ABSTRACT
Anthocyanins and proanthocyanidins are widespread in the plant kingdom and are involved in important bioactivities, such as antioxidation. These flavonoids can reduce the risk of cardiovascular diseases, cancer, high blood pressure, hyperlipidemia, and diabetes; consequently, they are widely studied for disease prevention and treatment. In this review, we summarize recent advances in our understanding of the chemical structures, food sources, and bioactivities of these compounds. We also discuss recent developments and trends related to anthocyanin- and proanthocyanidin-derived products. This review provides insights to inform related research and inspire future studies to help realize the potential of these plant pigments.
Introduction
Anthocyanins are natural water-soluble pigments that are commonly found in the sap of plant cells, and they are the main factors underlying the red, blue, and purple colors of certain vegetables, fruits, and grains.[Citation1] The content of anthocyanidin (the aglycone form) is particularly prominent in fruits such as grapes and blueberries. Anthocyanidin not only gives plants a unique flavor and color but also helps them to attract insects to complete the pollination process. Currently, over 550 types of anthocyanidins are recognized, of which six are widely distributed in plants, namely, cyanidin, delphinidin, pelargonidin, peonidin, malvidin, and petunidin, accounting for approximately 50%, 12%, 12%, 12%, 7%, and 7% of the anthocyanidin content in nature, respectively.[Citation2–4] Anthocyanidins are classified as flavonoids and exhibit diverse pharmacological activities that are primarily manifested in their anticancer, antioxidant, and anti-inflammatory properties.[Citation5]
Proanthocyanidins, also known as condensed tannins, are primarily found in fruits, nuts, bark, chocolate, wine, and some plant seeds and flowers.[Citation6] According to the degree of polymerization, proanthocyanidins can be divided into oligomeric and highly polymeric proanthocyanidins, with the former considered to be safe and effective natural antioxidants that can effectively scavenge free radicals from the body and maintain homeostasis.[Citation6] Numerous in vivo and in vitro experimental studies have demonstrated that proanthocyanidins are beneficial to human health and exhibit anti-inflammatory and immune regulatory functions, hypoglycemic and hypolipidemic effects, metabolic regulation, DNA repair, and anticancer effects.[Citation7] Accordingly, the potential of proanthocyanidins to prevent chronic diseases has attracted the attention of the food industry and public health institutions.
Castañeda-Ovando et al.[Citation3] have reviewed the chemical properties of anthocyanins, and Rauf et al.[Citation8] have reviewed the biological activities of proanthocyanidins. However, no literature on a systematic summary or comparison of the food sources and the biological activities between anthocyanins and proanthocyanidins exists. In addition, a series of new studies on the biological activities of anthocyanins and procyanidins has been reported in recent years. Considering this, the present review provides an overview of the chemical structures, food sources, bioactivities, and related product development for anthocyanidins and proanthocyanidins, aiming to provide an updated reference for the future research and development of anthocyanidins and proanthocyanidins as natural antioxidants as well as their utilization in health-related products including drugs, functional foods, and cosmetics.
Chemical structures of anthocyanins and proanthocyanidins
Chemical structures of anthocyanins
Anthocyanins belong to the flavonoid group of phenolic compounds. The basic structure of their parent nucleus is a highly conjugated 2-phenylbenzopyran cation. The two benzene rings are connected by three carbon atoms to form a C6–C3–C6 skeleton, which is the anthocyanin motif. Alternate substituents on the carbon positions of the two benzene rings leads to the formation of anthocyanidins with diverse structures; the six most common types are cyanidin, pelargonidin, delphinidin, peonidin, petunidin, and malvidin (). Free anthocyanidin is rare in natural conditions and it often exists in the form of glycosides in fruits. Anthocyanidin is usually combined with one or more glucose, galactose, arabinose, or rhamnose moiety to form anthocyanins through glycosidic bonds.[Citation9] Moreover, the glycosidic bonds in anthocyanins can also form acylated anthocyanins with an organic acid of one or more molecule through ester bonds.[Citation10] Glycosyl derivatives are esterified by aromatics or fatty acids. Different numbers of substitutions, sites of hydroxylation on different rings, patterns of methylation, and the number of glycosylation sites are the primary differences responsible for the various anthocyanidin chemical structures and colors found in nature.[Citation9]
The stability and color of anthocyanidins is affected by internal and external factors, primarily the chemical structure and the presence of oxygen, illumination, temperature, pH, metal ions, and enzymes, respectively. Generally, anthocyanidin is easily degraded under light conditions, and can readily oxidize and fade under heating or high temperature conditions, but is more stable under low temperature and acidic conditions.[Citation10] Anthocyanidins change color as the pH value of the cell sap changes, being reddish at pH < 7, purple between pH 7–8, and blue at pH > 11.[Citation11] In addition, the anthocyanidin color is also affected by the number and position of the hydroxyl, methoxy, and aglycone moieties. Moreover, the color rendering effect of anthocyanidin is enhanced under acidic conditions, and the color can be stabilized via a complex reaction with metal ions. The anthocyanidin molecule contains both an acidic group and a basic group and is readily soluble in polar solvents such as water and alcohol compounds (e.g., methanol and ethanol), but it is almost insoluble in nonpolar solvents (e.g., chloroform). Finally, anthocyanidin has two main absorption wavelength ranges, with one in the visible region (465–560 nm) and the other in the ultraviolet region (270–280 nm).[Citation12]
Chemical structure of proanthocyanidins
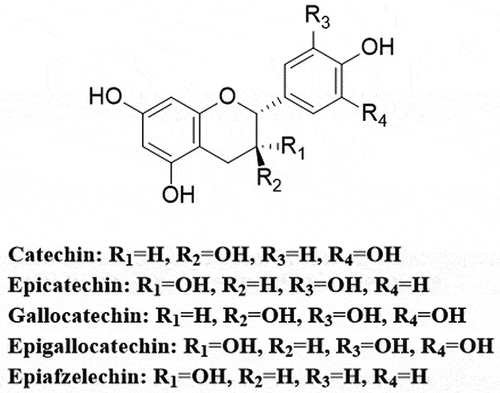
Proanthocyanidins are compounds formed by the polymerization of flavan-3-ol as a structural unit via a C–C bond. The structure depends on five aspects including the type of flavan-3-ol unit, connection method, degree of polymerization (number of constituent units), spatial configuration, and whether the hydroxyl group is substituted.[Citation13] Common flavan-3-ol structural units include catechin, epicatechin, epigallocatechin, and epiafzelechin (). Flavan-3-ol monomers can be polymerized to form oligomers or multimers of proanthocyanidins[Citation14] that are named according to the size of the polymer, with dimers, trimers, and tetramers usually termed oligomers and those composed of more than five sub-units called multimers. Among these, dimers are the most widely distributed, researched, and relevant, and the simplest types of proanthocyanidins primarily comprise catechin and epicatechin homo- or heterodimers.[Citation6] Notably, as secondary metabolites formed from a combination of flavanol monomers, proanthocyanidins are the most abundant plant phenolic compounds, second only to lignin and they constitute the second major phenolic compound in the human diet.[Citation15]
Proanthocyanidins are generally observed in fruits and grains with colors ranging from deep rose red to light red. Functionally, they are recognized as a new type of natural antioxidant with high efficiency and strong in vivo activity. The anti-radical oxidation ability of oligomeric proanthocyanidins is 50 times that of vitamin E and 20 times that of vitamin C.[Citation16] Furthermore, they are rapidly and completely absorbed by the body, with >90% bioavailability.[Citation16] Proanthocyanidins can produce red anthocyanidin under acidic and heating conditions, a property that can be exploited for their qualitative and quantitative analysis.[Citation8] In particular, the stability of proanthocyanidins with various degrees of polymerization differs substantively.[Citation8] For example, oligomeric proanthocyanidins have a better light and heat resistance, and higher stability (pH 2.0–6.0); in contrast, high polymeric proanthocyanidins have poor stability and are prone to condensation and precipitation. Structurally, proanthocyanidins contain numerous hydroxyls, and consequently they exhibit large polarity and are easily soluble in polar solutions such as water, methanol, and ethanol but are insoluble in nonpolar solutions including petroleum ether and benzene.[Citation8] Proanthocyanidins also have a maximum absorption wavelength of 280 nm and strong UV absorption capabilities owing to the presence of benzene rings in their structure.[Citation17]
Food sources of anthocyanins and proanthocyanidins
Anthocyanins and proanthocyanidins are usually found in fruits, vegetables, and colored grains. Although their content and composition can vary considerably among different plants, in general, they are most abundant in fruits.[1] Commonly consumed foods with higher anthocyanin contents include grapes, blueberries, blackberries, black goji berries, mulberries, bayberries, purple cabbage, purple sweet potato, purple potato, purple corn, black rice, and wild rice (); foods with higher proanthocyanidin contents include grape seeds, cranberries, Chinese wolfberries, red goji berries, pomegranates, and red rice ().[Citation18] At present, the main sources of anthocyanins and proanthocyanidins on the market are grapes and grape seeds, respectively. Representative foods are briefly introduced in the following sections.
Grapes and grape seeds
Grapes constitute a rich source of anthocyanins, whereas grape seeds contain high quantities of proanthocyanidins. Grapes are rich in nutrients, containing various fruit acids, vitamins, minerals, flavonoids, and essential amino acids required by the human body. These agents can effectively scavenge free radicals and prevent senility, protect against cardiovascular and cerebrovascular diseases, and also relieve fatigue, promote digestion, infection resistance, and blood circulation, and improve neurosthenia.[Citation19] In particular, the anthocyanins and resveratrol found in grapes endow grape-derived products with health-promoting functions such as antioxidation, enhancement of blood vessel elasticity, and improvement of skin radiance.[Citation19]
Grape seeds harbor a higher level and richer variety of proanthocyanidins. The proanthocyanidins extracted from grape seeds comprise oligomers formed from varying amounts of epicatechin or catechin, usually in the form of glycosides.[Citation8] Numerous in vitro and in vivo studies have also shown that grape seed proanthocyanidins can exert anti-oxidative, anti-microbial, antiobesity, antidiabetic, anti-neurodegenerative, anti-osteoarthritic, anticancer, and heart and eye protective effects, along with various other pharmacological effects.[Citation13] Grapes consequently offer considerable benefits for the market economy; moreover, the majority of proanthocyanidin products currently on the market are derived from grape seeds. Consumer education has also increased the appreciation for wine, grape seed oil, and grape products as being rich in anthocyanins and proanthocyanidins.
Blueberry
Blueberries are sweet and juicy dark blue berries that constitute one of the five healthy fruits recommended by the World Food and Agriculture Organization; they are often referred to as the “king of berries”. The unique dark blue skin of the blueberry fruit is rich in flavonoids such as flavanols, anthocyanins, and lignin, along with phenolic compounds such as phenolic acid and tannin. The skin also contains vitamin A, vitamin E, minerals, niacin, and microelements, and exerts functions classified as anticancer, pro-heart, anti-cardiovascular disease, visual fatigue relieving, and anti-aging as well as skin care promoting effects.[Citation20] The anthocyanin content in blueberries is high (3.87–4.87 mg/g) compared with that of strawberries (0.35 mg/g), raspberries (1.16 mg/g), and blackberries (2.45 mg/g).[Citation21] Blueberries are also rich in active ingredients such as proanthocyanidins, and are considered to be among the fruits with the highest antioxidant activity levels. The anthocyanins contained in blueberries are primarily distributed in the skin, with levels nearly 200 times those in the flesh.[Citation21] To date, 25 types of anthocyanins have been detected in blueberries, with most existing in the form of glucose, galactose, and arabinoside.[Citation22,Citation23] Comparing the content, composition, and antioxidant capacity of the different types of anthocyanins in blueberry will help to improve our understanding of the antioxidant activities of this fruit. Overall, blueberries are suitable for all ages, and they have a high nutritional value and are favored by consumers. Furthermore, they have a high economic value and broad development prospects as they can be eaten both directly and when processed into jam, juice, wine, and other primary and nutritional health products.
Black goji berry
A total of 46 types of flavonoids have been isolated from black goji berries, including 37 types of anthocyanins.[Citation24] Anthocyanin content comparisons have revealed that black goji berries contain 1.60–6.25 mg/g anthocyanin, whereas raspberries and grapes contain 0.3–0.6 mg/g and 0.40–0.70 mg/g anthocyanin, respectively, and this provides justification for the black goji berry nickname “black soft gold”.[Citation25,Citation26] Furthermore, Tian et al.[Citation27] reported that dry black goji berries contain eight types of anthocyanins with pelargonidin, petunidin, malvidin, and delphinidin as the parent cores and petunidin as the main component. In addition, although proanthocyanidins exist in most plants, as of 2016, natural, wild black goji berries are reported to harbor the highest known content of proanthocyanidins. Moreover, black goji berries are also rich in nutrients such as vitamins and organic acid, they have strong antioxidant properties, and could potentially be utilized as antioxidants in the research and development of functional foods and medicines.
Mulberry
Mulberry is a perennial woody plant native to central China. It has a wide planting range and a long cultivation history. China ranks first worldwide for having the highest variety of mulberry trees and mulberry yields.[Citation28] Mulberry is the fruit of the mulberry treeand can be eaten as a sweet and juicy fruit; it is also used in Chinese medicine. It was listed in the first list of medicinal and edible plants by the National Health Commission of the People’s Republic of China.[Citation28] Mulberry is not only rich in organic acids, vitamins, essential amino acids, and other nutrients, but it also contains bioactive components such as anthocyanins and phenols.[Citation29] Traditional Chinese medicine believes that mulberry is helpful for the treatment of conditions such as insomnia, neurasthenia, hypertension, and diabetes. It has also been reported to exert anti-fatigue effects and help prevent constipation.[Citation30] Studies have also found that anthocyanins have physiological functions such as free radical scavenging, anticancer, and fat reduction.[Citation31–34] At present, solvent extraction is widely used to extract mulberry anthocyanins in China and overseas, and the content of anthocyanins extracted with 70% ethanol as the extractant is 170.5 mg/100 g.[Citation35] Studies have shown that ripe mulberries are abundant in anthocyanins, and predominantly cyanidin-3-O-glucoside (C3G) and cyanidin-3-rutinoside.[Citation36,Citation37] Mulberry is currently developed and utilized predominantly by food industries for the production of products such as fruit juice, vinegar, wine, and jam, as well as in other industries such as for natural dye production and cosmetics. The medicinal value of the anthocyanins in mulberry, however, has been less widely developed and utilized.[Citation38] Moreover, studies have found that mulberry anthocyanins have a strong sensitivity to factors such as temperature, light, pH, and enzymes, owing to their strong activity and low levels of stability. This limits, to a certain extent, the production of mulberry anthocyanins.[Citation39,Citation40] Therefore, optimizing the extraction process conditions for anthocyanins is key to enabling their wider use.
Bayberry
Bayberry is a subtropical evergreen tree that produces an economically important fruit crop in southern China.[Citation41] Its ripe berries can be purple, red, pink, or white, depending on the variety.[Citation42] Bayberry is sweet and sour, with abundant juice and a high nutritive value. A high consumer demand exists for this fruit both in China and overseas. It can be eaten fresh or used to make products such as juices, preserves, jams, and wines.[Citation42] Bayberry is utilized in medicines as it is rich in nutrients such as vitamins and dietary fiber, as well as eight types of amino acids and 17 types of mineral elements, which are necessary for the human body.[Citation43] Bayberry also contains phenolic compounds such as anthocyanins, phenolic acids, and flavonols, which are involved in functions such as antioxidation, anticancer, and alleviating hyperglycemia.[Citation44–46] The anthocyanins in bayberry are predominantly C3G.[Citation47] The content of anthocyanins in bayberry is as high as 76.2 mg/100 g (in terms of fresh fruit). The C3G content of bayberry (64.8 mg/100 g) accounts for at least 85% of the anthocyanins in the fruit, which is similar to that of blackberries, and is markedly higher than the proportion found in cranberries and blueberries.[Citation47,Citation48] In addition, bayberry leaves and bark are also rich in compounds such as flavonoids and proanthocyanidins, and they have a strong oxidation resistance.[Citation49] Researchers used natural berries such as blueberries, strawberries, cranberries, mulberries, and bayberries as research objects and found that berry anthocyanins have significant α-glucosidase inhibitory activity, which is significant for controlling blood sugar after meals.[Citation50]
Purple cabbage
Purple cabbage is easy to plant and pick, has a strong adaptability and large yield, is affected by few pests and diseases, is easy to transport and store, and is commonly used in cooking. Purple cabbage is rich in nutrients, contains many B, C, and E vitamins, and is rich in anthocyanins and cellulose. Among the large quantity of natural pigments, the prominent purple component primarily comprises anthocyanins, with 24 anthocyanin components detected in a purple cabbage extract, of which acylated cyanidin was the primary type.[Citation51] Purple cabbage anthocyanins exert numerous physiological functions such as antioxidation, free radical scavenging, mutation resistance, cardiovascular and cerebrovascular disease prevention, lowering blood lipid and blood sugar, and inhibiting tumor cell growth. Therefore, purple cabbage is not only an important natural pigment resource but may have beneficial health effects.
Purple sweet potato and purple potato
Purple sweet potato is widely cultivated in China, both as a food and cash crop. As it is rich in anthocyanins, and has physiological functions such as blood lipid lowering, antioxidation, and anti-aging, it has become of increasing interest to consumers and researchers.[Citation52] The chemical components of purple sweet potato anthocyanins are predominantly cyanidin and peonidin.[Citation53] Purple sweet potato has a higher anthocyanin content than fruits such as eggplant and cherry, and anthocyanins from this plant have a higher stability against light and heat than those extracted from other plants (e.g., strawberries and red cabbages).[Citation54] Purple sweet potato is thus a rich source of stable anthocyanins.
The purple potato is a newly introduced variety in China, but its cultivation and breeding practices are relatively advanced. The purple potato contains the same nutrients found in white potatoes, such as starch, protein, minerals, and vitamins, but is also rich in anthocyanins.[Citation55] Purple potatoes have 3–4 times greater anthocyanins content and 2.5–3.0 times greater free radical scavenging ability than white potatoes.[Citation56] Studies have shown that purple potato also has multiple health benefits including liver protection and anticancer, blood sugar lowering, anti-aging, and weight loss promoting activities.[Citation57] Purple potato can thus be used as a rich source of anthocyanins owing to its low cost, strong adaptability, and large outputs.
Wild rice
Wild rice is an important aquatic cereal crop in North America and East Asia and is regarded as a whole grain with health benefits. Its antioxidant activity and health-related effects have attracted widespread attention, and they include inhibiting oxidative stress, relieving hyperlipidemia, and preventing atherosclerosis, type II diabetes, and obesity.[Citation58,Citation59] Wild rice is a primary source of phenolic compounds, with phenolic acids, flavonoid glycosides, and proanthocyanidin compounds representing the key factors underlying the efficacy of this grain.[Citation60,Citation61] In particular, the content and antioxidant activity of the total phenols, flavonoids, and proanthocyanidins in wild rice are significantly higher than those in indica, japonica, and red rice.[Citation62] Comparison of the composition type and content of wild rice and rice flavonoids using ultra-high performance liquid chromatography coupled with a triple quadrupole mass spectrometry–based metabolomics method identified a total of 159 flavonoids, of which 78 were differentially expressed (72 were upregulated, while six were downregulated in wild rice).[Citation62] In turn, the Kyoto Encyclopedia of Genes and Genomes classification of annotations indicated that the differentially expressed flavonoids are primarily related to anthocyanin biosynthesis.[Citation62] Compared with colorless rice, the representative flavonoids in red rice are proanthocyanidins, whereas those in wild rice and black rice include both anthocyanins and proanthocyanidins.[Citation61]
Bioactivities of anthocyanins
Anthocyanins have attracted considerable attention owing to their notable health benefits, which involve a wide range of pharmacological activities. In addition to their potential applications in the medical field, anthocyanins have promising development prospects in other fields, such as those for food and health products, and in cosmetics as natural pigments to replace artificial synthetic pigments.[Citation63] The bioactive effects of anthocyanins are summarized in .
Table 1. Bioactivities of anthocyanins
Antioxidant activity
Anthocyanins and proanthocyanidins are both natural antioxidants. Moreover, anthocyanins are the most effective safe and natural water-soluble free radical scavengers identified to date.[Citation10] Their antioxidant activity is primarily related to their chemical structures, and generally, the simpler the chemical structure of the anthocyanins, the stronger their antioxidant activity. Furthermore, their health and therapeutic effects are primarily related to their antioxidant activity, which can be improved with methoxylation, hydroxylation, and glycosylation of the B ring structure. The antioxidant activity of anthocyanins is comparable to that of vitamin C and is unmatched in the body compared with other antioxidants. This capacity can enhance the human immune system and may play an important role in future developments in modern medicine. In particular, studies have found that the anthocyanins in black goji berries can effectively increase the activity of antioxidant enzymes in mice and improve the efficiency of free radical scavenging, thereby preventing radiation-induced damage.[Citation64] Anthocyanin-3-glucoside also relieves the effects of liver ischemia–reperfusion induced by oxidative stress in rats[Citation65] moreover, anthocyanin, delphinidin, and malvidin-induced antioxidant enzymes can induce the upregulation of the antioxidant response element (ARE) pathway in these animals.[Citation66]
Anticancer activity
Cancer remains a serious threat to human health. Cancer cells can spread and proliferate rapidly without limits, causing considerable harm to the human body and sometimes death. As both chemotherapy and radiation therapy have adverse side effects on human health, natural anticancer substances that effectively treat cancer without causing side effects are playing increasingly important roles in cancer treatments. Natural anthocyanin extracts from different plants used as anticancer substances have been extensively studied worldwide. The anticancer activity of anthocyanins is primarily attributed to the catechol structure on the B ring structure.[Citation89] Notably, the free radical scavenging ability of anthocyanins can prevent the diffusion of cancer cells, thereby reducing the likelihood of metastasis. A proposed cancer prevention mechanism includes stages of antimutagenesis, inhibition of oxidative DNA damage, and the inhibition of cancer activation and induction by carcinogens.[Citation67] Numerous investigations related to the anticancer effects of natural anthocyanin extracts from different plants have revealed that C3G, the main component of black bean skin anthocyanins, can inhibit both the autonomous and non-autonomous autophagy of tumor cells and affects the tumor growth environment, thereby significantly inhibiting tumor growth and invasion. In particular, the combined use of C3G and chloroquine is highly effective in inhibiting tumor growth and metastasis.[Citation67] Moreover, Shi et al.[Citation68]found that the addition of anthocyanin extracts in specific proportions to the daily feed of mice could inhibit the inflammatory reactions of tumors by regulating the expression of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase, and nuclear factor kappa-B (NF-κB), thereby inhibiting tumor cell proliferation. Anthocyanins could thus be promising medicines to help inhibit the proliferation and spread of cancer cells.
Prevention of cardiovascular disease
It is predicted that nearly 28 million people will die of cardiovascular disease every year by 2030.[Citation90] Cardiovascular and cerebrovascular diseases are a particularly serious threat for the elderly, as incidence rates generally increase in an a rapidly aging society. Multiple studies have shown that anthocyanins are beneficial against cardiovascular disease, as they can inhibit the inflammatory process, endothelial dysfunction, and the production of nitric oxide.[Citation91,Citation92] Anthocyanins can also maintain the stability of blood pressure by regulating the contraction of blood vessels in the body, thereby reducing the incidence of cardiovascular disease.[Citation93] Moreover, anthocyanins can also protect the cardiovascular wall and inhibit platelet aggregation. Notably, regular consumption of foods rich in anthocyanins can increase the antioxidant capacity of the serum. Studies conducted over 16 years on healthy menopausal women reveal that the incidence and mortality rates of coronary heart disease are generally reduced for women who regularly ingest foods containing anthocyanins. In addition, the incidence rate of myocardial infarction is also significantly reduced with the probability of myocardial infarction reduced by up to 32%.[Citation69] For cardiovascular disease, in vitro and in vivo studies have shown that anthocyanins can inhibit the oxidative stress that contributes to the atherosclerotic process.[Citation70] Such effects involve multiple mechanisms; for example, anthocyanins can inhibit the oxidation of low-density lipoproteins[Citation94] and reduce the oxidative damage of vascular endothelial cells.[Citation95] Graf et al.[Citation96] also confirmed the anti-atherosclerosis effects of grape and bilberry juices that are rich in anthocyanins, which can reduce the level of total cholesterol and triglycerides. Moreover, in a study by Mauray et al.,[Citation71] Apo-E-deficient mice fed anthocyanins for 16 weeks exhibited reduced atherosclerosis lesions regardless of whether the anthocyanins were derived from bilberries or blueberries. Other research has demonstrated that anthocyanin intake is negatively correlated to hypertension, indicating their ability to effectively reduce blood pressure.[Citation97,Citation98]
Bacteriostatic and anti-inflammatory effects
Polyphenols play an important role in plant resistance to bacterial pathogen invasion. Consequently, anthocyanins can also be used as preservatives and bacteriostatic agents to help improve the color and luster of food, as natural colorants, and to extend shelf life.[Citation99] Anthocyanins, as a natural functional substance, can also reduce inflammatory reactions, which involve a series of complex physiological responses that occur when tissues are damaged, including fever, swelling, and headache. Specifically, anthocyanins function by inhibiting cytokinins and histamines, with anti-inflammatory effects, resulting from the inhibition of cyclooxygenase, and bacteriostatic effects, inferred from their minimum inhibitory concentration and minimum bactericidal concentration against bacteria, fungus, and mildew. For example, in a study to evaluate the effects of dietary anthocyanins on the acute inflammatory responses in rats, Winter et al.[Citation72] found that the anti-inflammatory effects became more evident as the concentration of anthocyanins increased. Notably, anthocyanins can inhibit the expression of inflammatory factors in rats and reduce the production of inflammatory substances. Moreover, Lycium ruthenicum anthocyanin extract and petunidin-3-glucoside, the component with the highest anthocyanin content, could both effectively reduce the content of monosodium urate and prevent gouty arthritis in rats. Furthermore, both agents exhibited intestinal anti-inflammatory effects in a mouse model for colitis.[Citation73] In turn, Xu et al.[Citation74] found that blueberry anthocyanins significantly inhibited the expression of COX-2 and NF-κB, further confirming that these anthocyanins exerted anti-inflammatory effects via an NF-κB mechanism.
Neuroprotective effects
Neurodegenerative diseases constitute a major threat to the elderly; however, although their incidence is currently increasing,[Citation100] few effective methods are available for their prevention or treatment. Dietary intervention constitutes a feasible strategy for the treatment of neurodegenerative diseases. Notably, anthocyanins can relieve the symptoms of neurodegenerative diseases and they could thus possibly be useful as dietary supplements. Notably, anthocyanins can protect neurons, glial cells, and hippocampal nerve cells from being damaged by β-amyloid (Aβ), glutamate, and lipopolysaccharide, thereby reducing nerve injury such as cognitive and memory impairment.[Citation100] For example, Zuo et al.[Citation75] showed that feeding hypertoxic paraquat-induced Drosophila with black rice anthocyanin extracts could inhibit disease progression and reduce the paraquat lethality rate. Ali et al.[Citation76] found that black bean anthocyanins could reduce the reactive oxygen species and oxidative stress induced by amyloid β protein oligomers (AβO), thereby preventing apoptosis and neurodegeneration in a mouse model for Alzheimer’s disease. In addition, anthocyanins could reverse age-related cognitive deficits and neurological problems caused by neurodegenerative diseases.[Citation101,Citation102] For example, adding blueberry anthocyanins to the diet of mice or rats could increase short-term memory and motivation,[Citation77] and reverse the process of neuronal and behavioral aging.[Citation103] In clinical trials, patients with Alzheimer’s disease were administered daily supplemental cherry juice rich in anthocyanins, leading to significant improvements in their memory and cognitive ability after 12 weeks of treatment.[Citation78] In addition, after elderly patients with mild cognitive impairment received blueberry anthocyanins for 16 weeks, their blood oxygen level in the brain increased, indicating that blueberry anthocyanins could alleviate nervous system disease.[Citation79] Therefore, anthocyanins could be useful in future pharmaceutical applications for the treatment of neuronal diseases.
Antidiabetic effects
Diabetes is a non-infectious severe endocrine and metabolic disease that causes insufficient insulin secretion or insulin resistance owing to the destruction of islet cells. Diabetes is primarily characterized by chronic hyperglycemia[Citation104] and can induce complications in various organs leading to heart disease, kidney failure, liver dysfunction, nerve injury, stroke, and numerous other diseases.[Citation105,Citation106] Studies have shown that anthocyanins can regulate the glucose and lipid metabolism levels in the body and lower blood sugar; they can also reduce lipotoxicity-induced endothelial dysfunction, thereby minimizing diabetes-related complications. For example, Grace et al.[Citation80] found that the blood sugar level of hyperglycemic mice subject to gavage with blueberry anthocyanins was reduced by 33%–51%, whereas that of mice subject to gavage with the hypoglycemic drug metformin hydrochloride tablets was reduced by only 27%. Pranprawit et al.[Citation81] further demonstrated that blueberry anthocyanins could inhibit the activity of glucosidase and reduce the production of blood sugar by preventing the process of glucose metabolism in the body. Johnson et al.[Citation82] also found that blueberry anthocyanins could not only reduce the blood sugar level of diabetic mice but also relieve adverse symptoms such as polydipsia, hyperphagia, and polyuria, with an equivalent blood sugar lowering effect of blueberry anthocyanins to that of acarbose. In turn, Kurimoto et al.[Citation83] found that black soybean seed coat extract rich in anthocyanins could improve the blood sugar and insulin sensitivity of diabetic mice, via a mechanism related to the activation of adenylate-activated protein kinase. Other studies have confirmed that anthocyanins are effective inhibitors of α-amylase and α-glucosidase, and that they thus help to regulate diabetes.[Citation107,Citation108] Finally, Yan et al.[Citation84] demonstrated that mulberry anthocyanins relieved the hyperglycemic symptoms of diabetic mice, reduced the accumulation of triglycerides and cholesterol, and increased adiponectin levels.
Effects against intestinal diseases
Intestinal health is currently a hot topic in both research and public health arenas as intestinal functions play a central role in host health.[Citation109] C3G has considerable benefits for the protection intestinal health, including repairing the intestinal mucosal barrier by regulating the levels of colitis-related indicators and signaling pathways, thereby alleviating inflammatory bowel disease.[Citation110–112] Studies have indicated that the interaction between C3G and the intestinal mucosal immune system represents the core of mechanism by which C3G impacts intestinal diseases.[Citation113] Anthocyanins can also promote the growth of beneficial bacteria. Zhu et al.[Citation85] found that black rice anthocyanins and Cy-3-O-glucoside significantly increased the number of Bifidobacteria and Lactobacilli. Chen et al.[Citation86] found that black raspberry anthocyanins can promote the growth of Lactobacillus, Faecalibacterium prausnitzii, and Eubacterium rectale, and that they can also inhibit the growth of harmful bacteria such as Enterococcus spp. Zhou et al.[Citation21] showed that blueberry anthocyanins can increase the diversity and abundance of intestinal microorganisms in humans. Consistent with this, the results of in vitro experiments by Zhang et al.[Citation87] evaluating the regulatory effects of purple sweet potato anthocyanins on human intestinal flora suggested that ingesting purple sweet potato rich in anthocyanins is beneficial for intestinal flora and synergistically improves host health. Notably, the metabolism of anthocyanins in the body is divided into two stages: the biotransformation of intestinal absorption and the decomposition and metabolic transformation of intestinal bacteria.[Citation87] Given their low bioavailability, only a small proportion of anthocyanins are directly absorbed into the small intestine, and up to 65% are not absorbed after passing through the small intestine, and consequently, most must be further metabolized.[Citation87] Finally, Lee et al.[Citation88] revealed that anthocyanins could promote the reverse transport of cholesterol mediated by the intestinal flora metabolites of macrophages, thereby reversing intestinal atherosclerotic lesions.
Bioactivities of proanthocyanidins
Natural extracts obtained from plants often have strong bioactivity. For example, proanthocyanidins exhibit antioxidant activity, thereby effectively scavenging free radicals in the body.[Citation114] They also help to regulate the blood sugar balance and reduce the risk of diabetes, prevent cardiovascular and cerebrovascular diseases, exert anti-tumor activity, antihypertensive, hypolipidemic, anti-inflammatory, anti-allergic, anti-radiation, anti-mutation, and anti-viral effects, prevent senile dementia, and are useful in treating sports injuries. Consequently, they are widely used as active ingredients in medicine and as safe and non-toxic natural antioxidants in health foods and cosmetics.[Citation115] The bioactivities of proanthocyanidins are summarized in .
Table 2. Bioactivities of proanthocyanidins
Antioxidant activity
The human body constantly produces free radicals because of metabolism. During normal life activities, the human body has a certain ability to scavenge free radicals, thereby maintaining the dynamic balance of free radicals within the system. However, exposure to external stimulus can lead to excessive free radical production; if these cannot be cleared in a timely manner, the dynamic balance will be disrupted, leading to damage in the organism. Oxidative stress represents the main cause of cell damage. Even under normal physiological conditions, the use of antioxidants can effectively control cell damage and oxidative stress-induced metabolic disorders. Specifically, proanthocyanidins have strong antioxidant activity as they inhibit lipid peroxidase and lipoxygenase, and scavenge hydroxyl, superoxide, and peroxy radicals.[Citation141] By eliminating excessive free radicals in the body, proanthocyanidins prevents lipid oxidation and the blockage of free radical chain reactions, thereby maintaining the dynamic equilibrium between free radicals and antioxidant enzymes (oxidative homeostasis). Ultimately, this helps in the prevention of various diseases caused by free radicals or lipid peroxidation, such as fatigue, radiation damage, and cell aging.[Citation141] Thiruchenduran et al.[Citation116] found that the content of superoxide dismutase, glutathione, ascorbic acid, and α-tocopherol in the myocardial tissue of male rats induced with hypercholesterolemia increased significantly following treatment with grape seed proanthocyanidins, suggesting that these proanthocyanidins reduced lipid peroxidation by chelating active oxygen and enhanced the antioxidant defense system. Using peanut red coat extract (primarily composed of proanthocyanidins and their dimers) purified from different organic solvents, Larrauri et al.[Citation117] demonstrated that the extract exhibited a strong scavenging ability on free radicals. Notably, the free radical scavenging effect of proanthocyanidins is better than that of vitamin C, vitamin E, resveratrol, or ascorbic acid.[Citation142–144] Moreover, an appropriate intake of grape seed proanthocyanidin extract in rat models can reduce oxidative stress and improve mitochondrial function.[Citation118] Consistent with this, Puiggròs et al.[Citation119] found that grape seed proanthocyanidins exert a regulatory effect on cellular antioxidant function, improving the redox state of cells through the glutathione synthesis pathway.
Anticancer activity
Natural proanthocyanidins have a certain inhibitory effect on the dissemination and proliferation of cancer cells and can be utilized as anticancer functional foods or as drug components. Shirataki et al.[Citation120] found that proanthocyanidins could selectively kill human oral cancer cells and could also inhibit protein kinase C during tumor development. In addition, bile and gastric acid reflux are important causes of esophageal cancer. Conversely, activating autophagy and inhibiting bile acid metabolism have been shown to be potential treatment mechanisms of proanthocyanidins against esophageal cancer, which has an increasing incidence rate and a low 5-year survival rate following surgery. This mechanism also plays a role in reversing the imbalance of intestinal microorganisms.[Citation145] Cranberry proanthocyanidins can act as chemopreventive agents for esophageal cancer by inducing apoptosis and inhibiting proliferation.[Citation121] Proanthocyanidins can also reduce oxidative stress by rapidly inducing apoptosis in cancer cells, thereby helping to prevent lung cancer.[Citation122,Citation146] Proanthocyanidins from cocoa can also inhibit the growth of human breast cancer and colon cancer cells.[Citation147] In turn, Minker et al.[Citation123] found that proanthocyanidins can induce the apoptosis of rectal cancer cells to prevent their occurrence of this cancer, which is the third most predominant cancer after lung and breast cancer, and affects women at a higher rate than men as shown by epidemiological studies.[Citation123] Moreover, the proanthocyanidins extracted from Masson pine bark has a positive effect against ovarian and cervical cancers, deriving protection through the activation of mitochondrial-related apoptosis pathways including, for cervical cancer, increased expression of pro-apoptotic proteins.[Citation124,Citation148] Numerous additional studies further support the anti-tumor effects of proanthocyanidins, including for the treatment and prevention of breast, bladder, stomach, and skin cancer.[Citation149,Citation150] Therefore, eating a certain amount of proanthocyanidins appears to have certain preventive and therapeutic effects against cancer.
Prevention of cardiovascular disease
Cardiovascular disease is also known as “triple high disease” or “rich man’s disease”, and is primarily prevented by a healthy diet and lifestyle. Proanthocyanidins also play a key role in the prevention of cardiovascular disease. Mortality from cardiovascular disease represents approximately one third of global deaths, with the associated morbidity and mortality rates increasing annually.[Citation151] Epidemiological studies also strongly indicate that proanthocyanidins can prevent cardiovascular disease[Citation152] Notably, proanthocyanidins exert anti-atherosclerosis effects by protecting vascular endothelial cells and reducing lipid deposition in blood vessels. Studies have shown that proanthocyanidins improve blood circulation by strengthening arteries, veins, and capillaries,[Citation153] and relieve cardiovascular disease by vasodilation and inhibiting low-density lipoprotein oxidation.[Citation154] Proanthocyanidins can also directly scavenge peroxy radicals and hydroxyls, reducing the oxidative stress response during myocardial ischemia–reperfusion, thereby exerting a protective effect on myocardial ischemia–reperfusion injury.[Citation115] Specifically, the release of endothelin 1 (ET-1) is reduced in rats treated with grape seed proanthocyanidins, confirming that proanthocyanidins can protect against increased blood pressure caused by cardiovascular remodeling by inhibiting the reactive oxygen species/mitogen-activated protein kinase pathway.[Citation125] Consistent with this, an evaluation of the effects of ingesting red grape seed proanthocyanidin extract in a clinical trial involving 52 patients with mild hyperlipidemia revealed reductions in low-density lipoprotein cholesterol, total cholesterol, and oxidized low-density lipoprotein particles after two months, thereby reducing the risk of atherosclerosis and cardiovascular disease.[Citation126] In a hamster model of atherosclerosis, the administration of grape seed proanthocyanidin extracts reduced triglyceride levels by 34% and plasma cholesterol levels by 25%, as well as significantly reducing plasma lipid peroxidation levels.[Citation127] Moreover, rats fed a high-fat diet exhibited reduced triglyceride synthesis following grape seed proanthocyanidin extract administration.[Citation128] Finally, Lee et al.[Citation155] showed that the microbial metabolites of proanthocyanidins could potentially prevent atherosclerosis by reducing the adhesion of tumor necrosis factor on vascular cells.
Hypoglycemic and hypolipidemic effects
Although living standards continue to improve, poor lifestyles and habits such as high-sugar, high-salt, and high-oil diets, smoking, drinking alcohol, and lack of exercise increase the occurrences of chronic diseases such as hyperlipidemia, hyperglycemia, hypertension, obesity, and diabetes, seriously limiting the ability of affected individuals to live a healthy life. Conversely, studies have found that in mice fed proanthocyanidins, liver cholesterol and lipid storage are significantly reduced, which is helpful for weight control, as are blood cholesterol and triglyceride levels, thereby reducing overall blood lipids.[Citation129] Proanthocyanidins do not only inhibit the formation of advanced glycosylation end products in the human body,[Citation130] but also effectively inhibit the activity of hydrolytic enzymes that play a key role in the carbohydrate digestion process, such as α-amylase and α-glucosidase. This slows the food hydrolysis process, thus avoiding sharp rises in blood sugar in the body after meals to help prevent and assist in the treatment of diabetes.[Citation156] In particular, the inhibitory effect of grape seed proanthocyanidins on α-amylase activity is equivalent to four times that of an equivalent amount of acarbose.[Citation130] Consistent with this, Tsujita et al.[Citation131] showed that the oral administration of proanthocyanidin extracts to rats could inhibit increases in blood sugar. Additional animal studies have also demonstrated that proanthocyanidins can improve blood pressure. For example, the treatment of hypertensive rats with grape seed proanthocyanidins led to significantly reduced systolic blood pressure, increased nitric oxide production, and an improved endothelium, which inhibited increases in blood pressure.[Citation132,Citation157] Together, the above studies indicate that proanthocyanidins have promising application prospects as new drugs for the prevention and treatment of hyperlipidemia, diabetes, and obesity, and could also be used in nutritional health products.
Bacteriostatic and anti-inflammatory effects
Bacteria constitute a diverse range of organisms that exist in large numbers, and many bacterial pathogens pose serious risks to human health. Inflammation, such as in response to bacterial infection, generally manifests in symptoms such as fever, pain, and swelling. Numerous studies have indicated that proanthocyanidins exhibit satisfactory anti-inflammatory effects; for example, proanthocyanidin trimers can destroy the cell membrane and cell wall integrity of Bacillus cereus, thereby curing food poisoning.[Citation158] Low doses of proanthocyanidins also inhibit the expression of proinflammatory cytokines in the liver, muscle, and mesenteric fat tissues. Furthermore, grape seed proanthocyanidins have been demonstrated to have anti-inflammatory effects not only in adipose tissue but also in intestine, skeletal muscle, and lung tissue.[Citation133] La et al.[Citation134] found that cranberry proanthocyanidins could inhibit the activity of periodontal bacteria and the production of matrix metalloproteinases inhibited Streptococcus mutans by affecting proteins in the biofilm, and exerted anti-inflammatory and bacteriostatic effects. Feldman et al.[Citation135] in turn revealed that cranberry proanthocyanidins could prevent oral epithelial cell inflammation by inhibiting Candida albicans in the oral cavity. Lee et al.[Citation136] demonstrated that feeding grape seed proanthocyanidins to mice with acute or chronic asthma could reduce pulmonary inflammation. Proanthocyanidin extracts could also mediate the reduction of interleukin-1β and tumor necrosis factor in female mice with rheumatoid arthritis, yielding an inflammation inhibition rate of up to 73%.[Citation159] Based on these reports and other similar reports, applications of the anti-inflammatory functions of proanthocyanidins has gradually expanded to other areas. For example, studies have identified a relationship between increased levels of neuroinflammation and cytokines and depression, whereas proanthocyanidins can inhibit lipopolysaccharide-induced depression in mice via anti-inflammatory effects.[Citation137] In conclusion, proanthocyanidins exhibit satisfactory anti-inflammatory properties in vivo and in vitro and have broad development prospects as anti-inflammatory drugs.
Regulation of intestinal flora
The main function of the intestine is to digest food and provide a physical barrier protecting against contact with the environment. Intestinal flora is an important component of body health and plays a critical role in maintaining the normal physiological functions of the intestines and regulates host immune function. Proanthocyanidins have a positive effect on intestinal flora, which is primarily reflected by increased microbial diversity, improved ability to tolerate oxidative stress, and the regulation of intestinal homeostasis.[Citation138] Proanthocyanidins can enhance the microbiota composition by promotingvarious probiotics, such as Bifidobacterium and Lactobacillus; they also inhibit a portion of the harmful intestinal flora, which helps to maintain intestinal health.[Citation138] Intestinal flora can in turn absorb the proanthocyanidins ingested through the diet and transform them into more biologically active compounds. Han et al.[Citation138] found that by acting on intestinal microorganisms, proanthocyanidins enhanced the ability of weaned piglets to resist intestinal oxidative stress and improved the mucosal barrier. In addition, probiotics such as Lactobacillus rhamnosus promote the metabolism and biotransformation of proanthocyanidins, with metabolites including 4-hydroxyphenylacetic acid and water cinnamic acid exhibiting enhanced anticancer activity during in vitro experiments with liver cancer HepG2 cells, thereby enabling proanthocyanidins to better exert their pharmacological activity.[Citation139] Furthermore, grape seed proanthocyanidins can reduce the proportion of intestinal Firmicutes/Bacteroidetes and increase the plasma glucagon-like peptide-1 content to increase satiety, thereby improving glucose tolerance and helping to prevent obesity and diabetes.[Citation140]
Anthocyanin and proanthocyanidin product development
Food and health products
Fruits and vegetables are generally rich in anthocyanins, proanthocyanidins, vitamins, and other nutrients, suggesting that their consumption will result in health benefits, such as reducing the occurrence of some chronic diseases. During food processing and production, the addition of edible pigments is generally also required to improve sensory properties to stimulate the desire of consumers to purchase products, thus increasing sales. At present, the majority of pigments used in the food industry are chemically synthesized, cheap, easy to obtain, and harbor a certain toxicity.[Citation160] Compared with synthetic pigments, natural pigments are safer and may even have some medicinal value.[Citation161] With the general improvement of living standards and the deepening awareness of the side effects associated with synthetic chemical additives and the knowledge that long-term consumption will harm human health and may even cause cancer, consumers are increasingly pursuing natural, green, and healthy lifestyles. Therefore, there is a growing need for research and development regarding natural pigments. As anthocyanins can be utilized as safe and non-toxic food additives and nutrition enhancers, they are expected to be widely used in the food industry in the future.
Proanthocyanidins, as a source of safe, reliable, stable, efficient, cheap, and readily available natural antioxidants, could have considerable development and application value. Proanthocyanidins are natural dyes that are currently widely used in foods such as yogurt, fruit juice, tea, drinks, cakes, jams, and condiments. Proanthocyanidins can also be used as natural preservatives to extend the shelf life of food, which not only meets the requirement of being natural but also eliminates the food safety hazards that may be affiliated with synthetic chemical preservatives. As certain side effects are associated with all synthetic antioxidants currently in use, countries and regions are gradually restricting or prohibiting their use in food products. However, development opportunities for proanthocyanidins are increasing.
Anthocyanins and proanthocyanidins can also be used in the research and development of health foods with age-delaying, blood pressure and blood lipids regulating, anti-tumor, and brain strengthening effects. Numerous health foods in the domestic and foreign markets primarily utilize the biological activities of proanthocyanidins and anthocyanins to prevent and treat diseases. At present, in medical and health product markets, proanthocyanidins extracted from grape seeds are actively sought by consumers because of their anti-aging, antioxidation, and blood vessel protection effects together with their satisfactory safety record.
Cosmetics
Consumer requirements for cosmetics are ever increasing, requiring the ability to not only to protect and improve the skin but also contain safe and reliable ingredients. Anthocyanins are known as “oral skin cosmetics” in Europe. They are strong absorbers of both visible and ultraviolet light and can reduce the damage of ultraviolet rays to the skin, which is helpful for antioxidation and improving skin inflammation to a certain extent. Thus, anthocyanins have the potential for wide use in cosmetics as well as in food industries, such as for sunscreen, hair dyes, lipstick, and rouge as a replacement to chemically synthesized pigments.[Citation161] Moreover, as proanthocyanidins are natural antioxidants with an antioxidant capacity far exceeding that of vitamin C, and they exhibit strong abilities to scavenge free radicals and inhibit lipid peroxidation, they meet the prerequisites for widespread application in cosmetics. Proanthocyanidins can also inhibit the activity of tyrosinase and help to delay skin aging, whiten skin, provide anti-wrinkle moisturization, and maintain skin elasticity. Consequently, they have become a focus of research and development in the related cosmetics fields.
Moreover, proanthocyanidins and other plant extracts such as vitamin C and vitamin E can be compounded with each other or other ingredients to exert a synergistic effect, which can further enhance effects such as whitening. Pycnogenol in the United Kingdom and the Lumene Sisu series of products in Finland afford satisfactory effects related to whitening, and many cosmetic brands such as UNIFON currently on the market have also developed proanthocyanidin-containing creams and essence waters. Notably, proanthocyanidins can inhibit the cross-linking effect of collagenase and elastase on connective tissue, maintain skin elasticity, and exert anti-aging and anti-wrinkle effects. As proanthocyanidins contain several phenolic hydroxyl structures, they can also serve as a natural active ingredient with rapid and distinct effects related to moisturization and hydration. They can not only lock in the original moisture of the skin but also replenish lost moisture. In addition, proanthocyanidins have the ability to absorb ultraviolet rays, which is related to the inclusion of benzene rings in their structure. However, the technology for developing sunscreen products containing proanthocyanidins is not yet mature, as most studies are in the experimental research stage. Nevertheless, the data indicate that anthocyanins and proanthocyanidins have considerable research and development potential for use in whitening, spot-removing, anti-wrinkle and anti-aging, anti-sun, and moisturizing products.
Medicine
Anthocyanins and proanthocyanidins exhibit strong antioxidation and free radical scavenging abilities and have medicinal value, for purposes such as regulating lipid metabolism, tumor resistance, treatment of eye diseases, immunoregulation, treatment of diabetes, and lowering blood pressure and anti-inflammatory functions. As an anti-inflammatory agent in macrophages stimulated by endotoxin, proanthocyanidins are effective inhibitors of nitric oxide synthesis.[Citation162] Proanthocyanidins have exhibited satisfactory anti-inflammatory properties in both in vivo and in vitro experiments, supporting their broad application potential as anti-inflammatory and anti-allergic drugs. Moreover, the pharmaceutical industry is also investigating the use of anthocyanins as a replacement for chemically synthesized pigments such as carmine, lemon yellow, and indigo, which are often used as drugs dyes to help distinguish and identify them, as their regular consumption has adverse effects on human health. It is therefore expected that the application of anthocyanins as natural colorants in different products will increase significantly in coming years. In turn, demand for the development of new natural bacteriostatic agents is increasing sharply, as the side effects associated with drug-resistant bacterial strains and conventional antibiotic drugs are now seriously endangering human safety. In particular, proanthocyanidins can be used to inhibit bacteria such as Staphylococcus aureus, Escherichia coli, and Bacillus subtilis, they can destroy the integrity of the combined cell membrane and wall of Bacillus cereus to treat food poisoning, and they are suitable for use in restoring intestinal flora.[Citation130] In turn, anthocyanins can also be used as effective drugs to prevent various diseases and can inhibit inflammation and control obesity. Compared with drugs, anthocyanins are effective alternatives; however, further research is needed prior to their application in humans, especially to determine the exact dosages required to achieve the desired effects.[Citation163]
Conclusions and future trends
In summary, the chemical structures of anthocyanins and proanthocyanidins determine their chemical properties. Extensive research has indicated that anthocyanins and proanthocyanidins exhibit strong biological activity and health-promoting effects, which are relevant for a wide range of experimental and practical applications. In-depth research related to the mechanisms and functions of anthocyanins and proanthocyanidins has led to new breakthroughs and has revealed new challenges in the fields of medicine, food, and cosmetics. Anthocyanins and proanthocyanidins are present in various fruits, vegetables, grains, and other foods that are consumed daily, with many foods containing both. However, additional research and exploration are warranted to better understand their bioactivities owing to their relative instability, low extraction rate, and inability to fully exert their bioactivity in the digestive pathways of the body. If plants containing anthocyanins and proanthocyanidins can be fully utilized, it would not only increase the income of growers but also the overall social and ecological benefits. Moreover, it is anticipated that the advances derived from the numerous years of exploration and research to improve our understanding of the characteristics and bioactivities of plant anthocyanins and proanthocyanidins and their considerable application potentials will stimulate researchers and investors to conduct more in-depth studies and exploration to help promote their utilization and relevant product development.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Burton-Freeman, B.; Brzeziński, M.; Park, E.; Sandhu, A.; Xiao, D.; Edirisinghe, I. A Selective Role of Dietary Anthocyanins and Flavan-3-Ols in Reducing the Risk of Type 2 Diabetes Mellitus: A Review of Recent Evidence. Nutrients. 2019, 11, 841. DOI: 10.3390/nu11040841.
- Gowd, V.; Jia, Z.-Q.; Chen, W. Anthocyanins as Promising Molecules and Dietary Bioactive Components against Diabetes–A Review of Recent Advances. Trends Food Sci. Tech. 2017, 68, 1–13. DOI: 10.1016/j.tifs.2017.07.015.
- Castañeda-Ovando, A.; de Lourdespacheco-hernández, M.; Páez-Hernández, M.-E.; Rodríguez, J.-A. Galán-Vidal, C. A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. DOI: 10.1016/j.foodchem.2008.09.001.
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.-G. Comparison of Anthocyanins and Phenolics in Organically and Conventionally Grown Blueberries in Selected Cultivars. Food Chem. 2015, 125, 201–208. DOI: 10.1016/j.foodchem.2010.08.063.
- Reis, J.-F.; Monteiro, -V.-V.-S.; de Souza Gomes, R.; Do Carmo, -M.-M.; Da Costa, G.-V.; Ribera, P.-C.; Monteiro, M.-C. Action Mechanism and Cardiovascular Effect of Anthocyanins: A Systematic Review of Animal and Human Studies. J. Transl. Med. 2016, 14, 315. DOI: 10.1186/s12967-016-1076-5.
- Ou, K.; Gu, L. Absorption and Metabolism of Proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. DOI: 10.1016/j.jff.2013.08.004.
- Ma, Z.-F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants. 2017, 6, 71. DOI: 10.3390/antiox6030071.
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Ul-Haq, I.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.-A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. DOI: 10.1016/j.biopha.2019.108999.
- Manolescu, B.-N.; Oprea, E.; Mititelu, M.; Ruta, -L.-L. Farcasanu, I.-C. Dietary Anthocyanins and Stroke: A Review of Pharmacokinetic and Pharmacodynamic Studies. Nutrients 2019, 11, 1479. DOI: 10.3390/nu11071479.
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of Anthocyanins and Derivatives. J. Funct. Foods. 2014, 7, 54–66. DOI: 10.1016/j.jff.2013.05.010.
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017, 214, 119–128. DOI: 10.1016/j.foodchem.2016.07.073.
- Shi, J.; Simal-Gandara, J.; Mei, J.; Ma, W.; Peng, Q.; Shi, Y.; Xu, Q.; Lin, Z.; Lv, H. Insight into the Pigmented Anthocyanins and the Major Potential Co-Pigmented Flavonoids in Purple-Coloured Leaf Teas. Food Chem. 2021, 363, 130278. DOI: 10.1016/j.foodchem.2021.130278.
- Unusan, N. Proanthocyanidins in Grape Seeds: An Updated Review of their Health Benefits and Potential Uses in the Food Industry. J. Funct. Foods. 2020, 67, 103861. DOI: 10.1016/j.jff.2020.103861.
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.-T.; Terra, X. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients. 2020, 12, 130. DOI: 10.3390/nu12010130.
- Alejo-Armijo, A.; Salido, S.; Altarejos, J. Synthesis of A-type Proanthocyanidins and their Analogues: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 8104–8118. DOI: 10.1021/acs.jafc.0c03380.
- Lu, Y.; Foo, L.-Y. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68, 81–85. DOI: 10.1016/S0308-8146(99)00167-3.
- Rao, L.-J.-M.; Yada, H.; Ono, H.; Ohnishi-Kameyama, M.; Yoshida, M. Occurrence of Antioxidant and Radical Scavenging Proanthocyanidins from the Indian Minor Spice Nagkesar (Mammea longifolia planch and triana syn). Bioorg. Med. Chem. 2004, 12, 31–36. DOI: 10.1016/j.bmc.2003.10.052.
- Hellström, J.-K.; Törrönen, A.-R.; Mattila, P.-H. Proanthocyanidins in Common Food Products of Plant Origin. J. Agric. Food Chem. 2009, 57, 7899–7906. DOI: 10.1021/jf901434d.
- Wang, X.; Tong, H.; Chen, F.; Gangemi, J.-D. Chemical Characterization and Antioxidant Evaluation of Muscadine Grape Pomace Extract. Food Chem. 2010, 123, 1156–1162. DOI: 10.1016/j.foodchem.2010.05.080.
- Martín-Gómez, J.; Varo, M.-A.; Mérida, J.; Serratosa, M.-P. Influence of Drying Processes on Anthocyanin Profiles, Total Phenolic Compounds and Antioxidant Activities of Blueberry (Vaccinium corymbosum). LWT-Food Sci. Technol. 2020, 120, 108931. DOI: 10.1016/j.lwt.2019.108931.
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant Activity of High Purity Blueberry Anthocyanins and the Effects on Human Intestinal Microbiota. LWT-Food Sci. Technol. 2020, 117, 108621. DOI: 10.1016/j.lwt.2019.10862.
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.-A.; Bagchi, D. Berry Anthocyanins as Novel Antioxidants in Human Health and Disease Prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. DOI: 10.1002/mnfr.200700002.
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry. 2003, 64, 923–933. DOI: 10.1016/S0031-9422(03)00438-2.
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional Constituents and Antioxidant Activities of Eight Chinese Native Goji Genotypes. Food Chem. 2016, 200, 230–236. DOI: 10.1016/j.foodchem.2016.01.046.
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins Composition and Antioxidant Activity of Wild Lycium Ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. DOI: 10.1016/j.foodchem.2010.11.052.
- Zhang, Y.; Liao, X.; Chen, F.; Wu, J.; Hu, X. Isolation, Identification, and Color Characterization of Cyanidin-3-Glucoside and Cyanidin-3-Sophoroside from Red Raspberry. Eur. Food Res. Technol. 2008, 226, 395–403. DOI: 10.1007/s00217-006-0550-3.
- Tian, Z.; Aizezijiang, A.; Pang, H.; Du, S.; Feng, M.; Ma, K.; Gao, S.; Bai, G.; Ma, C. Constituent Analysis and Quality Control of Anthocyanin Constituents of Dried Lycium Ruthenicum Murray Fruits by HPLC-MS and HPLC-DAD. J. Liq. Chromatogr. R. T. 2016, 39, 453–458. DOI: 10.1080/10826076.2016.1179201.
- Jiang, Y.; Nie, W.-J. Chemical Properties in Fruits of Mulberry Species from the Xinjiang Province of China. Food Chem. 2015, 174, 460–466. DOI: 10.1016/j.foodchem.2014.11.083.
- Lin, J.-Y.; Tang, C.-Y. Determination of Total Phenolic and Flavonoid Contents in Selected Fruits and Vegetables, as well as their Stimulatory Effects on Mouse Splenocyte Proliferation. Food Chem. 2007, 101, 140–147. DOI: 10.1016/j.foodchem.2006.01.014.
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and Antioxidant Properties of Anthocyanin-Rich Morus Nigra and Morus Rubra Fruits. Sci. Hortic. 2009, 119, 275–279. DOI: 10.1016/j.scienta.2008.08.007.
- Krishna, P.-G.-A.; Sivakumar, T.-R.; Jin, C.; Li, S.-H.; Weng, Y.-J.; Yin, J.; Gui, -Z.-Z. Antioxidant and Hemolysis Protective Effects of Polyphenol-Rich Extract from Mulberry Fruits. Pharmacogn. Mag. 2018, 14, 103. DOI: 10.4103/pm.pm_491_16.
- Raman, S.-T.; Ganeshan, A.-K.-P.-G.; Chen, C.; Jin, C.; Li, S.-H.; Chen, H.-J.; Gui, Z. In Vitro and in Vivo Antioxidant Activity of Flavonoid Extracted from Mulberry Fruit (Morus alba L.). Pharmacogn. Mag. 2016, 12, 128. DOI: 10.4103/0973-1296.177910.
- Huang, H.-P.; Chang, Y.-C.; Wu, C.-H.; Hung, C.-N.; Wang, C.-J. Anthocyanin-Rich Mulberry Extract Inhibit the Gastric Cancer Cell Growth in Vitro and Xenograft Mice by Inducing Signals of p38/p53 and c-jun. Food Chem. 2011, 129, 1703–1709. DOI: 10.1016/j.foodchem.2011.06.035.
- Chang, -J.-J.; Hsu, M.-J.; Huang, H.-P.; Chung, D.-J.; Chang, Y.-C.; Wang, C.-J. Mulberry Anthocyanins Inhibit Oleic Acid Induced Lipid Accumulation by Reduction of Lipogenesis and Promotion of Hepatic Lipid Clearance. J. Agric. Food Chem. 2013, 61, 6069–6076. DOI: 10.1021/jf401171k.
- Suh, H.-J.; Noh, D.-O.; Kang, C.-S.; Kim, J.-M.; Lee, S.-W. Thermal Kinetics of Color Degradation of Mulberry Fruit Extract. Mol. Nutr. Food Res. 2010, 47, 132–135. DOI: 10.1002/food.200390024.
- Kabi, F.; Bareeba, F.-B. Herbage Biomass Production and Nutritive Value of Mulberry (Morus alba) and Calliandra Calothyrsus Harvested at Different Cutting Frequencies. Anim. Feed Sci. Tech. 2008, 140, 178–190. DOI: 10.1016/j.anifeedsci.2007.02.011.
- Wu, X.; Liang, L.; Zou, Y.; Zhao, T.; Zhao, J.; Li, F.; Yang, L. Aqueous Two-Phase Extraction, Identification and Antioxidant Activity of Anthocyanins from Mulberry (Morus atropurpurea Roxb.). Food Chem. 2011, 129, 443–453. DOI: 10.1016/j.foodchem.2011.04.097.
- Liang, L.; Wu, X.; Zhu, M.; Zhao, W.; Li, F.; Zou, Y.; Yang, L. Chemical Composition, Nutritional Value, and Antioxidant Activities of Eight Mulberry Cultivars from China. Pharmacogn. Mag. 2012, 8, 215. DOI: 10.4103/0973-1296.99287.
- Jia, S.-J.; Tang, M.-S.; Wu, J.-M. The Determination of Flavonoid Contents in Mulberry and their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. DOI: 10.1016/S0308-8146(98)00102-2.
- Dastmalchi, K.; Dorman, H.-D.; Koşar, M.; Hiltunen, R. Chemical Composition and in Vitro Antioxidant Evaluation of A Water-Soluble Moldavian Balm (Dracocephalum moldavica L.) Extract. LWT-Food Sci. Technol. 2007, 40, 239–248. DOI: 10.1016/j.lwt.2005.09.019.
- Chen, K.-S.; Xu, C.-J.; Zhang, B.; Ferguson, I.-B. Red Bayberry: Botany and Horticulture. Hortic. Rev. 2004, 30, 83–114. DOI: 10.1002/9780470650837.ch3.
- Cheng, H.; Chen, J.; Chen, S.; Wu, D.; Liu, D.; Ye, X. Characterization of Aroma-Active Volatiles in Three Chinese Bayberry (Myrica rubra) Cultivars Using GC–MS–Olfactometry and an Electronic Nose Combined with Principal Component Analysis. Food Res. Int. 2015, 72, 8–15. DOI: 10.1016/j.foodres.2015.03.006.
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. A. Flavonols, and Free Radical Scavenging Activity of Chinese Bayberry (Myrica rubra) Extracts and their Color Properties and Stability. J. Agric. Food Chem. 2005, 53, 2327–2332. DOI: 10.1021/jf048312z.
- Fang, Z.; Zhang, M.; Wang, L. HPLC-DAD-ESIMS Analysis of Phenolic Compounds in Bayberries (Myrica rubra Sieb. et Zucc.). Food Chem. 2007, 100, 845–852. DOI: 10.1016/j.foodchem.2005.09.024.
- Sun, C.; Huang, H.; Xu, C.; Li, X.; Chen, K. Biological Activities of Extracts from Chinese Bayberry (Myrica rubra Sieb. et Zucc.): A Review. Plant Foods Hum. Nutr. 2013, 68, 97–106. DOI: 10.1007/s11130-013-0349-x.
- Sun, C.-D.; Zhang, B.; Zhang, J.-K.; Xu, C.-J.; Wu, Y.-L.; Li, X.; Chen, K.-S. Cyanidin-3-Glucoside-rich Extract from Chinese Bayberry Fruit Protects Pancreatic β Cells and Ameliorates Hyperglycemia in Streptozotocin-Induced Diabetic Mice. J. Med. Food. 2012, 15, 288–298. DOI: 10.1089/jmf.2011.1806.
- Zhang, W.-S.; Li, X.; Zheng, J.-T.; Wang, G.-Y.; Sun, C.-D.; Ferguson, I.-B.; Chen, K.-S. Bioactive Components and Antioxidant Capacity of Chinese Bayberry (Myrica rubra Sieb. and Zucc.) Fruit in Relation to Fruit Maturity and Postharvest Storage. Eur. Food Res. Technol. 2008, 227, 1091–1097. DOI: 10.1074/jbc.M301930200.
- Bao, J.-S.; Cai, Y.-Z.; Sun, M.; Wang, G.-Y.; Corke, H. Anthocyanins, Flavonols, and Free Radical Scavenging Activity of Chinese Bayberry(Myrica rubra) Extracts and their Color Properties and Stability. J. Agric. Food Chem. 2005, 53, 2327–2332. DOI: 10.1021/jf048312z.
- Zhang, Y.; Chen, S.; Wei, C.; Gong, H.; Li, L.; Ye, X. Chemical and Cellular Assays Combined with In Vitro Digestion to Determine the Antioxidant Activity of Flavonoids from Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Leaves. PLoS ONE. 2016, 11, 0167484. DOI: 10.1371/journal.pone.0167484.
- Xu, Y.; Xie, L.; Xie, J.; Liu, Y.; Chen, W. Pelargonidin-3-O-rutinoside as a Novel Α-Glucosidase Inhibitor for Improving Postprandial Hyperglycemia. Chem. Commun. 2019, 55, 39–42. DOI: 10.1039/c8cc07985d.
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low Temperature Promotes Anthocyanin Biosynthesis and Related Gene Expression in the Seedlings of Purple Head Chinese Cabbage (Brassica rapa L.). Genes. 2020, 11, 81. DOI: 10.3390/genes11010081.
- Lila, M.-A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. DOI: 10.1155/S111072430440401X.
- Terahara, N.; Shimizu, T.; Takashige, K.; Nakamura, M.; Maitani, T.; Yamaguchi, M.-A.; Goda, Y. Six Diacylated Anthocyanins from the Storage Roots of Purple Sweet Potato, Ipomoea Batatas. Biosci. Biotechnol. Biochem. 1999, 63, 1420–1424. DOI: 10.1271/bbb.63.1420.
- Hwang, Y.-P.; Choi, J.-H.; Han, E.-H.; Kim, H.-G.; Wee, J.-H.; Jung, K.-O.; Jeong, H.-G.; Kwon, K.-I.; Jeong, T.-C.; Chung, Y.-C. Purple Sweet Potato Anthocyanins Attenuate Hepatic Lipid Accumulation Through Activating Adenosine Monophosphate–Activated Protein Kinase in Human Hepg2 Cells and Obese Mice. Nutr. Res. 2011, 31, 896–906. DOI: 10.1016/j.nutres.2011.09.026.
- Jansen, G.; Flamme, W. Coloured Potatoes (Solanum tuberosum L.) —Anthocyanin Content and Tuber Quality. Genet. Resour. Crop Evol. 2006, 53, 1321–1331. DOI: 10.1007/s10722-005-3880-2.
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial Phytochemicals in Potato—A Review. Food Res. Int. 2013, 50, 487–496. DOI: 10.1016/j.foodres.2011.04.025.
- Reddivari, L.; Vanamala, J.; Safe, S.-H.; Miller, J.-C., Jr. The Bioactive Compounds Αlpha-Chaconine and Gallic Acid in Potato Extracts Decrease Survival and Induce Apoptosis in LNCaP and PC3 Prostate Cancer Cells. Nutr. Cancer. 2010, 62, 601–610. DOI: 10.1080/01635580903532358.
- Chu, M.-J.; Du, Y.-M.; Liu, X.-M.; Yan, N.; Wang, F.-Z.; Zhang, Z.-F. Extraction of Proanthocyanidins from Chinese Wild Rice (Zizania latifolia) and Analyses of Structural Composition and Potential Bioactivities of Different Fractions. Molecules. 2019, 24, 1681. DOI: 10.3390/molecules24091681.
- Yan, N.; Du, Y.-M.; Liu, X.-M.; Chu, C.; Shi, J.; Zhang, H.-B.; Liu, Y.-H.; Zhang, Z.-F. Morphological Characteristics, Nutrients, and Bioactive Compounds of Zizania latifolia, and Health Benefits of Its Seeds. Molecules. 2018, 23, 1561. DOI: 10.3390/molecules23071561.
- Sumczynski, D.; Kotásková, E.; Orsavová, J.; Valášek, P. Contribution of Individual Phenolics to Antioxidant Activity and in Vitro Digestibility of Wild Rices (Zizania aquatica L.). Food Chem. 2017, 218, 107–115. DOI: 10.1016/j.foodchem.2016.09.060.
- Chu, M.-J.; Liu, X.-M.; Yan, N.; Wang, F.-Z.; Du, Y.-M.; Zhang, Z.-F. Partial Purification, Identification, and Quantitation of Antioxidants from Wild Rice (Zizania latifolia). Molecules. 2018, 23, 2782. DOI: 10.3390/molecules23112782.
- Yu, X.-T.; Yang, T.; Qi, -Q.-Q.; Du, Y.-M.; Shi, J.; Liu, X.-M.; Liu, Y.-H.; Zhang, H.-B.; Zhang, Z.-F.; Yan, N. Comparison of the Contents of Phenolic Compounds Including Flavonoids and Antioxidant Activity of Rice (Oryza sativa) and Chinese Wild Rice (Zizania latifolia). Food Chem. 2021, 344, 128600. DOI: 10.1016/j.foodchem.2020.128600.
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red Raspberry and Its Anthocyanins: Bioactivity Beyond Antioxidant Capacity. Trends Food Sci. Technol. 2017, 66, 153–165. DOI: 10.1016/j.tifs.2017.05.015.
- Duan, Y.; Chen, F.; Yao, X.; Zhu, J.; Wang, C.; Zhang, J.; Li, X. Protective Effect of Lycium Ruthenicum Murr. against Radiation Injury in Mice. Int. J. Environ. Res. Public Health. 2015, 12, 8332–8347. DOI: 10.3390/ijerph120708332.
- Tsuda, T.; Horio, F.; Osawa, T. The Role of Anthocyanins as an Antioxidant under Oxidative Stress in Rats. Biofactors. 2000, 13, 133–139. DOI: 10.1002/biof.5520130122.
- Thoppil, R.-J.; Bhatia, D.; Barnes, K.-F.; Haznagy-Radnai, E.; Hohmann, J.; Darvesh, A.-S.; Bishayee, A. Black Currant Anthocyanins Abrogate Oxidative Stress through NRF2-mediated Antioxidant Mechanisms in a Rat Model of Hepatocellsular Carcinoma. Curr. Cancer Drug Tar. 2012, 12, 1244–1257. DOI: 10.2174/156800912803987968.
- Wei, T.; Ji, X.; Xue, J.; Yan, G.; Zhu, X.; Xiao, G. Cyanidin-3-O-Glucoside Represses Tumor Growth and Invasion in Vivo by Suppressing Autophagy via Inhibition of the JNK Signaling Pathways. Food Funct. 2021, 12, 387–396. DOI: 10.1039/D0FO02107E.
- Shi, N.; Riedl, K.-M.; Schwartz, S.-J.; Zhang, X.; Clinton, S.-K.; Chen, T. Efficacy Comparison of Lyophilised Black Raspberries and Combination of Celecoxib and PBIT in Prevention of Carcinogen-induced Oesophageal Cancer in Rats. J. Funct. Foods. 2016, 27, 84–94. DOI: 10.1016/j.jff.2016.08.044.
- Cassidy, A.; Mukamal, K.-J.; Liu, L.; Franz, M.; Eliassen, A.-H.; Rimm, E.-B. High Anthocyanin Intake is Associated with a Reduced Risk of Myocardial Infarction in Young and Middle-Aged Women. Circulation 2013, 127, 188–196. DOI: 10.1161/CIRCULATIONAHA.112.122408.
- Seeram, N.-P.; Zhang, Y.; Nair, M.-G. Inhibition of Proliferation of Human Cancer Cells and Cyclooxygenase Enzymes by Anthocyanidins and Catechins. Nutr. Cancer. 2003, 46, 101–106. DOI: 10.1207/S15327914NC4601_13.
- Mauray, A.; Milenkovic, D.; Besson, C.; Caccia, N.; Morand, C.; Michel, F.; Mazur, A.; Scalbert, A.; Felgines, C. Atheroprotective Effects of Bilberry Extracts in Apo E-Deficient Mice. J. Agric. Food Chem. 2009, 57, 11106–11111. DOI: 10.1021/jf9035468.
- Winter, A.-N.; Ross, E.-K.; Wilkins, H.-M.; Stankiewicz, T.-R.; Wallace, T.; Miller, K.; Linseman, D.-A. An Anthocyanin-Enriched Extract from Strawberries Delays Disease Onset and Extends Survival in the hSOD1G93A Mice Model of Amyotrophic Lateral Sclerosis. Nutr. Neurosci. 2018, 21, 414–426. DOI: 10.1080/1028415X.2017.1297023.
- Zhang, G.; Chen, S.; Zhou, W.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Anthocyanin Composition of Fruit Extracts from Lycium ruthenicum and their Protective Effect for Gouty Arthritis. Ind. Crop Prod. 2019, 129, 414–423. DOI: 10.1016/j.indcrop.2018.12.026.
- Xu, W.; Zhou, Q.; Yao, Y.; Li, X.; Zhang, J.-L.; Su, G.-H.; Deng, A.-P. Inhibitory Effect of Gardanblue Bluberry Anthocyanin Extracts on Liposaccharide-Stimulated Inflammation Response in RAW 264. 7 cells. J. Zheinjiang Uni-Sci. B 2016, 17, 425–436. DOI: 10.1631/jzus.B1500213.
- Zuo, Y.; Peng, C.; Liang, Y.; Ma, K.-Y.; Yu, H.; Chan, E. H.-Y.; Chen, Z.-Y. Black Rice Extract Extends the Lifespan of Fruit Flies. Food Funct. 2012, 3, 1271–1279. DOI: 10.1039/c2fo30135k.
- Ali, T.; Kim, T.; Rehman, S.-U.; Khan, M.-S.; Amin, F.-U.; Khan, M.; Ikram, M.; Kim, M.-O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mice Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 7, 6076–6093. DOI: 10.1007/s12035-017-0798-6.
- Papandreou, M.-A.; Dimakopoulou, A.; Linardaki, Z.-I.; Cordopatis, P.; Klimis-Zacas, D.; Margarity, M.; Lamari, F.-N. Effect of a Polyphenol-Rich Wild Blueberry Extract on cognitive Performance of Mice, Brain Antioxidant Markers and acetylcholinesterase Activity. Behav. Brain Res. 2009, 198, 352–358. DOI: 10.1016/j.bbr.2008.11.013.
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of Anthocyanin-Rich Cherry Juice for 12 Weeks Improves Memory and Cognition in Older Adults with Mild-to-Moderate Dementia. Eur. J. Nutr. 2017, 56, 333–341. DOI: 10.1007/s00394-015-1083-y.
- Boespflug, E.-L.; Eliassen, J.-C.; Dudley, J.-A.; Shidler, M.-D.; Kalt, W.; Summer, -S.-S.; Stein, A.-L.; Stover, A.-N.; Krikorian, R. Enhanced Neural Activation with Blueberry Supplementation in Mild Cognitive Impairment. Nutr. Neurosci. 2018, 21, 297–305. DOI: 10.1080/1028415X.2017.1287833.
- Grace, M.-H.; Ribnicky, D.-M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, -G.-G.; Raskin, I.; Lila, M.-A. Hypoglycemic Activity of a Novel Anthocyanin-Rich Formulation from Lowbush Blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009, 16, 406–415. DOI: 10.1016/j.phymed.2009.02.018.
- Pranprawit, A.; Heyes, J.-A.; Molan, A.-L.; Kruger, M.-C. Antioxidant Activity and Inhibitory Potential of Blueberry Extracts against Key Enzymes Relevant for Hyperglycemia. J. Food Biochem. 2015, 39, 109–118. DOI: 10.1111/jfbc.12094.
- Johnson, M.-H.; Lucius, A.; Meyer, T.; Gonzalez de Mejia, E. Cultivar Evaluation and Effect of Fermentation on Antioxidant Capacity and in Vitro Inhibition of α-Amylase and α-Glucosidase by Highbush Blueberry (Vaccinium corombosum). J. Agric. Food Chem. 2011, 59, 8923–8930. DOI: 10.1021/jf201720z.
- Kurimoto, Y.; Shibayama, Y.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H. Black Soybean Seed Coat Extract Ameliorates Hyperglycemia and Insulin Sensitivity via the Activation of AMP-Activated Protein Kinase in Diabetic Mice. J. Agric. Food Chem. 2013, 61, 5558–5564. DOI: 10.1021/jf401190y.
- Yan, F.; Dai, G.; Zheng, X. Mulberry Anthocyanin Extract Ameliorates Insulin Resistance by Regulating PI3K/AKT Pathway in HepG2 Cells and db/db Mice. J. Nutr. Biochem. 2016, 36, 68–80. DOI: 10.1016/j.jnutbio.2016.07.004.
- Zhu, Y.; Sun, H.; He, S.-D.; Lou, Q.-Y.; Yu, M.; Tang, -M.-M.; Tu, L.-J. Metabolism and Prebiotics Activity of Anthocyanins from Black Rice (Oryza sativa L.) in Vitro. PLoS ONE. 2018, 13, 0195754. DOI: 10.1371/journal.pone.0195754.
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of Colorectal Cancer by Black Raspberry Anthocyanins Involved the Modulation of Gut Microbiota and SFRP2 Demethylation. Carcinogenesis. 2018, 39, 471–481. DOI: 10.1093/carcin/bgy009.
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota in Vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. DOI: 10.1021/acs.jafc.6b00586.
- Lee, S.; Keirsey, K.-I.; Kirkland, R.; Grunewald, Z.-I.; Fischer, J.-G.; de La Serre, C.-B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. DOI: 10.1093/jn/nxx027.
- Marko, D.; Puppel, N.; Tjaden, Z.; Jakobs, S.; Pahlke, G. The Substitution Pattern of Anthocyanidins Affects Different Cellsular Signaling Cascades Regulating Cells Proliferation. Mol. Nutr. Food Res. 2004, 4, 318–325. DOI: 10.1002/mnfr.200400034.
- Wallace, T.-C.; Slavin, M.; Frankenfeld, C.-L. Systematic Review of Anthocyanins and Markers of Cardiovascular Disease. Nutrients. 2016, 8, 32. DOI: 10.3390/nu8010032.
- Juránek, I.; Bezek, S. Controversy of Free Radical Hypothesis: Reactive Oxygen Species-Cause or Consequence of Tissue Injury?. Gen. Physiol. Biophys. 2005, 24, 263–278. DOI: 10.1016/j.colsurfb.2005.04.016.
- Hori, M.; Nishida, K. Oxidative Stress and Left Ventricular Remodelling after Myocardial Infarction. Cardiovasc. Res. 2009, 81, 457–464. DOI: 10.1093/cvr/cvn335.
- Cutler, B.-R.; Petersen, C.; Anandh Babu, P.-V. Mechanistic Insights into the Vascular Effects of Blueberries: Evidence from Recent Studies. Mol. Nutr. Food Res. 2017, 61, 1600271. DOI: 10.1002/mnfr.201600271.
- Muñoz-Espada, A.-C.; Watkins, B.-A. Cyanidin Attenuates PGE2 Production and Cyclooxygenase-2 Expression in LNCaP Human Prostate Cancer Cells. J. Nutr. Biochem. 2006, 17, 589–596. DOI: 10.1016/j.jnutbio.2005.10.007.
- Mulabagal, V.; Lang, G.-A.; DeWitt, D.-L.; Dalavoy, -S.-S.; Nair, M.-G. Anthocyanin Content, Lipid Peroxidation and Cyclooxygenase Enzyme Inhibitory Activities of Sweet and Sour Cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. DOI: 10.1021/jf8032039.
- Graf, D.; Seifert, S.; Jaudszus, A.; Bub, A.; Watzl, B.; Gaetani, S. Anthocyanin-Rich Juice Lowers Serum Cholesterol, Leptin, and Resistin and Improves Plasma Fatty Acid Composition in Fischer Rats. PLoS ONE. 2013, 8, 66690. DOI: 10.1371/journal.pone.0066690.
- Cassidy, A.; O’Reilly, É.-J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.-P.; Curhan, G.; Rimm, E.-B. Habitual Intake of Flavonoid Subclasses and Incident Hypertension in Adults. Am. J. Clin. Nutr. 2011, 93, 338–347. DOI: 10.3945/ajcn.110.006783.
- Shaughnessy, K.-S.; Boswall, I.-A.; Scanlan, A.-P.; Gottschall-Pass, K.-T.; Sweeney, M.-I. Diets Containing Blueberry Extract Lower Blood Pressure in Spontaneously Hypertensive Stroke-Prone Rats. Nutr. Res. 2009, 29, 130–138. DOI: 10.1016/j.nutres.2009.01.001.
- Yoshimoto, M.; Okuno, S.; Yamaguchi, M.; Osamu, Y. Antimutagenicity of Deacylated Anthocyanins in Purple-Fleshed Sweet potato. Biosci. Biotechnol. Biochem. 2011, 65, 1652–1655. DOI: 10.1271/bbb.65.1652.
- Li, P.; Feng, D.; Yang, D.; Li, X.; Sun, J.; Wang, G.; Tian, L.; Jiang, X.; Bai, W. Protective Effects of Anthocyanins on Neurodegenerative Diseases. Trends Food Sci. Technol. 2021, 117, 205–217. DOI: 10.1016/j.tifs.2021.05.005.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules. 2020, 25, 3809. DOI: 10.3390/molecules25173809.
- Shishtar, E.; Rogers, G.-T.; Blumberg, J.-B.; Au, R.; Jacques, P.-F. Long-term Dietary Flavonoid Intake and Change in Cognitive Function in the Framingham Offspring Cohort. Public. Health. Nutr. 2020, 23, 1576–1588. DOI: 10.1017/S136898001900394X.
- Ramirez, M.-R.; Izquierdo, I.; Do Carmo Bassols Raseira, M.; Zuanazzi, J.-A.; Barros, D.; Henriques, A.-T. Effect of Lyophilised Vaccinium Berries on Memory, Anxiety and Locomotion in Adult Rats. Pharmacol. Res. 2005, 52, 457–462. DOI: 10.1016/j.phrs.2005.07.003.
- Zhang, C.; Lu, X.; Tan, Y.; Li, B.; Miao, X.; Jin, L.; Shi, X.; Zhang, X.; Miao, L.; Li, X.-K.; et al. Diabetes-induced Hepatic Pathogenic Damage, Inflammation, Oxidative Stress, and Insulin Resistance was Exacerbated in Zinc Deficient Mice Model. PLoS ONE. 2017, 7, 49257. DOI: 10.1371/journal.pone.0049257.
- Manna, P.; Das, J.; Ghosh, J.; Sil, P.-C. Contribution of Type 1 Diabetes to Rat Liver Dysfunction and Cellsular Damage Via Activation of NOS, PARP, IκBα/NF-κB, MAPKs, and Mitochondria-Dependent Pathways: Prophylactic Role of Arjunolic Acid. Free Radical Biol. Med. 2010, 48, 1465–1484. DOI: 10.1016/j.freeradbiomed.2010.02.025.
- Kam, J.; Puranik, S.; Yadav, R.; Manwaring, H.-R.; Pierre, S.; Srivastava, R.-K.; Yadav, R.-S. Dietary Interventions for Type 2 Diabetes: How Millet Comes to Help. Front. Plant Sci. 2016, 7, 1454. DOI: 10.3389/fpls.2016.01454.
- Sui, X.; Zhang, Y.; Zhou, W. In Vitro and in Silico Studies of the Inhibition Activity of Anthocyanins against Porcine Pancreatic Alpha-Amylase. J. Funct. Foods. 2016, 21, 50–57. DOI: 10.1016/j.jff.2015.11.042.
- Adisakwattana, S.; Ngamrojanavanich, N.; Kalampakorn, K.; Tiravanit, W.; Roengsumran, S.; Yibchok-Anun, S. Inhibitory Activity of Cyanidin-3-Rutinoside on Alpha-Glucosidase. J. Enzyme Inhib. Med. Chem. 2004, 19, 313–316. DOI: 10.1080/14756360409162443.
- Staudacher, H.-M.; Loughman, A. Gut health: Definitions and Determinants. Lancet Gastroenterol. Hepatol. 2021, 6, 269. DOI: 10.1016/S2468-1253(21)00071-6.
- Gan, Y.; Fu, Y.; Yang, L.; Chen, J.; Lei, H.; Liu, Q. Cyanidin-3-O-Glucoside and Cyanidin Protect against Intestinal Barrier Damage and 2, 4, 6-Trinitrobenzenesulfonic Acid-Induced Colitis. J. Med. Food. 2019, 23, 90–99. DOI: 10.1089/jmf.2019.4524.
- Xia, Y.; Tian, L.-M.; Liu, Y.; Guo, K.-S.; Lv, M.; Li, Q.-T.; Hao, S.-Y.; Ma, C.-H.; Chen, Y.-X.; Tanaka, M. Low Dose of Cyanidin-3-O-Glucoside Alleviated Dextran Sulfate Sodium–Induced Colitis, Mediated by CD169+ Macrophage Pathway. Inflammatory Bowel Dis. 2019, 25, 1510–1521. DOI: 10.1093/ibd/izz090.
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.-S.; Bashllari, R.; Busà, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the in Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediators Inflamm. 2017, 2017, 1–8. DOI: 10.1155/2017/3454023.
- Cheng, Z.; Si, X.; Tan, H.; Zang, Z.; Tian, J.; Shu, C.; Sun, X.; Li, Z.; Jiang, Q.; Meng, X. Cyanidin-3-O-Glucoside and Its Phenolic Metabolites Ameliorate Intestinal Diseases via Modulating Intestinal Mucosal Immune System: Potential Mechanisms and Therapeutic Strategies. Crit. Rev. Food Sci. Nutr. 2021, Online early access]. DOI: 10.1080/10408398.2021.1966381. Published Online: August 23, 2021.
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients. 2019, 11, 2435. DOI: 10.3390/nu11102435.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Brit. J. Pharmacol. 2017, 174, 1244–1262. DOI: 10.1111/bph.13630.
- Thiruchenduran, M.; Vijayan, N.-A.; Sawaminathan, J.-K.; Devaraj, S.-N. Protective Effect of Grape Seed Proanthocyanidins against Cholesterol Cholic Acid Diet-Induced Hypercholesterolemia in Rats. Cardiovasc. Pathol. 2011, 20, 361–368. DOI: 10.1016/j.carpath.2010.09.002.
- Larrauri, M.; Zunino, M.-P.; Zygadlo, J.-A.; Grosso, N.-R.; Nepote, V. Chemical Characterization and Antioxidant Properties of Fractions Separated from Extract of Peanut Skin Derived from Different Industrial Processes. Ind. Crop Prod. 2016, 94, 964–971. DOI: 10.1016/j.indcrop.2016.09.066.
- Pajuelo, D.; Quesada, H.; Díaz, S.; Fernández-Iglesias, A.; Arola-Arnal, A.; Bladé, C.; Salvadó, J.; Arola, L. Chronic Dietary Supplementation of Proanthocyanidins Corrects the Mitochondrial Dysfunction of Brown Adipose Tissue Caused by Diet-Induced Obesity in Wistar Rats. Brit. J. Nutr. 2012, 107, 170–178. DOI: 10.1017/S0007114511002728.
- Puiggròs, F.; Llópiz, N.; Ardévol, A.; Bladé, C.; Arola, L.; Salvadó, M.-J. Grape Seed Procyanidins Prevent Oxidative Injury by Modulating the Expression of Antioxidant Enzyme Systems. J. Agric. Food Chem. 2005, 53, 6080–6086. DOI: 10.1021/jf050343m.
- Shirataki, Y.; Kawase, M.; Saito, S.; Kurihara, T.; Tanaka, W.; Satoh, K.; Sakagami, H.; Motohashi, N. Selective Cytotoxic Activity of Grape Peel and Seed Extracts against Oral Tumor Cells Lines. Anticancer Res. 2000, 20, 423–426. DOI: 10.1016/S0010-2180(00)00136-X.
- Kresty, L.-A.; Howell, A.-B.; Baird, M. Cranberry Proanthocyanidins Induce Apoptosis and Inhibit Acid-Induced Proliferation of Human Esophageal Adenocarcinoma Cells. J. Agric. Food Chem. 2008, 56, 676–680. DOI: 10.1021/jf071997t.
- Kresty, L.-A.; Howell, A.-B.; Baird, M. Cranberry Proanthocyanidins Mediate Growth Arrest of Lung Cancer Cells through Modulation of Gene Expression and Rapid Induction of Apoptosis. Molecules. 2011, 16, 2375–2390. DOI: 10.3390/molecules16032375.
- Minker, C.; Duban, L.; Karas, D.; Järvinen, P.; Lobstein, A.; Muller, C.-D.; Vauzour, D. Impact of Procyanidins from Different Berries on Caspase 8 Activation in Colon Cancer. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. DOI: 10.1155/2015/154164.
- Chen, Q.; Liu, X.-F.; Zheng, P.-S.; Yang, B.-B. Grape Seed Proanthocyanidins (GSPs) Inhibit the Growth of Cervical Cancer by Inducing Apoptosis Mediated by the Mitochondrial Pathway. PLoS ONE. 2014, 9, 107045. DOI: 10.1371/journal.pone.0107045.
- Huang, -L.-L.; Pan, C.; Wang, L.; Ding, L.; Guo, K.; Wang, H.-Z.; Xu, A.-M.; Gao, S. Protective Effects of Grape Seed Proanthocyanidins on Cardiovascular Remodeling in DOCA-salt Hypertension Rats. J. Nutr. Biochem. 2015, 26, 841–849. DOI: 10.1016/j.jnutbio.2015.03.007.
- Razavi, S. -M.; Gholamin, S.; Eskandari, A.; Mohsenian, N.; Ghorbanihaghjo, A.; Delazar, A.; Rashtchizdeh, N.; Keshtkar-Jahromi, M.; Argani, H. Red Grape Seed Extract Improves Lipid Profiles and Decreases Oxidized Low-Density Lipoprotein in Patients with Mild Hyperlipidemia. J. Med. Food. 2013, 16, 255–258. DOI: 10.1089/jmf.2012.2408.
- Vinson, J.-A.; Mandarano, M.-A.; Shuta, D.-L.; Bagchi, M.; Bagchi, D. Beneficial Effects of a Novel IH636 Grape Seed Proanthocyanidin Extract and Aniacin-Bound Chromium in a Hamster Atherosclerosis Model. Mol. Cell. Biochem. 2002, 240, 99–103. DOI: 10.1023/A:1020611925819.
- Baiges, I.; Palmfeldt, J.; Bladé, C.; Gregersen, N.; Arola, L. Lipogenesis is Decreased by Grape Seed Proanthocyanidins According to Liver Proteomics of Rats Fed a High Fat Diet. Mol. Cell. Proteomics. 2010, 9, 1499–1513. DOI: 10.1074/mcp.M000055-MCP201.
- Toomer, O.-T.; Vu, T.; Pereira, M.; Williams, K. Dietary Supplementation with Peanut Skin Polyphenolic Extracts (PSPE) Reduces Hepatic Lipid and Glycogen Stores in Mice Fed an Atherogenic Diet. J. Funct. Foods. 2019, 55, 362–370. DOI: 10.1016/j.jff.2019.02.041.
- Gonzalez-Albuin, N.; Pinent, M.; Casanova-Marti, A.; Arola, L.; Blay, M.; Ardevol, A. Procyanidins and their Healthy Protective Effects Against Type 2 Diabetes. Curr. Med. Chem. 2015, 22, 39–50. DOI: 10.2174/0929867321666140916115519.
- Tsujita, T.; Shintani, T.; Sato, H. Preparation and Characterisation of Peanut Seed Skin Polyphenols. Food Chem. 2014, 151, 15–20. DOI: 10.1016/j.foodchem.2013.11.072.
- Liu, X.; Qiu, J.; Zhao, S.; You, B.; Ji, X.; Wang, Y.; Cui, X.; Wang, Q.; Gao, H. Grape Seed Proanthocyanidin Extract Alleviates Ouabain-Induced Vascular Remodeling through Regulation of Endothelial Function. Mol. Med. Rep. 2012, 6, 949–954. DOI: 10.3892/mmr.2012.1026.
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory Effect of Grape-Seed Procyanidins on Local and Systemic Inflammation in Diet-Induced Obesity Rats. J. Nutr. Biochem. 2011, 22, 380–387. DOI: 10.1016/j.jnutbio.2010.03.006.
- La, V.-D.; Howell, A.-B.; Grenier, D. Cranberry Proanthocyanidins Inhibit MMP Production and Activity. J. Dent. Res. 2009, 88, 627–632. DOI: 10.1177/0022034509339487.
- Feldman, M.; Tanabe, S.; Howell, A.; Grenier, D. Cranberry Proanthocyanidins Inhibit the Adherence Properties of Candida albicans and Cytokine Secretion by Oral Epithelial Cells. BMC Complem. Altern. M. 2012, 12, 6. DOI: 10.1186/1472-6882-12-6.
- Lee, T.; Kwon, H.-S.; Bang, B.-R.; Lee, Y.-S.; Park, M.-Y.; Moon, K.-A.; Kim, T.-B.; Lee, K.-Y.; Moon, H.-B.; Cho, Y.-S. Grape Seed Proanthocyanidin Extract Attenuates Allergic Inflammation in Murine Models of Asthma. J. Clin. Immunol. 2012, 32, 1292–1304. DOI: 10.1007/s10875-012-9742-8.
- Jiang, X.; Liu, J.; Lin, Q.; Mao, K.; Tian, F.; Jing, C.; Wang, C.; Ding, L.; Pang, C. Proanthocyanidin Prevents Lipopolysaccharide-Induced Depressive-Like Behavior in Mice Via Neuroinflammatory Pathway. Brain Res. Bull. 2017, 135, 40–46. DOI: 10.1016/j.brainresbull.2017.09.010.
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.-A.; Ma, X. Dietary Grape Seed Proanthocyanidins (Gsps) Improve Weaned Intestinal Microbiota and Mucosal Barrier Using a Piglet Model. Oncotarget. 2016, 7, 80313–80326. DOI: 10.18632/oncotarget.13450.
- Rupasinghe, H.-P.-V.; Parmar, I.; Neir, S.-V. Biotransformation of Cranberry Proanthocyanidins to Probiotic Metabolites by Lactobacillus rhamnosus Enhances their Anticancer Activity in HepG2 Cells in Vitro. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. DOI: 10.1155/2019/4750795.
- Casanova-Martí, À.; Serrano, J.; Portune, K.-J.; Sanz, Y.; Blay, M.-T.; Terra, X.; Ardévol, A.; Pinent, M. Grape Seed Proanthocyanidins Influence Gut Microbiota and Enteroendocrine Secretions in Rats. Food Funct. 2018, 9, 1672–1682. DOI: 10.1039/c7fo02028g.
- Lai, R.; Xia, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel Treatment for Psoriasis that Reduces Oxidative Stress and Modulates Th17 and Treg Cells. Redox Rep. 2018, 23, 130–135. DOI: 10.1080/13510002.2018.1462027.
- Bagchi, D.; Bagchi, M.; Stohs, S.-J.; Das, D.-K.; Ray, S.-D.; Kusszynski, C.-A.; Joshi, -S.-S.; Pruess, H.-G. Free radicals and Grape Seed Proanthocyanidin Extract: Importance in Human Health and disease Prevention. Toxicology. 2000, 148, 187–197. DOI: 10.1016/S0300-483X(00)00210-9.
- Bagchi, D.; Swaroop, A.; Preuss, H.-G.; Bagchi, M. Free radicals Scavenging, Antioxidant and Cancer Chemoprevention by Grape Seed Proanthocyanidin: An Overview. Mutat. Res. 2014, 768, 69–73. DOI: 10.1016/j.mrfmmm.2014.04.004.
- Maldonado, P.-D.; Rivero-Cruz, I.; Mata, R.; Pedraza-Chaverrí, J. Antioxidant Activity of A-type Proanthocyanidins from Geranium niveum (Geraniaceae). J. Agric. Food Chem. 2005, 53, 1996–2001. DOI: 10.1021/jf0483725.
- Weh, K.-M.; Salzman, N.-H.; Howell, A.-B.; Clarke, J.-L.; Tripp, B.-A.; Kresty, L.-A. Cranberry Proanthocyanidins Reverse Microbial Dysbiosis and inhibit Bile Acid Metabolism in Association with Esophageal Cancer Prevention. Cancer Res. 2017, 77, 5250. DOI: 10.1158/1538-7445.AM2017-5250.
- Vaid, M.; Katiyar, S.-K. Grape Seed Proanthocyanidins Inhibit Cigarette Smoke Condensate–Induced Lung Cancer Cells Migration through Inhibition of NADPH Oxidase and Reduction in the Binding of p22phox and p47phox Proteins. Mol. Carcinog. 2015, 54, 61–71. DOI: 10.1002/mc.22173.
- Ramljak, D.; Romanczyk, L.-J.; Metheny-Barlow, L.-J.; Thompson, N.; Knezevic, V.; Galperin, M.; Ramesh, A.; Dickson, R.-B. Pentameric Procyanidin from Theobroma Cacao Selectively Inhibits Growth of Human Breast Cancer Cells. Mol. Cancer Ther. 2005, 4, 537–546. DOI: 10.1158/1535-7163.MCT-04-0286.
- Liu, J.; Bai, J.; Jiang, G.; Li, X.; Wang, J.; Wu, D.; Owusu, L.; Zhang, E.; Li, W. Anti-tumor Effect of Pinus massoniana Bark Proanthocyanidins on Ovarian Cancer through Induction of Cells Apoptosis and Inhibition of Cells Migration. PLoS ONE. 2015, 10, 0142157. DOI: 10.1371/journal.pone.0142157.
- Nie, C.; Zhou, J.; Qin, X.; Shi, X.; Zeng, Q.; Liu, J.; Yan, S.; Zhang, L. Reduction of Apoptosis by Proanthocyanidin-Induced Autophagy in Thehuman Gastric Cancer Cells Line MGC-803. Oncol. Rep. 2016, 35, 649–658. DOI: 10.3892/or.2015.4419.
- Luan, -Y.-Y.; Liu, Z.-M.; Zhong, J.-Y.; Yao, R.-Y.; Yu, H.-S. Effect of Grape Seed Proanthocyanidins on Tumor Vasculogenic Mimicry in Human Triple-Negative Breast Cancer Cells. Asian Pac. J. Cancer P. 2015, 16, 531–535. DOI: 10.7314/APJCP.2015.16.2.531.
- Al-Mallah, M.-H.; Sakr, S.; Al-Qunaibet, A. Cardiorespiratory Fitness and Cardiovascular Disease Prevention: An Update. Curr. Atheroscler. Rep. 2018, 20, 1–9. DOI: 10.1007/s11883-018-0711-4.
- Bladé, C.; Arola, L.; Salvadó, M.-J. Hypolipidemic Effects of Proanthocyanidins and their Underlying Biochemical and Molecular Mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. DOI: 10.1002/mnfr.200900476.
- Shi, J.; Yu, J.; Pohorly, J.-E.; Kakuda, Y. Polyphenolics in Grape Seeds-Biochemistry and Functionality. J. Med. Food. 2003, 6, 291–299. DOI: 10.1089/109662003772519831.
- Kruger, M.-J.; Davies, N.; Myburgh, K.-H.; Lecour, S. Proanthocyanidins, Anthocyanins and Cardiovascular Diseases. Food Res. Int. 2014, 59, 41–52. DOI: 10.1016/j.foodres.2014.01.046.
- Lee, -C.-C.; Kim, J.-H.; Kim, J.-S.; Oh, Y.-S.; Han, S.-M.; Park, J. H.-Y.; Lee, K.-W.; Lee, C.-Y. 5-(3′, 4′-Dihydroxyphenyl-γ-Valerolactone), A Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion. Int. J. Mol. Sci. 2017, 18, 1363. DOI: 10.3390/ijms18071363.
- Liu, T.; Song, L.; Wang, H.; Huang, D. A High-Throughput Assay for Quantification of Starch Hydrolase Inhibition Based on Tuidity Measurement. J. Agric. Food Chem. 2011, 59, 9756–9762. DOI: 10.1021/jf202939d.
- Cui, X.; Liu, X.; Feng, H.; Zhao, S.; Gao, H. Grape Seed Proanthocyanidin Extracts Enhance Endothelial Nitric Oxide Synthase Expression through 5’-AMP Activated Protein Kinase/Surtuin 1-Krupple like Factor 2 Pathway and Modulate Blood Pressure in Ouabain Induced Hypertensive Rats. Biol. Pharm. Bull. 2012, 35, 2192–2197. DOI: 10.1248/bpb.b12-00598.
- Tamura, T.; Ozawa, M.; Tanaka, N.; Arai, S.; Mura, K. Bacillus cereus Response to a Proanthocyanidin Trimer, A Transcriptional and Functional Analysis. Curr. Microbiol. 2016, 73, 115–123. DOI: 10.1007/s00284-016-1032-x.
- Salinas-Sánchez, D.-O.; Jiménez-Ferrer, E.; Sánchez-Sánchez, V.; Zamilpa, A.; González-Cortazar, M.; Tortoriello, J.; Herrera-Ruiz, M. Anti-Inflammatory Activity of a Polymeric Proanthocyanidin from Serjania schiedeana. Molecules. 2017, 22, 863. DOI: 10.3390/molecules22060863.
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety Evaluation of Fungal Pigments for Food Applications. J. Fungi. 2021, 7, 692. DOI: 10.3390/jof7090692.
- Huang, X.-D.; Liang, J.-B.; Tan, H.-Y.; Rosiyah, Y.; Long, R.-J.; Ho, Y.-W. Protein-Binding Affinity of Leucaena Condensed Tannins of Differing Molecular Weights. J. Agric. Food Chem. 2011, 59, 10677–100682. DOI: 10.1021/jf201925g.
- Taverniti, V.; Fracassetti, D.; Del Bo’, C.; Lanti, C.; Minuzzo, M.; Klimis-Zacas, D.; Riso, P.; Guglielmetti, S. Immunomodulatory Effect of a Wild Blueberry Anthocyanin-Rich Extract in Human Caco-2 Intestinal Cells. J. Agric. Food Chem. 2014, 62, 8346–8351. DOI: 10.1021/jf502180j.
- Gomes, J.-V.-P.; Rigolon, T.-C.-B.; Da Silveira Souza, M.-S.; Alvarez-Leite, J.-I.; Lucia, C.-M.-D.; Martino, H.-S.-D.; Rosa, C. D. O. B. Antiobesity Effects of Anthocyanins on Mitochondrial Biogenesis, Inflammation, and Oxidative Stress: A Systematic Review. Nutrition. 2019, 66, 192–202. DOI: 10.1016/j.nut.2019.05.005.