ABSTRACT
Ultrasound technology is an emerging food processing technology that helps food processing/preservation shifting away from conventional thermal technologies due to their detrimental effects on food quality, composition, and sensory attributes. This review highlights the applications of ultrasound technology in food processing, and its prospects for eliminating microbial spores, important agents of food spoilage and foodborne intoxication/infection. Ultrasound (sound waves between 20–100 kHz and 2–10 MHz), when applied to foods, inhibits the proliferation of micro-organisms, extends shelf-life, and sustain food quality/sensory attributes. The generated sound waves induce intermolecular forces within the food medium and microbial cells, leading to cell wall rupture and cell lysis, and consequently inactivation. It has found application in the processing of various food items, including beef muscles, juices, oils, etc. The mode of microbial or bacterial spores’ inactivation includes cell wall and membrane disruption, inhibiting enzymatic activities and damaging DNA materials. As a tool for eliminating spores in foods, the technology is commonly used in combination with other treatments such as high temperature (thermosonication), high pressure (manosonication), and UV radiations. These combinations have been shown to improve foods’ physicochemical attributes while increasing spore permeability and sensitivity to other treatments, although through unclear mechanisms.
KEYWORDS:
Overview
In recent times, consumers demand minimally processed food, and changing food regulations have propelled several novel technologies with extensive food processing and production applications. Ultrasound technology is among the safe and fast-evolving green technologies used in the food industry with multiple applications. It is an advanced non-thermal food-processing technology that has attracted significant interest as an alternative or adjuvant method to conventional processing techniques.[Citation1–3] This method employs ultrasonic sound waves with frequencies above the threshold of human audibility. Typical frequency limit used in ultrasound technology ranges between 20 kHz and 500 MHz. Its application in food processing includes freezing/crystallization, homogenization, extraction, drying, sterilization, degassing, filtration, defoaming, emulsification, and preservation. The technology can be categorized as power ultrasound or high-intensity ultrasound with low frequency (20–100 kHz) and high-frequency ultrasound or low-intensity ultrasound with frequencies ranging between 2–10 MHz.[Citation4] The growing quest in the food industry to maintain the nutritional value and sensory characteristics of food products and at the same time ensure food safety by destroying spore-forming microbes has shifted attention to non-thermal novel food processing techniques. This is because the use of thermal processes such as high-temperature and short-time (HTST), low-temperature long-time (LTLT), and ultra-high temperature (UHT) denatures protein components, affect nutritional values and impact adversely on sensory attributes of foods.[Citation5] When pores-forming microbes adhere to food surfaces under harsh environmental conditions, they form spores that survive treatments that the foods receive and can potentially shorten food shelf-life and safety upon spore germination, resulting in foodborne illness when such food products are consumed. The bacteria endospore comprises protective layers such as coat, cortex and inner membrane, making them resistant to certain heat and radiation processing methods.[Citation6] This novel ultrasound technology offers a means to selectively inactivate spores without adversely impacting the food nutritional and sensory characteristics. Furthermore, ultrasonication can enhance spores’ detachment, thereby decreasing their ability to adhere to foods’ surface. This review highlights the emerging application of ultrasound treatment in food processing for the elimination of the vegetative and spore forms of microorganisms to elongate shelf-life and safety of foods.
Ultrasound technology
In the past, high-frequency ultrasound was used mainly for quality assessment and for physicochemical characterization of food.[Citation7] However, recent developments of the technology extend across various processing technologies, production, and preservation. A study on the use of ultrasound technology against foodborne pathogenic such as Escherichia coli O157:H7 and Listeria monocytogenes in almond milk suggested that the ultrasound treatment could exert a sub-lethal injury on the pathogens, as evidenced by the extended lag phase in L. monocytogenes and growth reduction of E. coli O157:H7.[Citation8] In 2019, decontamination of black pepper grains and tapioca starch inoculated with Bacillus subtilis vegetative cells and spores using ultrasonic technology showed 2.19 log CFU/g and 2.01 log CFU/g reductions in vegetative cell count after 30 min of treatment. However, the results showed no significant reduction in spore count after only ultrasound treatments were applied.[Citation9] Hence, studies involving a combination of ultrasound and other techniques such as ultrasonication with thermal treatment (i.e., thermosonication) and pressure (i.e., manosonication), have demonstrated synergistic effects with enhanced microbial inactivation. In the work of Lv et al.,[Citation10] they observed a significant sporicidal effect on Bacillus cereus spores following manothermosonication treatment. Whilst, minor sporicidal effects were observed for Bacillus cereus spores exposed to ultrasound alone, the combination with acidic electrolyzed water produced a synergistic effect, resulting in remarkable inactivation. The analysis of the spores’ structure revealed that ultrasound hydrolyzed the cortex while acidic electrolyzed water damaged cellular inner membrane integrity.[Citation11]
Furthermore, ultrasound treatment in the presence of mild heat was employed in the inactivation of thermoacidophilic B. subtilis suspended in Chinese bayberry juice, it was found using flow cytometric analysis that the combination compromised the cell membrane integrity, inactivated esterase activity, and destroyed nucleic acids.[Citation10] Therefore, it is clear that thermosonication damage the inner membrane proteins of Bacillus subtilis spores, resulting in altered plasma membrane permeability, leakage of intracellular substances, and a spore.[Citation12,Citation13] Palanisamy et al.,[Citation14] investigated the inactivation of thermophilic bacilli (Geobacillus spp. and Anoxybacillus flavithermus) vegetative cell and spores by low-frequency ultrasound. The results revealed a significant reduction in the vegetative cell count with extensive external and internal damage. However, when combined with sodium hydroxide and hydrogen peroxide, a synergistic sporicidal effect was observed. These studies have suggested that ultrasound combined with other preservation techniques promotes microbial inactivation of both vegetative cells and spores. . shows the extensive applications of ultrasound technology in combination with other methods in food processing.
Table 1. Other applications of ultrasound in industrial processing.
The combination of ultrasound with these technologies enhances its efficacy in food processing and preservation. In light of this, Fan et al.[Citation56] reported the study of the combined inhibitory effect of ultrasound and antimicrobial peptides such as nisin/carvacrol (0.01%, 0.02% (w/v)) on Bacillus subtilis spores germination, outgrowth, and subsequent growth of vegetative cells in laboratory medium and milk at 20 kHz frequency, temperature 23°C and power 100 W. It was found that in the laboratory medium, the synergistic effect of the ultrasound pre-treatment (3.33 W/mL, 15 min) and nisin/carvacrol (0.01%, 0.02%) inhibited Bacillus subtilis spores’ germination and outgrowth. Furthermore, the combined effect of ultrasound and surfactants (0.03–0.3%) on Bacillus cereus (10876, ATCC 13061, and W-1) spores on lettuce and carrots at a frequency of 40 kHz, power 30 W/L and duration 5 min has been reported by Sagong et al.[Citation57] While ultrasound treatment alone produced less than 0.7 log reduction, the combined effect of both ultrasound and surfactant increased the effectiveness as a function of the hydrophile-lipophile balance (HLB), resulting in a decrease of 2.49 and 2.22 log CFU/g on lettuce and carrots, respectively, with little or no deterioration of quality. It was found that an acoustic wave and pressure in air with frequencies ranging from ~50 kHz to 10 MHz without contact with the food could inactivate about 99.9% of bacterial spores such as those of Bacillus thuringiensis.[Citation58]
Mechanism of microbial inactivation
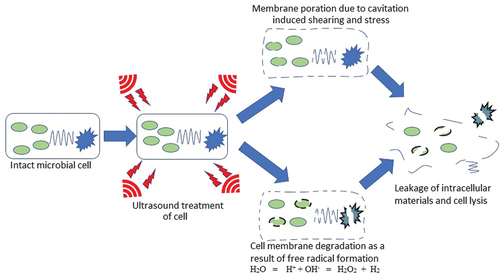
It is well known that spores are dormant and structurally complex compared with vegetative cells. Unlike vegetative cells, the protective layers provided by the spores’ coat, cortex, and inner membrane features give them additional protection against unfavourable conditions such as heat, radiation, and chemical. As a result, techniques that offer detachment, destruction of spores’ multi-protective sheets and or degradation of protein content and deactivation of enzymatic activities will result in their inactivation. Ultrasound technology is widely employed in the food industries either alone or in combination with other technologies for the inactivation of spoilage and foodborne pathogenic microorganisms.[Citation12,Citation59,Citation60] Though the inactivation mechanism is still a subject of debate, it is believed that the acoustic waves generate cavitations, pressure and friction. However, two categories of acoustic cavitation are generated during ultrasonic processing. The stable cavitation results from the regular oscillation of bubbles for several acoustic cycles, whereas transient cavitation results from irregular oscillations causing rapid alternation of temperature and pressure. Both stable and transient cavitations promote the inactivation of microbial cells through cell damage and microstreaming. Therefore, the physical, mechanical and chemical effects of the generated acoustic cavitation resulted in inactivation of bacteria and deagglomerated bacterial clusters or flocs.[Citation61] These shockwaves generated from cavitation destroy microbial spores’ coat, cell wall and inner membrane, destabilizing structural integrity, resulting in the release of core or nuclear materials.[Citation62] When applied on the bulk of a material or the surface, the waves travel through the material at a speed characterized by the nature of the wave and the bulk material generating alternating compression and expansion cycles of particles. These cycles create a negative pressure that overcomes the medium’s intermolecular forces, leading to the creation of small gas-filled bubbles, or cavities, as shown in .
Effects of acoustic cavitation
Acoustic cavitation mediates microbial inactivation through physical/mechanical effects, thermal effects, and chemicals effects during processing. . illustrates the mechanisms of microbial inactivation by ultrasonic cavitation. The transient cavitation leads to micro jets, localized heating hotspots, and the generation of free radical molecules. On the other hand, stable cavitation generates shear force induced by micro streaming, triggering stress on microbial cells and enzymes.[Citation61] Moreover, the contraction, expansion, and implosion of cavities with alternation in the expansion/compression cycle of the sound waves form the primary antimicrobial mechanism of ultrasound.[Citation63] Depending on the ultrasound frequency, alternations in the positive and negative pressures results in expansion or compression of the material, leading to cell wall rupture and subsequent cell lysis.[Citation64] During this process, the change in the average distance between molecules and decrease in pressure leads to the formation of gas bubbles or vapours which builds and eventually collapses upon transiting to areas of high pressure as shown in , resulting in pressures 1000 atm and high temperatures 80°C.[Citation7] Hence, cavitation causes severe damage to the cell walls, pitting and eroding the cell surface, resulting in microbial inactivation.
Figure 2. Ultrasound Cavitation.[Citation7].
![Figure 2. Ultrasound Cavitation.[Citation7].](/cms/asset/4c396c2f-ee09-4b6c-8679-d0e8f9ad5301/lfri_a_2013255_f0002_b.gif)
Furthermore, the localized extreme temperature generated during cavitation dissociates water molecules into free radicals (H+ and OH−) that mediate DNA damage, disruption of enzymatic activity, liposomal damage, and cell disruption structurally and functional components.[Citation65] It has also been proven that the technology could detach microbial spores from food surfaces without interfering with its quality.[Citation36] The increased detachment of exosporium and decreased hydrophobicity of Bacillus cereus spores were observed with ultrasound treatment including a reduction in the size of microbial spores from 2017.67 to 960.53 nm after 1 min duration at 20 kHz (frequency), 200 W (power) and temperature 25°C.[Citation36]
Effects of ultrasound treatment on Microbial spores
Microbial inactivating effects of ultrasound treatment extend beyond the damage of vegetative microbial cells. It can negatively impact the spores of foodborne bacteria, including Bacillus spp. and Clostridium spp. The use of ultrasound technology, combined with other technologies or as an adjuvant preservation treatment, enhances the deagglomeration of cells and inactivate resistant spores of foodborne spoilage and pathogenic microorganism. In combination with heat, the ultrasound pre-treatment enhanced C. perfringens spores’ thermal inactivation in beef slurry.[Citation2] The technique also improved inactivation of Alicyclobacillus acidoterrestris spores in orange juice when used in synergy with thermal processing technologies.[Citation60] Likewise, the combination of heat with manosonication enhanced the inactivation B. subtilis spore.[Citation12] In contrast, the combination of ultrasound and Tween 20 effectively reduced B. cereus spores’ levels on lettuce and carrots.[Citation59]
Although numerous researchers have reported the spore inactivating effects of ultrasound treatment alone or in combination with other green preservation techniques, the spore inactivation mechanism of ultrasound is still not well elaborated. However, few reports have suggested that the sporicidal effects of ultrasound treatment are evidently mediated by a series of mechanisms, initiated by the detachment of the spore exosporium, followed by core hydration and cortex degradation, damage of spores intracellular structure, coat destruction and cortex damaged. Other reported mechanisms include interference with metabolic enzymes and disruption of protein and nucleic acid synthesis. A study on the mechanisms of ultrasound assisted extraction reported a chain detexturation mechanism involving local erosion, shear forces, sonoporation, fragmentation, capillary effect, and detexturation.[Citation66] Similar mechanisms might mediate the sporicidal activities of ultrasound, leading to an alteration in permeability and spore degradation.
SDS-PAGE results revealed a reduction of protein bands intensity after ultrasound treatment. It was also observed that ultrasound could detach exosporium and decrease spores’ hydrophobicity.[Citation63] Furthermore, visual images analysis showed that thermosonication targeted multiple sites, including coat, cortex, and inner membrane of B. subtilis spores. Flow cytometry also showed physical compromise of the spore’s inner membrane and partially hydrolyzed cortex and damage of key enzymes involved in intermediary metabolism.[Citation64] It was discovered that inactivated spores showed loss of core proteins and altered plasma membrane permeability. This damage to spores’ inner membrane proteins or the inner membrane led to the leakage of intracellular substances and eventually death. It is worth noting that thermosonication did not induce DNA damage in treatment spores but led to the degradation of important proteins and enzymes such as α/β-type small, acid-soluble spore proteins SASPs, damage of germination enzymes such as GRs and cortex fragments transferase were involved in the process of B. subtilis spores inactivation.[Citation65] Furthermore, flow cytometry analysis of B. cereus spores treated with a combination of acidic electrolyzed water and ultrasonic treatment using fluorophores SYTO 9 and propidium iodide (PI) staining revealed that the sound waves generated by ultrasound hydrolyzed the spore cortex, promoted the detachment of exosporium and destroyed the spore structure. When combined with electrolyzed water, a synergistic sporicidal effect on spores was observed, resulting in the release of cell contents and more considerable disruption of coat and cortex.[Citation7] . shows the Transmission Electron Microscopy (TEM) image of the ultrasound treated and untreated E. coli cells. It is clear that the untreated cell has the wall and membrane intact (), while the ultrasound treated cell experienced ruptured cell wall and membrane (), the release of cellular material () and disruption of cell structure ().[Citation67] The rupture wall, membrane, and structure can be attributed to the acoustic wave and cavitation’s impact due to the ultrasound technique. These results demonstrate and confirm the mechanism of vegetable cell and spore’s inactivation during ultrasound processing. This observed disruption of the cell wall and structure in the TEM image allows the penetration of chemical, heat or radiation, resulting in effective inactivation of spores and vegetative cells when combined with other emerging food processing technologies.
Figure 3. TEM photographs of E. coli cells. (a) Untreated bacteria. (b, c, and d) Bacteria treated with ultrasound for 20 min.[Citation67].
![Figure 3. TEM photographs of E. coli cells. (a) Untreated bacteria. (b, c, and d) Bacteria treated with ultrasound for 20 min.[Citation67].](/cms/asset/62c6de1b-8c65-45d7-9ba1-a7c2ce7632b0/lfri_a_2013255_f0003_b.gif)
The adherence of spore-forming microbes and bacteria to food surfaces can shorten food shelf-life and cause safety concerns. Thermal methods of inactivating bacteria, spores and spore-forming microorganisms can cause deteriorations in nutritional and sensory attributes of foods such as proteins denaturation and flavour deterioration. In light of this, non-thermal alternative approaches are being researched on their ability to effectively inactivate bacteria and their spores while preserving nutritional and sensory attributes, ensuring food safety and extending shelf-life. The ultrasound technique has proven useful in detaching bacteria, spores and spore-forming microbes attached to food surfaces without interfering with its quality.[Citation36] Bacteria and other spore-forming microorganisms are sensitive to ultrasound treatment; however, it has been reported that the technique demonstrated a small fraction of effect on highly resistant spores.[Citation56] The shockwaves generated due to acoustic waves, cavitation, pressure and friction during ultrasound processing can significantly destroy bacteria such as Bacillus atrophaeus and Saccharomyces cerevisiae, and other spore-forming microbes’ cell wall, cellular structure and functional materials.[Citation62] Consequently, it has been found that without contact with the food, in the frequency range of ~50 kHz to 10 MHz, the technique can inactivate about 99.9% of bacteria and their spores such as Bacillus thuringiensis.[Citation58]
Furthermore, the technique has demonstrated the ability to inactivate Escherichia coli and Listeria in whole and skimmed milk at power 85 W/cm[Citation2] and temperature 35°C.[Citation68] In 2019, Fan et al.[Citation13] reported the inactivation and prevention of Bacillus subtilis spores’ germination, outgrowth, and subsequent growth of vegetative cells following treatment at 20 kHz frequency, temperature 23°C and power 100 W. In an earlier study, it was reported that the effect of ultrasound alone on Bacillus cereus spores at a frequency of 40 kHz, power 30 W/L and time 5 min, resulted in less than a 0.7 log reduction.[Citation57] This suggests that the combine impact and effect of ultrasound and other technique or solvent will greatly inactivate bacteria, spores and other spore-forming microorganisms attached to food.
Factors affecting microbial inactivation by ultrasound treatment
The appropriateness and effectiveness of microbial inactivation by ultrasound treatment depends on several factors, including treatment conditions, microbial characteristics, and environmental factors. Hence, the treatment Conditions or operational parameters such as time, ultrasonic amplitude, hydrostatic pressure, and temperature are necessary factors that must be optimized for effective results. However, the inactivation of microbial cells increases as the duration of treatment increases. In light of this, the effect of treatment time on ultrasonication of both green and purple cactus pear juice was investigated, for 1 min reduced bacteria counts by 1 and 3 log CFU/mL, whereas treatment for 3 min resulted in a 3–4 log CFU/mL reduction. Further treatment for an extended time of 5 min inactivated E. coli below the detection limit.[Citation61] Also, increasing ultrasonication time of a nonfat milk sample spiked with G. stearothermophilus spores from 1 to 10 min significantly increased (P < .0001) spore inactivation. The authors attributed this remarkable reduction to the significant interaction effect between acoustic wave amplitude and time.[Citation61] On the other hand, the microbial cells and spores’ characteristics have been reported to influence the outcome of ultrasonic treatment, in which microbes with a thicker and soft capsule demonstrated high resistance to deactivation process.[Citation69]
Impact of ultrasound technology on food quality and nutritional composition
As the search for novel technologies in food science continues to advance, the goal of treatments aimed for preservation has broadened to accommodate consumers’ demands, desires, and changes in food regulation. Thus, ideal food treatment should preserve the nutritional, sensory and compositional properties of the food. Therefore, it is required that emerging green technologies fulfil the basic preservative needs without impacting the food product’s inherent properties. Hence, the effects of ultrasound treatment and other evolving technologies on the nutritional, sensory, and compositional food properties are attracting attention from food scientist, technologist, and industries. Researchers are relentlessly investigating these technologies’ impact on food products to enable them to adopt the most suitable and recommending same to the food industries. The use of ultrasound technology in food processing for the inactivation of food microorganisms has demonstrated minimal impact on food products, including proteins, enzymes, and bioactive polyphenols. It had been discovered that the treatment of olive paste with low-frequency, high-power ultrasound increased the yield of extractable but did not impact the quality of the oil.[Citation70] Instead, ultrasound was shown to improve food quality, such as tenderness and water holding capacity of beef muscles.[Citation71]
Nonetheless, research results suggest that parameters such as frequency, intensity, and treatment time might be necessary considerations that should be optimized to suit the food type. Analogously, bayberry juice exposure to ultrasound treatment at a lower intensity and short time did not impair antioxidant compounds and activity. At increased ultrasonic intensity and extended treatment time, the antioxidant activity was progressively decreased.[Citation72] In 2019, Sun et al.[Citation73] reported that at a low ultrasonic intensity and short treatment time, β‐d ‐glucosidase was activated, whereas at high ultrasonic intensity and long treatment time, the enzyme was inhibited. In another work, the quality parameters, antioxidant activity, and β-carotene content of apple-carrot juice blend were reported unaltered after ultrasound treatment.[Citation74]
Similarly, the application of ultrasound processing technique on cape gooseberry juice increased the availability of carotenoids, total phenols, and retinol activity equivalent, but reduced the chromaticity, yellowing index (IY), and ascorbic acid content of the juice.[Citation75] Another study involving the combined effect of ultrasound and ultraviolet treatment on mango juice was also discovered to have preserved the physicochemical features stability, colour, total polyphenol content, carotenoids, reducing sugar, protein content and antioxidant activity.[Citation76] In contrast, treatment of mango juice with ultrasound radiation (10 min, 600 W) exerted a positive effect on nutritional value and quality parameters. Furthermore, the treatment inactivated polyphenol oxidation enzyme, peroxidase, and pectin methylesterase.[Citation76] SDS-PAGE revealed the absence of changes in protein bands of egg yolk after high-intensity ultrasonic treatment; however, particles size analysis of egg yolk components in solution demonstrated that high-intensity ultrasonic treatment caused the aggregation of low-density lipoprotein and partial dissociation of yolk granules.[Citation77] These studies show the minimal adverse impact of the ultrasound processing technique on food quality, especially inherent nutritional and sensory attributes, while inactivating vegetative cells and spores in food.
Advantages of ultrasound technology in food processing and preservation
The use of ultrasound technology is fast expanding in various fields, including medicine, pharmaceuticals, environmental, food science, and technology. In food science and processing, ultrasound technology applications provide unmatched solutions to previously unresolved industrial challenges due to its numerous advantages over conventional technologies. Assessing the benefits of ultrasound technology in food processing and production, however, depends on specific applications. The use of ultrasound technology as a non-thermal food processing technique and effective inactivation of food microorganisms has been found to preserve liable heat components, including proteins, lipids, and enzymes. The technique also facilitates solvents’ penetration, resulting in an improved extraction yield without altering heat-sensitive compounds.[Citation78]
Additionally, ultrasonic treatments are safe, non-time consuming and do not constitute a risk to food scientist or industrialist. It is also a flexible technique, adaptable and can be combined with other techniques such as thermal and pressure treatments and chemical treatments without interference. Conversely, in fermentation processes, the integration of ultrasound technique produced a superior performance compared to conventional fermentation processes by enhancing microbial growth rate, enzymatic activities, and mass transfer processes.[Citation79] Moreover, it has been demonstrated that the technology enhances the thawing rate and improve the quality of food, reduce drying time and energy consumption, shorten the freezing time, and improve the quality of frozen food, reduce the cooking time with increased food quality, improve emulsion stability, and reduce the usage of cleaning chemicals thus prolong the service life of the filtration membrane. With this technique, on-line measurements can be performed and are useful for monitoring food processing operations. In terms of economics, low-intensity ultrasound is affordable and offer energy saving with practical uses in the food industry. Unlike most conventional food processing methods, green technologies such as ultrasound, when employed singularly or in combinations like manosonication and thermosonication conserve time, energy, solvent and water consuming.[Citation1] Ultrasonic extraction processes reduce energy consumption, allow the use of alternative solvents and renewable natural products, and ensure a safe and high-quality product while ensuring optimum recovery of components such as proteins and lipids.[Citation1,Citation15] When conventional extraction methods proof incompatible due to solvent suitability and polarity, technologies such as microwave, ultrasound, subcritical and supercritical fluid extraction, along with other enabling technologies, can provide the efficient extraction with low solvent and energy consumption.[Citation80,Citation81]
Challenges to ultrasound technology in food processing
Irrespective of the numerous advantages of ultrasound technology, applications of this technology in food industries remain limited due to various challenges. The lack of easily accessible scale-up technique and lack of proper technical knowledge are basic drawbacks. The absence of standardized reporting also hinders techno-economic assessment before industrial application. Moreover, the activated ultrasound zone is restricted to a limited zone within the ultrasound emitter vicinity. Furthermore, it is believed that the attenuation of ultrasound by molecules and bubbles prevents the propagation of an acoustic wave through the sample. The technique might also impact the texture and nature of a product. For instance, ultrasound power density can decrease the texture of peeled fruit and activate the degradative pectin methylesterase and polygalacturonase enzymes in the tissue resulting in softened texture during peeling process.[Citation82] Continued usage of high-intensity ultrasound will depend on affordable instrumentation availability with proven significant advantages over alternative technologies.[Citation83]
Conclusion and Future Directives
The application of ultrasound technology in food processing and industries provides a novel solution to problems encountered in the food sector. A significant advantage of this technology is its diverse range of potential applications for food processing, and it can be used together with other technologies. The use of ultrasound technology for the inactivation of spoilage and foodborne pathogenic microorganisms remains unmatched in its ability to preserve food organoleptic, sensory, nutritional, and compositional properties. Furthermore, the technology has shown potential for use in the inactivation of microbial spores without subjecting the food to harsh, destructive treatments, negatively affecting food quality. Compared with other non-thermal processes, ultrasonic processing is a relatively cheap technology. Future applications of ultrasound technology will explore fusion with other non-thermal processes for enhanced and effective results.
Author contribution
All authors were involved in the preparation and writing of the manuscript. Conceptualization: HO and OFN, Initial draft: HO. OFN and CA, Revising and review: TM and AH, References: CA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Chemat, F.; Vian, M. A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A. R.; Munekata, P. E.; Lorenzo, J. M.; Barba, F. J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22(8), pp. 2325–2353.
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and Sono-chemical Effects of Ultrasound: Primary Applications in Extraction and Freezing Operations and Influence on Food Components. Ultrason. Sonochem. 2020, 60, p. 104726.
- Misra, N.; Martynenko, A.; Chemat, F.; Paniwnyk, L.; Barba, F. J.; Jambrak, A. R. Thermodynamics, Transport Phenomena, and Electrochemistry of External Field-assisted Nonthermal Food Technologies. Crit. Rev. Food Sci. Nutr. 2018, 58(11), pp. 1832–1863.
- Herrero, A.; Romero, D. A. M. Innovations in Food Processing: Nonthermal Methods. Revista de medicina de la Universidad de Navarra 2006, 50(4), pp. 71–74.
- Yu, T. Y.; Morton, J. D.; Clerens, S.; Dyer, J. M. Cooking‐induced Protein Modifications in Meat. Compr. Rev. Food. Sci. Food Saf. 2017, 16(1), pp. 141–159.
- Laue, M.; Han, H.-M.; Dittmann, C.; Setlow, P. Intracellular Membranes of Bacterial Endospores are Reservoirs for Spore Core Membrane Expansion during Spore Germination. Sci. Rep. 2018, 8(1), pp. 1–12.
- Soria, A. C.; Villamiel, M. Effect of Ultrasound on the Technological Properties and Bioactivity of Food: A Review. Trends Food Sci. Technol. 2010, 21(7), pp. 323–331.
- Iorio, M. C.; Bevilacqua, A.; Corbo, M. R.; Campaniello, D.; Sinigaglia, M.; Altieri, C. A Case Study on the Use of Ultrasound for the Inhibition of Escherichia Coli O157: H7 and Listeria Monocytogenes in Almond Milk. Ultrason. Sonochem. 2019, 52, pp. 477–483.
- Charoux, C. M.; O’Donnell, C. P.; Tiwari, B. K. Effect of Airborne Ultrasonic Technology on Microbial Inactivation and Quality of Dried Food Ingredients. Ultrason. Sonochem. 2019, 56, pp. 313–317.
- Lv, R.; Zou, M.; Chantapakul, T.; Chen, W.; Muhammad, A. I.; Zhou, J.; Ding, T.; Ye, X.; Liu, D. Effect of Ultrasonication and Thermal and Pressure Treatments, Individually and Combined, on Inactivation of Bacillus Cereus Spores. Appl. Microbiol. Biotechnol. 2019, 103(5), pp. 2329–2338.
- Lv, R.; Muhammad, A. I.; Zou, M.; Yu, Y.; Fan, L.; Zhou, J.; Ding, T.; Ye, X.; Guo, M.; Liu, D. Hurdle Enhancement of Acidic Electrolyzed Water Antimicrobial Efficacy on Bacillus Cereus Spores Using Ultrasonication. Appl. Microbiol. Biotechnol. 103, 2020, pp. 4505–4513.
- Fan, L.; Hou, F.; Muhammad, A. I.; Ruiling, L.; Watharkar, R. B.; Guo, M.; Ding, T.; Liu, D. Synergistic Inactivation and Mechanism of Thermal and Ultrasound Treatments against Bacillus Subtilis Spores. Food Res. Int. 2019, 116, pp. 1094–1102.
- Fan, L.; Ismail, B. B.; Hou, F.; Muhammad, A. I.; Zou, M.; Ding, T.; Liu, D. Thermosonication Damages the Inner Membrane of Bacillus Subtilis Spores and Impels Their Inactivation. Food Res. Int. 2019, 125, p. 108514.
- Palanisamy, N.; Seale, B.; Turner, A.; Hemar, Y. Low Frequency Ultrasound Inactivation of Thermophilic Bacilli (Geobacillus Spp. And Anoxybacillus Flavithermus) in the Presence of Sodium Hydroxide and Hydrogen Peroxide. Ultrason. Sonochem. 2019, 51, pp. 325–331.
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A. S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, pp. 248–263.
- Panadare, D. C.; Gondaliya, A.; Rathod, V. K. Comparative Study of Ultrasonic Pretreatment and Ultrasound Assisted Three Phase Partitioning for Extraction of Custard Apple Seed Oil. Ultrason. Sonochem. 2020, 61, p. 104821.
- Sharma, P.; Wichaphon, J.; Klangpetch, W. Antimicrobial and Antioxidant Activities of Defatted Moringa Oleifera Seed Meal Extract Obtained by Ultrasound-assisted Extraction and Application as a Natural Antimicrobial Coating for Raw Chicken Sausages. Int. J. Food Microbiol. 2020, 332, p. 108770.
- Stevanato, N.; Da Silva, C. Radish Seed Oil: Ultrasound-assisted Extraction Using Ethanol as Solvent and Assessment of Its Potential for Ester Production. Ind. Crops Prod. 2019, 132, pp. 283–291.
- Vernes, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of Ultrasound for Green Extraction of Proteins from Spirulina. Mechanism, Optimization, Modeling, and Industrial Prospects. Ultrason. Sonochem. 2019, 54, pp. 48–60.
- Ma, X.; Zhang, L.; Wang, W.; Zou, M.; Ding, T.; Ye, X.; Liu, D. Synergistic Effect and Mechanisms of Combining Ultrasound and Pectinase on Pectin Hydrolysis. Food Bioprocess. Technol. 2016, 9(7), pp. 1249–1257.
- Shabana, S.; Prasansha, R.; Kalinina, I.; Potoroko, I.; Bagale, U.; Shirish, S. Ultrasound Assisted Acid Hydrolyzed Structure Modification and Loading of Antioxidants on Potato Starch Nanoparticles. Ultrason. Sonochem. 2019, 51, pp. 444–450.
- Wang, D.; Hou, F.; Ma, X.; Chen, W.; Yan, L.; Ding, T.; Ye, X.; Liu, D. Study on the Mechanism of Ultrasound-accelerated Enzymatic Hydrolysis of Starch: Analysis of Ultrasound Effect on Different Objects. Int. J. Biol. Macromol. 2020, 148, pp. 493–500.
- Zhang, Y.; Li, T.; Shen, Y.; Wang, L.; Zhang, H.; Qian, H.; Qi, X. Extrusion Followed by Ultrasound as a Chemical-free Pretreatment Method to Enhance Enzymatic Hydrolysis of Rice Hull for Fermentable Sugars Production. Ind. Crops Prod. 2020, 149, p. 112356.
- Leong, T. S. H.; Manickam, S.; Martin, G. J.; Li, W.; Ashokkumar, M. Ultrasonic Production of Nano-emulsions for Bioactive Delivery in Drug and Food Applications; Cham: Springer, 2018.
- Ma, X.; Yan, T.; Hou, F.; Chen, W.; Miao, S.; Liu, D. Formation of Soy Protein Isolate (Spi)-citrus Pectin (CP) Electrostatic Complexes under a High-intensity Ultrasonic Field: Linking the Enhanced Emulsifying Properties to Physicochemical and Structural Properties. Ultrason. Sonochem. 2019, 59, p. 104748.
- Meirelles, A. A. D.; Costa, A. L. R.; Cunha, R. L. The Stabilizing Effect of Cellulose Crystals in O/W Emulsions Obtained by Ultrasound Process. Food Res. Int. 2020, 128, p. 108746.
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan Nanoemulsions by Ultrasound-mediated Emulsification: Formulation, Characterization and Antimicrobial Activity. Carbohydr. Polym. 2018, 193, pp. 144–152.
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of Frequency Ultrasound on the Properties of Zein-chitosan Complex Coacervation for Resveratrol Encapsulation. Food Chem. 2019, 279, pp. 223–230.
- Shahgholian, N.; Rajabzadeh, G. Preparation of BSA Nanoparticles and Its Binary Compounds via Ultrasonic Piezoelectric Oscillator for Curcumin Encapsulation. J. Drug Delivery Sci. Technol. 2019, 54, p. 101323.
- Taksima, T.; Limpawattana, M.; Klaypradit, W. Astaxanthin Encapsulated in Beads Using Ultrasonic Atomizer and Application in Yogurt as Evaluated by Consumer Sensory Profile. LWT-Food Sci. Technol. 2015, 62(1), pp. 431–437.
- Wang, W.; Feng, Y.; Chen, W.; Wang, Y.; Wilder, G.; Liu, D.; Yin, Y. Ultrasonic Modification of Pectin for Enhanced 2‐furfurylthiol Encapsulation: Process Optimization and Mechanisms. J. Sci. Food Agric. 2020, 100(1), pp. 110–118.
- Tao, Y.; Wu, Y.; Han, Y.; Chemat, F.; Li, D.; Show, P. L. Insight into Mass Transfer during Ultrasound-enhanced Adsorption/desorption of Blueberry Anthocyanins on Macroporous Resins by Numerical Simulation considering Ultrasonic Influence on Resin Properties. Chem. Eng. J. 2020, 380, p. 122530.
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea Lotus L. Using Ultrasonic-assisted Extraction and Macroporous Resin Adsorption. Antioxidants. 2020, 9(7), p. 576.
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Ultrasound Assisted Purification of Polyphenols of Apple Skins by Adsorption/desorption Procedure. Ultrason. Sonochem. 2019, 55, pp. 18–24.
- Wu, Y.; Han, Y.; Tao, Y.; Fan, S.; Chu, D.-T.; Ye, X.; Ye, M.; Xie, G. Ultrasound Assisted Adsorption and Desorption of Blueberry Anthocyanins Using Macroporous Resins. Ultrason. Sonochem. 2018, 48, pp. 311–320.
- Lv, R.; Zou, M.; Chen, W.; Zhou, J.; Ding, T.; Ye, X.; Liu, D. Ultrasound: Enhance the Detachment of Exosporium and Decrease the Hydrophobicity of Bacillus Cereus Spores. LWT. 2019, 116, p. 108473.
- Lva, R.; Liub, D.; Lua, X. Hurdle Enhancement of Antimicrobial Efficacy of Acidic Electrolyzed Water on Bacillus Cereus Spores Using Ultrasonication. Ultrason. Sonochem. 2019, 38, pp. 711–719.
- Zhang, Y.; Li, Y.; Wang, H.; Oladejo, A. O.; Zhang, H.; Liu, X. Effects of Ultrasound-assisted Freezing on the Water Migration of Dough and the Structural Characteristics of Gluten Components. J. Cereal Sci. 2020, 94, p. 102893.
- Zhu, Z.; Sun, D.-W.; Zhang, Z.; Li, Y.; Cheng, L. Effects of Micro-nano Bubbles on the Nucleation and Crystal Growth of Sucrose and Maltodextrin Solutions during Ultrasound-assisted Freezing Process. LWT. 2018, 92, pp. 404–411.
- Merone, D.; Colucci, D.; Fissore, D.; Sanjuan, N.; Carcel, J. Energy and Environmental Analysis of Ultrasound-assisted Atmospheric Freeze-drying of Food. J. Food Eng. 2020, 283, p. 110031.
- Tian, Y.; Chen, Z.; Zhu, Z.; Sun, D.-W. Effects of Tissue Pre-degassing Followed by Ultrasound-assisted Freezing on Freezing Efficiency and Quality Attributes of Radishes. Ultrason. Sonochem. 2020, 67, p. 105162.
- Iyer, S. R.; Gogate, P. R. Ultrasound Assisted Crystallization of Mefenamic Acid: Effect of Operating Parameters and Comparison with Conventional Approach. Ultrason. Sonochem. 2017, 34, pp. 896–903.
- Mohod, A. V.; Gogate, P. R. Improved Crystallization of Ammonium Sulphate Using Ultrasound Assisted Approach with Comparison with the Conventional Approach. Ultrason. Sonochem. 2018, 41, pp. 310–318.
- Prasad, R.; Dalvi, S. V. Sonocrystallization: Monitoring and Controlling Crystallization Using Ultrasound. Chem. Eng. Sci. 2020, 226, p. 115911.
- Sharma, A.; Gogate, P. R. Improvements in Crystallization of Mefenamic Acid Using Ultrasonic Bath Operating at Two Frequencies. Chem Eng Processing-Process Intensif. 2020, 147, p. 107768.
- Da Silva, L. F. R.; Gomes, A. D. S.; Castro, D. R. G.; Souza, F. D. C. D. A.; Mar, J. M.; Da Silva, L. S.; Sanches, E. A.; Bezerra, J. D. A.; Bakry, A. M.; Campelo, P. H. Ultrasound‐assisted Homogenization and Gum Arabic Combined to Physicochemical Quality of Cupuaçu Juice. J. Food Process. Preserv. 2019, 43(9), p. e14072.
- Monteiro, S. H.; Silva, E. K.; Guimarães, J. T.; Freitas, M. Q.; Meireles, M. A. A.; Cruz, A. G. High-intensity Ultrasound Energy Density: How Different Modes of Application Influence the Quality Parameters of a Dairy Beverage. Ultrason. Sonochem. 2020, 63, p. 104928.
- Plazzotta, S.; Manzocco, L. Effect of Ultrasounds and High Pressure Homogenization on the Extraction of Antioxidant Polyphenols from Lettuce Waste. Innovative Food Sci. Emerg. Technol. 2018, 50, pp. 11–19.
- Vélez-Erazo, E. M.; Consoli, L.; Hubinger, M. D. Spray Drying of Mono-and Double-layer Emulsions of PUFA-rich Vegetable Oil Homogenized by Ultrasound. Dry. Technol. 2021, 39(7), pp. 868–881.
- Ghanbarian, D.; Torki-Harchegani, M.; Sadeghi, M.; Pirbalouti, A. G. Ultrasonically Improved Convective Drying of Peppermint Leaves: Influence on the Process Time and Energetic Indices. Renewable Energy. 2020, 153, pp. 67–73.
- Mello, R. E.; Fontana, A.; Mulet, A.; Correa, J. L.; Cárcel, J. A., G. Ultrasound-assisted Drying of Orange Peel in Atmospheric Freeze-dryer and Convective Dryer Operated at Moderate Temperature. Dry. Technol. 2020, 38(1–2), pp. 259–267.
- Milani, E. A.; Silva, F. V. Ultrasound Assisted Thermal Pasteurization of Beers with Different Alcohol Levels: Inactivation of Saccharomyces Cerevisiae Ascospores. J. Food Eng. 2017, 198, pp. 45–53.
- Pan, Y.; Zhang, Y.; Cheng, J.-H.; Sun, D.-W. Inactivation of Listeria Monocytogenes at Various Growth Temperatures by Ultrasound Pretreatment and Cold Plasma. LWT. 2020, 118, p. 108635.
- Paniagua-Martínez, I.; Mulet, A.; García-Alvarado, M.; Benedito, J. Orange Juice Processing Using a Continuous Flow Ultrasound-assisted Supercritical CO2 System: Microbiota Inactivation and Product Quality. Innovative Food Sci. Emerg. Technol. 2018, 47, pp. 362–370.
- Scudino, H.; Silva, E. K.; Gomes, A.; Guimarães, J. T.; Cunha, R. L.; Sant’Ana, A. S.; Meireles, M. A. A.; Cruz, A. G. Ultrasound Stabilization of Raw Milk: Microbial and Enzymatic Inactivation, Physicochemical Properties and Kinetic Stability. Ultrason. Sonochem. 2020, 67, p. 105185.
- Fan, L.; Ismail, B. B.; Hou, F.; Muhammad, A. I.; Ding, T.; Liu, D. Ultrasound Pretreatment Enhances the Inhibitory Effects of Nisin/carvacrol against Germination, Outgrowth and Vegetative Growth of Spores of Bacillus Subtilis ATCC6633 in Laboratory Medium and Milk: Population and Single-cell Analysis. Int. J. Food Microbiol. 2019, 311, p. 108329.
- Sagong, H.-G.; Cheon, H.-L.; Kim, S.-O.; Lee, S.-Y.; Park, K.-H.; Chung, M.-S.; Choi, Y.-J.; Kang, D.-H. Combined Effects of Ultrasound and Surfactants to Reduce Bacillus Cereus Spores on Lettuce and Carrots. Int. J. Food Microbiol. 2013, 160(3), pp. 367–372.
- Hoover, K.; Bhardwaj, M.; Ostiguy, N.; Thompson, O. Destruction of Bacterial Spores by Phenomenally High Efficiency Non-contact Ultrasonic Transducers. Mater. Res. Innovations. 2002, 6(5), pp. 291–295.
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of Combination Treatments of Lysozyme and High Power Ultrasound on the Salmonella Typhimurium Inactivation and Quality of Liquid Whole Egg. Ultrason. Sonochem. 2020, 60, p. 104763.
- Tremarin, A.; Brandão, T. R.; Silva, C. L. Application of Ultraviolet Radiation and Ultrasound Treatments for Alicyclobacillus Acidoterrestris Spores Inactivation in Apple Juice. Lwt. 2017, 78, pp. 138–142.
- Joyce, E.; Phull, S.; Lorimer, J.; Mason, T. The Development and Evaluation of Ultrasound for the Treatment of Bacterial Suspensions. A Study of Frequency, Power and Sonication Time on Cultured Bacillus Species. Ultrason. Sonochem. 2003, 10(6), pp. 315–318.
- Ganesan, B.; Martini, S.; Solorio, J.; Walsh, M. K. Determining the Effects of High Intensity Ultrasound on the Reduction of Microbes in Milk and Orange Juice Using Response Surface Methodology. Int. J. Food Sci. 2015, p. 1–7.
- Ngadi, M. O.; Latheef, M. B.; Kassama, L. Emerging Technologies for Microbial Control in Food Processing. In Green Technologies in Food Production and Processing eds. Boye, Joyce, and Arcand, Yves; Boston, MA: Springer, 2012; pp. 363–411.
- Majid, I.; Nayik, G. A.; Nanda, V. Ultrasonication and Food Technology: A Review. Cogent Food Agric. 2015, 1(1), p. 1071022.
- Gabriel, A. A. Inactivation Behaviors of Foodborne Microorganisms in Multi-frequency Power Ultrasound-treated Orange Juice. Food Control. 2014, 46, pp. 189–196.
- Khadhraoui, B.; Turk, M.; Fabiano-Tixier, A.; Petitcolas, E.; Robinet, P.; Imbert, R.; El Maâtaoui, M.; Chemat, F. Histo-cytochemistry and Scanning Electron Microscopy for Studying Spatial and Temporal Extraction of Metabolites Induced by Ultrasound. Towards Chain Detexturation Mechanism. Ultrason. Sonochem. 2018, 42, pp. 482–492.
- Li, J.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Evaluation of Ultrasound-induced Damage to Escherichia Coli and Staphylococcus Aureus by Flow Cytometry and Transmission Electron Microscopy. Appl. Environ. Microbiol. 2016, 82(6), pp. 1828–1837.
- Gera, N.; Doores, S. Kinetics and Mechanism of Bacterial Inactivation by Ultrasound Waves and Sonoprotective Effect of Milk Components. J. Food Sci. 2011, 76(2), pp. M111–M119.
- Qiu, L.; Zhang, M.; Chitrakar, B.; Bhandari, B. Application of Power Ultrasound in Freezing and Thawing Processes: Effect on Process Efficiency and Product Quality. Ultrason. Sonochem. 2020, 68, p. 105230.
- Servili, M.; Veneziani, G.; Taticchi, A.; Romaniello, R.; Tamborrino, A.; Leone, A. Low-frequency, High-power Ultrasound Treatment at Different Pressures for Olive Paste: Effects on Olive Oil Yield and Quality. Ultrason. Sonochem. 2019, 59, p. 104747.
- Gonzalez-Gonzalez, L.; Alarcon-Rojo, A. D.; Carrillo-Lopez, L. M.; Garcia-Galicia, I. A.; Huerta-Jimenez, M.; Paniwnyk, L. Does Ultrasound Equally Improve the Quality of Beef? an Insight into Longissimus Lumborum, Infraspinatus and Cleidooccipitalis. Meat Sci. 2020, 160, p. 107963.
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. Effects of Ultrasound Processing on Physicochemical Parameters, Antioxidants, and Color Quality of Bayberry Juice. J. Food Qual. 2019, p. 1–12.
- Sun, Y.; Zeng, L.; Xue, Y.; Yang, T.; Cheng, Z.; Sun, P. Effects of Power Ultrasound on the Activity and Structure of β‐D‐glucosidase with Potentially Aroma‐enhancing Capability. Food Sci. Nutr. 2019, 7(6), pp. 2043–2049.
- Jingfei, G.; Vasantha, H. P.; Nutritional, R. Physicochemical and Microbial Quality of Ultrasound-treated Apple-carrot Juice Blends. Food Nutr. Sci. 3 2012, p. 212–218.
- Ordóñez-Santos, L. E.; Martínez-Girón, J.; Arias-Jaramillo, M. E. Effect of Ultrasound Treatment on Visual Color, Vitamin C, Total Phenols, and Carotenoids Content in Cape Gooseberry Juice. Food Chem. 2017, 233, pp. 96–100.
- Wang, J.; Liu, Q.; Xie, B.; Sun, Z. Effect of Ultrasound Combined with Ultraviolet Treatment on Microbial Inactivation and Quality Properties of Mango Juice. Ultrason. Sonochem. 2020, 64, p. 105000.
- Xie, Y.; Wang, J.; Wang, Y.; Wu, D.; Liang, D.; Ye, H.; Cai, Z.; Ma, M.; Geng, F. Effects of High-intensity Ultrasonic (HIU) Treatment on the Functional Properties and Assemblage Structure of Egg Yolk. Ultrason. Sonochem. 2020, 60, p. 104767.
- Ojha, K. S.; Aznar, R.; O’Donnell, C.; Tiwari, B. K. Ultrasound Technology for the Extraction of Biologically Active Molecules from Plant, Animal and Marine Sources. TrAC Trends Anal. Chem. 2020, 122, p. 115663.
- Pagnossa, J. P.; Rocchetti, G.; Ribeiro, A. C.; Piccoli, R. H.; Lucini, L. Ultrasound: Beneficial Biotechnological Aspects on Microorganisms-mediated Processes. Curr. Opin. Food Sci. 2020, 31, pp. 24–30.
- Dávila, I.; Robles, E.; Egüés, I.; Labidi, J.; Gullón, P. The Biorefinery Concept for the Industrial Valorization of Grape Processing By-products. In Handbook of Grape Processing By-Products Galanakis, Charis; Austria: Elsevier, 2017; pp. 29–53.
- Bordiga, M. Valorization of Wine Making By-products; Boca Raton: CRC Press, 2015.
- O’donnell, C.; Tiwari, B.; Bourke, P.; Cullen, P. Effect of Ultrasonic Processing on Food Enzymes of Industrial Importance. Trends Food Sci. Technol. 2010, 21(7), pp. 358–367.
- Rastogi, N. K. Opportunities and Challenges in Application of Ultrasound in Food Processing. Crit. Rev. Food Sci. Nutr. 2011, 51(8), pp. 705–722.