathway in Heart and Ameliorates Cardiac Oxidative Stress and Apoptosis. Toxicol. Appl. Pharmacol. 2012, 258(2), 296–308. DOI: 10.1016/j.taap.2011.11.009.
- Figueroa, A. L.; Figueiredo, H.; Rebuffat, S.; Vieira, E.; Gomis, R. Taurine Treatment Modulates Circadian Rhythms in Mice Fed A High Fat Diet. Sci. Rep. 2016, 6(November), 1–13. DOI: 10.1038/srep36801.
- Kim, K. S.; Oh, D. H.; Kim, J. Y.; Lee, B. G.; You, J. S.; Chang, K. J.; Chung, H. J.; Yoo, M. C.; Yang, H. I.; Kang, J. H.; et al. Taurine Ameliorates Hyperglycemia and Dyslipidemia by Reducing Insulin Resistance and Leptin Level in Otsuka Long-Evans Tokushima Fatty (OLETF) Rats with Long-term Diabetes. Exp. Mol. Med. 2012, 44(11), 665–673.
- Miyazaki, T.; Sasaki, S.; Toyoda, A.; Shirai, M.; Ikehami, T.; Matsuzaki, Y.; Honda, A. The Effects of Taurine Depletion on Bile Acid Composition and Its Amino Acid‐conjugation in the Bile of Cats. Faseb J. 2019, 33(S1).
- Nakamura-Yamanaka, Y.; Tsuji, K.; Ichikawa, T. Effect of Dietary Taurine on Cholesterol 7 Alpha-hydroxylase Activity in the Liver of Mice Fed a Lithogenic Diet. J. Nutr. Sci. Vitaminol. (Tokyo). 1987, 33(3), 239–243. DOI: 10.3177/jnsv.33.239.
- Bass, J.; Takahashi, J. S. Circadian Integration of Metabolism and Energetics. Science. 2010, 330(6009), 1349–1354. DOI: 10.1126/science.1195027.
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, Clock Genes, and Chrononutrition. Curr. Nutr. Rep. 2014, 3(3), 204–212. DOI: 10.1007/s13668-014-0082-6.
- Qian, J.; Scheer, F. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trens. Endocrinol. Metab. 2016, 27(5), 282–293. DOI: 10.1016/j.tem.2016.03.005.
- Parsons, M. J.; Lester, K. J.; Barclay, N. L.; Archer, S. N.; Nolan, P. M.; Eley, T. C.; Gregory, A. M. Polymorphisms in the Circadian Expressed Genes PER3 and ARNTL2 are Associated with Diurnal Preference and GNβ3 with Sleep Measures. J. Sleep Res. 2014, 23(5), 595–604. DOI: 10.1111/jsr.12144.
- Laermans, J.; Depoortere, I. Chronobesity: Role of the Circadian System in the Obesity Epidemic. Obes. Rev. 2016, 17(2), 108–125. DOI: 10.1111/obr.12351.
- Elvevoll, E. O.; Eilertsen, K. E.; Brox, J.; Dragnes, B. T.; Falkenberg, P.; Olsen, J. O.; Kirkhus, B.; Lamglait, A.; Osternud, B. Seafood Diets: Hypolipidemic and Antiatherogenic Effects of Taurine and N-3 Fatty Acids. Atherosclerosis. 2008, 200(2), 396–402. DOI: 10.1016/j.atherosclerosis.2007.12.021.
- Mikami, N.; Hosokawa, M.; Miyashita, K. Dietary Combination of Fish Oil and Taurine Decreases Fat Accumulation and Ameliorates Blood Glucose Levels in Type 2 Diabetic/Obese KK-A Y Mice. J. Food Sci. 2012, 77(6), 114–120. DOI: 10.1111/j.1750-3841.2012.02687.x.
- United States Department of Agriculture. Agricultural Research Service; National Nutrient Database for Standard Reference Legacy Release. https://www.usda.gov/(accessed July 24, 2019).
- Gormley, T. R.; Neumann, T.; Fagan, J. D.; Brunton, N. P. Taurine Content of Raw and Processed Fish Fillets/portions. Eur. Food Res. Technol. 2007, 225(5–6), 837–842. DOI: 10.1007/s00217-006-0489-4.
- Connell, J. J.; saff of Torry Research Station. Advances in Fish Science and Technology; Press: United Kingdom, 1980.
- Balfegó, M. Diabetis mellitus tipus 2: Impacte metabòlic duna dieta rica en sardina. Ph.D. Biomedicine thesis, Universitat de Barcelona, Barceona, Spain, 2016.
- Vuković, G.; Romanić, S. H.; Babić, Z.; Mustać, B.; Štrbac, M.; Deljanin, I.; Antanasijević, D. Persistent Organic Pollutants (Pops) in Edible Fish Species from Different Fishing Zones of Croatian Adriatic. Mar. Pollut. Bull. 2018, 137, 71–80. DOI: 10.1016/j.marpolbul.2018.10.014.
- Kljaković-Gašpić, Z.; Romanić, S. H.; Klinčić, D.; Tičina, V. Chlorinated Compounds in the Muscle Tissue of Fish from the Croatian Adriatic: Preliminary Data on Contamination and the Associated Health Risks. Arch. Ind. Hyg. Toxicol. 2015, 66(4), 299–308.
- Bocio, A.; Domingo, J. L.; Falcó, G.; Llobet, J. M. Concentrations of PCDD/PCDFs and PCBs in Fish and Seafood from the Catalan (Spain) Market: Estimated Human Intake. Environ. Int. 2007, 33(2), 170–175. DOI: 10.1016/j.envint.2006.09.005.
- Affane, F.; Benahmed Daidj, N. B.; Louala, S.; Munezero, A. N.; Lamri-Senhadji, M. Y. Effects of an Obesogenic Diet Enriched in Sardine By-products on Pro-atherogenic Markers in Wistar Rats. Ann. Cardiol. Angeiol. 2016, 65(3), 214–218. DOI: 10.1016/j.ancard.2016.04.011.
- Rodrigues, P. O.; Martins, S. V.; Lopes, P. A.; Ramos, C.; Miguéis, S.; Alfaia, C. M.; Pinto, R. M.; Rolo, E. A.; Bispo, P.; Batista, I.; et al. Influence of Feeding Graded Levels of Canned Sardines on the Inflammatory Markers and Tissue Fatty Acid Composition of Wistar Rats. Br. J. Nutr. 2014, 112(3), 309–319.
- Balfegò, M.; Canivell, S.; Hanzu, F. A.; Sala-Vila, A.; Martínez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.; Linares, F.; Porras, N.; et al. Effects of Sardine-enriched Diet on Metabolic Control, Inflammation and Gut Microbiota in Drug-naïve Patients with Type 2 Diabetes: A Pilot Randomized. Lipids Health Dis. 2016, 15(1), DOI: 10.1186/s12944-016-0245-0.
- Moreira, A. C.; Gaspar, A.; Serra, M. A.; Simões, J.; Lopes da Cruz, J.; Amaral, T. F. Effect of a Sardine Supplement on C-reactive Protein in Patients Receiving Hemodialysis. J. Ren. Nutr. 2007, 17(3), 205–213. DOI: 10.1053/j.jrn.2007.02.005.
- Madani, Z.; Louchami, K.; Sener, A.; Malaisse, W. J.; Ait Yahia, D. Dietary Sardine Protein Lowers Insulin Resistance, Leptin and TNF- α and Beneficially Affects Adipose Tissue Oxidative Stress in Rats with Fructose-induced Metabolic Syndrome. Int. J. Mol. Med. 2012, 29(2), 311–318. DOI: 10.3892/ijmm.2011.836.
- Hamza-Reguig, S.; Benahmed Daidj, N. B.; Louala, S.; Boualga, A.; Lamri-Senhadji, M. Effect of Replacing Sardine Oil with Margarine on Dyslipidemia, Dysglycemia and Redox Status of Adipose Tissue in High-fat Diet-induced Obesity in Wistar Rats. Nutr. Food Sci. 2017, 47(1), 2–17. DOI: 10.1108/NFS-04-2016-0041.
- BelHadj, S.; Hentati, O.; Baccouch, N.; Ben Salah, H.; Boudaouara, T.; Ben Hadj, A.; Allouch, N.; El Feki, A. F. Effect of Sardina Pilchardus Oil on Alloxan-induced Diabetic Rats. J. Metab. Dis. 2016, 122(1), 27–35.
- Aguilera, A. A.; Díaz, G. H.; Barcelata, M. L.; Guerrero, O. A.; Ros, R. M. Effects of Fish Oil on Hypertension, Plasma Lipids, and Tumor Necrosis factor-α in Rats with Sucrose-induced Metabolic Syndrome. J. Nutr. Biochem. 2004, 15(6), 350–357. DOI: 10.1016/j.jnutbio.2003.12.008.
- Venturini, D.; Simão, A. N.; Urbano, M. R.; Dichi, I. Effects of Extra Virgin Olive Oil and Fish Oil on Lipid Profile and Oxidative Stress in Patients with Metabolic Syndrome. Nutrition. 2015, 31(6), 834–840. DOI: 10.1016/j.nut.2014.12.016.
- Benaicheta, N.; Labbaci, F. Z.; Bouchenak, M.; Boukortt, F. O. Effect of Sardine Proteins on Hyperglycaemia, Hyperlipidaemia and Lecithin:cholesterol Acyltransferase Activity, in High-fat Diet-induced Type 2 Diabetic Rats. Br. J. Nutr. 2016, 115(1), 6–13. DOI: 10.1017/S0007114515004195.
