ABSTRACT
The growing interest in plant-based proteins is driven by sustainability concerns and potential adverse reactions to animal protein consumption. Plant proteins, while promising, are known for their inferior digestibility compared to animal counterparts. Solid-state fermentation (SSF) offers an ancient, cost-effective approach to address this limitation. SSF enhances plant-based foods by augmenting protein content through microbial hydrolysis, thereby improving overall nutritional quality. This method not only boosts protein digestibility but also reduces larger polypeptides, generates bioactive peptides, refines amino acid profiles, and eliminates anti-nutritional factors such as trypsin inhibitors and tannins. Further investigation is essential to optimize the purification of protein isolates and examine their behaviour during digestion and absorption using in vitro, cellular, and in vivo models. In conclusion, SSF represents a promising, cost-effective, and high-yield processing method to produce nutritionally enhanced plant proteins, aligning with the demands of both the industry and consumers.
Introduction
Fermentation is an ancient food processing technology used to extend the shelf-life of food and enhance its nutritional and organoleptic properties.[Citation1] Numerous biochemical changes occur during fermentation, leading to altered nutritive and anti-nutritive compositions and affecting product properties such as bioactivity and digestibility.[Citation2] Submerged (SmF) and solid-state fermentations (SSF) are two types of fermentation processes, where microorganisms are grown in liquid form for SmF but on a solid support for SSF. SSF offers numerous advantages over conventional SmF systems, such as lower water and energy requirements, higher protein productivity and less stringent requirement for sterile medium.[Citation3]
Recently, there has been a notable surge in the application of fermentation technology in various industries such as food, chemicals and pharmaceuticals.[Citation4] When considering the application of SSF to improve the nutritional properties of the plant foods such as cereals, grains, and oilseeds examination of protein content is imperative as they represent a substantial source of protein. The fermentation process may also modify the protein’s composition and nutritional profile. Prior studies have established that fermentation leads to improvements in the quality and bioavailability of protein, owing to reducing anti-nutritional factors (ANFs) and the degradation of complex macromolecular proteins to simpler smaller proteins, peptides and free amino acids.[Citation5]
While a significant body of literature exists on the fermentation of animal-derived proteins, mainly milk products, shifting consumer preferences and health considerations have shifted the focus towards exploring plant-based proteins.[Citation6] Recent trends highlight the rising demand for plant-based proteins, exemplified by a 44% increase in the consumption of lentils in the USA in 2020 compared to 2019.[Citation7] Previous reviews on protein fermentation focused on the bioactive peptide derived from the fermentation process and the structure-function relationship of protein.[Citation7,Citation8] Specific protein sources or fermentation methods, such as plant-derived protein and SSF, were not covered. Sadh et al.[Citation9] discussed the general influence of SSF on proteins without referring to the biological activity of the peptides and amino acids. Up to date, no comprehensive review has yet addressed SSF of plant proteins and its fermentation products.
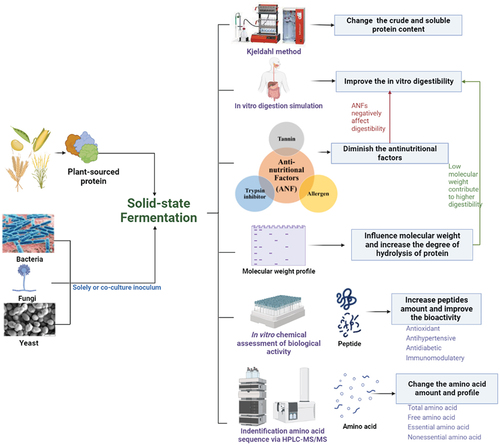
This review bridges this gap by summarizing the effect of SSF on various plant proteins, especially on its influence on protein content, molecular weight (MW), degree of hydrolysis, and in vitro digestibility. This paper compares the effects of different starter cultures such as bacteria, mold, yeast, and their co-culture on plant-based protein fermentation, and explores the mechanism of action. We further highlight on the reduction of antinutritional factors related to protein digestibility, changes in amino acid profile, production of peptides and modification of their bioactivity (). This review aims to provide insights for future research on SSF of plant-derived proteins and to promote practical application of fermented proteins into the food or feed system with significantly improved nutritional value.
Impact of starter cultures on protein content in plant foods during SSF
The protein content of SSF products varies significantly due to several factors. These factors include the type of microorganism, the availability of carbon and nutrients, and the cultivation conditions such as fermentation temperature and time.[Citation10] Influences of SSF by bacteria, moulds, yeast and their co-culture on the total protein content of the plant substrates are discussed in this section ().
Table 1. Effect of solid-state fermentation on protein content of selected plant-based substrates.
Various protein content expression formats and assay methods have been used in protein fermentation studies, where the three most prevalent formats are crude protein content, soluble protein content and trichloroacetic acid-soluble protein content. Crude protein content focus on the total nitrogen content within the sample with the application of either the Kjeldahl method or the Dumas combustion method.[Citation53] On the other hand, soluble protein content gauges the portion of protein which can be dissolved in different solvents (e.g. water or buffers), and can be measured via multiple methods such as Bradford protein assay,[Citation54] Pierce BCA assay or the Folin-Ciocalteu method (Lowry protein assay),[Citation55] with bovine serum albumin serving as the standard. The third way to express protein content is trichloroacetic acid-soluble protein content, which employs trichloroacetic acid for protein precipitation, followed by nitrogen content quantification in the supernatant using the Kjeldahl method. This method usually measures the small peptides with 2–20 residues and free amino acids that can be directly absorbed by the gastrointestinal tract.[Citation51]
Impact of bacterial start culture on protein content
As summarized in , bacteria such as Bacillus amyloliquefaciens, Bacillus subtilis, and Lactobacillus plantarum increased the crude protein content of soybean meal, cottonseed meal, maize flour, jatropha seed cake, and moringa oleifera leaf meal after fermentation.[Citation11–17,Citation19–21] In these studies, Bacillus subtilis was the most used start culture, and soybean meal was the most common fermented substrate.[Citation18] However, the addition of Bifidobacterium longum CRL 849 to soybean meal decreased the protein concentration. Variations in fermentation conditions, such as inoculum size, initial moisture, fermentation temperature and time, can also influence the fermentation outcomes.
The augmentation of crude protein content within bacterial Solid-State Fermentation (SSF) processes can be ascribed to two primary factors: the concentration of nitrogen and the generation of enzymes, which inherently comprise proteins. For instance, Zhang et al.[Citation16] found the increase in crude protein content from 507.2 to 557.1 mg/g during SSF of soybean by Bacillus subtilis. natto is mainly due to the relative change in the loss of fiber and lipid as a result of microbial hydrolysis and the metabolism during fermentation. Likewise, Li et al.[Citation11] expounded that carbohydrates serve as substrates for bacterial growth and proliferation during the fermentation of soybean meal with B. amyloliquefaciens, consequently elevating the nitrogen concentration and, in turn, the crude protein content. Furthermore, the production of extracellular enzymes, inherently proteinaceous, by bacteria during SSF contributes to the escalation of crude protein content. For example, Bacillus spp. have been found to produce industrially valuable enzymes such as amylases, catalases, lipases, proteases, and xylanase.[Citation56] Additionally, other bacterial species, such as Lactobacillus plantarum, secretes enzymes during SSF. Terefe et al.[Citation21] observed a remarkable 55% increase in crude protein content in maize flour through Lactobacillus plantarum SSF as opposed to a 49% increase in Saccharomyces cerevisiae SSF. The superior increase in crude protein content can be attributed to the unique enzymes produced by Lactobacillus plantarum, such as α-amylase, esterase, lipase, α-glucosidase, β-glucosidase, enolase, phosphoketolase, lactase dehydrogenase, etc.[Citation57,Citation58]
The increase in soluble protein content may be due to the enzymatic degradation of insoluble macromolecular proteins into soluble peptides and amino acids by bacterial enzymes. Su et al.[Citation51] reported that proteases secreted by B. subtilis BSWF hydrolysed macromolecular proteins (>35 kDa) and resulted in a dramatic increase in soluble protein in solid-state fermented rice bran. Conversely, it is noteworthy that the soluble protein content may exhibit a decrease following SSF. de Olmos et al.[Citation18] applied Bifidobacterium longum CRL 849 to soybean meal and observed a decrease in protein content after 24 h fermentation. This decrease could be related to the B. longum CRL 849 not being able to hydrolyze the soybean protein and instead consuming free amino acid during the fermentation progress.[Citation18]
Impact of mould start culture on protein content
SSF with various fungal species, including Aspergillus niger, Beauveria bassiana, Fusarium flocciferum, Rhizodiscina cf. lignyota, Rhizopus oligosporus, Trichoderma viride, Trichoderma reesei, Pleurotus ostreatus, and Lentinula edodes has been conducted with the most prevalent choices being Aspergillus niger and Rhizopus oligosporus.[Citation23–34,Citation36–40] The increased crude protein content has been shown in various plant-based materials such as ginkgo leaves, vegetable waste powder, cake from olive, pressing seeds or grains, quinoa seeds, wheat, common bean flour, mesocarp flour, cassava leaves and bagasse, palm kernel cake, pineapple peels, orange peels, sugar beet pulp, lentils, and chia seeds. The increase in protein content depending on the type of plant material, fungal species, and fermentation conditions such as temperature, moisture, and time.
The increased crude protein content during mould dominated SSF is derived from different sources. Initially, mould may use carbon as energy sources to synthesize protein-containing mycelia during fermentation.[Citation36] For example, Asensio-Grau et al.[Citation38] fermented lentils flour with Pleurotus ostreatus and reported a net protein increment of 18.5% with a concurrent 6% decrease for carbohydrates. The growth of Pleurotus ostreatus led to the increase of unicellular protein biomass. Furthermore, the mould-secreted lignocellulases can partially degrade lignocellulose including lignin, cellulose, and hemicellulose and release small organic molecules, such as sugars. These small organic molecules can then be utilized by the fungi as a source of energy and nutrients, leading to the growth of the fungi.[Citation59] Wang et al.[Citation23] fermented ginkgo leaves with Aspergillus niger and attributed the relatively higher crude protein content of fermented sample (16.28%) compared to the unfermented one (14.26%) to the same reason. In addition, some mould strains such as Pleurotus ostreatus and Trichoderma species have specific mechanism to influence protein content. The acidophil nature of Pleurotus species and its ability to reduce pH by releasing organic acid could prevent ammoniac volatilisation driven nitrogen losses.[Citation60] The Trichoderma species has the ability to capture the nitrogen and urea by aerobic fermentation in a mineral-salt medium, which contributes to the significant increase of crude protein content.[Citation37] Moreover, similar as bacteria, mould such as Rhizopus oligosporus, Aspergillus niger can secrete extracellular enzymes, which are proteins in nature and contribute to higher crude protein content.[Citation30] Aspergillus niger has been found to produce proteases, pectinase, catalase during fermentation and Rhizopus oligosporus has superior protease and amylase activity.[Citation30] Finally, Firdaus et al.[Citation35] found that the size of the substrate impacted crude protein in palm kernel cake fermented by Rhizopus oryzae ME01, where smaller and uniformly sized palm kernels resulted in a higher increase in crude protein, possibly due to the larger surface area of smaller kernels that enhances protein extraction efficiency.
Reduction of crude protein content was also observed in some mould-induced SSF due to prolonged fermentation time. Aruna[Citation36] found that in the initial 72 hours of fermentation, the crude and soluble protein contents of pineapple peels significantly increased, but then both declined when the fermentation time reached 96 hours. The author suggested that this decline was due to either proteolysis or the consumption of protein by the mould during the later stage of SSF.[Citation36]
Impact of yeast start culture on protein content
Yeast fermentation has been shown to increase protein content in various food ingredients, such as wheat bran, brown rice flour, soybean okara, guar and copra meal, maize flour, cassava leaf and the mixture of groundnut oil cake and Pistia leaves, with Saccharomyces cerevisiae being the most common starter culture.[Citation21,Citation22,Citation41–46]
The increase in protein content can be attributed to an escalation in yeast biomass.[Citation41,Citation43] Yeast is frequently applied in fermentation processes due to their facile cultivation, rapid growth rate, and inherently high protein content, making them ideal for producing single-cell proteins from agricultural by-products.[Citation61] Ilowefah et al.[Citation42] found that fermenting brown rice flour with three commercial baker’s yeasts led to a protein content increase from 77.1 to 88.4, 86.6, and 86.4 mg/g. Su et al.[Citation51] reported a similar increase in crude protein levels, which was due to the decrease in dry matter (mainly carbohydrates) and the elevation in proteins produced by S. cerevisiae. In contrast, Vong et al.[Citation45] reported an absence of significant change in protein content of okara fermented by Yarrowia lipolytica, despite an increase in total free amino acids. The lack of significant change in protein content may be due to the Kjeldahl method conducted, which measures the total nitrogen content of a sample without distinguishing between protein and free amino acids.
Impact of multiple start culture on protein content
also summarizes the results of using multi-strain cultures in SSF with various plant-based substrates, including drumstick leaf flour, milk thistle fruit, Moringa oleifera leaf meal, lupin flour, soybean meal, cottonseed meal, defatted rice bran, cassava leaf, maize flour, and dehusked barley.[Citation10,Citation21,Citation22,Citation28,Citation47–52,Citation62]
Previous studies have indicated that several fungal, yeast and bacterial strains can grow synergistically. Wang et al.[Citation52] fermented de-husked barley with a combination of Lactobacillus plantarum and Rhizopus oryzae and observed a significant increase in soluble protein content. During SSF, Lactobacillus species secrete lactic acid, lowering the pH value of the entire system. The dominant protease, aspartic protease, secreted by Rhizopus exhibited improved activity and stability in acidic environments, further contributing to the heightened soluble protein content.[Citation63] In a separate study fermenting Moringa oleifera leaf meal by Aspergillus niger, Candida utilis and Bacillus subtilis co-cultures, the effective fermentation is attributed in part to greater cellulase, pectinase, and amylase activities of A. niger.[Citation48] This facilitates the degradation of cellulose and starch to support the growth of both C. utilis and B. subtilis. Moreover, the bacteria convert non-protein nitrogen into microbial protein and secrete enzymes that accelerate cellulose and starch degradation, thereby promoting the mycelial growth of A. niger.
Recent research also suggests that several biochemical reactions may be hindered if only a single microorganism strain is used for SSF. Co-culture fermentation is required for optimal results as all available substrates can be used in a multi-strain fermentation process.[Citation64,Citation65] For example, Olukomaiya et al.[Citation28] found that co-culture fermentation of lupin flour with Aspergillus sojae and Aspergillus ficuum co-culture resulted in the greatest increase in crude protein content than single culture fermentation. In contrast, several studies have reported a reduction in crude protein content during co-culture SSF. For instance, Terefe et al.[Citation22] found that instead of co-culture SSF, Lactobacillus plantarum fermentation resulted in the largest increase in crude protein content when fermented with cassava leaves. Likewise, Terefe et al.[Citation21] observed that the increase in protein contents of maize flour fermented solely by L. plantarum is higher than L. plantarum and S. cerevisiae coculture-fermentation. This phenomenon is not yet fully understood, but it may be linked to the increased consumption of nitrogen-containing substances like proteins, peptides, or amino acids to support the growth and metabolism of microorganisms. Further research is needed to elucidate this mechanism.
As discussed above, the selection of the starting culture is critical to the substrate protein levels. Taking soybeans as an example, Bacillus amyloliquefaciens, Bacillus subtilis, Lactobacillus plantarum, Saccharomyces cerevisiae r. f. bayanus, and Bacillus velezensis 157/Lactobacillus plantarum BLCC2–0015 co-culture have been shown to increase protein levels. However, it is noteworthy that each of these strains elicits varying increments in protein content, whereas the utilization of Bifidobacterium longum leads to a decrease in soluble protein levels. Fermentation conditions such as temperature, initial moisture, and time also play important role. Moreover, it is worth emphasizing that the majority of studies in this domain have commonly employed protein content as the primary metric to assess the nutritional value of the fermented product (). While this choice is certainly justifiable when the primary objective is to achieve an elevated protein level in the end product. However, when the focus is on the production of hydrolyzed products such as peptides and amino acids and their consequent impact on nutritional or bioactivity value, it is advisable to consider the degree of protein hydrolysis as a more informative parameter for analysis.
Overall, SSF is a complex microorganism-guided process, influenced by several factors such as timing of inoculation, fermentation time and temperature. The growth of microorganism could further be influenced by the plant substrates, making it challenging to compare different microorganisms in their capacity to alter plant protein content. Most studies only observed the effects of SSF on the protein content of substrates without follow-up experiments to explore the mechanisms. For example, the increase in soluble protein content may be due to hydrolytic enzymes produced by microorganisms, whereas the specific enzyme(s) responsible for this action was not well studied. Furthermore, the influence of co-culture fermentation on protein content and their mechanism of action is rarely studied. Future study may investigate the interaction between fungi, yeast, and bacteria during co-culture fermentation.
Degree of protein hydrolysis and molecular weight of protein after SSF
During SSF, the degree of protein hydrolysis and molecular weight (MW) of plant-based food can be influenced by various factors such as the choice of microorganisms, fermentation conditions, and the composition of the substrate. The degree of protein hydrolysis refers to the extent to which the protein molecules are broken down into smaller peptides and amino acids,[Citation66] while the MW of the protein molecules may undergo change as a result of the fermentation process. It is well known that both the degree of protein hydrolysis and MW have a significant impact on the bioavailability and functional properties of the fermented product.[Citation51] Consequently, understanding the factors that influence these parameters during SSF is crucial for optimizing the quality of plant-based food and ensuring its maximum nutritional value.
Degree of hydrolysis of plant protein after SSF
The degree of hydrolysis (DH) measures the extent of peptide bonds cleaved in the protein substrate by a proteolytic agent: the higher the value, the higher number of amino groups released. Proteolytic enzymes, primarily endogenous enzymes, hydrolyze large and intact proteins into small polypeptides and intermediate peptides.[Citation67] Microorganisms such as lactic acid bacteria, yeasts, and mould further catalyze the hydrolysis of intermediate peptides into small peptides and amino acids.[Citation67] This process significantly impacts the nutritional values and health-promoting benefits of plant food products, as most small peptides and free amino acids can be directly absorbed by the gastrointestinal tract into the body.[Citation51] The o-phthalaldehyde (OPA) and trinitrobenzenesulphonic acid (TNBS) amino acid determination methods are commonly employed to assess DH with results being expressed as percentage of hydrolysis.[Citation66]
The alteration of DH in plant-based food after SSF is shown in . Research has shown that the DH can vary greatly depending on the type of substrate employed. Lupin and quinoa, for instance, resulting in higher DH compared to wheat when fermented by Lactobacillus spp.[Citation68] This was attributed to the greater proteolytic activity of endogenous proteases present in lupine and quinoa, and/or the higher susceptibility of lupine and quinoa proteins to the proteolytic enzymes produced by Lactobacillus spp. Strain selection is also critical, as distinct strains of Lactobacillus spp can produce varying levels of proteolytic enzymes and exert differential effects on the DH.[Citation68,Citation73] Cereals fermented by Lactobacillus reuteri K×88177 were observed to have higher DH than those by Lactobacillus plantarum DSM and Lactobacillus plantarum KX881779.[Citation68] Furthermore, co-culture fermentation has been shown to be beneficial for enhancing DH due to the diverse microbial enzymes present, thereby accelerating the rate of protein proteolysis.[Citation63,Citation74] It is also noteworthy that the activities of proteinases and peptidases are time-dependent, with prolonged fermentation time is beneficial to the enhancement of DH.[Citation69,Citation73]
Table 2. Effect of solid-state fermentation on the degree of hydrolysis and protein molecular weight profile of selected plant-based substrates.
One advantage of implementing SSF for protein rich foods is that it can easily achieve the same DH as commercial enzymes with being cost-effective, albeit at the expense of longer incubation times.[Citation30] Chin et al.[Citation30] reported that after 3 days of SSF with R. oligosporus, proteins in brewers’ spent grains were hydrolysed to a degree of 14.6%, which is comparable to the highest DH of 13% obtained from an enzymatic hydrolysis using alcalase.[Citation75] The DH of the proteins can be further demonstrated from the change of the protein MW profile, as discussed below.
Changes in protein MW profile of plant protein after SSF
The MW represents another important parameter reflecting the solubility and bioactivity of proteins. Proteases and peptidases secreted by microorganisms during fermentation breakdown proteins into smaller fragments, thus affecting the distribution of their molecular mass.[Citation76] This alteration in molecular mass can be determined by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and gel filtration chromatography.[Citation77] summarizes the studies that examine changes in the MW distribution of plant protein after SSF.
SSF typically induce the degradation of macromolecular proteins, yielding increased quantities of smaller proteins and peptides.[Citation28,Citation52,Citation70,Citation71] Smaller MW peptide and amino acids are related to enhanced antioxidant and antihypertensive activities of fermented protein-rich materials.[Citation78] Additionally, reducing molecular sizes increases the digestibility of protein, improving both the nutritional value and flavor of fermented foods.[Citation10,Citation52] However, the level of low MW protein can decrease during certain SSF conditions. For instance, Zhang et al.[Citation72] reported a 4.6% decrease in the concentration of low MW peptides (<1 kDa) in soybeans fermented by a B. animalis 937 and B. subtilis natto co-culture for 24 hours. The reduction may be due to the poor protease activity at the initial stage of the fermentation, where the soybean protein was only partly hydrolyzed and most peptides were utilized by B. animalis.[Citation72] In other cases, higher MW proteins may be produced in SSF. Shi et al.[Citation10] studied the fermentation of Moringa oleifera leaf meal by Candida utilis. In this study, proteins with MW of 60–140 kDa appeared from the third day, which could be due to the production of yeast proteins as the yeast cells proliferate. Similarly, Wang et al.[Citation52] observed an increase in proteins with MW > 25 kDa in fermented dehusked barley by Lactobacillus plantarum and Rhizopus oryzae, which may also be caused by the increased microbial metabolic activity. However, The effect of SSF on protein MW can sometimes be limited. Amadou et al.[Citation20] reported the influence of SSF by Lactobacillus plantarum Lp6 on the high MW polypeptides in soybean meal protein was restricted.
The effect of SSF on the MW changes is dependent on the substrate and fermentation time. For example, in the study of Sun et al.,[Citation77] Bacillus subtilis BJ-1 fermented cottonseed meal contained 56.52% low MW peptides (<1 kDa), which is comparable to a value of 46% for similar MW peptides in the fermented soybean extracts,[Citation79] but notably smaller than the 86.89% content observed in fermented peanut.[Citation80] Furthermore, Zhang et al.[Citation72] reported that the level of soybean low MW peptides (<1 kDa) decreased by 4.6% after 24 h of co-culture fermentation with B. animalis 937 and B. subtilis natto, but increased by 30.7% at 48 h. This indicates that fermentation time has contributed to the variation of protein molecular profile.
So far, numerous studies have explored the impact of SSF on DH and MW distribution. Future research could investigate the optimal fermentation conditions for enhancing the activity of endogenous and microbial proteases and peptidases to boost the DH of specific plant substrates. Additionally, it may be valuable to compare the DH achieved by SSF with that obtained through alternative techniques, such as enzymatic or chemical hydrolysis. This would allow developing the most cost-effective and sustainable method for industrial application. Furthermore, future studies may explore the relationship between protein MW distribution and physical properties, such as solubility, emulsification, foaming, and gelation, which could provide insight into the impact of SSF on protein functionality and offer values for practical applications.
Effect of SSF on the in vitro protein digestibility and antinutritional factors (ANF)
Plant proteins are widely recognized to possess a lower nutritional value to animal proteins due to their reduced digestibility and lower content of essential amino acids.[Citation81] During SSF, the enhancement of DH and the reduction of molecular weight MW of proteins have a beneficial impact on their digestibility. Digestibility is an important indicator to evaluate the nutritional value of proteins, which is depended on internal factors such as the amino acid profile and protein structure, as well as external factors such as the presence of antinutritional factors (ANF).[Citation82,Citation83] Enhancing the digestibility of plant proteins reduces the levels of undigested protein that cause gastrointestinal distress and protein excretion in the stool, and eliminate gastro-intestinal immune reactions from immunogenic peptides.[Citation7,Citation84]
Modification of plant protein digestibility by SSF
Several studies have reported that SSF can increase protein digestibility of plant products (). In simulating the human digestive process, in vitro pepsin-trypsin digestion is commonly employed to assess protein digestibility.[Citation89,Citation92] Research has revealed that the inclusion of bacteria in SSF imparts an overall improvement in protein digestibility. For example, Bacillus amyloliquefaciens, Bacillus subtilis, Lactobacillus plantarum, Pediococcus acidilactici, Pediococcus pentosaceus, and Lactobacillus sakei applied for SSF can significantly increase the protein digestibility of soybean meal, cottonseed meal, soy seeds, lupin seeds, and rapeseed meal.[Citation11,Citation17,Citation71,Citation85,Citation87,Citation88] The improvement in protein digestibility can be attributed to several factors. Firstly, bacterial proteolysis partially degrades complex proteins in the substrate into simple and soluble products such as peptides and amino acids, consequently bolstering digestibility.[Citation51] Secondly, cellulase secreted by bacteria in the substrates degrades cellulose, exposing starch granules or cell wall proteins to digestion.[Citation93] Thirdly, some bacteria such as lactic acid bacteria can produce organic acids, lowering the pH value of the fermented product and enhancing the activity of pepsin, leading to improved protein digestion.[Citation94] Finally, the rise in protein digestibility is linked to reduction of anti-nutritional factors (ANF). These ANF include inhibitors that inhibit digestive enzymes like trypsin and chymotrypsin, as well as phenolics and tannins which exacerbate the crosslinking of proteins.[Citation7]
Table 3. Effect of solid-state fermentation on the protein digestibility of selected plant-based substrates.
Nevertheless, there exist instances where no discernible improvement in protein digestibility is evident. For example, there was no significant difference in the protein digestibility of lupine wholemeal fermented with Pediococcus acidilactici, contrasting with the notable increase in protein digestibility in lupine protein isolates.[Citation86] This divergence may due to the presence of polyphenols in the lupin wholemeal, which binds to proteins and decreases their digestibility.[Citation86] Polyphenols can also directly impact protein digestibility by inhibiting proteases in the digestive tract after food intake.[Citation95] It is important to note, however, that certain studies, the protein digestibility of lupin seeds was shown to increase after fermentation with Pediococcus acidilactici in the studies by Krunglevičiute et al.[Citation87] and Bartkiene et al..[Citation88]
also summarizes the results of several studies applied fungi starter culture. The food substrates encompassed a diverse array of plant-based materials, including soya bean, flaxseed oil cake, de-oiled rice bran, common bean flour, lentils, and red bean flour. The fungi species applied in the studies were Aspergillus oryzae, Neurospora intermedia, Rhizopus oryzae, Rhizopus oligosporus, Pleurotus ostreatus, and Cordyceps militaris.[Citation27,Citation33,Citation38,Citation89–91] The conditions for the fermentation process, such as temperature, moisture content, and pH, exhibited variations across each of these studies, reflecting the context-dependent nature of SSF and its influence on protein digestibility.
SSF with fungi has demonstrated its capacity to enhance protein digestibility, as indicated by several studies.[Citation27,Citation33,Citation38,Citation89,Citation91] Similar to bacteria, the improvement in protein in vitro digestibility by fungal SSF is due to the reduced levels of anti-nutritive compounds as well as partial pre-digestion of the substrate by fungal proteases.[Citation96,Citation97] Flaxseed oil cake shown increased digestibility when fermented with both Aspergillus oryzae and Neurospora intermedia. However, with longer fermentation time, the product fermented with Aspergillus oryzae showed higher digestibility while that fermented with Neurospora intermedia did not change significantly.[Citation27] The discrepancy was explained by changes in the proteolytic activity of different fungal mycelium, which is strongly dependent on the culture age.
Nevertheless, certain studies have reported a reduction in protein digestibility following fungal SSF. For instance, lupin flour subjected to SSF with Aspergillus sojae, Aspergillus ficuum and their co-cultures showed lower protein digestibility.[Citation28] This reduction was attributed to proteins being locked within the lupin fibre matrix, thus, reducing accessibility by proteases. The authors also stated that partial protein denaturation during drying process might have also lowered the protein dispersibility and solubility, thereby resulting in a reduced protein digestibility, noted a physical change in processing prior to digestion that should be wary about. This result is aligned with the findings by Ranjan et al.[Citation90] that the SSF with R. oryzae has negative effects on the protein digestibility of dry defatted rice bran.
The effect of co-fermentation on protein digestibility is presented in as well. Although co-culture SSF generally improves the protein digestibility of the substrates, it is noteworthy that the achieved increase is often less pronounced than that observed in single-strain SSF. In the study of Terefe et al.,[Citation21] the protein digestibility of maize flour was increased significantly after 48 h of SSF, while the highest increase was observed when employing the Lactobacillus plantarum, followed by the Saccharomyces cerevisiae, with co-culture fermentation showing the least improvement. Furthermore, in a study involving the co-fermentation of grass pea seeds with Rhizopus microsporus var. chinensis and Aspergillus oryzae, it was noted that protein digestibility exhibited improvement only when the inoculum size of A. oryzae was less than that of R. microsporus var. chinensis.[Citation69] This observation suggests that within co-culture fermentation, competition among microorganisms may inhibit the growth of those with a stronger capacity to enhance protein digestibility. Consequently, co-culture fermentation may not be as effective as individual fermentation in optimizing protein digestibility.
Elimination of ANF in plant food after SSF
ANF refers to chemical entities in plant products that negatively affect the digestibility of proteins or other macronutrients such as starch, hence their bioavailability.[Citation98] The reduction of ANF during SSF can indirectly increases the accessibility of proteins by enzymes and this in turn increases the protein digestibility.[Citation21] Tannins and trypsin inhibitors represent key ANFs linked to reduced protein digestibility.
Tannins represent a diverse group of phenolic compounds known for their ability to interact with proteins, thereby altering protein structure and functionality while impeding the digestibility.[Citation99] Several studies have shown the reduction of tannins by SSF. For instance, the tannin content was reduced significantly from 49.84% to 12.3% in maize flour fermented by co-culture of Lactobacillus plantarum and Saccharomyces cerevisiae.[Citation21] This reduction in tannin content can be attributed to the activities of tannases and mono- and di-oxygenases produced by the microbes during the SSF, which hydrolyze tannin-protein complexes, including both hydrolysable and condensed tannins.[Citation100,Citation101] Similar result was found by Dileep et al.[Citation44] where the tannin content was reduced by 29.74% and 20.00% in Saccharomyces cerevisiae fermented guar and copra meal, respectively, with concurrent increase in protein digestibility.
Trypsin inhibitors are compounds that impede protein digestion by inhibiting the activity of trypsin.[Citation102] They form a stable complex with trypsin, leading to its inactivation and thereby reducing the availability of protein and amino acids.[Citation16] Multiple studies have highlighted the capacity of SSF to diminish trypsin inhibitor levels in plant-based foods. Teng et al.[Citation15] observed that trypsin inhibitor content in fermented soybean meal with B. subtilis SB102 and A. oryzae AO3042 was reduced by 95.33% and 81.33%, respectively, after 72 h fermentation. Similarly, Zhang et al.[Citation16] reported decrease in trypsin inhibitor content by 53.7% in Bacillus subtilis natto fermented soybean. Dileep et al.[Citation44] also reported that trypsin inhibitor activity was decreased by 56.81% and 58.02% in Saccharomyces cerevisiae fermented guar and copra meal, respectively.
The reduction of antigenic proteins through SSF is not directly linked to protein digestibility; however, it can significantly enhance digestion by mitigating gastrointestinal discomfort caused by these proteins. Antigenic proteins are known to trigger the body’s adaptive immune response, and in soybeans, glycinin (11S) and β-conglycinin (7S) are the two primary antigenic proteins, constituting 70–80% of the total seed protein.[Citation103] Previous studies showed that soybean meal after SSF could enhance intestinal barrier function, weaken undesirable intestinal mucosal immune response, and reduce the incidence of diarrhea of animals, which was related to the reduction of soybean meal antigenic proteins.[Citation104,Citation105] For example, Li et al.[Citation11] reported that 92.32% of glycinin and 85.05% of β-conglycinin in soybean meal were removed by Bacillus amyloliquefaciens fermentation for 24 h. Similarly, Zhang et al.[Citation106] found that 84.77% of glycinin and 87.07% of β-conglycinin were degraded after 24 h fermentation with B. subtilis BS12. However, another B. subtilis strain KCCM11438P eliminated only 42% of glycinin and 70% of β-conglycinin after 24 h fermentation.[Citation107] Compared to B. subtilis, Bacillus amyloliquefaciens’s superior ability to decompose antigenic protein is attributed to its stronger ability to secrete several extracellular hydrolases including serine proteases, acid proteases, endoglucanases, acetylxylan and esterases. These hydrolases promote the breakdown of cell wall polymers and degradation of antigenic protein of soybean meal.[Citation11]
Effect of SSF on the peptide bioactivity of plant protein
Compared to the non-fermented counterparts, fermented foods show multiple benefits to human health such as reducing the risks of a number of chronic diseases including cardiovascular disease, arthritis, Type 2 diabetes, periodontitis, and bladder disorders.[Citation108] The bioactivities of fermented plant products by bacterial and fungi can be attributed to bioactive small peptides.[Citation109] This section discusses the production of bioactive peptides produced by SSF and their biological activities such as antioxidant, antihypertensive, antidiabetic, antiproliferative and immunomodulatory effects. Selected studies on the influence of SSF on the quantity and bioactivity of the peptides from plant substrates are shown in .
Table 4. Selective studies on production of bioactive peptides via solid-state fermentation of plant-based substrates.
Antioxidant peptides from plant protein produced by SSF
While relatively limited research has been conducted on the extraction and purification of antioxidant peptides from solid-state fermented products, some studies have indicated that the antioxidant properties of these products are related to the increase of peptide contents. Substrates such as quinoa seeds, rapeseed, drumstick leaf, cottonseed, soybean, okara, barley and mixture of corn gluten and wheat bran have been subjected to SSF to produce antioxidant peptides.[Citation10,Citation52,Citation62,Citation71,Citation77,Citation110,Citation112]
The antioxidant peptides in plant material are encrypted within their protein sequence, which are released by microbial enzymes during SSF, especially in the case of small peptides with a molecular weight of less than 3 kDa, which have been shown to have higher bioactivity than larger peptides.[Citation115,Citation116] For example, Wang et al.[Citation71] reported that SSF of rapeseed meal by B. subtilis YY-4 and L. plantarum CICC6026 did not cause a significant change in total phenolic content, but it significantly increased DPPH radical scavenging, Fe2+ chelation, and reducing power with an elevated peptide content, implying the generation of antioxidant peptides during fermentation. Similarly, SSF significantly improved the antioxidant capacity of okara, which was attributed to the generation of peptides through yeast proteolysis.[Citation45] Furthermore, Starzyńska-Janiszewska et al.[Citation62] fermented pumpkin seeds cake with R. oligosporus and observed a gradual increase in peptide generation over 96 h fermentation, and the content of peptides was significantly correlated with the level of ABTS˙+-scavenging activity. The rapeseed peptide produced by SSF with B. subtilis 10160[Citation111] and B. subtilis YY-4 and L. plantarum CICC6026[Citation71] also showed an increase in radical scavenging activity, as well as in cottonseed fermented with B. subtilis.[Citation77] In addition, cotton seed fermentation produced peptides showed protective effect against H2O2-induced oxidant death of raw 264.7 macrophage cells in in-vitro culture at 2.5 mg/mL of protein.[Citation77]
However, the increased antioxidant activity of SSF products may also result from other factors. One such factor is the deglycosylation of phenolic glycosides during fermentation, leading to the release of more phenolic aglycone, thus augmenting antioxidant activity, as seen in fermented soybean with A. oryzae.[Citation89] Furthermore, the release of polyphenols from protein complexes can also contribute to the increased antioxidant capacity of fermented products.[Citation89] Additionally. Certain amino acids such as tyrosine, cysteine and methionine have been found to contribute to antioxidant activity. For instance, the increases in antioxidant capacity of the fermented rapeseed meal were correlated with increases in these amino acids. (Wang et al., 2022)
Antihypertensive peptides produced by SSF
Antihypertensive peptides is of great research interests and the majority of the reported antihypertensive peptides from food are products of enzymatic hydrolysis.[Citation117] Angiotensin-converting enzyme (ACE) plays an important role in blood pressure regulation in the body. It converts the inactive decapeptide angiotensin I to the active angiotensin II, which induces increased blood pressure through narrowing of blood vessels.[Citation118] Synthetic ACE inhibitors have been shown to lower blood pressure in in vivo clinical trials, and their development as antihypertensive drugs play an important role in promoting cardiovascular health.[Citation118] However, antihypertensive drugs have side effects such as fatigue, dizziness and headaches, and these side-effects are exacerbated by their requirement for lifetime use. Consequently, there has been increasing interest in natural sources of ACE-inhibiting peptides that are considered safer with less side effects.[Citation119] ACE inhibitory activity is measured in vitro to assess the antihypertensive properties of foods. During SSF, microbial proteases can release bioactive peptides with ACE inhibitory activity, potentially serving as antihypertensive agents.[Citation120]
The amino acid composition within the peptide sequence plays a crucial role in determining its activity. For a potent ACE inhibitory peptide, the most favorable N-terminus residues would be branched amino acids such as valine and isoleucine.[Citation121] Nawaz et al.[Citation113] identified a competitive ACE inhibitory octapeptide Val-Val-Ser-Leu-Ser-Ile-Pro-Arg with a molecular mass of 869.53 Da from pigeon pea fermented with A. niger (KR535626). Larger active peptides containing these octapeptide sequence were shown to be less inhibitory, which could be due to weaker binding of the peptide to the active site restricted by the extra amino acids.[Citation122]
Solid-state co-fermentation involving R. oryzae and L. plantarum B1–6 demonstrated a significant enhancement in ACE inhibitory activity in oat proteins. Over 24 and 72 hours of fermentation, the ACE inhibitory activity increased from 9.90% to 59.45% and 77.81%, respectively.[Citation63] Comparing to the co-fermentation system, extracts from R. oryzae single fermentation showed slightly lower ACE inhibitory activity (51.31% and 63.64% in 24 and 72 h, respectively). The better results obtained from co-fermentation is most likely due to the presence of multiple microorganisms that facilitate greater hydrolysis of proteins. In the study of Wu et al.,[Citation63] the IC50 values (half maximal inhibitory concentration) of co-fermentation product was significantly reduced to 0.42 mg protein/mL compared to unfermented samples at 1.26 mg protein/mL. This value is similar to the IC50 of the enzymatic hydrolysates of lentils protein (0.44 mg protein/mL)[Citation123] and red bean (0.63 mg protein/mL),[Citation91] but lower than that of fermented soybean meal (1.38 mg protein/mL).[Citation124] The observed differences in the ACE inhibitory activity may be attributed to a variety of causes, such as different proteins, protein extraction procedures, peptide mixture compositions, fermentation or digestion parameters (strain, fermentation method, time, temperature, pH, enzyme, substrate/enzyme ratio, etc.), or analytical methods used for the determination of ACE inhibitory activity.[Citation125]
In addition, Ayyash et al.[Citation68] found that lupin and quinoa subjected to SSF by Lactobacillus spp. showed higher ACE-inhibition and antioxidant activity than the control (without SSF). However, the authors cautioned that the bioactive fragments possessing the antioxidant activities are not necessarily the same as those that manifested the ACE-inhibition.[Citation68] Future studies should focus on identifying and purifying the ACE-inhibitory peptides in fermented plant, which could serve as a precursor for further research and potential clinical trials.
Antiproliferative peptides from plant protein produced by SSF
Indeed, research into anticancer peptides produced by SSF is quite limited, as reflected in . To date, only two studies have shown that SSF products possess antiproliferative activity. Xie et al.[Citation114] demonstrated that peptide extracts from solid-state fermented rapeseed inhibited the proliferation of human HepG2 cells, human HeLa cervical cancer cells, and human MCF-7 breast cancer cells. Additionally, they found that the rapeseed antitumor peptide participated in mitochondria-mediated apoptosis, leading to the inhibition of human HepG2 hepatoma cell proliferation.[Citation126] Another study by Ayyash et al.[Citation68] reported that lupin fermented by Lactobacillus strains exhibited greater antiproliferative activities than fermented quinoa and wheat against Caco-2 colon cancer and MCF-7 breast cancer cell lines. The author attributed the superior antiproliferative activity of fermented lupins to the higher quantity and lower MW of protein hydrolysates during fermentation. Nevertheless, none of these studies isolated nor identified the sequence of the peptides contributing to the nominated functions, which warrants further investigation.
Antidiabetic and immunomodulatory peptides from plant protein produced by SSF
Antidiabetic compounds that inhibit α-amylase and α-glucosidase activities are valuable for managing postprandial hyperglycemia and have potential applications for diabetic treatment.[Citation127] However, there is limited literature on the application of SSF for the generation of antidiabetic peptides from plant proteins. Ayyash et al.[Citation68] observed a sharp increase in α-glucosidase inhibition after 24 h fermentation of lupin, quinoa and wheat, which was attributed to the production α-glucosidase inhibitory peptides during fermentation.
For immunomodulatory capability, Liu et al.[Citation50] demonstrated that oligopeptides derived from SSF of cottonseed meal significantly affect the immunomodulatory ability of BALB/c mice treated with cyclophosphamide. The authors suggested that treatment with the oligopeptides protected the mice immune organs by reversing the immunosuppression caused by cyclophosphamide treatment. Also, the oligopeptide significantly increased the levels of IgG and IgM in cyclophosphamide-treated immunosuppressed mice, which indicated that the intake of oligopeptide could enhance humoral immunity effectively.[Citation50]
At present, the research on the production of bioactive peptides through SSF of plant-derived proteins is still limited, with a predominant focus on the antioxidant and anti-hypertensive activities. Most of the existing literature does not report on the separation, purification and identification of the bioactive peptides; instead, thy rather establish a correlation between the concentration of peptides and the increased biological activities. This highlights a big research gap in the area and further research is warranted. In addition, in vitro digestion and animal studies are needed to demonstrate the retention of these bioactive activities through processes of absorption and digestion. Such endeavours would significantly contribute to our understanding of the potential applications and benefits of SSF-derived bioactive peptides from plant sources.
The effect of SSF on amino acid profile of plant-based food
Adequate intake of dietary amino acids is essential for human health.[Citation128] Thus, the content of total amino acids (TAA), free amino acids (FAA), essential amino acids (EAA), and non-essential amino acids (NEAA) in a food matrix has a profound effect on its nutritional value. TAA analysis reveals the overall amino acid content, encompassing those integrated into proteins and those existing as unbound units, while FAA analysis quantifies the quantity of amino acids that are not part of a protein structure.[Citation129] Plant proteins, although capable of providing EAA, often present an imbalanced amino acid profile and a restricted range of amino acids when compared to animal proteins.[Citation87] Animal proteins, for example, often contain higher proportions of the EAA leucine, an amino acid required for efficient protein synthesis.[Citation130] Therefore, it is necessary to apply suitable food processing methods to improve availability of specific amino acids originated from plant proteins, and SSF has been shown to be an effective method, with selective studies summarised in .
Table 5. Effect of solid-state fermentation on the amino acid profile of plant sourced protein.
The effect of SSF on total amino acid of plant-based food
In most cases, SSF lead to an increment in TAA content.[Citation10,Citation11,Citation17,Citation19,Citation23,Citation49,Citation91,Citation133,Citation134] The most prevalent substrate choice is soybean, however, with different choice of starter culture, the result can be different. Bacillus amyloliquefaciens, Bacillus velezensis 157 and Lactobacillus plantarum BLCC2–0015 as starter culture tend to increase TAA content, whereas Bifidobacterium longum CRL 849 and Lactobacillus plantarum Lp6 tend to decrease TAA content.[Citation11,Citation18,Citation49,Citation131] In addition, with same substrate moringa oleifera leaf, using Aspergillus niger solely for SSF resulted in a greater increase in TAA content than co-culture of Aspergillus niger, Candida utilis and Bacillus subtilis, although fermentation conditions such as fermentation time may also play a significant role.[Citation48,Citation134] Prolonged fermentation time may lead to the consumption of amino acids by microbes.[Citation45]
The observed Increase in TAA content resulting from SSF can be attributed to the biosynthesis of amino acids by microorganisms utilizing carbohydrates within the substrate via the glycerate-3P and pyruvate pathway TCA cycle (oxaloacetate and α-ketoglutarate).[Citation51] However, there were also instances where the TAA content was reduced or remained unchanged. de Olmos et al.[Citation18] fermented soybean meal with Bifidobacterium longum CRL 849 and found that total amino acid was decreased by 33.2%. The authors attributed this decrease to nutrient losses during fermentation due to leaching, destruction by light, heat or oxygen, or from microbial utilization.[Citation139] Starzynska-Janiszewska et al.[Citation132] reported that the amino acid profile remained unchanged when unhulled common bean was fermented with Rhizopus var. chinensis and Lactobacillus plantarum. However, this may be an artifact of the short fermentation time (31 h compared with 96 h in other studies) of the study and the use of whole, unhulled common beans rather than crushed or ground beans, where the nutrients are largely locked in the whole bean matrix.
The effect of SSF on free amino acid of plant-based food
In addition to TAA content, changes in free amino acid (FAA) composition have also been discussed by several researchers.[Citation13,Citation14,Citation16,Citation20,Citation23,Citation29,Citation45,Citation71,Citation131,Citation134] Except for one study which found no change in FAA content during the fermentation of moringa oleifera leaf with Aspergillus niger, all other studies have shown that SSF increased the TAA content of final products. FAA and peptides play a crucial role in producing taste and flavour in food, and among them, FAA are more favourable for human absorption.[Citation23,Citation140] In addition, improved digestibility is tie to the process of protein hydrolysis into FAA.[Citation45]
The increase in FAA content may result from the breakdown of large proteins into smaller amino acids through protease hydrolysis by microorganism. Zhang et al.[Citation16] attributed the significant increase in FAA in soybean meal to the proteolytic activity of B. subtilis natto. Wang et al.[Citation71] indicated that the extracellular protease hydrolytic activity of Lactobacillus plantarum can be a contributor for the increased FAA in fermented rapeseed meal, while Vong et al.[Citation45] stated that the increase of FAA in okara fermented by Yarrowia lipolytica was due to the superior proteolytic ability of extracellular acidic and alkaline proteases produced by the yeast.
Although amino acid degradation and deamidation may also occur during fermentation, the rate of protein hydrolysis is more likely to exceed the rate of amino acid catabolism resulting in a net increase in FAA.[Citation45] There are also studies discussing the decline of specific FAAs. For example, Shiu et al.[Citation13] applied B. subtilis to ferment soybean meal and found that most of the free amino acids in the fermented soybean meal were increased, while some of them, such as hydroxyproline, asparagine, and β-alanine, were decreased. The reduction in individual amino acid levels after fermentation can be linked to metabolism of specific amino acid by B. subtilis.[Citation141]
The effect of SSF of plant-based food on essential and non-essential amino acids
The quality of the protein is directly related to protein digestibility and EAA contents, which contributes to the bioavailability.[Citation40] Elevated consumption of EAA has health benefits such as enhancing the level of lean body mass, muscle strength, and physical function in elderly.[Citation142] During SSF, the conversion of amino acids by the action of transaminases could partly contribute to the increase of certain EAA or NEAA,[Citation33,Citation96] and fermented foods enriched in EAA represent a valuable dietary source.[Citation86] The impact of SSF on EAA and NEAA has been summarized in .
The effect of SSF on EAA and NEAA profile of the same substrate varies depending on the starter culture employed. Frias et al.[Citation135] conducted SSF of B. subtilis, A. oryzae, R. oryzae on cracked soybean seed under the same fermentation condition. They found that R. oryzae fermentation only significantly increased the content of non-essential alanine (Ala) and essential threonine (Thr), while the other microorganisms significantly increased the levels of almost all amino acids. This discrepancies may be attributable to the proteinase profiles and proteinase secretion abilities of different microorganisms.[Citation143] Furthermore, even identical starter cultures may produce varying effects when applied to different cultivars of plants within the same species. Krunglevičiute et al.[Citation87] employed Lactobacillus sakei KTU05–6, Pediococcus acidilactici KTU05–7, and Pediococcus pentosaceus KTU05–8 for SSF on two species of lupin seed (Lupinus luteus L. and Lupinus albus L.) and observed an increase in leucine content in all fermented L. luteus samples, but a decrease in all fermented L. albus samples. The same study further identified increased lysine and histidine levels in all fermented L. luteus samples but decreased lysin in all L. albus samples. These results underscore the complexity of SSF outcomes, which are influenced by plant cultivar.
Selection of an appropriate starter culture is critical to achieve the target EAA/NEAA content. For example, level of methionine, a limiting EEAs present in the soybean, was not significantly changed by SSF using B. subtilis E20.[Citation13] However, an increase in methionine was reported when soybean was fermented using Bacillus subtilis natto[Citation16] and Bacillus amyloliquefaciens.[Citation11] Likewise, lysine, a limiting EAA in cereals[Citation144] was significantly reduced in common bean flour when fermented using Rhizopus oligosporus,[Citation33] and in drumstick leaf flour fermented with Aspergillus niger GIM 3.576, Candida utilis GIM 1.427 and Bacillus subtilis CICC 31,188 co-culture.[Citation10] However, a significant increase (1.14% vs. 0.68%) was reported by Jiang et al.[Citation112] using Lactobacillus delbrueckii subsp. bulgaricus to ferment corn gluten-wheat bran mixture. In contrast, lysine amount remained unaltered after fermentation of soybean meal with Bifidobacterium longum.[Citation18] These examples highlight how the amino acid composition of fermented plant proteins can be influenced by both the specific starter culture and the nature of the substrate. Researchers must carefully select the combination of microorganism and substrate to achieve their desired target for improving amino acid content in the final product.
It is promising to see that the EAA content in plant proteins can meet the requirements for WHO[Citation145] human nutrition after biological treatments like fermentation. For instance, cottonseed subjected to SSF by B. subtilis contained 296.2 mg/g protein of EAAs, which is close to the amino acid requirements for human adult nutrition (277 mg/g protein).[Citation77] Wang et al.[Citation134] also stated that the individual EAA contents in fermented moringa oleifera leaf were higher than the FAO reference protein and comparable to those in soybeans. The protein qualities of fermented teff/grass-pea mixtures have similar EAA composition as the recommended level for 2 − 5 year old children.[Citation138]
The content of NEAAs is also important in formulating balanced diets to support overall body growth and health.[Citation146] Several studies have shown SSF partially or fully increase all NEAAs.[Citation10,Citation16,Citation17,Citation86] However, Rajesh et al.[Citation24] fermented a mixture of dried vegetable waste powder and oil cake mixture with Aspergillus niger and revealed an increase in the levels of certain NEAA including aspartic acid, serine, alanine, glycine, and tyrosine, but a decrease for other NEAA such as arginine, cysteine, glutamine, and proline. This outcome may be attributed to the utilization of these amino acids for the production of enzymes and other organic compounds by the fungal strain.[Citation147]
The effect of SSF on sulphur-containing and aromatic amino acids of plant-based food
Sulfur-containing amino acid, cysteine and methionine, are important for sulfur homeostasis and various cellular processes in the body.[Citation148] Morales et al.[Citation40] reported that cysteine and methionine (20.84 mg EAA/g of protein) in fermented cassava bagasse and leaves could reach 83.7% of daily requirements (25 mg EAA/g of protein) according to FAO/WHO pattern. Similarly, the total sulphur-containing amino acids of fermented milk thistle fruit with Aspergillus niger ATCC16404 and Candida tropicalis ACCC21145 co-culture were significantly increased compared to that without fermentation.[Citation47]
Aromatic amino acids, including tyrosine, phenylalanine, and tryptophan, also play important roles in various physiological processes. Tyrosine is essential for synthesizing important compounds like epinephrine, norepinephrine, thyroid hormones, and melanin.[Citation149] Phenylalanine intake is associated with neurological development and function.[Citation149] A previous study showed that tyrosine, phenylalanine, and tryptophan were significantly increased in SSF of milk thistle fruit.[Citation47] Similarly, Morales et al.[Citation40] observed that fermented cassava bagasse and leaves (51.44 mg EAA/g of protein) can provide approximately 81.7% of the daily requirements (63 mg EAA/g of protein) of Phe and Tyr, which is higher than that found in animal protein such as in raw bacon.
In general, the increase in amino acid content benefits the nutritional value of the fermented products. However, it is important to note that the selection of the starter culture in SSF is crucial when aiming to produce specific amino acids, such as methionine and lysine. Different microorganisms compass varying impacts on the amino acid profile of the final product, and careful consideration must be given to selecting the culture that will yield the desired amino acid composition. Additionally, some amino acids such as histidine, tyrosine, methionine, and cysteine, have been reported as antioxidants. Particularly, histidine exhibits strong radical scavenging activity due to the decomposition of its imidazole ring.[Citation150] Therefore, the functionalities of amino acids should also be taken into consideration when developing protein fermentation products.
Conclusion
Due to the concerns about environment, cost-effectiveness, sustainability, ethical issues, and health problems such as high cholesterol and lactose intolerance relevant to animal protein-based foods, the preference of plant protein-based foods is increasing. Fermentation is a process with a long history and SSF has the advantage of being less costly and more productive than submerged fermentation, making it a promising method for producing high-nutritional and functional foods from plant protein sources.
Generally, both soluble and total protein contents are increased after SSF. Proteins may come from substrate hydrolysis, microorganism growth, or enzyme secretion to break down macromolecules into small peptides and amino acids. The increment in protein content suggests higher nutritional quality of the final products. Plant proteins inherently have the disadvantage of being less digestible than animal proteins. SSF enhances the hydrolysis of plant-derived proteins by breaking down large molecules into smaller ones, thereby increasing protein digestibility. In addition, SSF can simultaneously eliminate anti-nutritional factors such as trypsin inhibitors and tannins, further increasing the protein digestibility. While SSF can produce bioactive peptides from plant proteins, research in this area is limited. Further research is required on isolating and purifying peptides from fermentation products and assessing their impact on in vitro digestion, cellular models, and animal models. The challenge in industrial production lies in optimizing various protein properties simultaneously. In addition, the development of SSF and its industry application are closely related to the evolution of SSF bioreactor. Innovative bioreactor design is needed to provide precise control, maximize yields, and enhance cost-effectiveness, thereby facilitating the application of SSF in large-scale industrial production. Furthermore, depending on the choice of starter culture and plant protein substrate, a fermented product may exhibit different protein content, protein digestibility, anti-nutritional factor content, bioactive peptides, and amino acid composition. Achieving the desirable combination of these properties requires further research, especially to develop large-scale applications of SSF for plant-based protein production. In conclusion, SSF holds the potential to improve the nutritional value and biological activity of plant proteins in a low-cost and high-yield manner, although more research is needed to identify the specific proteins and peptides that have contributed to the nutritional value and biological activities of the fermented foods.
Author contribution
Xiaoyu Feng: Conceptualization, Investigation, Writing – Original Draft Pangzhen Zhang: Conceptualization, Investigation, Resources, Writing – Review & Editing, Supervision. Ken Ng: Writing – Review & Editing, Supervision. Said Ajlouni: Writing – Review & Editing, Supervision. Zhongxiang Fang: Conceptualization, Resources, Writing – Review & Editing, Supervision.
Acknowledgments
The authors thank the support of University of Melbourne Research Scholarship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Frias, J.; Miranda, M. L.; Doblado, R.; Vidal-Valverde, C. Effect of Germination and Fermentation on the Antioxidant Vitamin Content and Antioxidant Capacity of Lupinus Albus L. Var. Multolupa. Food Chem. 2005, 92(2), 211–220. DOI: 10.1016/j.foodchem.2004.06.049.
- Zhang, Z.; Lv, G.; Pan, H.; Fan, L.; Soccol, C. R.; Pandey, A. Production of Powerful Antioxidant Supplements via Solid-State Fermentation of Wheat (Triticum Aestivum Linn.) by Cordyceps Militaris. Food Technol. Biotechnol. 2012, 50(1), 32–39.
- Dey, T. B.; Chakraborty, S.; Jain, K. K.; Sharma, A.; Kuhad, R. C. Antioxidant Phenolics and Their Microbial Production by Submerged and Solid-State Fermentation Process: A Review. Trends Food Sci. Technol. 2016, 53, 60–74. DOI: 10.1016/j.tifs.2016.04.007.
- Martins, S.; Mussatto, S. I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C. N.; Teixeira, J. A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnol. Adv. 2011, 29(3), 365–373. DOI: 10.1016/j.biotechadv.2011.01.008.
- Rayaprolu, S. J.; Hettiarachchy, N. S.; Chen, P.; Kannan, A.; Mauromostakos, A. Peptides Derived from High Oleic Acid Soybean Meals Inhibit Colon, Liver and Lung Cancer Cell Growth. Food. Res. Int. 2013, 50(1), 282–288. DOI: 10.1016/j.foodres.2012.10.021.
- Hur, S. J.; Lee, S. Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. DOI: 10.1016/j.foodchem.2014.03.112.
- Alrosan, M.; Tan, T.-C.; Koh, W. Y.; Easa, A. M.; Gammoh, S.; Alu’datt, M. H. Overview of Fermentation Process: Structure-Function Relationship on Protein Quality and Non-Nutritive Compounds of Plant-Based Proteins and Carbohydrates. Crit. Rev. Food Sci. Nutr. 2022, 63(25), 1–15. DOI: 10.1080/10408398.2022.2049200.
- Chai, K. F.; Voo, A. Y. H.; Chen, W. N. Bioactive Peptides from Food Fermentation: A Comprehensive Review of Their Sources, Bioactivities, Applications, and Future Development. Compr. Rev. Food Sci. Food Saf. 2020, 19(6), 3825–3885. DOI: 10.1111/1541-4337.12651.
- Sadh, P. K.; Duhan, S.; Duhan, J. S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresources Bioprocess. 2018, 5(1), 1–15. DOI: 10.1186/s40643-017-0187-z.
- Shi, H.; Yang, E.; Li, Y.; Chen, X.; Zhang, J. Effect of Solid-State Fermentation on Nutritional Quality of Leaf Flour of the Drumstick Tree (Moringa Oleifera Lam.). Front. Bioeng. Biotechnol. 2021, 9, 626628. DOI: 10.3389/fbioe.2021.626628.
- Li, Y.; Guo, B.; Li, C.; Wang, W.; Wu, Z.; Liu, G.; Cai, H. Isolation of a Highly Efficient Antigenic-Protein-Degrading Bacillus Amyloliquefaciens and Assessment of Its Safety. Animals. 2020, 10(7), 1–15. DOI: 10.3390/ani10071144.
- Zhang, M.; Huang, Y.; Zhao, H.; Wang, T.; Xie, C.; Zhang, D.; Wang, X.; Sheng, J. Solid-State Fermentation of Moringa Oleifera Leaf Meal Using Bacillus Pumilus Cicc 10440. J. Chem. Technol. Biotechnol. 2017, 92(8), 2083–2089. DOI: 10.1002/jctb.5203.
- Shiu, Y. L.; Wong, S. L.; Guei, W. C.; Shin, Y. C.; Liu, C. H. Increase in the Plant Protein Ratio in the Diet of White Shrimp, Litopenaeus Vannamei (Boone), Using Bacillus Subtilis E20-Fermented Soybean Meal as a Replacement. Aquacult. Res. 2015, 46(2), 382–394. DOI: 10.1111/are.12186.
- Wang, J.; Liu, Z.; Wang, Y.; Cheng, W.; Mou, H. Production of a Water-Soluble Fertilizer Containing Amino Acids by Solid-State Fermentation of Soybean Meal and Evaluation of Its Efficacy on the Rapeseed Growth. J. Biotechnol. 2014, 187, 34–42. DOI: 10.1016/j.jbiotec.2014.07.015.
- Teng, D.; Gao, M.; Yang, Y.; Liu, B.; Tian, Z.; Wang, J. Bio-Modification of Soybean Meal with Bacillus Subtilis or Aspergillus Oryzae. Biocatal Agric. Biotechnol. 2012, 1(1), 32–38. DOI: 10.1016/j.bcab.2011.08.005.
- Zhang, Y. K.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W. L.; Zhang, X. X.; Shadrack, R. S.; Mzengereza, K.; Zhu, K. H., et al. Optimization of Soybean Meal Fermentation for Aqua-Feed with Bacillus Subtilis Natto Using the Response Surface Methodology. Fermentation. 2021, 7(4), 306. DOI: 10.3390/fermentation7040306.
- Sun, H.; Tang, J. W.; Yao, X. H.; Wu, Y. F.; Wang, X.; Feng, J. Improvement of the Nutritional Quality of Cottonseed Meal by Bacillus Subtilis and the Addition of Papain. Int. J. Agric. Biol. 2012, 14(4), 563–568.
- de Olmos, A. R.; Garro, O. A.; Garro, M. S. Behavior Study of Bifidobacterium Longum Using Solid State Fermentation from Commercial Soybean Meal. LWT. 2022, 157, 157. DOI: 10.1016/j.lwt.2022.113101.
- Zhang, X. Y.; Yang, Z. H.; Liang, J.; Tang, L.; Chen, F. Detoxification of Jatropha Curcas Seed Cake in Solid-State Fermentation of Newly Isolated Endophytic Strain and Nutrition Assessment for Its Potential Utilizations. Int. Biodeterior. Biodegrad. 2016, 109, 202–210. DOI: 10.1016/j.ibiod.2016.02.001.
- Amadou, I.; Le, G. W.; Shi, Y. H.; Gbadamosi, O. S.; Kamara, M. T.; Jin, S. Optimized Lactobacillus Plantarum Lp6 Solid‐State Fermentation and Proteolytic Hydrolysis Improve Some Nutritional Attributes of Soybean Protein Meal. J. Food Biochem. 2011, 35(6), 1686–1694. DOI: 10.1111/j.1745-4514.2010.00493.x.
- Terefe, Z. K.; Omwamba, M. N.; Nduko, J. M. Effect of Solid State Fermentation on Proximate Composition, Antinutritional Factors and in vitro Protein Digestibility of Maize Flour. Food Sci. Nutr. 2021, 9(11), 6343–6352. DOI: 10.1002/fsn3.2599.
- Terefe, Z. K.; Omwamba, M.; Nduko, J. M. Effect of Microbial Fermentation on Nutritional and Antinutritional Contents of Cassava Leaf. J. Food Saf. 2022, 42(3), 3. DOI: 10.1111/jfs.12969.
- Wang, J. H.; Cao, F.; Su, E.; Zhao, L.; Qin, W. Improvement of Animal Feed Additives of Ginkgo Leaves Through Solid-State Fermentation Using Aspergillus Niger. Int. J. Biol. Sci. 2018, 14(7), 736–747. DOI: 10.7150/ijbs.24523.
- Rajesh, N.; Imelda, J.; Paul Raj, R. Value Addition of Vegetable Wastes by Solid-State Fermentation Using Aspergillus Niger for Use in Aquafeed Industry. Waste Manag. 2010, 30(11), 2223–2227. DOI: 10.1016/j.wasman.2009.12.017.
- Chebaibi, S.; Leriche Grandchamp, M.; Burgé, G.; Clément, T.; Allais, F.; Laziri, F. Improvement of Protein Content and Decrease of Anti-Nutritional Factors in Olive Cake by Solid-State Fermentation: A Way to Valorize This Industrial By-Product in Animal Feed. J. Biosci. Bioeng. 2019, 128(3), 384–390. DOI: 10.1016/j.jbiosc.2019.03.010.
- Duhan, J. S.; Chawla, P.; Kumar, S.; Bains, A.; Sadh, P. K. Proximate Composition, Polyphenols, and Antioxidant Activity of Solid State Fermented Peanut Press Cake. Prep. Biochem. Biotechnol. 2021, 51(4), 340–349. DOI: 10.1080/10826068.2020.1815060.
- Stodolak, B.; Starzynska-Janiszewska, A.; Baczkowicz, M. Aspergillus Oryzae (Koji Mold) and Neurospora Intermedia (Oncom Mold) Application for Flaxseed Oil Cake Processing. LWT. 2020, 131, 131. DOI: 10.1016/j.lwt.2020.109651.
- Olukomaiya, O. O.; Adiamo, O. Q.; Fernando, W. C.; Mereddy, R.; Li, X. H.; Sultanbawa, Y. Effect of Solid-State Fermentation on Proximate Composition, Anti-Nutritional Factor, Microbiological and Functional Properties of Lupin Flour. Food Chem. 2020, 315, 315. DOI: 10.1016/j.foodchem.2020.126238.
- Starzynska-Janiszewska, A.; Baczkowicz, M.; Sabat, R.; Stodolak, B.; Witkowicz, R. Quinoa Tempe as a Value-Added Food: Sensory, Nutritional, and Bioactive Parameters of Products from White, Red, and Black Seeds. Cereal Chem. 2017, 94(3), 491–496. DOI: 10.1094/CCHEM-07-16-0186-R.
- Chin, Y. L.; Chai, K. F.; Chen, W. N. Upcycling of Brewers’ Spent Grains via Solid-State Fermentation for the Production of Protein Hydrolysates with Antioxidant and Techno-Functional Properties. Food Chem. X. 2022, 13, 100184. DOI: 10.1016/j.fochx.2021.100184.
- Starzyńska-Janiszewska, A.; Stodolak, B.; Socha, R.; Mickowska, B.; Wywrocka-Gurgul, A. Spelt Wheat Tempe as a Value-Added Whole-Grain Food Product. LWT. 2019, 113, 113. DOI: 10.1016/j.lwt.2019.108250.
- Lucke, F. K.; Fritz, V.; Tannhauser, K.; Arya, A. Controlled Fermentation of Rapeseed Presscake by Rhizopus, and Its Effect on Some Components with Relevance to Human Nutrition. Food. Res. Int. 2019, 120, 726–732. DOI: 10.1016/j.foodres.2018.11.031.
- Reyes-Bastidas, M.; Reyes-Fernández, E. Z.; López-Cervantes, J.; Milán-Carrillo, J.; Loarca-Piña, G. F.; Reyes-Moreno, C.; Physicochemical. Nutritional and Antioxidant Properties of Tempeh Flour from Common Bean (Phaseolus Vulgaris L.). Food Sci. Technol. Int. 2010, 16(5), 427–434. DOI: 10.1177/1082013210367559.
- Morales, E. M.; Domingos, R. N.; Angelis, D. F. Improvement of Protein Bioavailability by Solid-State Fermentation of Babassu Mesocarp Flour and Cassava Leaves. Waste Biomass Valorization. 2018, 9(4), 581–590. DOI: 10.1007/s12649-016-9759-y.
- Firdaus, O. M.; Rohaya, M. H.; Miskandar, M. S.; Aa, A. Nutrient Enhancement of Palm Kernel Cake via Solid State Fermentation by Locally Isolated Rhizopus Oryzae Me01. J. Oil Palm Res. 2022, 34(1), 92–103. DOI: 10.21894/jopr.2021.0022.
- Aruna, T. Production of Value-Added Product from Pineapple Peels Using Solid State Fermentation. Innovative Food Sci. Emerging Technol. 2019, 57, 102193. DOI: 10.1016/j.ifset.2019.102193.
- Ahmadi, F.; Zamiri, M. J.; Khorvash, M.; Banihashemi, Z.; Bayat, A. R. Chemical Composition and Protein Enrichment of Orange Peels and Sugar Beet Pulp After Fermentation by Two Trichoderma Species. Iran J Vet Res. 2015, 16(1), 25–30.
- Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. Enhancing the Nutritional Profile and Digestibility of Lentil Flour by Solid State Fermentation With: Pleurotus Ostreatus. Food Funct. 2020, 11(9), 7905–7912. DOI: 10.1039/d0fo01527j.
- Calvo-Lerma, J.; Asensio-Grau, A.; Garcia-Hernandez, J.; Heredia, A.; Andres, A. Exploring the Impact of Solid-State Fermentation on Macronutrient Profile and Digestibility in Chia (Salvia Hispanica) and Sesame (Sesamum Indicum) Seeds. Foods. 2022, 11(3), 3. DOI: 10.3390/foods11030410.
- Morales, E. M.; Zajul, M.; Goldman, M.; Zorn, H.; Angelis, D. F. Effects of Solid-State Fermentation and the Potential Use of Cassava By-Products as Fermented Food. Waste Biomass Valorization. 2020, 11(4), 1289–1299. DOI: 10.1007/s12649-018-0479-3.
- Moore, J.; Cheng, Z. H.; Hao, J. J.; Guo, G.; Liu, J. G.; Lin, C. J.; Yu, L. L. Effects of Solid-State Yeast Treatment on the Antioxidant Properties and Protein and Fiber Compositions of Common Hard Wheat Bran. J. Agric. Food. Chem. 2007, 55(25), 10173–10182. DOI: 10.1021/jf071590o.
- Ilowefah, M.; Bakar, J.; Ghazali, H. M.; Muhammad, K. Enhancement of Nutritional and Antioxidant Properties of Brown Rice Flour Through Solid-State Yeast Fermentation. Cereal Chem. 2017, 94(3), 519–523. DOI: 10.1094/CCHEM-08-16-0204-R.
- Queiroz Santos, V. A.; Nascimento, C. G.; Schimidt, C. A. P.; Mantovani, D.; Dekker, R. F. H.; da Cunha, M. A. A. Solid-State Fermentation of Soybean Okara: Isoflavones Biotransformation, Antioxidant Activity and Enhancement of Nutritional Quality. LWT. 2018, 92, 509–515. DOI: 10.1016/j.lwt.2018.02.067.
- Dileep, N.; Pradhan, C.; Peter, N.; Kaippilly, D.; Sashidharan, A.; Sankar, T. V. Nutritive Value of Guar and Copra Meal After Fermentation with Yeast Saccharomyces Cerevisiae in the Diet of Nile Tilapia, Oreochromis Niloticus. Trop. Anim. Health Prod. 2021, 53(4). DOI: 10.1007/s11250-021-02855-4.
- Vong, W. C.; Au Yang, K. L.; Liu, S. Q. Okara (Soybean Residue) Biotransformation by Yeast Yarrowia Lipolytica. Int. J. Food Microbiol. 2016, 235, 1–9. DOI: 10.1016/j.ijfoodmicro.2016.06.039.
- Mandal, S.; Ghosh, K. Optimization of Tannase Production and Improvement of Nutritional Quality of Two Potential Low-Priced Plant Feedstuffs Under Solid State Fermentation by Pichia Kudriavzevii Isolated from Fish Gut. Food Biotechnol. 2013, 27(1), 86–103. DOI: 10.1080/08905436.2012.755929.
- Li, F.; Zhao, T.; Mao, G.; Zou, Y.; Zheng, D.; Takase, M.; Feng, W.; Wu, X.; Yang, L. Solid-State Fermentation of Industrial Solid Wastes from the Fruits of Milk Thistle Silybum Marianum for Feed Quality Improvement. Appl. Microbiol. Biotechnol. 2013, 97(15), 6725–6737. DOI: 10.1007/s00253-013-5002-y.
- Shi, H.; Su, B.; Chen, X.; Pian, R. Solid State Fermentation of Moringa Oleifera Leaf Meal by Mixed Strains for the Protein Enrichment and the Improvement of Nutritional Value. PeerJ. 2020, 8, 8. DOI: 10.7717/peerj.10358.
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional Quality Improvement of Soybean Meal by Bacillus Velezensis and Lactobacillus Plantarum During Two-Stage Solid- State Fermentation. AMB Express. 2021, 11(1), 23. DOI: 10.1186/s13568-021-01184-x.
- Liu, J. C.; Sun, H.; Nie, C. X.; Ge, W. X.; Wang, Y. Q.; Zhang, W. J. Oligopeptide Derived from Solid-State Fermented Cottonseed Meal Significantly Affect the Immunomodulatory in Balb/C Mice Treated with Cyclophosphamide. Food Sci. Biotechnol. 2018, 27(6), 1791–1799. DOI: 10.1007/s10068-018-0414-1.
- Su, W. F.; Jiang, Z. P.; Wang, C.; Xu, B. C.; Lu, Z. Q.; Wang, F. Q.; Zong, X.; Jin, M. L.; Wang, Y. Z. Dynamics of Defatted Rice Bran in Physicochemical Characteristics, Microbiota and Metabolic Functions During Two-Stage Co-Fermentation. Int. J. Food Microbiol. 2022, 362, 362. DOI: 10.1016/j.ijfoodmicro.2021.109489.
- Wang, K.; Niu, M.; Song, D.; Liu, Y.; Wu, Y.; Zhao, J.; Li, S.; Lu, B. Evaluation of Biochemical and Antioxidant Dynamics During the Co-Fermentation of Dehusked Barley with Rhizopus Oryzae and Lactobacillus Plantarum. J. Food Biochem. 2020, 44(2), e13106. DOI: 10.1111/jfbc.13106.
- Onyango, C.; Ochanda, S.; Mwasaru, M.; Ochieng, J.; Mathooko, F. M.; Kinyuru, J. Effects of Malting and Fermentation on Anti-Nutrient Reduction and Protein Digestibility of Red Sorghum, White Sorghum and Pearl Millet. J Food Res. 2013, 2(1), 41. DOI: 10.5539/jfr.v2n1p41.
- Bradford, M. M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72(1–2), 248–254. DOI: 10.1016/0003-2697(76)90527-3.
- Lowry, O. H.; Rosebrough, N.; Farr, A. L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193(1), 265–275. DOI: 10.1016/S0021-9258(19)52451-6.
- Liu, X.; Kokare, C. Microbial Enzymes of Use in Industry. Biotechnol. Microbial Enzymes. 2017, 267–298.
- Siezen, R. J.; van Hylckama Vlieg, J. E. Genomic Diversity and Versatility of Lactobacillus Plantarum, a Natural Metabolic Engineer. Microb. Cell Fact. 2011, 10(1), 1–13. DOI: 10.1186/1475-2859-10-S1-S3.
- Behera, S. S.; Ray, R. C.; Zdolec, N. Lactobacillus Plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed Res. Int. 2018, 2018, 1–18. DOI: 10.1155/2018/9361614.
- Salgado, J. M.; Abrunhosa, L.; Venâncio, A.; Domínguez, J. M.; Belo, I. Enhancing the Bioconversion of Winery and Olive Mill Waste Mixtures into Lignocellulolytic Enzymes and Animal Feed by Aspergillus Uvarum Using a Packed-Bed Bioreactor. J. Agric. Food. Chem. 2015, 63(42), 9306–9314. DOI: 10.1021/acs.jafc.5b02131.
- Cohen, R.; Persky, L.; Hadar, Y. Biotechnological Applications and Potential of Wood-Degrading Mushrooms of the Genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 58(5), 582–594. DOI: 10.1007/s00253-002-0930-y.
- Hezarjaribi, M.; Ardestani, F.; Ghorbani, H. R. Single Cell Protein Production by Saccharomyces Cerevisiae Using an Optimized Culture Medium Composition in a Batch Submerged Bioprocess. Appl. Biochem. Biotechnol. 2016, 179(8), 1336–1345. DOI: 10.1007/s12010-016-2069-9.
- Starzyńska-Janiszewska, A.; Duliński, R.; Stodolak, B. Fermentation with Edible Rhizopus Strains to Enhance the Bioactive Potential of Hull-Less Pumpkin Oil Cake. Molecules. 2020, 25(24), 25 (24. DOI: 10.3390/molecules25245782.
- Wu, H.; Rui, X.; Li, W.; Xiao, Y.; Zhou, J.; Dong, M. Whole-Grain Oats (Avena Sativa L.) as a Carrier of Lactic Acid Bacteria and a Supplement Rich in Angiotensin I-Converting Enzyme Inhibitory Peptides Through Solid-State Fermentation. Food Funct. 2018, 9(4), 2270–2281. DOI: 10.1039/c7fo01578j.
- Xie, P. J.; Huang, L. X.; Zhang, C. H.; Zhang, Y. L. Nutrient Assessment of Olive Leaf Residues Processed by Solid-State Fermentation as an Innovative Feedstuff Additive. J. Appl. Microbiol. 2016, 121(1), 28–40. DOI: 10.1111/jam.13131.
- Del Fresno, J. M.; Morata, A.; Loira, I.; Bañuelos, M. A.; Escott, C.; Benito, S.; González Chamorro, C.; Suárez-Lepe, J. A. Use of Non-Saccharomyces in Single-Culture, Mixed and Sequential Fermentation to Improve Red Wine Quality. Eur. Food Res. Technol. 2017, 243(12), 2175–2185. DOI: 10.1007/s00217-017-2920-4.
- Nielsen, P.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66(5), 642–646. DOI: 10.1111/j.1365-2621.2001.tb04614.x.
- Gänzle, M. G.; Loponen, J.; Gobbetti, M. Proteolysis in Sourdough Fermentations: Mechanisms and Potential for Improved Bread Quality. Trends Food Sci. Technol. 2008, 19(10), 513–521. DOI: 10.1016/j.tifs.2008.04.002.
- Ayyash, M.; Johnson, S. K.; Liu, S.-Q.; Mesmari, N.; Dahmani, S.; Al Dhaheri, A. S.; Kizhakkayil, J. In vitro Investigation of Bioactivities of Solid-State Fermented Lupin, Quinoa and Wheat Using Lactobacillus Spp. Food Chem. 2019, 275, 50–58. DOI: 10.1016/j.foodchem.2018.09.031.
- Starzyńska-Janiszewska, A.; Stodolak, B.; Wikiera, A. Proteolysis in Tempeh-Type Products Obtained with Rhizopus and Aspergillus Strains from Grass Pea (Lathyrus Sativus) Seeds. Acta Sci. Pol. Technol. Aliment. 2015, 14(2), 125–132. DOI: 10.17306/J.AFS.14.
- Zhang, N.; Li, D.; Zhang, X.; Shi, Y.; Wang, H. Solid-State Fermentation of Whole Oats to Yield a Synbiotic Food Rich in Lactic Acid Bacteria and Prebiotics. Food Funct. 2015, 6(8), 2620–2625. DOI: 10.1039/c5fo00411j.
- Wang, Y.; Sun, H.; Liu, X. A Novel Fermented Rapeseed Meal, Inoculated with Selected Protease-Assisting Screened B. Subtilis Yy-4 and L. Plantarum 6026, Showed High Availability and Strong Antioxidant and Immunomodulation Potential Capacity. Foods. 2022, 11(14), 2118. DOI: 10.3390/foods11142118.
- Zhang, S. T.; Shi, Y.; Zhang, S. L.; Shang, W.; Gao, X. Q.; Wang, H. K. Whole Soybean as Probiotic Lactic Acid Bacteria Carrier Food in Solid-State Fermentation. Food Control. 2014, 41, 1–6. DOI: 10.1016/j.foodcont.2013.12.026.
- Leclerc, P.-L.; Gauthier, S. F.; Bachelard, H.; Santure, M.; Roy, D. Antihypertensive Activity of Casein-Enriched Milk Fermented by Lactobacillus Helveticus. Int. Dairy. J. 2002, 12(12), 995–1004. DOI: 10.1016/S0958-6946(02)00125-5.
- Tsai, J.-S.; Lin, Y.; Pan, B.; Chen, T. Antihypertensive Peptides and Γ-Aminobutyric Acid from Prozyme 6 Facilitated Lactic Acid Bacteria Fermentation of Soymilk. Process Biochem. 2006, 41(6), 1282–1288. DOI: 10.1016/j.procbio.2005.12.026.
- Celus, I.; Brijs, K.; Delcour, J. A. Enzymatic Hydrolysis of Brewers’ Spent Grain Proteins and Technofunctional Properties of the Resulting Hydrolysates. J. Agric. Food. Chem. 2007, 55(21), 8703–8710. DOI: 10.1021/jf071793c.
- Chi, C.-H.; Cho, S.-J. Improvement of Bioactivity of Soybean Meal by Solid-State Fermentation with Bacillus Amyloliquefaciens versus Lactobacillus Spp. And Saccharomyces Cerevisiae. LWT - Food Sci. Technol. 2016, 68, 619–625. DOI: 10.1016/j.lwt.2015.12.002.
- Sun, H.; Yao, X. H.; Wang, X.; Wu, Y. F.; Liu, Y.; Tang, J. W.; Feng, J. Chemical Composition and in vitro Antioxidant Property of Peptides Produced from Cottonseed Meal by Solid-State Fermentation. Cyta–J. Food. 2015, 13(2), 264–272. DOI: 10.1080/19476337.2014.948072.
- Amadou, I.; Le, G.-W.; Shi, Y.-H. Evaluation of Antimicrobial, Antioxidant Activities, and Nutritional Values of Fermented Foxtail Millet Extracts by Lactobacillus Paracasei Fn032. Int. J. Food. Prop. 2013, 16(6), 1179–1190. DOI: 10.1080/10942912.2011.579673.
- Lee, J.-S.; Rho, S.-J.; Kim, Y.-W.; Lee, K. W.; Lee, H. G. Evaluation of Biological Activities of the Short-Term Fermented Soybean Extract. Food Sci. Biotechnol. 2013, 22(4), 973–978. DOI: 10.1007/s10068-013-0172-z.
- Zhang, Y.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Influence of the Degree of Hydrolysis (Dh) on Antioxidant Properties and Radical-Scavenging Activities of Peanut Peptides Prepared from Fermented Peanut Meal. Eur. Food Res. Technol. 2011, 232(6), 941–950. DOI: 10.1007/s00217-011-1466-0.
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship Between Digestibility and Secondary Structure of Raw and Thermally Treated Legume Proteins: A Fourier Transform Infrared (Ft-Ir) Spectroscopic Study. Amino Acids. 2012, 43(2), 911–921. DOI: 10.1007/s00726-011-1151-4.
- Joye, I. Protein Digestibility of Cereal Products. Foods. 2019, 8(6), 199. DOI: 10.3390/foods8060199.
- Sá, A. G. A.; Moreno, Y. M. F.; Carciofi, B. A. M. Food Processing for the Improvement of Plant Proteins Digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60(20), 3367–3386. DOI: 10.1080/10408398.2019.1688249.
- Untersmayr, E.; Jensen-Jarolim, E. The Role of Protein Digestibility and Antacids on Food Allergy Outcomes. J. Allergy Clin. Immunol. 2008, 121(6), 1301–1308. DOI: 10.1016/j.jaci.2008.04.025.
- Rui, X.; Wang, M.; Zhang, Y.; Chen, X.; Li, L.; Liu, Y.; Dong, M. Optimization of Soy Solid‐State Fermentation with Selected Lactic Acid Bacteria and the Effect on the Anti‐Nutritional Components. J. Food Process Preserv. 2017, 41(6), e13290. DOI: 10.1111/jfpp.13290.
- Bartkiene, E.; Sakiene, V.; Lele, V.; Bartkevics, V.; Rusko, J.; Wiacek, C.; Ruzauskas, M.; Braun, P. G.; Matusevicius, P.; Zdunczyk, Z., et al. Perspectives of Lupine Wholemeal Protein and Protein Isolates Biodegradation. Int. J. Food Sci. Technol. 2019, 54(6), 1989–2001. DOI: 10.1111/ijfs.13901.
- Krunglevičiute, V.; Starkute, V.; Bartkiene, E.; Bartkevics, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Design of Lupin Seeds Lactic Acid Fermentation - Changes of Digestibility, Amino Acid Profile and Antioxidant Activity. Vet. Med. Zoot. 2016, 73(95), 47–53.
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid State Fermentation with Lactic Acid Bacteria to Improve the Nutritional Quality of Lupin and Soya Bean. J. Sci. Food Agric. 2015, 95(6), 1336–1342. DOI: 10.1002/jsfa.6827.
- Chen, Y. Q.; Su, H. J.; Ouyang, Y.; Wang, J. M.; Yang, X. Q.; Hu, W. F. Preparation and Characterisation of Glyceollin-Enriched Soya Bean Protein Using Solid-State Fermentation. Int. J. Food Sci. Technol. 2017, 52(8), 1878–1886. DOI: 10.1111/ijfs.13463.
- Ranjan, A.; Sahu, N. P.; Deo, A. D.; Kumar, S. Solid State Fermentation of De-Oiled Rice Bran: Effect on in vitro Protein Digestibility, Fatty Acid Profile and Anti-Nutritional Factors. Food. Res. Int. 2019, 119, 1–5. DOI: 10.1016/j.foodres.2019.01.054.
- Xiao, Y.; Sun, M.; Zhang, Q.; Chen, Y.; Miao, J.; Rui, X.; Dong, M. Effects of Cordyceps Militaris (L.) Fr. Fermentation on the Nutritional, Physicochemical, Functional Properties and Angiotensin I Converting Enzyme Inhibitory Activity of Red Bean (Phaseolus Angularis [Willd.] W.F. Wight.) Flour. J. Food Sci. Technol. 2018, 55(4), 1244–1255. DOI: 10.1007/s13197-018-3035-z.
- Boisen, S.; Fernández, J. Prediction of the Total Tract Digestibility of Energy in Feedstuffs and Pig Diets by in vitro Analyses. Anim. Feed Sci. Technol. 1997, 68(3–4), 277–286. DOI: 10.1016/S0377-8401(97)00058-8.
- Yang, Y.; Kiarie, E.; Slominski, B.; Brûlé-Babel, A.; Nyachoti, C. Amino Acid and Fiber Digestibility, Intestinal Bacterial Profile, and Enzyme Activity in Growing Pigs Fed Dried Distillers Grains with Solubles-Based Diets. J. Anim. Sci. 2010, 88(10), 3304–3312. DOI: 10.2527/jas.2009-2318.
- Su, W.; Jiang, Z.; Hao, L.; Li, W.; Gong, T.; Zhang, Y.; Du, S.; Wang, C.; Lu, Z.; Jin, M., et al. Variations of Soybean Meal and Corn Mixed Substrates in Physicochemical Characteristics and Microbiota During Two-Stage Solid-State Fermentation. Front. Microbiol. 2021, 12, 688839. DOI: 10.3389/fmicb.2021.688839.
- Cirkovic Velickovic, T. D.; Stanic‐Vucinic, D. J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17(1), 82–103. DOI: 10.1111/1541-4337.12320.
- Angulo-Bejarano, P. I.; Verdugo-Montoya, N. M.; Cuevas-Rodríguez, E. O.; Milán-Carrillo, J.; Mora-Escobedo, R.; Lopez-Valenzuela, J. A.; Garzón-Tiznado, J. A.; Reyes-Moreno, C. Tempeh Flour from Chickpea (Cicer Arietinum L.) Nutritional and Physicochemical Properties. Food Chem. 2008, 106(1), 106–112. DOI: 10.1016/j.foodchem.2007.05.049.
- Cabuk, B.; Stone, A. K.; Korber, D. R.; Tanaka, T.; Nickerson, M. T. Effect of Lactobacillus Plantarum Fermentation on the Surface and Functional Properties of Pea Protein-Enriched Flour. Food Technol. Biotechnol. 2018, 56(3), 411–420. DOI: 10.17113/ftb.56.03.18.5449.
- Thakur, A.; Sharma, V.; Thakur, A. An Overview of Anti-Nutritional Factors in Food. Int. J. Chem. Stud. 2019, 7(1), 2472–2479.
- Girard, A. L.; Bean, S. R.; Tilley, M.; Adrianos, S. L.; Awika, J. M. Interaction Mechanisms of Condensed Tannins (Proanthocyanidins) with Wheat Gluten Proteins. Food Chem. 2018, 245, 1154–1162. DOI: 10.1016/j.foodchem.2017.11.054.
- Dlamini, N. R.; Taylor, J. R.; Rooney, L. W. The Effect of Sorghum Type and Processing on the Antioxidant Properties of African Sorghum-Based Foods. Food Chem. 2007, 105(4), 1412–1419. DOI: 10.1016/j.foodchem.2007.05.017.
- Kuddus, M. Enzymes in Food Technology: Improvements and Innovations; Singapore: Springer, 2018.
- Cristina Oliveira de Lima, V.; Piuvezam, G.; Leal Lima Maciel, B.; Heloneida de Araújo Morais, A. Trypsin Inhibitors: Promising Candidate Satietogenic Proteins as Complementary Treatment for Obesity and Metabolic Disorders? J. Enzyme Inhib. Med. Chem. 2019, 34(1), 405–419. DOI: 10.1080/14756366.2018.1542387.
- He, L.; Han, M.; Qiao, S.; He, P.; Li, D.; Li, N.; Ma, X. Soybean Antigen Proteins and Their Intestinal Sensitization Activities. Curr. Protein & Peptide Sci. 2015, 16(7), 613–621. DOI: 10.2174/1389203716666150630134602.
- Zhang, Y.; Chen, S.; Zong, X.; Wang, C.; Shi, C.; Wang, F.; Wang, Y.; Lu, Z. Peptides Derived from Fermented Soybean Meal Suppresses Intestinal Inflammation and Enhances Epithelial Barrier Function in Piglets. Food Agric. Immunol. 2020, 31(1), 120–135. DOI: 10.1080/09540105.2019.1705766.
- Zhu, J. J.; Gao, M. X.; Zhang, R. L.; Sun, Z. J.; Wang, C. M.; Yang, F. F.; Huang, T. T.; Qu, S. Q.; Zhao, L.; Li, Y. W., et al. Effects of Soybean Meal Fermented by L. Plantarum, B. Subtilis and S. Cerevisieae on Growth, Immune Function and Intestinal Morphology in Weaned Piglets. Microb. Cell Fact. 2017, 16(1), 16. DOI: 10.1186/s12934-017-0809-3.
- Zhang, Y.; Shi, C.; Wang, C.; Lu, Z.; Wang, F.; Feng, J.; Wang, Y. Effect of Soybean Meal Fermented with Bacillus Subtilis Bs12 on Growth Performance and Small Intestinal Immune Status of Piglets. Food Agric. Immunol. 2018, 29(1), 133–146. DOI: 10.1080/09540105.2017.1360258.
- Seo, S.-H.; Cho, S.-J. Changes in Allergenic and Antinutritional Protein Profiles of Soybean Meal During Solid-State Fermentation with Bacillus Subtilis. LWT. 2016, 70, 208–212. DOI: 10.1016/j.lwt.2016.02.035.
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G. I.; Cui, C.; Ruan, Z. Fermentation-Enabled Wellness Foods: A Fresh Perspective. Food Sci. Hum. Wellness. 2019, 8(3), 203–243. DOI: 10.1016/j.fshw.2019.08.003.
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, Biological Activities, and Applications in Foods and Health Benefits. A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. DOI: 10.1016/bs.afnr.2016.10.003.
- Starzyńska-Janiszewska, A.; Stodolak, B.; Gómez-Caravaca, A. M.; Mickowska, B.; Martin-Garcia, B.; Byczyński, Ł. Mould Starter Selection for Extended Solid-State Fermentation of Quinoa. LWT. 2019, 99, 231–237. DOI: 10.1016/j.lwt.2018.09.055.
- He, R.; Ju, X.; Yuan, J.; Wang, L.; Girgih, A. T.; Aluko, R. E. Antioxidant Activities of Rapeseed Peptides Produced by Solid State Fermentation. Food. Res. Int. 2012, 49(1), 432–438. DOI: 10.1016/j.foodres.2012.08.023.
- Jiang, X.; Liu, X.; Xu, H.; Sun, Y.; Zhang, Y.; Wang, Y. Improvement of the Nutritional, Antioxidant and Bioavailability Properties of Corn Gluten-Wheat Bran Mixture Fermented with Lactic Acid Bacteria and Acid Protease. LWT. 2021, 144, 111161. DOI: 10.1016/j.lwt.2021.111161.
- Nawaz, K. A. A.; David, S. M.; Murugesh, E.; Thandeeswaran, M.; Kiran, K. G.; Mahendran, R.; Palaniswamy, M.; Angayarkanni, J. Identification and in silico Characterization of a Novel Peptide Inhibitor of Angiotensin Converting Enzyme from Pigeon Pea (Cajanus Cajan). Phytomedicine. 2017, 36, 1–7. DOI: 10.1016/j.phymed.2017.09.013.
- Xie, H.; Wang, Y.; Zhang, J.; Chen, J.; Wu, D.; Wang, L. Study of the Fermentation Conditions and the Antiproliferative Activity of Rapeseed Peptides by Bacterial and Enzymatic Cooperation. Int J Of Food Sci Tech. 2015, 50(3), 619–625. DOI: 10.1111/ijfs.12682.
- Zheng, Z.; Shetty, K. Cranberry Processing Waste for Solid State Fungal Inoculant Production. Process Biochem. 1998, 33(3), 323–329. DOI: 10.1016/S0032-9592(97)87514-0.
- Alashi, A. M.; Blanchard, C. L.; Mailer, R. J.; Agboola, S. O.; Mawson, A. J.; He, R.; Girgih, A.; Aluko, R. E. Antioxidant Properties of Australian Canola Meal Protein Hydrolysates. Food Chem. 2014, 146, 500–506. DOI: 10.1016/j.foodchem.2013.09.081.
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and Bioavailability of Food Protein‐Derived Angiotensin‐I‐Converting Enzyme–Inhibitory Peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20(2), 1150–1187. DOI: 10.1111/1541-4337.12711.
- Torino, M. I.; Limón, R. I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and Antihypertensive Properties of Liquid and Solid State Fermented Lentils. Food Chem. 2013, 136(2), 1030–1037. DOI: 10.1016/j.foodchem.2012.09.015.
- Rui, X.; Wen, D.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enrichment of Ace Inhibitory Peptides in Navy Bean (Phaseolus Vulgaris) Using Lactic Acid Bacteria. Food Funct. 2015, 6(2), 622–629. DOI: 10.1039/C4FO00730A.
- Müntz, K.; Belozersky, M.; Dunaevsky, Y.; Schlereth, A.; Tiedemann, J. Stored Proteinases and the Initiation of Storage Protein Mobilization in Seeds During Germination and Seedling Growth. J. Exp. Bot. 2001, 52(362), 1741–1752. DOI: 10.1093/jexbot/52.362.1741.
- Hong, S.; WANG, F. L.; MA, O.; EF, S.; DW, C. Binding of Peptide Substrates and Inhibitors of Angiotensin-Converting Enzyme: Importance of the COOH-Terminal Dipeptide Sequence. J. Biol. Chem. 1980, 255, 401–407
- Natesh, R.; Schwager, S. L.; Sturrock, E. D.; Acharya, K. R. Crystal Structure of the Human Angiotensin-Converting Enzyme–Lisinopril Complex. Nature. 2003, 421(6922), 551–554. DOI: 10.1038/nature01370.
- Boye, J. I.; Roufik, S.; Pesta, N.; Barbana, C. Angiotensin I-Converting Enzyme Inhibitory Properties and Sds-Page of Red Lentil Protein Hydrolysates. LWT - Food Sci. Technol. 2010, 43(6), 987–991. DOI: 10.1016/j.lwt.2010.01.014.
- Dai, C.; Ma, H.; He, R.; Huang, L.; Zhu, S.; Ding, Q.; Luo, L. Improvement of Nutritional Value and Bioactivity of Soybean Meal by Solid-State Fermentation with Bacillus Subtilis. LWT. 2017, 86, 1–7. DOI: 10.1016/j.lwt.2017.07.041.
- Barbana, C.; Boye, J. I. Angiotensin I-Converting Enzyme Inhibitory Properties of Lentil Protein Hydrolysates: Determination of the Kinetics of Inhibition. Food Chemistry. 2011, 127(1), 94–101. DOI: 10.1016/j.foodchem.2010.12.093.
- Wang, L.; Zhang, J.; Yuan, Q.; Xie, H.; Shi, J.; Ju, X. Separation and Purification of an Anti-Tumor Peptide from Rapeseed (Brassica Campestris L.) and the Effect on Cell Apoptosis. Food Funct. 2016, 7(5), 2239–2248. DOI: 10.1039/c6fo00042h.
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-Diabetic Activity Peptides from Albumin Against Α-Glucosidase and Α-Amylase. Food Chem. 2012, 135(3), 2078–2085. DOI: 10.1016/j.foodchem.2012.06.088.
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of Dietary Arginine Supplementation on Reproductive Performance of Mice with Porcine Circovirus Type 2 Infection. Amino Acids. 2012, 42(6), 2089–2094. DOI: 10.1007/s00726-011-0942-y.
- Van Sadelhoff, J. H.; Mastorakou, D.; Weenen, H.; Stahl, B.; Garssen, J.; Hartog, A. Short Communication: Differences in Levels of Free Amino Acids and Total Protein in Human Foremilk and Hindmilk. Nutrients. 2018, 10(12), 1828. DOI: 10.3390/nu10121828.
- Paddon-Jones, D.; Campbell, W. W.; Jacques, P. F.; Kritchevsky, S. B.; Moore, L. L.; Rodriguez, N. R.; Van Loon, L. J. Protein and Healthy Aging. Am. J. Clin. Nutr. 2015, 101(6), 1339S–1345S. DOI: 10.3945/ajcn.114.084061.
- Amadou, I.; Jin, S.; Kamara, M. T.; Shi, Y. H.; Gbadamosi, O. S.; Guo-Wei, L. Characterization, in vitro Trypsin Digestibility and Antioxidant Activity of Fermented Soybean Protein Meal with Lactobacillus Plantarum Lp6. Am. J. Food Technol. 2009, 4(6), 286–276. DOI: 10.3923/ajft.2009.268.276.
- Starzynska-Janiszewska, A.; Stodolak, B.; Mickowska, B. Effect of Controlled Lactic Acid Fermentation on Selected Bioactive and Nutritional Parameters of Tempeh Obtained from Unhulled Common Bean (Phaseolus Vulgaris) Seeds. J. Sci. Food Agric. 2014, 94(2), 359–366. DOI: 10.1002/jsfa.6385.
- Tie, Y.; Li, L.; Liu, J.; Liu, C.; Fu, J.; Xiao, X.; Wang, G.; Wang, J. Two-Step Biological Approach for Treatment of Rapeseed Meal. J. Food Sci. 2020, 85(2), 340–348. DOI: 10.1111/1750-3841.15011.
- Wang, J. H.; Cao, F. L.; Zhu, Z. L.; Zhang, X. H.; Sheng, Q. Q.; Qin, W. S. Improvement of Quality and Digestibility of Moringa Oleifera Leaves Feed via Solid-State Fermentation by Aspergillus Niger. Int. J. Chem. React. Eng. 2018, 16(12). DOI: 10.1515/ijcre-2018-0094.
- Frias, J.; Song, Y. S.; Martinez-Villaluenga, C.; De Mejia, E. G.; Vidal-Valverde, C. Immunoreactivity and Amino Acid Content of Fermented Soybean Products. J. Agric. Food. Chem. 2008, 56(1), 99–105. DOI: 10.1021/jf072177j.
- Starkute, V.; Bartkiene, E.; Bartkevics, V.; Rusko, J.; Zadeike, D.; Juodeikiene, G. Amino Acids Profile and Antioxidant Activity of Different Lupinus Angustifolius Seeds After Solid State and Submerged Fermentations. J. Food Sci. Technol. 2016, 53(12), 4141–4148. DOI: 10.1007/s13197-016-2384-8.
- Zhang, D. Q.; Tan, B. Effects of Different Solid-State Fermentation Ratios of S. Cerevisiae and L. Plantarum on Physico-Chemical Properties of Wheat Bran and the Quality of Whole Wheat Bread. J. Sci. Food Agric. 2021, 101(11), 4551–4560. DOI: 10.1002/jsfa.11097.
- Yigzaw, Y.; Gorton, L.; Solomon, T.; Akalu, G. Fermentation of Seeds of Teff (Eragrostis Teff), Grass-Pea (Lathyrus Sativus), and Their Mixtures: Aspects of Nutrition and Food Safety. J. Agric. Food. Chem. 2004, 52(5), 1163–1169. DOI: 10.1021/jf034742y.
- Wang, J. H.; Guo, H.; Zhang, T. R.; Wang, H.; Liu, B. N.; Xiao, S. Growth Performance and Digestion Improvement of Juvenile Sea Cucumber Apostichopus Japonicus Fed by Solid-State Fermentation Diet. Aquacult. Nutr. 2017, 23(6), 1312–1318. DOI: 10.1111/anu.12506.
- Zhao, C. J.; Schieber, A.; Gänzle, M. G. Formation of Taste-Active Amino Acids, Amino Acid Derivatives and Peptides in Food Fermentations–A Review. Food. Res. Int. 2016, 89, 39–47. DOI: 10.1016/j.foodres.2016.08.042.
- Moses, S.; Sinner, T.; Zaprasis, A.; Stöveken, N.; Hoffmann, T.; Belitsky, B. R.; Sonenshein, A. L.; Bremer, E. Proline Utilization by Bacillus Subtilis: Uptake and Catabolism. J. Bacteriol. 2012, 194(4), 745–758. DOI: 10.1128/JB.06380-11.
- Wu, G. Amino Acids: Biochemistry and Nutrition; Boca Raton: CRC Press, 2021.
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian Australas. J. Anim. Sci. 2016, 29(11), 1523. DOI: 10.1007/s00253-013-5002-y.
- Yang, Q.-Q.; Suen, P. K.; Zhang, C.-Q.; Mak, W. S.; Gu, M.-H.; Liu, Q.-Q.; Sun, S. S.-M. Improved Growth Performance, Food Efficiency, and Lysine Availability in Growing Rats Fed with Lysine-Biofortified Rice. Sci. Rep. 2017, 7(1), 1–11. DOI: 10.1038/s41598-017-01555-0.
- J, W. H. O. Protein and Amino Acid Requirements in Human Nutrition. W. H. O. Tech. Rep. Ser. 2007, 935, 1.
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary Requirements of “Nutritionally Non-Essential Amino Acids” by Animals and Humans. Amino Acids. 2013, 44(4), 1107–1113. DOI: 10.1007/s00726-012-1444-2.
- Imelda, J.; Paulraj, R.; Bhatnagar, D. Effect of Solid State Fermentation on Nutrient Composition of Selected Feed Ingredients. Indian J. Fish. 2008, 55(4), 327–332.
- Townsend, D. M.; Tew, K. D.; Tapiero, H. Sulfur Containing Amino Acids and Human Disease. Biomed. Pharmacother. 2004, 58(1), 47–55. DOI: 10.1016/j.biopha.2003.11.005.
- Parthasarathy, A.; Cross, P. J.; Dobson, R. C.; Adams, L. E.; Savka, M. A.; Hudson, A. O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. DOI: 10.3389/fmolb.2018.00029.
- Udenigwe, C. C.; Aluko, R. E. Chemometric Analysis of the Amino Acid Requirements of Antioxidant Food Protein Hydrolysates. Int. J. Mol. Sci. 2011, 12(5), 3148–3161. DOI: 10.3390/ijms12053148.