ABSTRACT
In recent years, seeds and pseudocereals, such as chia, sesame, flax or quinoa, have been incorporated into different food products, improving their nutritional profile and providing benefits for human health due to their composition. However, the addition of these ingredients to new formulations of foods can also contribute to a higher exposure to compounds that are harmful to health such as chemical process contaminants or certain allergens, among others. This review provides an overview about the composition of these ingredients, the benefits/risks associated with their addition in bakery products, and their impact on sensory attributes of these new food formulations.
Introduction
In recent decades, there has been an increase in non-communicable diseases (NCDs), responsible for 41 million deaths per year. Nearly 80% of premature deaths from NCDs are due to cardiovascular disease (CVD), cancer and diabetes, all of them related to poor dietary habits.[Citation1] In 2015, CVDs accounted for 31% of all deaths recorded worldwide.[Citation2] Between 2000 and 2016, premature mortality due to diabetes increased by 5% and 1.5 million deaths worldwide were considered to be caused by this disease in 2019.[Citation3] In addition, in 2016, more than 1.9 billion adults over the age of 18 (39%) were overweight, of which 650 million (13%) were obese.[Citation4] Recent assessments suggest that overweight and obesity cause more than 1.2 million deaths annually in the WHO European Region, being the fourth most important cause of death (13% of all deaths).[Citation5] At this point, as unhealthy dietary habits can increase the incidence of overweight and obesity, acting on dietary habits appears to be urgent in reducing the risk of the population.
The population has moved away from traditional dietary habits that include a balanced intake of nutrients applying classical culinary techniques to more complex diets that increase the presence of industrially processed foods. For instance, the beneficial effects associated with higher adherence to the Mediterranean diet in reference to the prevention/management of age-associated NCDs are well-known and reinforced by many prospective observational studies and trials in diverse populations. However, countries from the Mediterranean area are getting away from this healthy diet pattern.[Citation6] The consumption of ultraprocessed foods (UPFs) is becoming dominant in the diet of the world population, contributing between 25–50% of energy intake.[Citation7,Citation8] These are foods with a poor nutritional quality made through intense processes such as extrusion or frying, with the addition of colorants, flavorings, inverted sugar or other additives, resulting in hyperpalatable products.[Citation9] UPFs present dietary risks such as high concentrations of sodium, trans and saturated fats and added sugars. Therefore, the consumption of UPFs together with a low intake of whole grains, fruits and vegetables and a high consumption of animal protein, can favor the development of NCDs.[Citation7]
Due to the increase in diseases associated with diet, consumers are demanding healthier products with beneficial properties for health, thus emerging functional foods.[Citation10] Functional foods are natural or processed foods that contain biologically active compounds, which in effective and non-toxic amounts have beneficial effects on health.[Citation11] One of these effects is the prevention of diseases such as diabetes or CVD linked to compounds such as phytochemicals (polyphenols, carotenoids or tocopherols), vitamins and minerals, carbohydrates, lipids or proteins. These compounds can also have an antioxidant, anti-inflammatory effect or prevent osteoporosis.[Citation12] All these health benefits may explain the consumer behavior toward functional foods, which can also be selected focusing on factors such as convenience, price, preferences, taste, and other sensory attributes.[Citation10,Citation13]
The food industry tends towards the inclusion of new ingredients in food formulations already on the market, thus improving their nutritional and healthy properties. In this sense, ingredients such as seeds, legumes, vegetables, spices, different types of cereals or various protein sources are incorporated into meats, snacks, dairy products or formulations of cereal-based foods.[Citation14–17] Among these new formulations are bakery products, which are easy to modify and allow the incorporation of new ingredients such as legumes, insects as a valuable source of protein, vegetables, seeds or pseudocereals.[Citation17]
Seeds and pseudocereals are often included in foods to meet the consumers’ demand for gluten-free products. This market has seen exponential growth in recent years due to the increase in diseases related to gluten intake (celiac disease), non-celiac sensitivity to gluten and wheat allergy, as well as a greater demand from sector of the population that opts for a gluten-free diet because it is considered healthier.[Citation18,Citation19] Gluten-free foods are known to have lower nutritional quality than their gluten-containing analogues, as they have higher concentrations of fats, sugars, and sodium, and low concentrations of protein, minerals, and dietary fiber.[Citation19] For this reason, new raw materials are being sought to improve the nutritional, technological and sensory characteristics of these products, thus including seeds and pseudocereals in the formulations, which are naturally gluten-free products with a high nutritional value.[Citation20]
Some seeds and pseudocereals such as chia, quinoa, sesame or flax are especially used in cereal-based foods. These seeds can be added as unprocessed seeds, roasted seeds, ground seed or as flour either fortifying wheat flour, as is the case of chia, sesame or flax,[Citation21–23] or completely replacing wheat flour, as in the case of quinoa.[Citation24] When seeds contain a high percentage of lipids, they are also usually added as defatted flour.[Citation25,Citation26] Due to their composition, the incorporation of these seeds into new formulations can have beneficial health effects and further improve the organoleptic properties because of the contribution of new aromas, tastes and flavors. However, at the same time, the addition of these ingredients can negatively impact the final product, such as the incorporation of antinutrients or facilitate the formation of chemical contaminants generated during food processing. These aspects need to be considered when designing new cereal-based products that aim to improve food from a health point of view without compromising food safety.
The increasing research on chia, quinoa, sesame, and flax as new ingredients of cereal foods in recent decades () indicates a growing interest, in which their technical properties as well as their nutritional properties are mainly evaluated in the last decades. However, there are fewer studies on the toxicological properties or the potential risk posed by the incorporation of these seeds into other food products, accounting for only 5–15% of all publications on this topic (). In this context, the present review aims at compiling the most relevant scientific information about these seeds as examples of new ingredients found in bakery products consumed by the general public on a daily basis. This article will improve the understanding of benefits/risks associated with the consumption of chia, quinoa, sesame, and flax seeds when incorporated in bakery products, and their impact on sensory attributes of these new food formulations.
Figure 1. Number of articles published in the last 20 years (2002–2022) containing the words “chia/quinoa/sesame/flax” and “bakery products”. Source = Web of Science Core Collection (WOS).
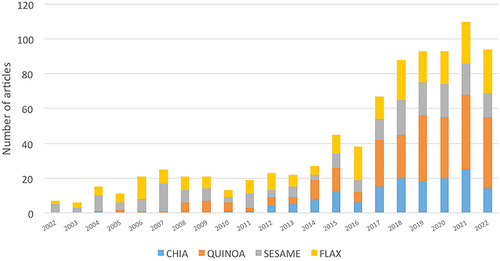
Figure 2. Percentage of scientific reports on chia, quinoa, sesame and flax seeds over the last 20 years (2002–2022) distributed in research areas (chemistry, toxicology, nutrition & dietetics, food science & technology, business & economics, and agriculture with the keywords “chia/quinoa/sesame/flax” and “bakery products”. Source = Web of Science Core Collection (WOS).
Chia
Chia (Salvia hispanica L.) is an herbaceous plant belonging to the Lamiaceae family. It can reach a meter in height and has toothed leaves, arranged in an opposite way with a length of 4–8 cm and between 3–5 cm wide.[Citation27] Chia grows naturally in tropical and subtropical areas such as Mexico, Argentina, Peru, Paraguay, Ecuador or Australia, although in Europe these crops are usually grown in greenhouses.[Citation28] It is optimally established at altitudes between 400 and 2,500 m[Citation29] and at optimum temperatures between 16–26°C.[Citation30] Chia has round fruits with small oval seeds 2 mm long and 1 mm wide, smooth surface and colors ranging from white to brown and, in some cases, irregularly arranged black spots.[Citation27,Citation31]
Chia has a high nutritional value mainly due to its high content of dietary fiber and polyunsaturated fatty acids. Chia seed contains approximately 18–40% fiber, of which 85–93% corresponds to insoluble fiber and the remaining 7–15% to soluble fiber[Citation27,Citation28,Citation31,Citation32] (). The fat content represents 25–40% of the seed, highlighting the high content of polyunsaturated fatty acids, especially from the ω-3 group. The most representative is α-linolenic acid, which constitutes 60% of the total fatty acids.[Citation28,Citation31] Seeds also contain 18–24% protein and are rich in amino acids like arginine, leucine, phenylalanine, glutamic acid, aspartic acid, alanine, serine and glycine. Regarding minerals and vitamins, high concentrations of phosphorus, calcium, potassium and magnesium and of group B vitamins such as thiamine (B1), riboflavin (B2) and niacin (B3) have been described.[Citation27,Citation28,Citation33] Compared to the chemical composition of wheat, chia seeds present a higher content of protein, fiber, and lipids, which can be very interesting when formulating new products where the nutritional composition wants to be improved.[Citation34,Citation35]
Table 1. Nutritional composition of chia, quinoa, sesame and flax seeds compared with wheat. Data are expressed per 100 g of sample.
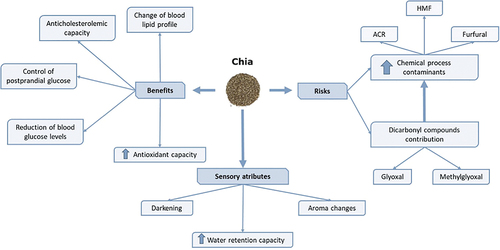
The great interest on chia is due to the fact that this seed is a source of bioactive compounds, predominating the polyphenols group, where phenolic compounds such as gallic, caffeic, chlorogenic, cinnamic acid, or ferulic acid stand out. Total phenol contents of 3.07 mg gallic acid equivalent (GAE)/g, with 31.14 mg/kg of caffeic acid as the most abundant individual phenolic compound.[Citation36] Furthermore, it is rich in isoflavones such as daidzein, glycitein or genistein and in tocopherols such as α-tocopherol, γ-tocopherol and δ-tocopherol.[Citation28,Citation31] On the other hand, chia contains mucilage, a highly viscous heteropolysaccharide made up of large molecules of sugars and uronic acids linked by glycosidic bonds.[Citation37,Citation38] The main constituent of mucilage is soluble fiber (72%) that gives it a high water retention capacity, in addition to proteins (10%) that favor emulsification and a small fraction of lipids (<1%).[Citation37,Citation39,Citation40] In recent years, because the high demand for vegan or low-fat products, chia mucilage has aroused great interest in the food industry since it has structuring, texturizing, emulsifying, and thickening properties[Citation38,Citation41] being used in numerous products as a fat substitute.[Citation39] Due to the water retention capacity of mucilage, it is being used in bakery products to prevent excessive water loss during baking. It also provides viscosity to the masses, preventing the loss of gases during cooking and improving then the specific volume of the products.[Citation42] In addition, chia mucilage is being used as an emulsifier due to the presence of lipids, contributing to the stability of products, and as an egg substitute, with similar emulsifying properties.[Citation38,Citation41]
Benefits associated with the consumption of chia seeds
The composition in nutrients and antioxidant compounds makes chia seeds to have beneficial properties for health. For that reason, the food industry is introducing these seeds in their products to improve the nutritional value. Numerous studies have shown that chia have a high antioxidant capacity associated with the composition in phenolic compounds, mainly chlorogenic acid, caffeic acid and flavonols,[Citation33] although certain fatty acids such as α-linolenic and linoleic acid may also be involved.[Citation31] Sargi et al.[Citation43] reported that chia seeds are able to deactivate 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) cationic radicals, reducing iron ions, and scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals. This property was also described by Coelho and Salas-Mellado[Citation44] who determined that phenolic compounds extracted from chia seeds could trap 70% of DPPH radicals. The high content of antioxidant compounds has prompted the food industry to use this seed in the development of new products where it is intended to protect lipids from lipid oxidation during storage or thermal processing.[Citation28] However, it must be taken into account that the initial contents of the raw material may be altered during processing, since some phytochemicals are sensitive to heat treatment and hence require proper attention during selection of a processing technique.[Citation36,Citation45]
Chia seeds also have anticholesterolemic capacity linked to the ability to modify the blood lipid profile by reducing cholesterol levels thanks to its content of fiber and ω-3 fatty acids. da Silva et al.[Citation46] observed that rats fed diets supplemented with chia seeds or flour had lower concentrations in blood of triglycerides, total cholesterol, low-density lipoproteins (LDL) and very low-density lipoproteins (VLDL) than those not consuming this supplementation. Similarly, rats including chia seeds in their diet increased their concentration of high-density lipoprotein (HDL) and ω-3 polyunsaturated fatty acids in blood plasma.[Citation47] Coelho et al.[Citation48] showed that the bioactive proteins and peptides present in chia seeds had the ability to block key markers in cholesterol synthesis such as 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase), reducing then the formation of corporal cholesterol.
Another positive property of chia seed is the control of postprandial glucose. Numerous studies have shown that the consumption of foods containing chia reduces blood glucose levels. Vuksan et al.[Citation49] demonstrated that the daily consumption of 24 g of chia reduced blood glucose levels 30 minutes after eating (from 2.10 mmol/L in with the control treatment to 1.52 mmol/L with these experimental conditions), confirming that chia intake could reduce postprandial glucose in healthy subjects. Ho et al.[Citation50] reported that the format in which chia is added to food (seed or flour) does not affect the reduction of glucose levels. This property is due to the content of high viscosity dietary fiber present mainly in the mucilage, which can alter the rate of digestibility and absorption of carbohydrates in a food, thus affecting the glycemic response.[Citation51] This type of fiber allows the formation of a gel that acts as a barrier between carbohydrates and digestive enzymes in stomach, facilitating a more gradual conversion of carbohydrates to glucose.[Citation32] In addition, the soluble fiber of chia seeds allows the stabilization of blood glucose levels thanks to the regulation of the speed of digestion and assimilation of sugars.[Citation33] This conclusion was also supported by da Silva et al.[Citation46] in studies where rats consuming diets supplemented with chia seeds for a period of 28 days lowered blood glucose levels compared to the control group (118.82 mg/dL and 172.33 mg/dL, respectively).
Risk associated with the consumption of chia seeds
In 2009, the European Union authorized the use of chia seeds in bread up to 5% in the context of the novel food regulation.[Citation52] In 2013, the use was extended in baked products, breakfast cereals and fruit, nut and seed mix up to 10% chia, considering that the intake of chia should not exceed 15 g/day.[Citation53] Years later, it was authorized in the following food categories: bread products, baked products, breakfast cereals, fruit, nut and seed mixes, fruit juice and fruit/vegetable blend beverages, pre-packaged chia seed as such, fruit spreads, yoghurt, sterilized ready-to-eat meals based on cereal grains, pseudocereals and/or pulses.[Citation54] In 2019, based on an investigation of Mesias et al.,[Citation55] the European Food Safety Authority (EFSA) stated that the use of chia should be limited to non-thermally treated foods due to the possible formation of chemical process contaminants such as acrylamide.[Citation56] Acrylamide is a contaminant formed when foods containing free asparagine and reducing sugars are cooked at temperatures above 120°C in low moisture conditions.[Citation57] It is identified by the International Agency for Research on Cancer as being probably carcinogenic to humans (group 2A)[Citation58] and its consumption through foods has been reported to increase the risk of developing cancer in all age groups.[Citation57] To respond to the lack of information about the presence of acrylamide and other contaminants in processed chia seeds, in 2020, EFSA published a scientific opinion affirming the need to further investigate the formation of chemical process contaminants in foods with chia seeds subjected to heat treatment.[Citation59]
Mesias et al.[Citation55] observed that the incorporation of chia flour into wheat biscuit formulations increased the formation of chemical process contaminants such as acrylamide, hydroxymethylfurfural (HMF) and furfural. The maximum levels of acrylamide (~1200 µg/kg) and HMF (~70 mg/kg) were reached when 10% of chia flour was added to the samples, exceeding the benchmark levels established by the European Regulation for acrylamide in biscuits.[Citation60] Chia flour was found to contain α-dicarbonyl compounds such as glyoxal (GO) (3.0 µg/g) or methylglyoxal (MGO) (371 µg/g), which were not present in wheat flour. This fact could explain the greater formation of the contaminants in chia biscuits since dicarbonyl compounds could be mainly responsible for the formation of the Maillard reaction products.[Citation57] Dicarbonyls are formed by the degradation of the Amadori compounds of the Maillard reaction or by caramelization reactions and have been related to diseases such as diabetes, obesity or cardiovascular diseases.[Citation61] Similarly, Galluzzo et al.[Citation62] studied the acrylamide content in wheat bread formulated with chia seeds. Maximum levels of the contaminant (156.50 µg/kg) were observed in breads containing 5% of seeds. However, no significant differences were found between control samples and bread with the different percentages of chia, concluding that the formation of acrylamide in chia samples is not completely clear. Definitely, more studies about safety for human consumption of bakery products supplemented with chia seeds are needed.
Sensory attributes of cereal-based foods formulated with chia seeds
The incorporation of chia as whole seeds, flour, mucilage or oil in bakery products can modify their technological and sensory characteristics, affecting the acceptability of the product by the consumer.[Citation63] Color of cereal-based foods is modified, presenting darker color as the concentration of chia increases or as it is incorporated as flour.[Citation23,Citation26] These browning may be due to the chia pigments themselves but also to the caramelization and Maillard reactions that can be promoted when food products are heat treated.[Citation64] The water retention capacity increases (10–33%) with higher percentages of chia in the product[Citation64,Citation65] since the mucilage can interact with the protein network.[Citation63] Higher values of moisture linked to higher fiber content have been described in bread enriched with chia seeds (38.8 g/100 g) as compared with control bread (37.2 g/100 g).[Citation44] Regarding sensory characteristics, significant differences have been observed in the aroma of cookies containing 10 and 20% defatted chia flour compared to a control cookie, although without changes in sweet taste and chewiness.[Citation26] Due to sensorial changes, products with 10% chia seeds in their formulation have been described to be more accepted by the consumer than those containing 20% seeds.[Citation23,Citation26,Citation44]
A summary of the risk/benefit associated with the consumption of chia seeds as well as sensory attributes of cereal-based foods formulated with these seeds is depicted in .
Quinoa
Quinoa (Chenopodium quinoa) is a pseudocereal belonging to the Chenopodiaceae family. It grows annually, from sea level to 3,800 m above sea and is a crop native to South America, cultivated in countries such as Colombia, Chile, Bolivia, Ecuador and Peru.[Citation66] In recent years, this crop has been introduced in Europe, North America, Asia and Africa.[Citation67] Seeds have 3 well-defined parts: the episperm, the embryo and the perisperm, where the embryo represents 30% of the total seed and the perisperm 60%. The color of these seeds can be very diverse, ranging from green, red or purple to white or even black.[Citation68] In 2021, 6,890 ha of quinoa were cultivated in Spain, representing an increase of 3.8% compared to 2004. In 2020, the production of quinoa in Spain was 24,691 tons[Citation69] and 175,188 tons were produced worldwide.[Citation70] The consumption of quinoa has increased in the last decades since this pseudocereal does not contain gluten, making it to be used to obtain new formulations intended for celiac population. This fact has prompted the study of its composition and possible beneficial effects on human health.
Quinoa seeds have a high protein content (approximately 12–23% of the total composition of the seed), comparable to the protein content of wheat (11.3–14.4%) (). It also contains all the essential amino acids, highlighting lysine, methionine and threonine.[Citation34] Carbohydrates represent 60–74% of the total seed,[Citation71] close to wheat (63–85%), with starch being the main component of this fraction (36–69% of total carbohydrates). Dietary fiber represents 7–10%, including soluble fiber (1.6–6.1%) and insoluble fiber (6.8–8.4%). Simple sugars represent 3% of carbohydrates, with maltose, D-galactose and D-ribose being the majority.[Citation66] The lipid fraction ranges between 1.9 and 14.5% of the total seed. Among the saturated fatty acids (12.3–19%) palmitic acid stands out, constituting 10% of the total fatty acids. Monounsaturated fatty acids represent 25–29.5% of the total fatty acids, highlighting the oleic acid that represents 19.7–29.5% of the total fatty acids. Finally, within the group of polyunsaturated fatty acids (~58%), linoleic acid constitutes 49–56.4% of the total fatty acids.[Citation34] Mineral content in quinoa represents 3–4% of the total seed,[Citation71] highlighting calcium, iron,[Citation34] potassium, phosphorus and magnesium. Regarding vitamins, quinoa contains some of the B group such as vitamin B6, B2, B3 and folic acid, apart from vitamin E and C, which are found in significant amounts.[Citation66,Citation72] Finally, phenolic compounds such as ferulic acid, p-coumaric acid and caffeic acid are also present in the quinoa seed.[Citation66]
Benefits associated with the consumption of quinoa
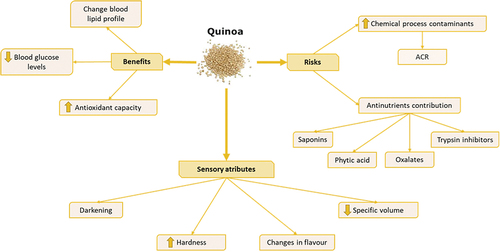
Quinoa has become a highly appreciated raw material by the food industry to formulate gluten-free bakery products. In addition, quinoa has functional and health beneficial characteristics due to its composition in phenolic compounds and saponins, which confer it antioxidant activity and the effect of reducing the risk of cardiovascular disease or diabetes.[Citation71]
Several studies have shown that quinoa could modify the blood lipid profile, causing a significant decrease in triglyceride and LDL cholesterol levels and increasing HDL cholesterol.[Citation72–74] Due to the soluble and insoluble fiber content of quinoa, levels of glucose, triglycerides and free fatty acids are reduced in blood after its consumption, suggesting quinoa could be used to control the levels of these compounds.[Citation73] A decrease in triglycerides, LDL (59.06% and 68.60%) and VLDL and an increase in HDL cholesterol of 20.39% and 29.21%, respectively have been described in rats fed with red and yellow quinoa for 4 weeks.[Citation72] In humans, mean serum triglyceride levels were also significantly reduced in overweight and obese people consuming 50 g quinoa/day for 12 weeks (from 1.14 to 0.72 mmol/L). However, the content in LDL and HDL were not significantly modified.[Citation74]
Quinoa contains fiber, phenolic compounds, tocopherols, proteins and bioactive peptides that may contribute to antidiabetic potential. To control blood glucose levels, α-amylase and α-glucosidase could be inhibited, thus preventing the breakdown of polysaccharides into monosaccharides. In vitro studies have shown that α-amylase and α-glucosidase can be inhibited by quinoa polysaccharides, in addition to the fact that they are polysaccharides with a low glycemic index, thus helping to reduce blood glucose levels.[Citation42] Due to its content of vitamin E, zinc, iron and magnesium, quinoa allows the release of insulin into the blood plasma, makings it a good controller of diabetes.[Citation73]
The high antioxidant capacity is associated to the polysaccharides, saponins and polyunsaturated fatty acids content, being even higher than that common cereals.[Citation75,Citation76] In vivo studies in animals have shown that the consumption of quinoa can increase the activity of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and catalase, thus reducing oxidative stress.[Citation72,Citation77,Citation78] The way in which quinoa grains are treated has been demonstrated to affect their antioxidant activity and, therefore, if they are germinated, cooked, fermented or ground, the antioxidant capacity can be modified.[Citation72,Citation79] Al-qabba et al.[Citation72] found that after 6 days of germination of quinoa grains, antioxidant capacity reached the maximum increase (from 105.16 to 293.35 mg GAE/100 g and from 112.42 to 259.02 mg GAE/100 g for total phenolic content in red and yellow quinoa, respectively), whereas cooking them could increase the levels by 5.9%.[Citation79]
Risk associated with the consumption of quinoa
Sazesh and Goli[Citation80] studied the effect of the addition of quinoa flour in different concentrations to wheat crackers on the formation of acrylamide, as well as the influence of bicarbonate in the formulation and the baking temperature of the product. At 0.1% sodium bicarbonate level, the highest amount of acrylamide was observed at both the highest content of wheat flour (161 µg/kg for 100% wheat crackers baked at 185ºC) and baking temperatures (292 µg/kg for 100% wheat crackers baked at 210ºC). In contrast, the lowest concentration of acrylamide was observed at the highest levels of quinoa flour (23 µg/kg for 100% quinoa crackers baked at 185ºC) with the lowest baking temperatures (22 µg/kg for 100% quinoa crackers baked at 160ºC). This finding may be explained because the low content of both reducing sugars, such as glucose and fructose, and asparagine in quinoa compared to wheat flour, reducing then the level of precursors of the Maillard reaction and consequently the formation of acrylamide.
Another potential risk derived from the addition of quinoa to new formulations of bakery products is linked to the antinutrients present in this pseudocereal, such as phytic acid, saponins, oxalates or trypsin inhibitors. These compounds are contained in plants and their consumption can reduce the nutritional value of foods, affect the digestibility or availability of nutrients or exert harmful effects on health if they are ingested in large quantities.[Citation76] Phytic acid is mainly found in the outermost parts of the seeds (38–41% of the total phytic acid of the seed).[Citation24,Citation81] Phytic acid chelates divalent minerals such as calcium, zinc, iron or magnesium, as well as starch, proteins and enzymes, leading to the formation of insoluble complexes at physiological pH which could compromise their bioavailability.[Citation76] Demir and Kilinç[Citation24] showed that adding quinoa flour to wheat cookies in percentages ranging between 0 and 50%, increased mean phytic acid content from 122.82 to 297.54 mg/100 g. To mitigate this negative aspect, several authors have recommended macerating quinoa seeds in water, reducing then the level of phytic acid.[Citation67] Saponin content in quinoa ranges between 0.2 and 11.3 g/kg of dry matter[Citation67] highlighting oleanolic acid, hederagenin, phytolacogenic acid and 30-O-methyl-spergulagenate.[Citation76] Saponins are glycoside terpenoids without a well-defined chemical formula. These compounds are soluble in water and in high amounts can be harmful to health since they can affect the absorption of nutrients (it can be complexed with zinc) or the inactivation of enzymes.[Citation81] Similar to phytic acid, the maceration of quinoa seeds in water can reduce the saponin content.[Citation82] Finally, quinoa contains oxalates, which can be harmful to health, accumulate in the kidney and be involved in the absorption of minerals or trace elements, forming oxalate stones.[Citation76]
Sensory attributes of cereal-based foods formulated with quinoa
The color of bakery products fortified with quinoa flour is darker as compared to foods made from wheat because the formation of melanoidins produced by the Maillard reaction[Citation83–85] or by the oxidation of the phenolic compounds contained in quinoa. This darkening will be more or less pronounced depending on the temperature and baking time and the sugar content of the product.[Citation84]
The hardness of bakery products is given by the protein and fiber content of their ingredients. Therefore, the higher concentrations of both compounds in quinoa flour make its incorporation increases the hardness as the percentage of quinoa is greater compared to products made with wheat flour.[Citation24,Citation83,Citation85] Another effect of the addition of quinoa to bakery products is related to the expansion of the product during baking. The specific volume of breads and cookies fortified with quinoa flour is reduced[Citation83,Citation85] as well as the diameter, weight and height of the sliced bread.[Citation85] Demir and Kilinç[Citation24] also showed that cookies added with quinoa were harder (from 36.37 N in control cookies to 39.46, 43.34, 48.79, 52.87 and 58.01 N with the addition of 10, 20, 30, 40 and 50% of quinoa flour) because this flour does not contain gluten and, consequently, the gas formed during the fermentation of the dough is not retained in the baked product.[Citation85]
The consumer acceptance of cereal based foods containing quinoa is also a very important attribute to consider. In this sense, El-Sohaimy et al.[Citation85] developed and characterized a functional pan bread supplemented with quinoa flour (5–30%) and found that consumers accepted all formulations, although there were significant differences in flavour and texture if compared with the control (without quinoa flour).
A summary of the risk/benefit associated with the consumption of quinoa as well as sensory attributes of cereal-based foods formulated with this pseudocereal is depicted in .
Sesame seeds
Sesame seeds (Sesamum indicum L.) are an oil seed crop of a fast-growing plant that can reach up to 3 m in height, originating in Ethiopia (Africa) from where it spread to tropical and subtropical areas such as India, Japan and China.[Citation86] Seeds are small flattened 2 to 4 mm long and 1 to 2 mm wide[Citation87] that can range from white to black, passing through grey shades. In 2020, 13.97 million hectares of sesame were cultivated worldwide, producing a total of 6.80 million tons.[Citation70]
Sesame oil represents 40–60% of the seed,[Citation88,Citation89] where 80–85% are unsaturated fatty acids[Citation90] (). Approximately 39% are monounsaturated fatty acids, mainly oleic acid, 46% are polyunsaturated fatty acids such as linoleic acid and linolenic acid, and saturated fatty acids represent 14% of total fatty acids, the majority being palmitic and stearic acids.[Citation86,Citation91] In addition, 15–25% of the seed are proteins and 13–19% carbohydrates, such as glucose (3.2%), fructose (2.6%) and sucrose (0.2%), the rest being dietary fibre.[Citation86,Citation89] Sesame has a humidity of 3–5% and 5–6% ash. Sesame is a cereal that, compared to wheat, has higher percentage of fats, lower carbohydrate content and similar protein content (). Magnesium, sodium, iron, zinc and to a lesser extent phosphorus, calcium or manganese are the most representative minerals and vitamin B1 and vitamin E are the most representative vitamins in this seed.[Citation89]
The consumption of sesame seeds has increased due to their content in bioactive compounds such as tocopherols, polyphenols or lignans (sesamin, semolina and pinoresinol) with beneficial health.[Citation88] The polyphenol content of black sesame is 1.38 mg/g and 2.88 mg/g in the case of white sesame and the flavonoid content is 0.05 mg/g and 0.12 mg/g for black and white sesame, respectively.[Citation92] Several studies have reported the high antioxidant capacity of sesame seeds associated with its content in polyphenols and flavonoids. According to the polyphenol content, ethanolic extracts of white sesame have shown greater capacity to trap DPPH radicals than those of black sesame (61.16% and 56.73%, respectively).[Citation92] In agreement with these results, Dravie et al.[Citation89] described that ethanolic extracts of sesame seeds trapped 55% of DPPH radicals.
It must be taken into account that the composition of the sesame seed can be modified during heat treatment, an important aspect to be considered when they are incorporated into cereal-based formulations that are going to be subjected to roasting or baking processes. In this sense, increases in the antioxidant activity, protein, oil, total phenolic, and tocopherol content, whereas reductions in moisture and linolenic acid content have been described in sesame seeds roasting under different oven and microwave conditions.[Citation93,Citation94]
Benefits associated with the consumption of sesame seeds
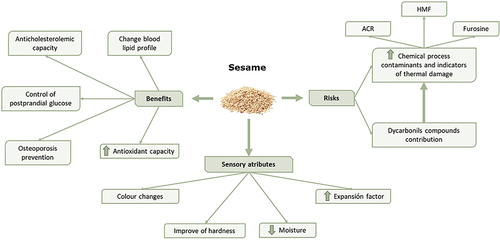
According to its composition, especially in unsaturated fatty acids, sesame seed has aroused great interest in the food industry for the development of new functional foods. Several authors have reported that the incorporation of sesame confers beneficial health properties such as the prevention of diabetes because of the low carbohydrate and the high fiber and protein content. Moreover, sesame seeds are rich in magnesium, which regulates blood glucose and insulin levels.[Citation95,Citation96] Bigoniya et al.[Citation97] studied the effect of feeding sesame seed cake to obese, hyperglycemic rats and observed that the rodents’ weight and blood glucose levels were reduced, concluding that the supplementation of the diet with sesame seeds could be a strategy to reduce the risk of type II diabetes.
The content of polyunsaturated fatty acids, fiber, phytosterols and lignans has been reported to reduce the risk of hypertension and thus CVDs.[Citation98] Sesame can reduce cholesterol presence in the blood by decreasing the absorption of cholesterol in the intestine.[Citation95,Citation99] Thereby, the supplementation with sesame seed has been demonstrated to have beneficial effects in hyperlipidaemic/hypercholesteraemic and oxidative stress conditions due to increased excretion of sterols and production of hepatic bile acids.[Citation99] This ability may be due to phytoconstituents such as fiber, sterols, polyphenols and flavonoids. Aslam et al.[Citation100] have also observed these effects when different amounts of sesame oil were supplied to rats with hypercholesterolemia. In this study, HDL levels increased proportionally with the amount of sesame consumed (from 26.04 in normal diet to 32.39 mg/dL with the addition of 8% of sesame seed oil) whereas the levels of LDL, VLDL, total cholesterol and triglycerides decreased (from 66.20 to 48.88 mg/dL, from 66.20 to 48.80 mg/dL, from 137.33 to 115.46 mg/dL and from 99.99 to 97.27 mg/dL, respectively). Authors associated this decrease in the blood lipid content with an increase in the concentration of lignans provided by sesame.
Sankar et al.[Citation101] carried out a study where hypertensive men and women between 45–65 years old consuming sesame oil for 45 days and another type of oil for other 45 days. After the use of sesame oil, a significant reduction in systolic (from 150 to 129.6 mm Hg) and diastolic pressure (from 98.3 to 80 mm Hg) was observed. However, the use of usual consumption oils led to an increase in the pressure almost to the initial levels (140 and 90.0 mm Hg, respectively), thus concluding that a diet supplemented with sesame oil could allow the patients’ hypertension to be controlled. Sesame can also prevent osteoporosis since its content of minerals such as zinc, calcium and phosphorus may prevent bone weakening.[Citation95] At this respect, studies with rats consuming 10% flax oil and 10% sesame oil for 4 weeks have exhibited reductions in bone markers such as alkaline phosphatase activity (bone formation marker) and tartrate-resistant acid phosphatase activity (bone resorption marker) together with an improvement in bone structure compared to control rats.[Citation102]
Risk associated with the consumption of sesame seeds
The incorporation of sesame seeds in different foods subjected to thermal treatment has promoted the formation of chemical process contaminants such as acrylamide or HMF. Berk et al.[Citation90] studied the development of Maillard reaction products such as acrylamide or furosine in baked sesame seeds, through different assays with different baking times and temperatures. Furosine is considered a heat-induced indicator of the nutritional quality of the protein since reflects the impairment of lysine residues of the protein with the extent of the thermal treatment.[Citation103,Citation104] Lysine is usually the limiting amino acid in cereals and, therefore, the lowest furosine content ensures the highest nutritional value of proteins from grain-derived foods.[Citation105] Acrylamide formation reached its maximum concentration in sesame seeds baked at 200ºC for 10 min (633 µg/kg) drastically decreasing at 220ºC (199 µg/kg) due to the asparagine depletion. For furosine, the maximum concentration was reached at 150ºC (more than 120 mg/kg).[Citation90] In successive studies, Berk et al.[Citation106] reported that the main sugar present in sesame seeds was sucrose (2.3 g/100 g). Throughout the roasting process, this sugar reduces its concentration, especially at 200–220ºC, just like the amino acids, leading to the formation of new compounds. The formation of HMF throughout the process was also evaluated, whose concentration raised as the baking time increased, reaching its maximum (75.6 mg/kg) with the treatment at 180ºC for 30 min.
Other reactive substances generated during the roasting of sesame seeds are α-dicarbonyl compounds, including 3-deoxyglucosone (3-DG), 1-deoxyglucosone (1-DG), MGO, GO or diacetyl (DA). They have not been detected in unroasted sesame seeds, except for 3-DG and MGO, which may be formed during storage or transport of the raw material. Berk et al.[Citation107] determined that the main α-dicarbonyl compound found in roasted sesame seeds was 3-DG, reaching its maximum concentration at 180ºC for 30 min, and in lower concentrations GO and MGO, reaching their maximum value at 220ºC for 10 min. 1-DG and DA were found in much lower concentrations, not showing large variations during roasting.
Sensory attributes of cereal-based foods formulated with sesame seeds
In recent decades numerous products have been formulated by the integration of this raw material in their composition in the form of flour or seeds. In this way, multiple studies have been carried out to determine the acceptability of these new foods by consumers. The moisture of formulations with sesame, mainly cookies, decreases compared to those made with wheat.[Citation22,Citation108,Citation109] In this line, Prakash et al.[Citation25] reported that the incorporation of defatted sesame flour into wheat biscuits caused a 7% reduction in moisture linked to the low water absorption capacity of these seeds.
One of the most important technological factors for biscuits is the expansion factor, which is obtained from the relationship between the diameter of the cookies and their weight. Similarly, spread factor is another important characteristic in determining the quality of cookies. It is calculated using the formula: diameter divided by height of cookies. Several authors have described that these parameters increase when sesame is incorporated into biscuits as compared to control samples (wheat biscuits). El-Enzi et al.[Citation109] reported an increase in diameter and a decline in weight in biscuits formulated with 40% of sesame flour, leading to an increase in the expansion factor from 0.468 (control biscuit) to 0.522 (sesame biscuit). In these samples, spread factor changed from 6.39 to 8.21 in control and sesame biscuits, respectively. Spread factor values of 2.61 and 4.13 have been also reported in control and sesame cookies. In this case, sesame peels flour replaced the 50% of wheat flour in cookie formulations.[Citation108]
Hardness, texture and color are three of the parameters that impact on consumer preferences. Sesame cookies present greater hardness compared to samples formulated with wheat.[Citation25,Citation101] This increase could be due to the release of sugars in the degreasing of sesame seeds, causing a network of sugars to form during baking and generating greater hardness in the final product.[Citation25] Sesame cookies have also been described to be more fragile than wheat samples, since they have less resistance to cutting associated to their lower gluten content.[Citation25,Citation110] The color of sesame cookies depends on the variety of seed used. In this sense, products made with white sesame have a color quite similar to that of wheat cookies[Citation25,Citation109,Citation110] whereas those made with black sesame have darker color, making it a less pleasant product for the consumer.[Citation25,Citation108]
A summary of the risk/benefit associated with the consumption of sesame seeds as well as sensory attributes of cereal-based foods formulated with these seeds is depicted in .
Flax seeds
Flax (Linum usitatissimum L.) is an annual plant belonging to the Linaceae family. It is indigenous from the eastern Mediterranean area to the Middle East. Around 2.6 million hectares of flax are currently cultivated, mainly in countries such as India, China, the United States and Ethiopia.[Citation111] Seeds are oval, flat and pointed, measure between 4 and 6 mm and have a shiny surface and colors ranging from dark brown to yellow.[Citation14] Anatomically, flax seeds are composed of 3 parts: the germ (4%), two cotyledons (55%) and the stanhell (36%).[Citation112] In 2020, 285.418 hectares of flax were harvested worldwide, producing a total of 976.113 tons.[Citation70]
Flax seeds have a high added value, especially due to their oil content (38–45% of the total seed)[Citation21] (), where α-linolenic acid (41–53%) is the main fatty acid, followed by linoleic acid (15–17%) and oleic acid (19%). To a lesser extent, saturated fatty acids represent 14%, being mainly stearic acid (3%) and palmitic acid (5%).[Citation111] Dietary fiber is another of their main compounds, representing 22–28% of the total seed. It is mostly insoluble fiber, which constitutes between 60–80% (cellulose and lignins) and the remaining 20–40% corresponds to soluble fiber. Simple sugars represent 1% of carbohydrates (20–29% of the total), where xylose, arabinose and galactose stand out and, in lower concentrations, L-rhamnose, D-galactose and D-galacturonic acid.[Citation111] The protein content constitutes 21–34%. The major amino acids are glutamic acid, aspartic acid, arginine and glycine[Citation21] although seeds are also rich in leucine, lysine and methionine.[Citation86] The main minerals are potassium, phosphorus, magnesium and calcium and among vitamin is the vitamin E, highlighting α-tocopherol, β-tocopherol and γ-tocopherol in addition to vitamin B3 or niacin.[Citation112]
Similar to previous seeds, flax seeds also contain bioactive compounds associated with healthy properties. These compounds mainly belong to the group of lignans and phenolic compounds, including phenolic acids such as ferulic acid, chlorogenic acid and gallic acid. Moreover, these seeds contain flavonoids such as pinoresinol[Citation21,Citation111] and lignans such as secoisolariciresinol diglucoside.[Citation113] Concentrations ranging between 0.14–255.71 µg/g (total carotenoid content), 0.66–424.29 mg/100 g (flavonoid content), 178.81–243.73 mg/100 (total phenolic content), 26.19–45.69 mg/100 g (quercetine), 10.82–25.44 mg/100 g (catechin) and 1.86–2.78 mg/100 g (ferulic acid) have been reported in several varieties of flax seeds.[Citation114]
Benefits associated with the consumption of flax seeds
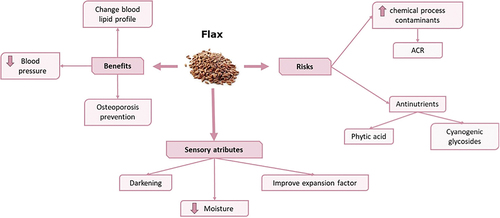
Flax has beneficial properties for health such as the prevention of osteoporosis, the modification of the lipid profile or the reduction of blood pressure. Abreu et al.[Citation115] investigated the effect of flax on osteoporosis in a group of rats fed a diet supplemented with flax meal and observed that were fed flax meal had higher bone mineral density, reducing the probability of bone loss. This fact could be due to the high concentrations of α-linolenic acid (ALA), since it can be transformed into docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) and also to the polyunsaturated fatty acids that act on the macrophages derived from the bone marrow, allowing the decrease in osteoclastogenesis and the increase in osteoblastogenesis.[Citation116] Furthermore, flax meal is rich in calcium, which, together with the high content of ALA, promotes greater absorption of this mineral in the intestine and greater deposition in the bones.[Citation117]
Several researchers have evaluated the effect of flax consumption on the reduction of blood lipids. The intake of flax has been demonstrated to reduce the total cholesterol in humans,[Citation118] percentages being 5–17% and 4–10% for reductions of total cholesterol and LDL, respectively, in the case of patients with hypercholesterolemia.[Citation119,Citation120] The fiber content of flax seeds may explain this effect, which increases satiety and reduces caloric intake, apart from the decrease in transit time and the increase in fecal excretion of cholesterol.[Citation121]
The FLAX-PAD study (FLAX effects in Peripheral Arterial Disease) determined that the consumption of flax could significantly lower blood pressure. The effects of incorporating flax in the diet on peripheral arterial disease (PAD) were studied over a period of 6 months, which is closely related to hypertension.[Citation122] A decrease of 10 mm Hg in systolic pressure and 7 mm Hg in diastolic was observed in the group of patients who had ingested flax, leading to decreases much greater than those observed with the use of antihypertensive drugs.[Citation113] The reduction in the blood pressure may be associated with the high content of ALA, since polyunsaturated fatty acid metabolites such as oxylipins are involved in hypertension, allowing inflammation and vascular tone to be controlled.[Citation123] In addition, flax seeds are rich in arginine, which can be transformed into nitric oxide, exerting a hypertensive effect due to its vasodilator compound.[Citation113]
Risk associated with the consumption of flax seeds
Flax seeds have many beneficial properties for health but, at the same time, also contain certain compounds that can be harmful, such as cyanogenic glycosides or phytic acid. Cyanogenic glycosides are present in concentrations of 250–550 mg/100 g of whole seed[Citation124] and the majority are linustatin (213–352 mg/100 g), neolinustatin (91–203 mg/100 g) and linamarin (32 mg/100 g). These compounds can be harmful since, upon reaching the intestine, they are transformed into hydrogen cyanide, which in turn, is transformed into thiocyanate through the intestinal enzyme β-glucosidase. This compound can interfere with the absorption of iodine by the thyroid gland, causing iodine deficiency, goiter or cretinism.[Citation125] Since these compounds are thermolabile, treating flax seeds with thermal processes such as autoclaving or microwave baking can reduce their content.[Citation126]
Phytic acid is found in concentrations of 23–33 g/kg of whole seed.[Citation124] This compound is considered an antinutrient due to its high chelating potential, forming complexes with proteins or minerals such as iron, magnesium, zinc, copper or calcium and reducing then its bioaccessibility.[Citation127] Despite these negative aspects, phytic acid present in flax seeds have not been associated with the detrimental effect found in other foods such as soybeans or rapeseed.[Citation125]
Other important negative consequence of the incorporation of flax seeds into bakery products is the formation of chemical process contaminants after baking, such as acrylamide. Flax seeds are rich in amino acids such as asparagine (12.5–15.2 mg/100 g) and reducing sugars (8.1 mg/100 g),[Citation112,Citation128] promoting then the Maillard reaction and consequently the formation of this contaminant. Bartkiene et al.[Citation128] evaluated the effect of incorporating flax flour and fermented lupine flour into wheat biscuits to study the acrylamide formation. The addition of flaxseed (25 g, 50 g, 75 g) increased acrylamide content in biscuits by 70.9, 74.2 and 78.3%, respectively, compared to control samples (100% wheat flour). Authors proposed fermentation of flax as a mitigation strategy, since when seeds are fermented, the content of asparagine and reducing sugars is reduced, decreasing then by 78–84% in the content of acrylamide.
Sensory attributes of cereal-based foods formulated with flax seeds
The incorporation of flax as seeds, flour or oil in the formulation of different bakery products can affect quality parameters such as moisture, color or hardness, and therefore influence the acceptability of the product by the consumer. The addition of flax flour into bakery products has been demonstrated to decrease the moisture content as compared to control products (made with 100% wheat).[Citation21,Citation129,Citation130] In this context, Khouryieh and Aramouni[Citation129] reported that moisture could decrease from 6.5% in biscuits made entirely from wheat to 4.8% in those containing 18% flax flour.
Dimensions (weight, thickness, diameter and expansion factor or spread factor) are physical properties evaluated when checking the quality of a product. At this respect, cookies made with flax flour are thicker (0.83–0.89 cm) than those made with wheat flour (0.71–0.75 cm).[Citation21,Citation131] As mentioned before, another factor evaluated when studying the quality of biscuits is the expansion factor. The weight of biscuits made with flax seeds has been observed to decrease as the concentration of flax increased (from 12.42 g in control samples to 10.56 g in cookies containing 30% of raw flaxseed flour). At the same time the diameter of the cookies increased (from 4.32 cm to 5.08 cm). Consequently, the expansion factor was greater in flax cookies (0.481) as compared with wheat samples (0.347).[Citation132]
Bakery products formulated with flax seeds have darker colors, which increase as the percentage of seeds is greater. This darkening is due to the high fiber content or the Maillard reaction generated during heating, resulting in colored compounds with brown tones.[Citation14,Citation120–124] Through sensory analysis, it has been observed that products containing 10% flax in the formulation were those most accepted by the consumer and from this percentage, the values of each analyzed attribute were decreased.[Citation21,Citation129,Citation130,Citation132,Citation133] This percentage can be increased up to 20–25% when the flour used for the preparation of bakery products is previously toasted.[Citation132,Citation133]
A summary of the risk/benefit associated with the consumption of flax seeds as well as sensory attributes of cereal-based foods formulated with these seeds is depicted in .
Conclusions
Chia, quinoa, flax or sesame seeds are being incorporated in the preparation of new formulations of foods, especially bakery products, looking for innovative products more attractive to the consumer. Their incorporation leads to modifications in the organoleptic profile of foods through changes in hardness, flavor or aroma, and in the nutritional properties, which in short, will provide benefits to population’s health like reducing the risk of have diabetes or modifying the lipid profile among others. However, the addition of these ingredients may also contribute the intake of antinutrients or promote the formation of compounds with a potential toxicological risk, such as acrylamide or HMF, during heat treatments. In conclusion, a global risk/benefit evaluation should be implemented when formulating new foods containing these seeds, considering not only nutritional or sensory improvements but also avoiding the increase of the presence of potentially harmful compounds to health that compromise the food safety of the bakery products.
Author contributions
Marta Mesías and Francisco J. Morales: conceptualization, validation and supervision. Francisco J. Morales; funding acquisition. Elena Olombrada, Marta Mesías and Francisco J. Morales: investigation. Elena Olombrada and Marta Mesías: data curation. Elena Olombrada: writing original draft preparation. Marta Mesías and Francisco J. Morales: writing – review and editing. All authors have read and agreed to the published version of the manuscript
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization (WHO). Diabetes. (accessed Feb 6, 2023). https://www.who.int/es/news-room/fact-sheets/detail/diabetes
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). (accessed Feb 6, 2023). https://www.who.int/es/news-room/fact-sheets/detail/cardiovascular-diseases-cvds
- World Health Organization (WHO). Non-Communicable Diseases (NCDs). (accessed Feb 8, 2023). https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
- World Health Organization (WHO). Obesity and Overweight. (accessed Feb 8, 2023). https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- World Health Organization (WHO). Who European Regional. Obesity Report 2022. (accessed Feb 10, 2023). https://apps.who.int/iris/bitstream/handle/10665/353747/9789289057738-eng.pdf
- Dominguez, L. J.; Di Bella, G.; Veronese, N.; Barbagalo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients. 2021, 13(6), 2028. DOI: 10.3390/nu13062028.
- Blanco-Rojo, R.; Sandoval-Insausti, H.; López-Garcia, E.; Graciani, A.; Ordovás, J. M.; Banegas, J. R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Consumption of Ultra-Processed Foods and Mortality: A National Prospective Cohort in Spain. Mayo Clin. Proc. 2019, 94(11), 2178–2188. DOI: 10.1016/j.mayocp.2019.03.035.
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients. 2020, 12(7), 1955. DOI: 10.3390/nu12071955.
- Srour, B.; Touvier, M. Processed and Ultra-Processed Foods: Coming to a Health Problem? Int. J. Food Sci. Nutr. 2020, 71(6), 653–655. DOI: 10.1080/09637486.2020.1807476.
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional Food. Product Development, Marketing and Consumer Acceptance. A Review. Appetite. 2008, 51(3), 456–467. DOI: 10.1016/j.appet.2008.05.060.
- Martirosyan, D.; Kanya, H.; Nadalet, C. Can Functional Foods Reduce the Risk of Disease? Advancement of Functional Food Definition and Steps to Create Functional Food Products. J. Funct. Food Health Dis. 2021, 11(5), 213–221. DOI: 10.31989/ffhd.v11i5.788.
- Ortega, I. Foodomics in Health: Advanced Techniques for Studying the Bioactive Role of Foods. TrAc. 2022, 150, 116589. DOI: 10.1016/j.trac.2022.116589.
- Urala, N.; Lähteenmäki, L. Reasons Behind Consumers’ Functional Food Choices. Nutr. Food Sci. 2003, 33(4), 148–158. DOI: 10.1108/00346650310488499.
- Kowaleski, A.; Quast, L. B.; Steffens, J.; Lovato, F.; dos Santos, L. R.; da Silva, S. Z.; de Souza, D. M.; Steffens, M. A. Functional Yogurt with Strawberries and Chia Seeds. Food Biosci. 2020, 37, 100726. DOI: 10.1016/j.fbio.2020.100726.
- Mesias, M.; Delgado-Andrade, C.; Morales, F. J. Risk/Benefit Evaluation of Traditional and Novel Formulations for Snacking: Acrylamide and Furfurals as Process Contaminants. J. Food Compost. Anal. 2019, 79, 114–121. DOI: 10.1016/j.jfca.2019.03.011.
- Owusu-Ansah, P.; Besiwah, E. K.; Bonah, E.; Amagloh, F. K. Non-Meat Ingredients in Meat Products: A Scoping Review. Appl. Food Res. 2022, 2(1), 100044. DOI: 10.1016/j.afres.2022.100044.
- Birch, C. S.; Bonwick, G. A. Ensuring the Future of Functional Foods. Int. J. Food Sci. Technol. 2019, 54(5), 1467–1485. DOI: 10.1111/ijfs.14060.
- Estévez, V.; Araya, M. Gluten-Free Diet and Gluten-Free Foods. Rev. Chil. Nutr. 2016, 43(4), 428–433. DOI: 10.4067/S0717-75182016000400014.
- Martinez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal Grains: Nutritional Value, Health Benefits and Current Applications for the Development of Gluten-Free Foods. Food. Chem. Toxicol. 2020, 137, 111178. DOI: 10.1016/j.fct.2020.111178.
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients. 2021, 13(7), 2244. DOI: 10.3390/nu13072244.
- Kaur, M.; Singh, V.; Kaur, R. Effect of Partial Replacement of Wheat Flour with Varying Levels of Flaxseed Flour on Physicochemical, Antioxidant and Sensory Characteristics of Cookies. Bioact. Carbohydr. Diet. Fibre. 2017, 9, 14–20. DOI: 10.1016/j.bcdf.2016.12.002.
- Kumar, M.; Bhardwaj, A. Optimization and Quality Evaluation of the Cookies Developed from Composite Dehulled Sesame Seed Flour Blend. Int. J. Adv. Sci. Res. Manag. 2017, 2(8), 1–8.
- Singh, J.; Sharma, B.; Madaan, M.; Sharma, P.; Kaur, T.; Kaur, N.; Kaur Bhamra, I.; Kaur, S.; Rasane, P. Chia Seed Based Nutri Bar: Optimization, Analysis and Shelf Life. Curr. Sci. 2020, 118(9), 1394–1400. DOI: 10.18520/cs/v118/i9/1394-1400.
- Demir, M. K.; Kilinç, M. Utilization of Quinoa Flour in Cookie Production. Int. Food Res. J. 2017, 24(6), 2394–2401.
- Prakash, K.; Naik, S. N.; Vadivel, D.; Hariprasad, P.; Gandhi, D.; Saravanadevi, S. Utilization of Defatted Sesame Cake in Enhancing the Nutritional and Functional Characteristics of Biscuits. J. Food Process Preserv. 2018, 42(9), e13751. DOI: 10.1111/jfpp.13751.
- Mas, A. L.; Brigante, F. I.; Salvucci, E.; Pigni, N. B.; Martinez, M. L.; Ribotta, P.; Wunderlin, D. A.; Baroni, M. V. Defatted Chia Flour as Functional Ingredient in Sweet Cookies. How Do Processing, Simulated Gastrointestinal Digestion and Colonic Fermentation Affect Its Antioxidant Properties? Food Chem. 2020, 316, 126279. DOI: 10.1016/j.foodchem.2020.126279.
- Muñoz, L. A.; Cobos, A.; Diaz, O.; Aguilera, J. M. Chia Seed (Salvia Hispanica): An Ancient Grain and a New Functional Food. Food Rev. Int. 2013, 29(4), 394–408. DOI: 10.1080/87559129.2013.818014.
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The Chemical Composition and Nutritional Value of Chia Seeds—Current State of Knowledge. Nutrients. 2019, 11(6), 1242. DOI: 10.3390/nu11061242.
- Orozco de Rosas, G.; Duran Puga, N.; González Eguiarte, D. R.; Zarazua Villaseñor, P.; Ramirez Ojeda, G.; Mena Munguía, S. Proyecciones de Cambio Climático y Potencial Productivo para Salvia Hispanica L. en las Zonas Agrícolas de México. Rev. Mexicana Cienc. Agríc. 2014, 5(10), 1831–1842. DOI: 10.29312/remexca.v0i10.1020.
- Ayerza, R.; Coates, W. Influence of Environment on Growing Period and Yield, Protein, Oil and A-Linolenic Content of Three Chia (Salvia Hispanica L.) Selections. Ind. Crops Prod. 2009, 30(2), 321–324. DOI: 10.1016/j.indcrop.2009.03.009.
- Ali, N. M.; Yeap, S. K.; Ho, W. Y.; Beh, B. K.; Tan, S. W.; Tan, S. G. The Promising Future of Chia, Salvia Hispanica L. J. Biotechnol. Biomed. 2012, 2012, 1–9. DOI: 10.1155/2012/171956.
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M. A. Dietary Fibre Content and Antioxidant Activity of Phenolic Compounds Present in Mexican Chia (Salvia Hispanica L.) Seeds. Food Chem. 2008, 107(2), 656–663. DOI: 10.1016/j.foodchem.2007.08.062.
- Santos Fernandes, S.; Prentice, C.; Salas-Mellado, M. M. Chapter 11. Chia Seed (Salvia Hispanica). In Oilseeds: Health Attributes and Food Applications;, Tanwar, B. and Goyal, A.; Eds.; Springer: Singapore, 2021; pp. 289–304. DOI:10.1007/978-981-15-4194-0.
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and Biological Value of Quinoa (Chenopodium Quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. DOI: 10.1016/j.cofs.2016.11.007.
- Villacrés, E.; Cueva, P.; Díaz, M.; Rosell, C. M. Replacing Wheat Flour with Debittered and Fermented Lupin: Effects on Bread’s Physical and Nutritional Features. Plant Foods Hum. Nutr. 2020, 75(4), 569–575. DOI: 10.1007/s11130-020-00844-w.
- Ghafoor, K.; Aljuhaimi, F.; Özcan, M. M.; Uslu, N.; Hussain, S.; Babiker, E. E.; Fadimu, G. Effects of Roasting on Bioactive Compounds, Fatty Acid, and Mineral Composition of Chia Seed and Oil. J. Food Process Preserv. 2018, 42(10). DOI: 10.1111/jfpp.13710.
- Goh, K. K. T.; Matia-Merino, L.; Chiang, J. H.; Quek, R.; Soh, S. J. B.; Lentle, R. G. The Physico-Chemical Properties of Chia Seed Polysaccharide and Its Microgel Dispersion Rheology. Carbohydr. Polym. 2016, 149, 297–307. DOI: 10.1016/j.carbpol.2016.04.126.
- Chiang, J. H.; Ong, D. S. M.; Kay Ng, F. S.; Hua, X. Y.; Tay, W. L. W.; Henry, C. J. Application of Chia (Salvia Hispanica) Mucilage as an Ingredient Replacer in Foods. Trends Food Sci. Technol. 2021, 115, 105–116. DOI: 10.1016/j.tifs.2021.06.039.
- Santos Fernandes, S.; Salas Mellado, M. M. Addition of Chia Seed Mucilage for Reduction of Fat Content in Bread and Cakes. Food Chem. 2017, 227, 237–244. DOI: 10.1016/j.foodchem.2017.01.075.
- Santos Fernandes, S.; Filipini, G.; Salas-Mellado, M. M. Development of Cake Mix with Reduced Fat and High Practicality by Adding Chia Mucilage. Food Biosci. 2021, 42, 101148. DOI: 10.1016/j.fbio.2021.101148.
- Santos Fernandes, S.; Salas Mellado, M. M. Development of Mayonnaise with Substitution of Oil or Egg Yolk by the Addition of Chia (Salvia Hispanica L.) Mucilage. J. Food Sci. 2018, 83(1), 74–83. DOI: 10.1111/1750-3841.13984.
- Tan, M.; Chang, S.; Liu, J.; Li, H.; Xu, P.; Wang, P.; Wang, X.; Zhao, M.; Zhao, B.; Wang, L., et al. Physicochemical Properties, Antioxidant and Antidiabetic Activities of Polysaccharides from Quinoa (Chenopodium Quinoa Willd.) Seeds. Molecules. 2020, 25(17), 3840. DOI: 10.3390/molecules25173840.
- Sargi, S. C.; Silva, B. C.; Santos, H. M. C.; Montanher, P. F.; Boeing, J. S.; Santos Júnior, O. O.; Souza, N. E.; Visentainer, J. V. Antioxidant Capacity and Chemical Composition in Seeds Rich in Omega-3: Chia, Flax, and Perilla. Food Sci. Technol. 2013, 33(3), 541–548. DOI: 10.1590/S0101-20612013005000057.
- Coelho, M. S.; Salas-Mellado, M. D. L. M. Effects of Substituting Chia (Salvia Hispanica L.) Flour or Seeds for Wheat Flour on the Quality of the Bread. LWT. Food Sci. Technol. 2015, 60(2), 729–736. DOI: 10.1016/j.lwt.2014.10.033.
- Özcan, M. M.; Al-Juhaimi, F. Y.; Ahmed, I. A. M.; Osman, M. A.; Gassem, M. A. Effect of Different Microwave Power Setting on Quality of Chia Seed Oil Obtained in a Cold Press. Food Chem. 2019, 278, 190–196. DOI: 10.1016/j.foodchem.2018.11.048.
- da Silva, B. P.; Dias, D. M.; de Castro Moreira, M. E.; Toledo, R. C. L. M.; da Matta, S. L. P.; Lucia, C. M. D.; Martino, H. S. D.; Pinheiro-Sant’ana, H. M. Chia Seed Shows Good Protein Quality, Hypoglycemic Effect and Improves the Lipid Profile and Liver and Intestinal Morphology of Wistar Rats. Plant Foods Hum. Nutr. 2016, 71(3), 225–230. DOI: 10.1007/s11130-016-0543-8.
- Ayerza, R., Jr.; Coates, W. Effect of Dietary α-Linolenic Fatty Acid Derived from Chia When Fed as Ground Seed, Whole Seed and Oil on Lipid Content and Fatty Acid Composition of Rat Plasma. Annals Nutr. Metab. 2007, 51(1), 27–34. DOI: 10.1159/000100818.
- Coelho, M. S.; Soares-Freitas, R. A. M.; Gomes, J. A.; Avila, E.; Salas-Mellado, M. D. L. M. Peptides from Chia Present Antibacterial Activity and Inhibit Cholesterol Synthesis. Plant Foods Hum. Nutr. 2018, 73(2), 101–107. DOI: 10.1007/s11130-018-0668-z.
- Vuksan, V.; Jenkins, A. L.; Dias, A. G.; Lee, A. S.; Jovanovski, E.; Rogovik, A. L.; Hanna, A. Reduction in Postprandial Glucose Excursion and Prolongation of Satiety: Possible Explanation of the Long-Term Effects of Whole Grain Salba (Salvia Hispanica L.). Eur. J. Clin. Nutr. 2010, 64(4), 436–438. DOI: 10.1038/ejcn.2009.159.
- Ho, H.; Lee, A. S.; Jovanovski, E.; Jenkins, A. L.; de Souza, R.; Vuksan, V. Effect of Whole and Ground Salba Seeds (Salvia Hispanica L.) on Postprandial Glycemia in Healthy Volunteers: A Randomized Controlled, Dose-Response Trial. Eur. J. Clin. Nutr. 2013, 67(7), 786–788. DOI: 10.1038/ejcn.2013.103.
- Vuksan, V.; Choleva, L.; Jovanovski, E.; Jenkins, A. L.; Au-Yeung, F.; Dias, A. G.; Ho, H.; Zurbau, A.; Duvnjak, L. Comparison of Flax (Linum Usitatissimum) and Salba-Chia (Salvia Hispanica L.) Seeds on Postprandial Glycemia and Satiety in Healthy Individuals: A Randomized, Controlled, Crossover Study. Eur. J. Clin. Nutr. 2017, 71(2), 234–238. DOI: 10.1038/ejcn.2016.148.
- European Commission (EC). Authorizing the Placing on the Market of Chia Seed (Salvia Hispanica) as Novel Food Ingredient Under Regulation (EC) No 258/97 of Council of the European Parliament. OJEU L. 2009, 294, 14–15.
- European Commission (EC). Commission Implementing Decision of 22 January 2013 Authorising an Extension of Use of Chia (Salvia Hispanica) Seed as a Novel Food Ingredient Under Regulation (EC) No 258/97 of the European Parliament and of the Council. OJEU L. 2013, 21(34), 34–35.
- European Commission (EC). Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. OJEU L. 2017, 351, 72.
- Mesias, M.; Holgado, F.; Márquez-Ruiz, G.; Morales, F. J. Risk/Benefit Considerations of a New Formulation of Wheat-Based Biscuit Supplemented with Different Amounts of Chia Flour. LWT. Food Sci. Technol. 2016, 73, 528–535. DOI: 10.1016/j.lwt.2016.06.056.
- EFSA. Safety of Chia Seeds (Salvia Hispanica L.) as a Novel Food for Extended Uses Pursuant to Regulation (EU) 2015/2283. Efsa J. 2019, 17(4). DOI: 10.2903/j.efsa.2019.5657.
- EFSA (European Food Safety Authority). Scientific Opinion on Acrylamide in Food. Efsa J. 2015, 13(6), 4104. DOI:10.2903/j.efsa.2015.4104.
- IARC (International Agency for Research on Cancer). IARC Monographs on the Evaluation for Carcinogenic Risk of Chemicals to Humans: Some Industrial Chemicals; IARC: Lyon, France, 1994; Vol. 60.
- EFSA. Outcome of a Public Consultation on the Draft Scientific Opinion on the Safety of Chia Seeds (Salvia Hispanica L.) Subject to Thermal Processing in Relation to the Formation of Process Contaminants as a Novel Food for Extended Uses. EFSA Support. Publ. 2020, 17(9). DOI: 10.2903/sp.efsa.2020.EN-1925.
- European Commission (EC). Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. OJEU L. 2017, L304, 24–44.
- Rabbani, N.; Thornalley, P. J. Dicarbonyl Proteome and Genome Damage in Metabolic and Vascular Disease. Biochem. Soc. Trans. 2014, 42(2), 425–432. DOI: 10.1042/BST20140018.
- Galluzzo, F. G.; Cammilleri, G.; Pantano, L.; Lo Cascio, G.; Pulvirenti, A.; Macaluso, A.; Vella, A.; Ferrantelli, V. Acrylamide Assessment of Wheat Bread Incorporating Chia Seeds (Salvia Hispanica L.) by LC-MS/MS. Food Addit. Contam. 2021, 38(3), 388–395. DOI: 10.1080/19440049.2020.1853823.
- Zettel, V.; Hitzmann, B. Applications of Chia (Salvia Hispanica L.) in Food Products. Trends Food Sci. Technol. 2018, 80, 43–50. DOI: 10.1016/j.tifs.2018.07.011.
- Sayed-Ahmad, B.; Talou, T.; Straumite, E.; Sabovics, M.; Kruma, Z.; Saad, Z.; Hijazi, A.; Merah, O. Evaluation of Nutritional and Technological Attributes of Whole Wheat Based Bread Fortified with Chia Flour. Foods. 2018, 7(9), 135. DOI: 10.3390/foods7090135.
- Švec, I.; Hrušková, M.; Jurinová, I. Pasting Characteristics of Wheat-Chia Blends. J. Food Eng. 2016, 172, 25–30. DOI: 10.1016/j.jfoodeng.2015.04.030.
- James, L. E. A. Quinoa (Chenopodium Quinoa Willd.): Composition, Chemistry, Nutritional and Functional Properties. Adv. Food Nutr. Res. 2009, 58(9), 1–31. DOI: 10.1016/S1043-4526(09)58001-1.
- Borges, J.; Bonomo, R.; De Paula, C. D.; Oliveira, L.; Cesário, M. Características Físico-Químicas, Nutricionais e Formas de Consumo da Quinoa (Chenopodium Quinoa Willd.). Temas Agrarios. 2010, 15(1), 9–23. DOI: 10.21897/rta.v15i1.815.
- Yang, X.; Zhu, K.; Guo, H.; Geng, Y.; Lv, W.; Wang, S.; Guo, Y.; Qin, P.; Ren, G. Characterization of Volatile Compounds in Differently Coloured Chenopodium Quinoa Seeds Before and After Cooking by Headspace-Gas Chromatography-Ion Mobility Spectrometry. Food Chem. 2021, 348, 129086. DOI: 10.1016/j.foodchem.2021.129086.
- MAPA (Ministerio de Agricultura, Pesca y Alimentación) Encuesta Sobre Superficies y Rendimientos Cultivos (ESYRCE). Estadística Digital. Anuario De Estadística. Ministerio de Agricultura, Pesca y Alimentación, Gobierno de España. (accessed Feb 15, 2023). https://www.mapa.gob.es/es/estadistica/temas/estadistica-digital/powerbi-cultivos.aspx
- FAOSTAT Statistical Database FAO. (accessed Feb 15, 2023). https://www.fao.org/faostat/es/#data/QCL
- Sezgin, A. C.; Sanlier, N. A New Generation Plant for the Conventional Cuisine: Quinoa (Chenopodium Quinoa Willd.). Trends Food Sci. Technol. 2019, 86, 51–58. DOI: 10.1016/j.tifs.2019.02.039.
- Al-Qabba, M. M.; El-Mowafy, M. A.; Althwab, S. A.; Alfheeaid, H. A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium Quinoa Sprouts Against Ccl4-Induced Oxidative Stress in Rats. Nutrients. 2020, 12(10), 2904. DOI: 10.3390/nu12102904.
- Farinazzi-Machado, F. M. V.; Barbalho, S. M.; Oshiiwa, M.; Goulart, R.; Pessan Junior, O. Use of Cereal Bars with Quinoa (Chenopodium Quinoa W.) to Reduce Risk Factors Related to Cardiovascular Diseases. Food Sci. Technol. 2012, 32(2), 239–244. DOI: 10.1590/s0101-20612012005000040.
- Navarro-Perez, D.; Radcliffe, J.; Tierney, A.; Jois, M. Quinoa Seed Lowers Serum Triglycerides in Overweight and Obese Subjects: A Dose-Response Randomized Controlled Clinical Trial. Curr. Dev. Nutr. 2017, 1(9), e001321. DOI: 10.3945/cdn.117.001321.
- Gorinstein, S.; Vargas, O. J. M.; Jaramillo, N. O.; Salas, I. A.; Ayala, A. L. M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The Total Polyphenols and the Antioxidant Potentials of Some Selected Cereals and Pseudocereals. Eur. Food Res. Technol. 2007, 225(3–4), 321–328. DOI: 10.1007/s00217-006-0417-7.
- Filho, A. M. M.; Pirozi, M. R.; Borges, J. T. D. S.; Pinheiro Sant’ana, H. M.; Chaves, J. B. P.; Coimbra, J. S. D. R. Quinoa: Nutritional, Functional, and Antinutritional Aspects. Crit. Rev. Food Sci. Nutr. 2017, 57(8), 1618–1630. DOI: 10.1080/10408398.2014.1001811.
- Ali, O. Nutritional Value of Germinated Quinoa Seeds and Their Protective Effects on Rats Health Injected by Nicotine. Egypt. J. Food Sci. 2019, 47(2), 227–241. DOI: 10.21608/ejfs.2019.15608.1014.
- Cisneros-Yupanqui, M.; Lante, A.; Mihaylova, D.; Krastanov, A. I.; Vílchez-Perales, C. Impact of Consumption of Cooked Red and Black Chenopodium Quinoa Willd. Over Blood Lipids, Oxidative Stress, and Blood Glucose Levels in Hypertension-Induced Rats. Cereal Chem. 2020, 97(6), 1254–1262. DOI: 10.1002/cche.10351.
- Nickel, J.; Spanier, L. P.; Botelho, F. T.; Gularte, M. A.; Helbig, E. Effect of Different Types of Processing on the Total Phenolic Compound Content, Antioxidant Capacity, and Saponin Content of Chenopodium Quinoa Willd Grains. Food Chem. 2016, 209, 139–143. DOI: 10.1016/j.foodchem.2016.04.031.
- Sazesh, B.; Goli, M. Quinoa as a Wheat Substitute to Improve the Textural Properties and Minimize the Carcinogenic Acrylamide Content of the Biscuit. J. Food Process Preserv. 2020, 44(8), e14563. DOI: 10.1111/jfpp.14563.
- Popova, A.; Mihaylova, D. Antinutrients in Plant-Based Foods: A Review. Open Biotechnol. J. 2019, 13(1), 68–76. DOI: 10.2174/1874070701913010068.
- Mhada, M.; Metougui, M. L.; El Hazzam, K.; El Kacimi, K.; Yasri, A. Variations of Saponins, Minerals and Total Phenolic Compounds Due to Processing and Cooking of Quinoa (Chenopodium Quinoa Willd.) Seeds. Foods. 2020, 9(5), 660. DOI: 10.3390/foods9050660.
- Brito, I. L.; de Souza, E. L.; Felex, S. S. S.; Madruga, M. S.; Yamashita, F.; Magnani, M. Nutritional and Sensory Characteristics of Gluten-Free Quinoa (Chenopodium Quinoa Willd)-Based Cookies Development Using an Experimental Mixture Design. J. Food Sci. Technol. 2015, 52(9), 5866–5873. DOI: 10.1007/s13197-014-1659-1.
- Jan, K. N.; Panesar, P. S.; Singh, S. Optimization of Antioxidant Activity, Textural and Sensory Characteristics of Gluten-Free Cookies Made from Whole Indian Quinoa Flour. LWT. 2018, 93, 573–582. DOI: 10.1016/j.lwt.2018.04.013.
- El-Sohaimy, A. S.; Shehata, G. M.; Djapparovec, T. A.; Mehany, T.; Zeitoun, A. M.; Zeitoun, M. A. Development and Characterization of Functional Pan Bread Supplemented with Quinoa Flour. J. Food Process Preserv. 2021, 45(2). DOI: 10.1111/jfpp.15180.
- Tufail, T.; Riaz, M.; Arshad, M. U.; Gilani, S. A.; Ains, H. B. U.; Khursheed, T.; Islam, Z.; Imran, M.; Bashir, S.; Shahid, M. Z., et al. A. Functional and Nutraceutical Scenario of Flaxseed and Sesame: A Review. Int. J. Biosci. 2020, 17(3), 173–190. DOI: 10.12692/ijb/17.3.173-190.
- FAO. Fichas Técnicas, Productos Frescos y Procesados. PRODAR (Programa de Desarrollo de La Agroindustria Rural de América Latina y El Caribe). (accessed Feb 16, 2023). www.fao.org/3/aae620s.pdf%0Ahttp://www.fao.org/fileadmin/templates/inpho/documents/PRODAR.pdf
- Abirached, C.; Bonifacino, C.; Dutto, E.; Velazco, L.; Jorge, F.; Vieitez, I. Study of Sesame Seeds Antioxidant and Emulsifying Properties: Original High-Quality Research Paper. J. Supercrit Fluids. 2020, 166, 104994. DOI: 10.1016/j.supflu.2020.104994.
- Dravie, E. E.; Kortei, N. K.; Essuman, E. K.; Tettey, C. O.; Boakye, A. A.; Hunkpe, G. Antioxidant, Phytochemical and Physicochemical Properties of Sesame Seed (Sesamum Indicum L). Sci. Afr. 2020, 8, e00349. DOI: 10.1016/j.sciaf.2020.e00349.
- Berk, E.; Hamzalloglu, A.; Gökmen, V. Investigations on the Maillard Reaction in Sesame (Sesamum Indicum L.) Seeds Induced by Roasting. J. Agric. Food. Chem. 2019, 67(17), 4923–4930. DOI: 10.1021/acs.jafc.9b01413.
- Gharby, S.; Harhar, H.; Bouzoubaa, Z.; Asdadi, A.; El Yadini, A.; Charrouf, Z. Chemical Characterization and Oxidative Stability of Seeds and Oil of Sesame Grown in Morocco. J. Saudi Soc. Agric. Sci. 2017, 16(2), 105–111. DOI: 10.1016/j.jssas.2015.03.004.
- Vishwanath, H. S.; Anilakumar, K. R.; Harsha, S. N.; Khanum, F.; Bawa, A. S. In vitro Antioxidant Activity of Sesamum Indicum Seeds. Asian J. Pharm. Clin. Res. 2012, 5(1), 56–60.
- Ahmed, I. A. M.; Uslu, N.; Özcan, M. M.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E. E.; Osman, M. A.; Alqah, H. A. S. Effect of Conventional Oven Roasting Treatment on the Physicochemical Quality Attributes of Sesame Seeds Obtained from Different Locations. Food Chem. 2021, 338, 128109. DOI: 10.1016/j.foodchem.2020.128109.
- Ahmed, I. A. M.; Özcan, M. M.; Uslu, N.; Juhaimi, F. A. L.; Osman, M. A.; Alqah, H. A. S.; Ghafoor, K.; Babiker, E. E. Effect of Microwave Roasting on Color, Total Phenol, Antioxidant Activity, Fatty Acid Composition, Tocopherol and Chemical Composition of Sesame Seed and Oils Obtained from Different Countries. J. Food Process Preserv. 2020, 44(10), e14807. DOI: 10.1111/jfpp.14807.
- Tripathy, S. K.; Kar, J.; Sahu, D. Advances in Sesame (Sesamum Indicum L.) Breeding. In Advances in Plant Breeding Strategies: Industrial and Food Crops;, Al-Khayri, J.M., Jain, S.M. and Johnson, D.V.; Eds.; Springer: Switzerland, 2019; Vol. 6pp. 577–635. DOI:10.1007/978-3-030-23265-8_15.
- Sharma, L.; Saini, C. S.; Punia, S.; Nain, V.; Sandhu, K. S. Chapter 12. Sesame (Sesamum indicum) Seed. In Oilseeds: Health Attributes and Food Applications;, Tanwar, B. and Goyal, A.; Eds.; Springer: Singapore, 2021; pp. 305–330. DOI:10.1007/978-981-15-4194-0_12.
- Bigoniya, P.; Nishad, R.; Singh, C. S. Preventive Effect of Sesame Seed Cake on Hyperglycemia and Obesity Against High Fructose-Diet Induced Type 2 Diabetes in Rats. Food Chem. 2012, 133(4), 1355–1361. DOI: 10.1016/j.foodchem.2012.01.112.
- Khosravi-Boroujeni, H.; Nikbakht, E.; Natanelov, E.; Khalesi, S. Can Sesame Consumption Improve Blood Pressure? A Systematic Review and Meta-Analysis of Controlled Trials. J. Sci. Food Agric. 2017, 97(10), 3087–3094. DOI: 10.1002/jsfa.8361.
- Visavadiya, N. P.; Narasimhacharya, A. V. R. L. Sesame as a Hypocholesteraemic and Antioxidant Dietary Component. Food. Chem. Toxicol. 2008, 46(6), 1889–1895. DOI: 10.1016/j.fct.2008.01.012.
- Aslam, M.; Shabbir, M. A.; Pasha, I.; Shukat, R.; Siddique, U.; Manzoor, M. F.; Ayub, S. Protective Effect of Sesame (Sesamum Indicum) Seed Oil Against Hypercholesterolemic in Sprague-Dawley Male Rats. Food Sci. Technol. 2021, 41(2), 741–745. DOI: 10.1590/fst.35320.
- Sankar, D.; Rao, M. R.; Sambandam, G.; Pugalendi, K. V. A Pilot Study of Open Label Sesame Oil in Hypertensive Diabetics. J. Med. Food. 2006, 9(3), 408–412. DOI: 10.1089/jmf.2006.9.408.
- Boulbaroud, S.; Mesfioui, A.; Arfaoui, A.; Ouichou, A.; el-Hessni, A. Preventive Effects of Flaxseed and Sesame Oil on Bone Loss in Ovariectomized Rats. Pak. J. Biol. Sci. 2008, 11(13), 1696–1701. DOI: 10.3923/pjbs.2008.1696.1701.
- Charissou, A.; Ait-Ameur, L.; Birlouez-Aragon, I. Kinetics of Formation of Three Indicators of the Maillard Reaction in Model Cookies: Influence of Baking Temperature and Type of Sugar. J. Agric. Food. Chem. 2007, 55(11), 4532–4539. DOI: 10.1021/jf063024j.
- Giannetti, V.; Mariani, M. B.; Mannino, P. Furosine as a Pasta Quality Marker: Evaluation by an Innovative and Fast Chromatographic Approach. J. Food Sci. 2013, 78(7), C994–999. DOI: 10.1111/1750-3841.12163.
- García-Baños, J. L.; Villamiel, M.; Olano, A.; Rada-Mendoza, M. Study on Nonenzymatic Browning in Cookies, Crackers and Breakfast Cereals by Maltulose and Furosine Determination. J. Cereal Sci. 2004, 39(2), 167–173. DOI: 10.1016/S0733-5210(03)00070-5.
- Berk, E.; Hamzalıoğlu, A.; Gökmen, V. Multiresponse Kinetic Modelling of 5-Hydroxymethylfurfural and Acrylamide Formation in Sesame (Sesamum Indicum L.) Seeds During Roasting. Eur. Food Res. Technol. 2020, 246(12), 2399–2410. DOI: 10.1007/s00217-020-03583-z.
- Berk, E.; Aktağ, I. G.; Gökmen, V. Formation of α-Dicarbonyl Compounds and Glycation Products in Sesame (Sesamum Indicum L.) Seeds During Roasting: A Multiresponse Kinetic Modelling Approach. Eur. Food Res. and Technol. 2021, 247(9), 2285–2298. DOI: 10.1007/s00217-021-03787-x.
- Zouari, R.; Besbes, S.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Cookies from Composite Wheat-Sesame Peels Flours: Dough Quality and Effect of Bacillus Subtilis SPB1 Biosurfactant Addition. Food Chem. 2016, 194, 758–769. DOI: 10.1016/j.foodchem.2015.08.064.
- El-Enzi, S. M.; Andigani, N. M.; Al-Tamimi, N. A.; Gabr, G. A. Physico Chemical and Sensory Evaluation of the Fortified Biscuits with Sesame Cake Flour. Asian Food Sci. J. 2018, 5(4), 1–8. DOI: 10.9734/afsj/2018/45232.
- Martínez, E.; García-Martínez, R.; Álvarez-Ortí, M.; Rabadán, A.; Pardo-Giménez, A.; Pardo, J. E. Elaboration of Gluten-Free Cookies with Defatted Seed Flours: Effects on Technological, Nutritional, and Consumer Aspects. Foods. 2021, 10(6), 1213. DOI: 10.3390/foods10061213.
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical Composition and Health Benefits of Flaxseed. Austin J. Nutri. Food Sci. 2014, 2(8), 1045.
- Bekhit, A. E. D. A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I. A.; Al-Juhaimi, F. Y.; Saeedi, P.; Bekhit, A. A. Flaxseed: Composition, Detoxification, Utilization, and Opportunities. Biocatal Agric. Biotechnol. 2018, 13, 129–152. DOI: 10.1016/j.bcab.2017.11.017.
- Parikh, M.; Netticadan, T.; Pierce, G. N. Flaxseed: Its Bioactive Components and Their Cardiovascular Benefits. Am. J. Physiol. Heart Circ. Physiol. 2018, 314(2), H146–159. DOI: 10.1152/ajpheart.00400.2017.
- Özcan, M. M.; Uslu, N. Investigation of Changes in Some Chemical Properties, Bioactive Compound, Antioxidant Activity, Phenolic and Fatty Acid Profiles of Flaxseed and Oils. J. Food Process Preserv. 2022, 46(11), e17091. DOI: 10.1111/jfpp.17091.
- Abreu, M. D. C.; Pessoa, L. R.; Costa, R. L.; Boueri, B. F. C.; Pessanha, C. R.; Pereira, A. D.; Ribeiro, D. C.; Silva, E. M.; Costa, C. A. S.; Boaventura, G. T. Flaxseed (Linum Usitatissimum) Flour Contributes to Bone Health in Adult Male Rats. Nutrition. 2018, 49, 48–50. DOI: 10.1016/j.nut.2017.11.025.
- Kruger, M. C.; Coetzee, M.; Haag, M.; Weiler, H. Long-Chain Polyunsaturated Fatty Acids: Selected Mechanisms of Action on Bone. Prog. lipid res. 2010, 49(4), 438–449. DOI: 10.1016/j.plipres.2010.06.002.
- Pessanha, C. R.; Da Camara Boueri, B. F.; Da Costa, L. R.; Ferreira, M. R.; De Abreu, M. D. C.; Pessoa, L. R.; Pereira, A. D. A.; Ribeiro, D. C.; da Silva, E. M.; Da Costa, C. A. S., et al. Flaxseed Flour, Compared to Flaxseed Oil, Contributes to Femoral Structure in Male Rats Subjected to Early Weaning. Food Funct. 2016, 7(3), 1296–1300. DOI: 10.1039/c6fo00021e.
- Kristensen, M.; Jensen, M. G.; Aarestrup, J.; Petersen, K. E.; Søndergaard, L.; Mikkelsen, M. S.; Astrup, A. Flaxseed Dietary Fibers Lower Cholesterol and Increase Fecal Fat Excretion, but Magnitude of Effect Depend on Food Type. Nutr. Metab. 2012, 9(1), 1–8. DOI: 10.1186/1743-7075-9-8.
- Mandaşescu, S.; Mocanu, V.; Dăscaliţa, A. -M.; Haliga, R.; Nestian, I.; Stitt, P. A.; Luca, V. Flaxseed Supplementation in Hyperlipidemic Patients. Rev Med Chir Soc Med Nat Iasi. 2005, 109(3), 502–506.
- Patade, A.; Devareddy, L.; Lucas, E. A.; Korlagunta, K.; Daggy, B. P.; Arjmandi, B. H. Flaxseed Reduces Total and LDL Cholesterol Concentrations in Native American Postmenopausal Women. J. Womens Health. 2008, 17(3), 355–366. DOI: 10.1089/jwh.2007.0359.
- Fechner, A.; Kiehntopf, M.; Jahreis, G. The Formation of Short-Chain Fatty Acids is Positively Associated with the Blood Lipid-Lowering Effect of Lupin Kernel Fiber in Moderately Hypercholesterolemic Adults. J. Nutr. 2014, 144(5), 599–607. DOI: 10.3945/jn.113.186858.
- Rodriguez-Leyva, D.; Weighell, W.; Edel, A. L.; Lavallee, R.; Dibrov, E.; Pinneker, R.; Maddaford, T. G.; Ramjiawan, B.; Aliani, M.; Guzman, R., et al. Potent Antihypertensive Action of Dietary Flaxseed in Hypertensive Patients. Hypertension. 2013, 62(6), 1081–1089. DOI: 10.1161/HYPERTENSIONAHA.113.02094.
- Gabbs, M.; Leng, S.; Devassy, J. G.; Monirujjaman, M.; Aukema, H. M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs 1,2. Adv Nutr. 2015, 6(5), 513–540. DOI: 10.3945/an.114.007732.
- Mazza, G. Production, Processing and Uses of Canadian Flax. Presented at the First CGNA International Workshop, Temuco, Chile, August 2008.
- Kajla, P.; Sharma, A.; Sood, D. R. Flaxseed—A Potential Functional Food Source. J. Food Sci. Technol. 2015, 52(4), 1857–1871. DOI: 10.1007/s13197-014-1293-y.
- Feng, D.; Shen, Y.; Chavez, E. R. Effectiveness of Different Processing Methods in Reducing Hydrogen Cyanide Content of Flaxseed. J. Sci. Food Agric. 2003, 83(8), 836–841. DOI: 10.1002/jsfa.1412.
- Akande, K. E.; Doma, U. D.; Agu, H. O.; Adamu, H. M. Major Antinutrients Found in Plant Protein Sources: Their Effect on Nutrition. Pak J. Nutr. 2010, 9(8), 827–832. DOI: 10.3923/pjn.2010.827.832.
- Bartkiene, E.; Jakobsone, I.; Pugajeva, I.; Bartkevics, V.; Zadeike, D.; Juodeikiene, G. Reducing of Acrylamide Formation in Wheat Biscuits Supplemented with Flaxseed and Lupine. LWT. Food Sci. Technol. 2016, 65, 275–282. DOI: 10.1016/j.lwt.2015.08.002.
- Khouryieh, H.; Aramouni, F. Physical and Sensory Characteristics of Cookies Prepared with Flaxseed Flour. J. Sci. Food Agric. 2012, 92(11), 2366–2372. DOI: 10.1002/jsfa.5642.
- Kaur, R.; Kaur, M.; Purewal, S. S. Effect of Incorporation of Flaxseed to Wheat Rusks: Antioxidant, Nutritional, Sensory Characteristics, and in vitro DNA Damage Protection Activity. J. Food Process Preserv. 2018, 42(4), 1–11. DOI: 10.1111/jfpp.13585.
- Man, S. M.; Stan, L.; Păucean, A.; Chiş, M. S.; Mureşan, V.; Socaci, S. A.; Pop, A.; Muste, S. Nutritional, Sensory, Texture Properties and Volatile Compounds Profile of Biscuits with Roasted Flaxseed Flour Partially Substituting for Wheat Flour. Appl. Sci. 2021, 11(11), 4791. DOI: 10.3390/app11114791.
- Kaur, P.; Sharma, P.; Kumar, V.; Panghal, A.; Kaur, J.; Gat, Y. Effect of Addition of Flaxseed Flour on Phytochemical, Physicochemical, Nutritional, and Textural Properties of Cookies. J. Saudi Soc. Agric. Sci. 2019, 18(4), 372–377. DOI: 10.1016/j.jssas.2017.12.004.
- Wirkijowska, A.; Zarzycki, P.; Sobota, A.; Nawrocka, A.; Blicharz-Kania, A.; Andrejko, D. The Possibility of Using By-Products from the Flaxseed Industry for Functional Bread Production. LWT. Food Sci. Technol. 2020, 118, 108860. DOI: 10.1016/j.lwt.2019.108860.