ABSTRACT
Sub-Saharan Africa has one of the world’s richest selections of indigenous fermented foods which constitute valuable cultural heritage with significant socio-economic impact. The review provides an in-depth examination of the diversity of indigenous fermented foods of Kenya in the cultural practices context, interlinked microbiome, associated nutritional and food security aspects while positing valorisation perspective. The traditional fermented foods are profiled into five clusters with significant valorisation potential for food systems in: (i) non-alcoholic cereal-based thin porridge (uji) and (ii) fermented milk products (mursik and suusac). This is informed by agro-productivity resilience and evolving consumption preferences. Whereas these foods are commonly processed via artisanal methods, often resulting in inconsistent products, locally tailored starter culture trials in uji and suusac have shown a promising model to guarantee microbiological safety, mitigate contamination and assure stable sensory characteristics. It is thus plausible to posit, that integrated microbial ecology of traditional fermentation and food systems policy-level research targeted at a reengineering of the unit operations with the intent of improving safety and nutritional quality while being cognizant of organoleptic traits and intertwined biocultural diversity is highly desirable. Particularly, with a broader foresight for promoting sustainable food systems at the base of the pyramid.
Introduction
The pursuit of agricultural productivity has led to the loss of food biocultural diversity coupled with a general standardization of the global foodscape owing to the industrialization of food production since the so-called “green revolution” after World War II.[Citation1–3] In response to this shift, a new perspective geared towards promoting indigenous varieties, safeguarding food and gastronomic heritage emerged. In the late 1960s, European design and enactment of legislation about origin certification in the wine industry was the first indication.[Citation4] The global north consumers’ attitudes towards food shifted to a greater emphasis on nutritional and health benefits, methods of production, traits tied to regional identity and sensory attributes.[Citation5–7] Interest in food products was further characterized by a new awareness that challenged the dominance of commercial, mass-produced food. The awareness entailed encouraging new forms of production and consumption as a means of developing more socially just and environmentally sustainable food systems alongside improving nutrition for the global vulnerable.[Citation8–11] Consequently, food heritage, the collection of tangible and intangible aspects of food cultures (such as ingredients, techniques, recipes, and food traditions) that are considered a common good in a given agroecology and sociocultural setting, has been revitalized.[Citation11–14] This is in a quest to build an all-inclusive capacity for food systems and food security at large[Citation11,Citation13]
Concurrently, studies have established a correlation between the ingestion of fermented foods and an array of health promoting benefits.[Citation15–17] That is in complementation to fermentation leverages in augmentation of the sensory characteristics, nutritional value and enrichment of foods’ shelf life.[Citation18] The convergence of these advancements has prompted a renewed emphasis on research and innovation concerning fermented foods. Specifically traditional fermented foods, in an effort to utilise this knowledge for the creation of novel fermented foodstuffs and ingredients that can be commercially available.[Citation17,Citation19] In the African context, milk, cereals, honey, fruits and starchy root crops are commonly used to make a range of traditional fermented dishes and beverages.[Citation14,Citation18,Citation20,Citation21] Across Africa, industrially prepared fermented food products coexist with indigenous fermented foods such as bushera, mahewu, mabisi, kwerionik, munkoyo, fufu and ogi which are produced in rural and ethnic regions utilising locally accessible raw materials and indigenous knowledge.[Citation17,Citation18,Citation21] The fermented foods are primarily produced at the household level via spontaneous fermentation.[Citation22]
In Kenya, most traditional fermented foods and beverages have socioeconomic value. Indigenous fermentation technologies are widely engrained in culture as a way of life of most rural communities.[Citation23] They provide a relatively inexpensive source of food and contribute significantly to the rural populations’ food, health and nutritional security.[Citation13,Citation24] Constrained structured upscaling to fulfil local and urban inhabitants’ budding demand for traditional products[Citation7,Citation24,Citation25] is a common phenomenon. Therefore, identification and prioritization of research to ensure the safety, quality, and upscaling to advance food security is essential[Citation25–27] Besides, the world population is predicted to exceed 9 billion by 2050, putting a growing strain on food supplies. Indigenous fermentation technology could be applied as a crucial pivot to reinforce reductions in food waste while boosting sustainable food systems.[Citation13,Citation27,Citation28]
Given the above, first, we provide a comprehensive and in-depth examination of the diversity of indigenous fermented foods and beverages of Kenya in the cultural practices context along with interlinked microbiomes. Second, we examine the microbial diversity nexus with health benefits, especially probiotic attributes and safety influences in the traditional fermented foods profiled. Cereals and milk emerge as the predominant substrates for the diverse traditional fermented foods identified. Consequently, we appraise agroproduction interplays that could influence traditional fermented foods’ sustainability and subsequent valorisation prospects in food systems. SSA has one of the world’s richest collections of traditional fermented foods which constitute a valuable cultural heritage. Across the region, the indigenous foods and beverages diversity has a sizeable socioeconomic influence among smallholder farmers and households food security.[Citation11,Citation13,Citation29] Particularly for countries like Kenya and South Africa where indigenous fermented foods are integrated into national nutrition guidelines.[Citation16,Citation30] Thus, this article concludes by highlighting the existent prospects of the systematic microbial ecology of natural fermentation research and interlinked food systems action cues for traditional fermented foods valorisation. Among the indigenous foods and beverages, alcoholic brews are historically highly entwined with substance use disorders.[Citation31,Citation32] Therefore, this review does not elaborate on alcoholic fermented beverages in detail.
Diversity of the traditional fermented food products and associated microbes
Indigenous fermented foods and beverages of Kenya can be clustered into five domains: (i) non-alcoholic cereal-based thin porridge (uji, kirario, kimere and ikii), (ii) fermented milk products (kule naoto, suusac/suusa, mursik, amabere amaruranu and iria ri matii), (iii) alcoholic beverages from maize and/or sorghum or millet malt (busaa), (iv) fermented fruit mashes/wine (mnazi and muratina), and (v) distilled spirits (chang’aa/nubian gin). The diversities and associated predominant microbiota characterized so far are depicted in . For a map of Kenya with regional diversity, see Supplementary material. The foods and beverages have varying adaptabilities as exemplified by the different tribes/ethnic groups of Kenya and/or region of origin () in conjunction with ingredients, techniques, recipes, and inherent food traditions. A vast majority of the fermentation process is via earthen pots, gourds or calabashes as preferred vessels. By extension, containers made of plastic are being embraced because of their durability, ease of use, low cost and availability. The microbial structure is not significantly impacted by the container material.[Citation79,Citation80]
Fermented uji is a thin gruel (porridge) widely prepared from unblended or composite flours (). The variability of recipes depends on cultural practices, the predominant crops grown in the area, and the price and availability of raw materials. After allowing the slurry (30% w/v solids) to ferment, it is diluted with water (to 8–10% w/v solids), boiled, sweetened, and consumed while still hot.[Citation39] Among the implicated microbiota (), Lactobacillus plantarum accounts for the majority of the lactobacilli in uji. Fermentation by both traditional backslopping or the use of starter cultures (as the preferred approach) with lactic acid bacteria (majorly Lactobacillus plantarum, L. brevis, L. buchneri, L. paracasei ssp. paracasei and Pediococcus pentosaceus) are effective in uji preparation.[Citation39,Citation40,Citation42,Citation81] The use of a starter culture is denoted to impede the occurrence of different pathogenic and spoilage microbes.[Citation39,Citation82,Citation83] Kirario, kimere and ikii variants of non-alcoholic cereal-based thin porridge () are uniquely characterized by exploiting wet-milling involving green maize as an ingredient, a combination of dry-milling + wet-milling and dry maize grits in the order of major consumption habits routinely or during special celebrations commonly observed in the respective ethnicities of origin[Citation43–49] Notably, kimere was more of a staple food before the initiation of maize.[Citation47] Regarding the distribution of microbiota for the cereal-based beverages, lactic acid bacteria were the most dominant microbial flora () except for busaa (an anaerobically fermented alcoholic beverage from maize) in which the lactic acid bacteria (LAB) are limited.[Citation38,Citation81]
For the traditional fermented milk products (), the LAB were the predominant microbial flora with some variations in the product traits. In kule naoto, wherein blood is added before fermentation, high levels of proteinCitation51 are present. Due to the high iron content in blood, which is a cofactor in various cellular physiological processes, the mixing of blood and milk for fermentation might impact microbial metabolism.[Citation84,Citation85] However, to our understanding, no evidence validates the notion of the influence of blood in the fermentation process regarding a correlation between microbial flora diversity and volatile compounds along with sensory characteristics.
Table 1. Kenyan traditional fermented foods and beverages and their associated microbiota.
The concept of thermophilic or mesophilic lactic acid cultures, being typically utilized in mini-production trials of fermented milk under regulated conditions, has been observed in suusac and cow milk fermentation mimicking mursik to a good extent.[Citation57,Citation86,Citation87] The mesophilic lactic cultured milk showed certain benefits in warm areas (as is the case of Kenya) on capacity to be incubated at room temperature (20–30°C) with optimum fermentation at 1–1.2% lactic acid, avoiding the requirement for refrigeration to stop additional souring as occurs with yoghurt.[Citation57,Citation86] It is therefore apparent that developments in sequence-based molecular technologies for exploring the diversity and functionality of microbiota in traditional fermented foods at metagenomic and metabolomics scale,[Citation88] offer the chance to enrich our understanding of traditional fermenting microbial ecosystems. Such findings could have practical implications for ecology-driven process design to upscale and standardize indigenous fermentation or for optimization of context-fit starter cultures.
In fermented dairy products like kule naoto, a definite correlation between the occurrence of yeasts and pathogenic and/or spoilage microbes like Enterobacteriaceae was noted where no Enterobacteriaceae were detected in samples where yeasts were profiled.[Citation50,Citation89] In addition to the yeasts being major players in the metabolism of lactic acid with a consequent increase in pH, thus supporting the growth interactively of less acid-tolerant microorganisms.[Citation56,Citation65,Citation90] As well, yeasts can produce proteolytic and lipolytic enzymes that are important in the formation of flavour compounds.[Citation56,Citation65,Citation89,Citation90] Still, the low pH of fermented milk provides a favourable environment for yeast growth, but is unfavourable for the majority of bacteria. Spoiling becomes apparent when the yeast population reaches 105–106 cells g−1[Citation89,Citation91,Citation92]. Yet, except for amabere amaruranu and suusac (in which the dominant species of yeast was S. cerevisiae, ), there is little data on the characterization of yeasts across the majority of the foods and beverages studied. They were mostly enumerated but not characterized. This limits the understanding of their interplays in the indigenous spontaneous fermentation setting, for instance, their interaction with LAB and the ensuing metabolic properties and safety effects.
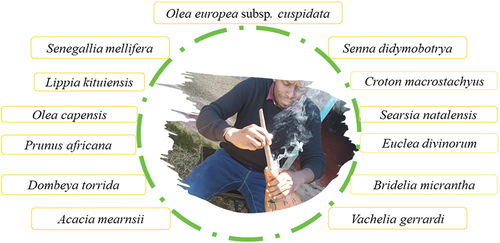
A fermentation vessel (i.e., gourd or calabash) for milk processing usually originates from the plant Lagenaria leucantha or Lagenaria siceraria. The gourd is commonly sooted on the inside with glowing splints of Olea Africana chopped stem as a preparatory step (). More diverse plant species () can be applied in mursik and kule naoto processing bearing in mind preferences and availability.[Citation68,Citation93,Citation94] The benefits of soot application are still debatable and wide-ranging: a) improves flavours, b) slows down the fermentation process and the growth of coliforms thus enhancing safety and extended shelf life of the fermented products c) functions to inactivate potential spoilage organisms in the milk, allowing lactic acid bacteria to establish as the dominant flora, d) suppresses naturally-occurring yeasts, moulds and bacterial contaminants brought in by the raw milk, and e) protects the gourd from wearing out fast and erases the gourd natural smell when milk is drunk from it.[Citation54,Citation68,Citation95–97] Notwithstanding, there is paucity of evidence on the origin of plant species used and the associated sooting impact on the microbial ecology interplays in the fermentation process.
Figure 1. Diversity of plant species for which chopped stems are used to prepare glowing splints for sooting the fermentation vessel (calabash/gourd) inner surface. A case example of the gourd smoking in progress (shown in the centre) with the charcoal from the burning wood. This process is repeated until the inside is smooth and even. After it has been suitably sooted, the extra dust from the charcoal is gently removed. The gourd is now regarded as ready for usage and is covered and left to cool.
(iii) The Kenyan traditional beers, wines and spirits are commonly denoted as “pombe” but distinctively differ from each other based on the substrate used () and methods of preparation besides variations in alcohol and/or lactic acid proportion. For busaa, which is a beer, spontaneous fermentation results in the formation of, among others, alcohol and lactic acid. The final product contains varying amounts of alcohol (2–4% v/v)(volume/volume) and lactic acid (0.5–1.0% w/v)(weight/volume). In chekwe beer, the final product has a pleasant, refreshing sour taste (acidity approximately 0.5–0.8% w/v as lactic acid) and usually contains 4–5% v/v alcohol. The alcohol content of marwa remains indeterminate. Among the wines, the alcohol content of muratina varies from 3–6% v/v with that of mnazi depending on the chemical composition of the sap, the duration of fermentation, and the temperature during the fermentation, thus typically varying between 0.5–7% v/v. Changaa, which is a spirit, contains 25–60% v/v alcohol, contingent on manufacturing conditions.
Microbiota health benefit aspects and safety influences in traditional fermented foods
We next examine the microbiota diversity nexus with health benefits, especially probiotic attributes and safety influences in the traditional fermented foods profiled. Indigenous fermentation takes place by a mixed colony of microorganisms such as moulds, bacteria and yeasts in a food substrate.[Citation98] The resulting microbiota are beneficial with enzymes such as proteases, amylases and lipases that hydrolyze food complexes into simple nontoxic products with desirable textures and aromas that make them palatable for consumption.[Citation17,Citation98–101]
In the traditional foods and beverages examined (majorly cereal and milk-based), LAB are the dominant microorganisms, which include those of the genera Leuconostoc, Lactococcus Streptococcus, Lactobacillus, Enterococcus, Aerococcus and Pediococcus. However, the genera Lactobacillus, Lactococcus and the yeast species Saccharomyces cerevisiae are predominant across ≥ 5 of the fermented products () four of which are milk-based. Overall, the yeasts isolated from different fermented products were mainly of the genus Saccharomyces and Candida. The reviewed literature lacks detailed descriptions of moulds but moulds that have been established in milk and cheese fermentation include Penicillium, Mucor, Geotrichum, and Rhizopus species.[Citation109,Citation110] Cereals of major importance as identified in the reviewed cereal-based fermented products () are maize (Zea mays), sorghum, and millets, especially pearl millet (Pennisetum glaucum) and finger millet (Eleusine coracana). For these cereal products, L. fermentum, Pediococcus pentosaceus, W. confusa, L. plantarum, L. salivarius, L. casei, L. acidophilus, and Leuconostoc spp. are some species that have been commonly isolated. Fermentation of cereals provides optimum pH conditions for enzymatic degradation of phytate and releases minerals such as manganese (which is an important growth factor of LAB), iron, zinc and calcium.[Citation111] Moreover, cereal and cereal component-based foods (like those herein profiled) contain water-soluble fibre (such as β-glucan and arabinoxylan), oligosaccharides (such as galacto- and fructo-oligosaccharides) and resistant starch, which are known for their prebiotic properties.[Citation111–113]
Figure 2. Bacteria and yeast genera that commonly co-occur in five major Kenyan traditional fermented foods. Microorganisms widely used as probiotics mostly belong to the genera Lactobacillus for bacteria and the yeast Saccharomyces.[Citation102,Citation103] in the five fermented foods identified, the LAB and the yeast Saccharomyces have a predominant co-occurrence despite varied substrate and processing variations that may shape microbial ecology attributes that influence mechanisms of microbial community formation.[Citation104] LAB and yeast co-exist synergistically in diverse traditional fermented foods by stimulating their growth and survival.[Citation64,Citation105] the co-occurrence and anticipated beneficial interactions of LAB and yeast across the five foods give credence to their distinctive probiotic viability while in their native forms and that, processing optimization through microbial ecology principles would positively influence valorisation prospects.[Citation17,Citation104,Citation105] Microorganisms commonly considered probiotics, and characterized and/or co-occurring in the five foods: Kule naota; Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus rhamnosus, Lactobacillus acidophilus, isolates of Lactococcus lactis and Saccharomyces cerevisiae. Mursik; Lactobacillus plantarum, Lactobacillus curvatus, Lactobacillus fermentum, Lactobacillus brevis, Lactobacillus casei as well as Lactococcus lactis, Enterococcus faecium, Leuconostoc mesenteroides and Saccharomyces cerevisiae. Amabere amaruranu; Lactobacillus plantarum, Lactobacillus bulgaricus, Lactococcus lactis, Leuconostoc mesenteroides, Streptococcus thermophilus and Saccharomyces cerevisiae. Suusac; Lactobacillus plantarum, Lactobacillus curvatus, Leuconostoc mesenteroides and Saccharomyces cerevisiae. Mnazi: Lactobacillus plantarum, Lactobacillus paracasei, Lactococcus lactis, and Saccharomyces cerevisiae. Strain specific data to justify precise probiotic traits is scanty, there is need for further investigations to delineate the subspecies and genetic differences if any and where necessary. Methods such as comparative genomics analysis have the potential to provide insights into the genetic variation and evolutionary relationships among subspecies, while also facilitating the precise detection of strains that display dual phenotypes.[Citation105–108]
![Figure 2. Bacteria and yeast genera that commonly co-occur in five major Kenyan traditional fermented foods. Microorganisms widely used as probiotics mostly belong to the genera Lactobacillus for bacteria and the yeast Saccharomyces.[Citation102,Citation103] in the five fermented foods identified, the LAB and the yeast Saccharomyces have a predominant co-occurrence despite varied substrate and processing variations that may shape microbial ecology attributes that influence mechanisms of microbial community formation.[Citation104] LAB and yeast co-exist synergistically in diverse traditional fermented foods by stimulating their growth and survival.[Citation64,Citation105] the co-occurrence and anticipated beneficial interactions of LAB and yeast across the five foods give credence to their distinctive probiotic viability while in their native forms and that, processing optimization through microbial ecology principles would positively influence valorisation prospects.[Citation17,Citation104,Citation105] Microorganisms commonly considered probiotics, and characterized and/or co-occurring in the five foods: Kule naota; Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus rhamnosus, Lactobacillus acidophilus, isolates of Lactococcus lactis and Saccharomyces cerevisiae. Mursik; Lactobacillus plantarum, Lactobacillus curvatus, Lactobacillus fermentum, Lactobacillus brevis, Lactobacillus casei as well as Lactococcus lactis, Enterococcus faecium, Leuconostoc mesenteroides and Saccharomyces cerevisiae. Amabere amaruranu; Lactobacillus plantarum, Lactobacillus bulgaricus, Lactococcus lactis, Leuconostoc mesenteroides, Streptococcus thermophilus and Saccharomyces cerevisiae. Suusac; Lactobacillus plantarum, Lactobacillus curvatus, Leuconostoc mesenteroides and Saccharomyces cerevisiae. Mnazi: Lactobacillus plantarum, Lactobacillus paracasei, Lactococcus lactis, and Saccharomyces cerevisiae. Strain specific data to justify precise probiotic traits is scanty, there is need for further investigations to delineate the subspecies and genetic differences if any and where necessary. Methods such as comparative genomics analysis have the potential to provide insights into the genetic variation and evolutionary relationships among subspecies, while also facilitating the precise detection of strains that display dual phenotypes.[Citation105–108]](/cms/asset/3040b6e0-ca99-4b2c-9837-62dfc8b6cdf5/lfri_a_2355992_f0002_oc.jpg)
Taken together, lactic acid fermentation is considered a major contributor to the beneficial characteristics of indigenous fermented foods and beverages.[Citation15,Citation17,Citation81] Probiotics isolated from a variety of sources including traditional fermented foods, mostly belong to genera Lactobacillus, Bifidobacterium, Lactococcus, Enterococcus, Leuconostoc, Streptococcus and the yeast Saccharomyces.[Citation102,Citation105,Citation114,Citation115] The respective strains, which are predominantly LAB and considered to be of importance to food and nutrition, have been characterized, and validated for safety, functionality and technological attributes[Citation52,Citation102,Citation114–116] with more yeast strains identified as having probiotic properties and capabilities.[Citation105,Citation106] A selective range of the reported microorganism species resonates with those that co-occur in traditional foods () while in their native forms of preparation. The utilization of wet-milling or a combination of dry-milling + wet-milling for the non-alcoholic cereal-based porridges (kirario, kimere and ikii) confers them good traits for prebiotics and probiotics.[Citation45,Citation102,Citation115,Citation117] Prebiotic and probiotic bacteria are beneficial in that they favourably alter the intestinal microflora balance, such as the reconstruction of normal intestinal microflora after disorders caused by diarrhoea and antibiotic therapy inhibiting the growth of harmful bacteria, promoting good digestion, boosting immune functions and increasing resistance to infections.[Citation111,Citation112] Further physiological benefits include the removal of carcinogens, lowering of cholesterol, immunostimulating and allergy-lowering effects, and synthesis and enhancement of the bioavailability of nutrients.[Citation111,Citation112]
In food and health safety, fermentation plays a crucial role in the reduction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP).[Citation118] FODMAP ingestion induces abdominal symptoms in people suffering from irritable bowel syndrome, which is a common gastrointestinal tract disorder.[Citation118] Though not conclusive owing to limited studies, fermented foods can support microbiota formation during pregnancy.[Citation30] Antimicrobial effects of LAB, inactivation and control of foodborne pathogens through fermentation can reduce the risk of pathogenic diarrhea, which is the leading cause of infant mortality in sub-Saharan Africa.[Citation17,Citation119] For instance, raw sorghum flour contains ~ 2,400 colony-forming units per gramme (cfu/g) of Escherichia coli, whereas fermented dough contains significantly fewer.[Citation17,Citation120] The fermented dough contains no Salmonella spp., which were found in several sorghum cultivars. Still, a variety of spoilage and pathogenic microorganisms have been identified in traditional fermented foods and beverages.[Citation121,Citation122] These microorganisms are primarily native microflora of fermenting foods in addition to those from poor handling and processing methods.[Citation122–124] They include non-spore-forming pathogenic bacteria (e.g., Listeria monocytogenes, Salmonella spp., and Shigella spp.), bacterial toxin producers (Staphylococcus aureus), yeasts (for instance Candida spp.), moulds (such as Rhizopus spp., Penicillium spp.), and toxigenic fungi (like Aspergillus spp.).[Citation123,Citation125–127]
Cereals are prone to contamination by toxigenic fungi that lead to mycotoxicosis.[Citation128,Citation129] As an indirect environmental influence, mycotoxins have been detected in milk as a result of feeding livestock with contaminated agricultural produce.[Citation129,Citation130] Interlinkages of direct and indirect types and sources of contaminants of milk and dairy products including those impacting the processing of foods like mursik, kule naota, suusac and amabere amaruranu have been examined.[Citation131,Citation132] Considering the significant burden of mycotoxins in Kenya’s food systems, the mainstay control strategy entails the use of chemical preservatives but their impact on health, nutritional and organoleptic qualities of food is a growing concern.[Citation128,Citation129] The LAB, including those from Lactobacillus, Lactococcus and yeast strains of Saccharomyces cerevisiae predominant in the traditional fermented foods herein profiled () are generally regarded as safe (GRAS). Apart from the LAB and the yeasts’ essential traits for application in food fermentation systems, their ability to produce bioactive metabolites or enzymes with synergistic antifungal activity has been reported.[Citation119,Citation128,Citation129,Citation133,Citation134] The traditional fermented foods thus act as reservoirs of beneficial LAB and yeasts that can aid in food preservation and control of mycotoxins.
Agroproduction determinants and traditional fermented foods valorisation prospects
An important component of national nutrition policies is dietary recommendations. The guidelines represent suggestions for optimal nutrition and overall health, devised by experts in the field of science and health, drawing upon the most recent scientific data and information on food consumption.[Citation16,Citation23] Fermented foods are consumed globally but national nutrition guidelines in the majority of countries do not provide recommendations concerning the consumption of fermented foods. Australia, Bulgaria, South Africa, India, Qatar, Kenya, Sri Lanka, and Oman are among the very few nations that have national nutrition guidelines that include indigenous and traditional fermented foods.[Citation16,Citation23,Citation30] Fermented foods that are indigenous and traditional to these nations are accessible and denote an integral part of the national ethos.[Citation16,Citation19]
While national dietary guidelines across the globe may contain similar recommendations, they remain distinct to the population and country that established them and are country-specific.[Citation16,Citation135] They are interconnected and impacted by the accessibility of food products within a particular nation, including dietary and cultural attributes that are specific to that country.[Citation16,Citation19] Cereals and milk are the predominant substrates for the diverse traditional fermented foods identified (). We, therefore, explore the agroproduction of these substrates that would conceivably influence traditional fermented foods’ sustainability and subsequent valorisation in food systems. Across many households in Kenya, the staple food crop is maize, often consumed as stiff porridge (ugali), alongside cooked vegetables and/or meat (with chicken as the most preferred meat in Kenya at 92%), beef (85%), fish (79%) or goat with milk and related products as common features in the diet.[Citation136–144] The Food Balance Sheet (FBS 2023) by the Kenya National Bureau of Statistics (KNBS) shows that milk and related products have a per capita consumption of 93.3 kg, followed by maize and maize products (69.5 kg), wheat products (41.3 kg) and vegetables at 32.6 kg.[Citation145,Citation146]
The projected value of Kenya’s dairy industry is 4% of its gross domestic product.[Citation147,Citation148] This vitality is supported by rising domestic milk production (an average of 5.3% annually), processing capacity (an average of 7% annually), yearly per capita milk consumption (an average of 5.8% annually, now 110 litres), well beyond those of other African nations.[Citation144,Citation147,Citation148] There is a growing dairy production cutting across smallholder farmers (who are the majority), dominating mixed crop-livestock (MCL) systems with small herds of superior dairy cattle breeds replacing the traditional breeds.[Citation144,Citation148,Citation149] Bovine and camel milk along with derivative products are the most produced and consumed animal-sourced foods while ∼70%–80% of the milk marketed in the country is sold through informal markets.[Citation144,Citation150–152] Of note, recent trends have positioned Kenya Arid and Semi-Arid Lands (ASALs) to be in the lead in camel milk production with an annual production volume of 1.165 million metric tonnes (MMT), followed by Somalia (0.958 MMT) and Saudi Arabia (0.271 MMT).[Citation152,Citation153]
The consumption of milk-based traditional fermented foods is at a rate of up to 2–3 litres per day per person for kule naoto, majorly among the Maasai, irrespective of income or number of cattle per household.[Citation50,Citation154–156] Comparatively, mursik consumption is estimated at 500 ml (2 cups) per person per day during main meals within the agropastoralists’ settings, coupled with the “traditional fermentation technology export” spanning the country () and ~ 53% of milk produced in cooler Kenyan highlands consumed as fermented milk products [Citation157–160] Camel milk products are equally gaining increased acceptability beyond the usual ASAL settings.[Citation59,Citation161,Citation162] Despite a robust legislative framework in the dairy sector, up to 80% of the milk is marketed informally providing livelihoods to households. Informal milk markets are ascribed for proficiency in delivering dairy products that satisfies sociocultural expectations in quality, at reduced prices and in smaller quantities fitting low-income consumers’ purchasing power.[Citation144] Mursik, is a archetypical case milk product bridging the pursuit for sustainable food systems especially at the bottom of the pyramid. It is plausible to note, with valorisation, milk based products like mursik and suusac have a good potential to add to food security as driven by the growing agroproduction sustained in the dairy sectors over time.
Figure 3. A case of rural Kenya experience in mursik production and consumption: (a) an enterprising local farmer who produces calabashes/gourd bottles for sale to the community and beyond, due to its high demand for use in the production of indigenous fermented milk (mursik). (b) a demonstration of how the calabash/gourd is coated on the inside by smoking with special tree species locally used for flavour and alluded medicinal benefits. (c) Fermented mursik in a calabash ready for consumption. (d) Mursik with the dark effect due to smoking is aliquoted from the calabash to an open container for further mixing before consumption to ensure the smoking effect from the fermentation process done in the calabash is properly mixed and for evenness.

Maize, sorghum, finger millet and cassava have a diverse role in the food chain other than the preparation of fermented products. The cereals are important raw materials for making thick (ugali) and thin (uji) porridge, githeri (a boiled mixture of maize+beans) mainly in school meals programme and mkarango (maize meal snacks) but their production is persistently less than the demand.[Citation34,Citation37,Citation163,Citation164] The annual production of white maize is about 3.6 million tons with insufficient production to meet national demand spanning all the way from the 1980s to date[Citation165,Citation166] with the shortage getting more severe. The productivity of sorghum is about 2.0 t/ha, which is below the inherent potential of 3–4 t/ha, even with farmers increasingly adopting varieties with improved genetic potential like Serena, Seredo, Gadam and E1291.[Citation34,Citation167] The average mean yield of finger millet in Kenya is 2.9 t/ha with yields of improved varieties (such as P224 and Katumani) reaching up to 3.3 t/ha.[Citation34,Citation168] Cassava, an ideal food security crop for semi-arid regions, has average yields of 15 to 20 t/ha against a potential yield of over 50 t/ha.[Citation34]
In contrasting the cereals’ productivity with fermented beverages utilizing the cereals or cereal flour as substrate (), uji is commonly used as a vital weaning food along with being consumed by individuals of all age groups as a breakfast meal or a refreshing beverage at any time of day.[Citation40,Citation169] It is a highly preferred food among invalids as they are often weak and unable to consume food that requires much energy to chew and swallow.[Citation34] Per capita consumption of uji is high in poor households, especially during a famine, because only a small amount of flour is necessary to produce a significant quantity of the product, which can be consumed throughout the day.[Citation34,Citation39] As earlier detailed (), although the main substrates for uji comprise unblended or composite flours of cassava and whole-milled grains of maize (cornmeal), sorghum or finger millet (wimbi), the most popular 1:1 composites are maize – sorghum, maize – finger millet, cassava – finger millet and cassava – sorghum. The growing adaptive premise of composites (blending of cereals) in traditional cereal-based foods (like uji) and enhanced productivity of alternative cereals (mainly sorghum and finger millet) is promising for food sufficiency in SSA where indigenous fermented foods continue to gain acceptance.[Citation29,Citation170]
Conclusion and perspective
It is apparent that the traditional fermented foods examined are widely accepted and within the socioeconomic means of the communities in which they are produced, based on the substrates used. As a way of mitigating wastage under ambient temperatures, improving the nutritional quality, organoleptic characteristics and enhancing the products’ shelf life at the production level, indigenous spontaneous fermentation persists as a mainstay approach across different ethnicities for varied substrates.
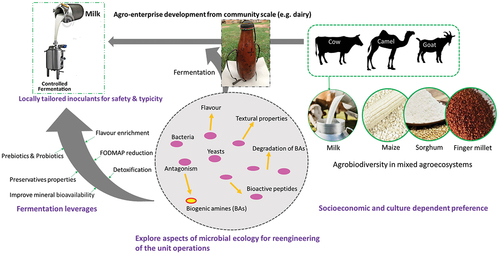
We profile the traditional fermented foods and beverages into five major clusters with significant valorisation potential for greater nutritional value and assurance for food system progression in two: (i) non-alcoholic cereal-based thin porridge (majorly uji) and (ii) fermented milk products (mainly mursik and suusac) as informed by agroproduction resilience and evolving consumption preferences, especially for the milk products. Likewise, some products such as uji, mursik and suusac have a likability that transcends their ethnic jurisdiction of origin as a consequence of widely perceived and/or partly established health benefits and organoleptic properties. Whereas these foods are commonly processed and produced using empirical and artisanal methods, often resulting in inconsistent products, locally tailored starter culture trials and related applications in products like uji and suusac have shown a promising prototype to guarantee safety, mitigate contamination, assure stable sensory characteristics and nutritional value. It is thus plausible to affirm, that integrated basic, applied and policy-level research () aimed at improving traditional processes by reengineering the unit operations with the intent of improving safety and nutritional quality while being cognizant of organoleptic traits and intertwined biocultural diversity is highly desirable. In the long run, this would potentially stimulate assurance of sustainable food systems, especially at the base of the pyramid.
Figure 4. Schematic model for interlinked microbial ecology of natural fermentation research and food systems policy action cues in traditional fermented foods: understanding food products’ preference and perception (e.g. cereal or milk-based and alcoholic or non-alcoholic) depending on the cultural context of the communities; investigating aspects of the microbial ecology of the traditional fermented foods (e.g. studying the diverse bacteria, yeasts, and moulds that contribute to the fermentation process) and its role in the substrates utilized; microbial ecology studies can assist in identifying biomarkers (measurable indicator of some biological state or condition) for assessing fermented food quality and aid in the development of optimal ecology driven process design to upscale and standardize fermentation while being cognizant of intertwined biocultural diversity (for instance preservation of organoleptic properties). In the case of microbial ecology-driven biomarkers identification, some microbial metabolites, enzymes, and proteins can be utilized to evaluate whether a food product has been properly fermented or contains harmful microbes. As an effect of the interlaced actions, stimulate agro-enterprise development (e.g. shift from calabash/ gourd fermentation (top centre) to milk grade controlled fermentation tank (top left) with a touch of typicity).
From the declining productivity of maize (Zea mays) that spans decades coupled with known unfavourable climate variability trends in the tropics and the growing adaptive premise of composites (blending of cereals) in cereal-based foods and beverages, promotion of the productivity of alternative cereals (mainly sorghum and finger millet) in the context of indigenous foods and beverages consumption habits, would go a long way in invigorating food sufficiency at community and households levels. Ultimately, such efforts would help alleviate or mitigate the overreliance on maize as the main substrate for different cereal-based indigenous foods and beverage preparation among other competing needs (like home-grown school meal programmes). In doing so, this would proportionally advance responsiveness to several SDGs, namely 2) Zero hunger, 3) Good health and well-being, 12) Responsible consumption and production and 13) Climate action.
Supplemental Material
Download MS Word (349.4 KB)Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/87559129.2024.2355992.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Patel, R. The Long Green Revolution. J. Peasant Stud. 2013, 40(1), 1–63. DOI: 10.1080/03066150.2012.719224.
- Bégin, C. Taste of the Nation: The New Deal Search for America’s Food; University of Illinois Press: Champaign, IL, 2016.
- Clark, J. K. Food by Jennifer Clapp Cambridge, UK: Polity Press, 2012. Econ. Geogr. 2013, 89(2), 195–196. DOI: 10.1111/ecge.12010.
- Addor, F.; Grazioli, A. Geographical Indications Beyond Wines and Spirits: A Roadmap for a Better Protection for Geographical Indications in the WTO/TRIPS Agreement. J. World Intell. Prop. 2002, 5(6), 865. DOI: 10.1111/j.1747-1796.2002.tb00185.x.
- Ilbery, B.; Morris, C.; Buller, H.; Maye, D.; Kneafsey, M. Product, Process and Place: An Examination of Food Marketing and Labelling Schemes in Europe and North America. Eur. Urban Reg. Stud. 2005, 12(2), 116–132. DOI: 10.1177/0969776405048499.
- Tregear, A.; Arfini, F.; Belletti, G.; Marescotti, A. Regional Foods and Rural Development: The Role of Product Qualification. J. Rural Stud. 2007, 23(1), 12–22. DOI: 10.1016/j.jrurstud.2006.09.010.
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented Food Products in the Era of Globalization: Tradition Meets Biotechnology Innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. DOI: 10.1016/j.copbio.2020.10.006.
- Murdoch, J.; Marsden, T.; Banks, J. Quality, Nature, and Embeddedness: Some Theoretical Considerations in the Context of the Food Sector. Econ. Geogr. 2000, 76(2), 107–125. DOI: 10.1111/j.1944-8287.2000.tb00136.x.
- Guptill, A. E.; Copelton, D. A.; Lucal, B. Food & Society: Principles and Paradoxes, 3rd ed.; John Wiley & Sons: Hoboken, New Jersey, 2022.
- Allen, P. Realizing Justice in Local Food Systems. Camb. J. Reg. Econ. Soc. 2010, 3(2), 295–308. DOI: 10.1093/cjres/rsq015.
- Materia, V. C.; Linnemann, A. R.; Smid, E. J.; Schoustra, S. Upscaling of Traditional Fermented Foods to Build Value Chains and to Promote Women Entrepreneurship. IFAD Rural Development Report-Background Paper, 2021.
- Bessière, J. Local Development and Heritage: Traditional Food and Cuisine As Tourist Attractions in Rural Areas. Sociol. Ruralis. 1998, 38(1), 21–34. DOI: 10.1111/1467-9523.00061.
- Materia, V. C.; Linnemann, A. R.; Smid, E. J.; Schoustra, S. E. Contribution of Traditional Fermented Foods to Food Systems Transformation: Value Addition and Inclusive Entrepreneurship. Food Secur. 2021, 13(5), 1163–1177. DOI: 10.1007/s12571-021-01185-5.
- Moonga, H. B.; Schoustra, S. E.; Linnemann, A. R.; Shindano, J.; Smid, E. J. Towards Valorisation of Indigenous Traditional Fermented Milk: Mabisi as a Model. Curr. Opin. Food Sci. 2022, 46, 100835. DOI: 10.1016/j.cofs.2022.100835.
- Salama, S. M.; Mariod, A. A. Significance of African Fermented Foods in Nutrition and Food Science. In African Fermented Food Products-New Trends; Springer: Berlin, Germany, 2022; pp. 37–44.
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A. G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-To-Date Systematic Review. Nutrients 2019, 11(5), 1189. DOI: 10.3390/nu11051189.
- Ghosh, S.; Bornman, C.; Meskini, M.; Joghataei, M. Microbial Diversity in African Foods and Beverages: A Systematic Assessment. Curr. Microbiol. 2024, 81(1), 19. DOI: 10.1007/s00284-023-03481-z.
- Obafemi, Y. D.; Oranusi, S. U.; Ajanaku, K. O.; Akinduti, P. A.; Leech, J.; Cotter, P. D. African Fermented Foods: Overview, Emerging Benefits, and Novel Approaches to Microbiome Profiling. Npj Sci. Food 2022, 6(1), 15. DOI: 10.1038/s41538-022-00130-w.
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E. M.; Kenny, J. G.; Cotter, P. D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. DOI: 10.3389/fnut.2022.902642.
- Franz, C. M.; Huch, M.; Mathara, J. M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W. H. African Fermented Foods and Probiotics. Int. J. Food Microbiol. 2014, 190, 84–96. DOI: 10.1016/j.ijfoodmicro.2014.08.033.
- Schoustra, S. E.; Kasase, C.; Toarta, C.; Kassen, R.; Poulain, A. J.; Smidt, H. Microbial Community Structure of Three Traditional Zambian Fermented Products: Mabisi, Chibwantu and Munkoyo. PLOS ONE 2013, 8(5), e63948. DOI: 10.1371/journal.pone.0063948.
- FAO. Current Status and Options for Biotechnologies in Food Processing and in Food Safety in Developing Countries. 2010. https://www.fao.org/3/k6993e/k6993e.pdf.
- National Guidelines for Healthy Diets and Physical Activity. Ministry of Health. 2017. https://www.fao.org/nutrition/education/food-dietary-guidelines/regions/kenya/en/.
- Watson, F.; Ngesa, A.; Onyang’o, J.; Alnwick, D.; Tomkins, A. Fermentation-A Traditional Anti-Diarrhoeal Practice Lost? The Use of Fermented Foods in Urban and Rural Kenya. Int. J. Food Sci. Nutr. 1996, 47(2), 171–179. DOI: 10.3109/09637489609012579.
- Holzapfel, W. H. Appropriate Starter Culture Technologies for Small-Scale Fermentation in Developing Countries. Int. J. Food Microbiol. 2002, 75(3), 197–212. DOI: 10.1016/S0168-1605(01)00707-3.
- Mokoena, M. P.; Mutanda, T.; Olaniran, A. O. Perspectives on the Probiotic Potential of Lactic Acid Bacteria from African Traditional Fermented Foods and Beverages. Food Nutr. Res. 2016, 60(1), 29630. DOI: 10.3402/fnr.v60.29630.
- Sybesma, W.; Kort, R.; Lee, Y.-K. Locally Sourced Probiotics, the Next Opportunity for Developing Countries? Trends Biotechnol. 2015, 33(4), 197–200. DOI: 10.1016/j.tibtech.2015.01.002.
- Steinkraus, K. Handbook of Indigenous Fermented Foods, Revised and Expanded; CRC press: Boca Raton, Florida, 2018.
- Ekpa, O.; Palacios-Rojas, N.; Kruseman, G.; Fogliano, V.; Linnemann, A. R. Sub-Saharan African Maize-Based Foods-Processing Practices, Challenges and Opportunities. Food Rev. Int. 2019, 35(7), 609–639. DOI: 10.1080/87559129.2019.1588290.
- Erçelik, H. C.; Kaya, V. The Effects of Fermented Food Consumption in Pregnancy on Neonatal and Infant Health: An Integrative Review. J. Pediatr. Nurs. 2024, 75, 173–179. DOI: 10.1016/j.pedn.2023.12.019.
- Nout, M. J. Aspects of the Manufacture and Consumption of Kenyan Traditional Fermented Beverages; Wageningen University and Research: Wageningen, Netherlands, 1981.
- Jaguga, F.; Kwobah, E. A Review of the Public Sector Substance Use Disorder Treatment and Prevention Systems in Kenya. Subst. Abuse. Treat Prev. Policy 2020, 15(1), 1–9. DOI: 10.1186/s13011-020-00291-5.
- Onyango, C.; Okoth, M. W.; Mbugua, S. K. The Pasting Behaviour of Lactic‐Fermented and Dried Uji (An East African Sour Porridge). J. Sci. Food Agric. 2003, 83(14), 1412–1418. DOI: 10.1002/jsfa.1550.
- Wanjala, W.; Onyango, A.; Makayoto, M.; Onyango, C. Indigenous Technical Knowledge and Formulations of Thick (Ugali) and Thin (Uji) Porridges Consumed in Kenya. Afr. J. Food Sci. 2016, 10(12), 385–396.
- Mbugua, S.; Njenga, J. The Antimicrobial Activity of Fermented Uji. Ecology of Food and Nutrition. Ecol. Food Nutr. 1992, 28(3), 191–198. DOI: 10.1080/03670244.1992.9991270.
- Mbugua, S.; Ahrens, R.; Kigutha, H.; Subramanian, V. Effect of Fermentation, Malted Flour Treatment and Drum Drying on Nutritional Quality of Uji. Ecology of Food and Nutrition. Ecol. Food Nutr. 1992, 28(4), 271–277. DOI: 10.1080/03670244.1992.9991280.
- De Groote, H.; Kimenju, S. C. Consumer Preferences for Maize Products in Urban Kenya. Food Nutr. Bull. 2012, 33(2), 99–110. DOI: 10.1177/156482651203300203.
- Mwizerwa, H.; Abongo, G. O.; Mbugua, S. K.; Okoth, M. W.; Gacheru, P.; Maina, M.; Obura, B.; Viljoen, B. Profiling of Microbial Content and Growth in Fermented Maize Based Products from Western Kenya. Curr. Res. Nutr. Food Sci. 2018, 6(2), 509–519. DOI: 10.12944/CRNFSJ.6.2.25.
- Masha, G.; Ipsen, R.; Petersen, M.; Jakobsen, M. Microbiological, Rheological and Aromaticcharacteristics of Fermented Uji (An East African Sour Porridge). World J. Microbiol. Biotechnol. 1998, 14(3), 451–456. DOI: 10.1023/A:1008889900944.
- Mbugua, S. Microbial Growth During Spontaneuos Uji Fermentation and Its Influence on the End Product. East African Agric. For. J. 1984, 50(1–4), 101–110. DOI: 10.1080/00128325.1984.11663450.
- Muyanja, C.; Narvhus, J. A.; Treimo, J.; Langsrud, T. Isolation, Characterisation and Identification of Lactic Acid Bacteria from Bushera: A Ugandan Traditional Fermented Beverage. Int. J. Food Microbiol. 2003, 80(3), 201–210. DOI: 10.1016/S0168-1605(02)00148-4.
- Onyango, C.; Bley, T.; Raddatz, H.; Henle, T. Flavour Compounds in Backslop Fermented Uji (An East African Sour Porridge). Eur. Food Res. Technol. 2004, 218(6), 579–583. DOI: 10.1007/s00217-003-0870-5.
- Kunyanga, C.; Mbugua, S.; Kangethe, E.; Imungi, J. Microbiological and Acidity Changes During the Traditional Production of Kirario: An Indigenous Kenyan Fermented Porridge Produced from Green Maize and Millet. Afr. J. Food Agric. Nutr. Dev. 2009, 9(6). DOI: 10.4314/ajfand.v9i6.46261.
- Kunyanga, C. Microbiological Studies of Kirario, an Indigenous Kenyan Fermented Porridge Based on Green Maize and Millet. 2006.
- Njeru, P.; Rösch, N.; Ghadimi, D.; Geis, A.; Bockelmann, W.; de Vrese, M.; Schrezenmeir, J.; Heller, K. Identification and Characterisation of Lactobacilli Isolated from Kimere, a Spontaneously Fermented Pearl Millet Dough from Mbeere, Kenya (East Africa). Beneficial. Microbes. 2010, 1(3), 243–252. DOI: 10.3920/BM2010.0019.
- Njeru, P.; Rösch, N.; Ghadimi, D.; Schrezenmeir, J.; de Vrese, M.; Heller, K. Isolation, Characterization and Identification of Potentially Probiotic Lactobacilli from Kimere; a Spontaneously Fermented Pearl Millet Dough from Mbeere, Kenya (East Africa). Akt. Ernähr. Med. 2007, 32(3), 3_6. DOI: 10.1055/s-2007-983414.
- Nduti, N.; Reid, G.; Sumarah, M.; Hekmat, S.; Mwaniki, M.; Njeru, P. Weissella Cibaria Nn20 Isolated from Fermented Kimere Shows Ability to Sequester AFB1 in vitro and Ferment Milk with Good Viscosity and Phin Comparison to Yogurt. Food Sci. Nutr. Technol. 2018, 3(1), 000137. DOI: 10.23880/FSNT-16000138.
- Kalui, C.; Mathara, J.; Kutima, P.; Kiiyukia, C.; Wongo, L. Functional Characteristics of Lactobacillus Plantarum and Lactobacillus Rhamnosus from Ikii, a Kenyan Traditional Fermented Maize Porridge. Afr. J. Biotechnol. 2009, 8(18).
- Kalui, C.; Mathara, J.; Kutima, P.; Kiiyukia, C.; Wongo, L. Partial Characterisation and Identification of Lactic Acid Bacteria Involved in Production of Ikii: A Traditional Fermented Maize Porridge by the Kamba in Kenya. J. Trop. Microbiol. Biotechnol. 2008, 4(1), 3–15. DOI: 10.4314/jtmb.v4i1.35461.
- Mathara, J. M.; Schillinger, U.; Kutima, P. M.; Mbugua, S. K.; Holzapfel, W. H. Isolation, Identification and Characterisation of the Dominant Microorganisms of Kule Naoto: The Maasai Traditional Fermented Milk in Kenya. Int. J. Food Microbiol. 2004, 94(3), 269–278. DOI: 10.1016/j.ijfoodmicro.2004.01.008.
- Mathara, J. M. Studies on Lactic Acid Producing Microflora in Mursik and Kule Naoto, Traditional Fermented Milks from Nandi and Masai Communities in Kenya; University of Nairobi: Nairobi, Kenya, 1999.
- Mathara, J. M.; Schillinger, U.; Guigas, C.; Franz, C.; Kutima, P. M.; Mbugua, S. K.; Shin, H.-K.; Holzapfel, W. H. Functional Characteristics of Lactobacillus Spp. from Traditional Maasai Fermented Milk Products in Kenya. Int. J. Food Microbiol. 2008, 126(1–2), 57–64. DOI: 10.1016/j.ijfoodmicro.2008.04.027.
- Mathara, J. M.; Schillinger, U.; Kutima, P. M.; Mbugua, S. K.; Guigas, C.; Franz, C.; Holzapfel, W. H. Functional Properties of Lactobacillus Plantarum Strains Isolated from Maasai Traditional Fermented Milk Products in Kenya. Curr. Microbiol. 2008, 56(4), 315–321. DOI: 10.1007/s00284-007-9084-6.
- Onyango, C.; Gakuya, L.; Mathooko, F. M.; Maina, J.; Nyaberi, M.; Makobe, M.; Mwaura, F. Preservative Effect of Various Indigenous Plants on Fermented Milk from Maasai Community of Kajiado County. J. Appl. Biosci. 2014, 73, 5935–5941.
- Lore, T. A.; Mbugua, S. K.; Wangoh, J. Enumeration and Identification of Microflora in Suusac, a Kenyan Traditional Fermented Camel Milk Product. LWT Food Sci. Technol. 2005, 38(2), 125–130. DOI: 10.1016/j.lwt.2004.05.008.
- Njage, P.; Dolci, S.; Jans, C.; Wangoh, J.; Lacroix, C.; Meile, L. Characterization of Yeasts Associated with Camel Milk Using Phenotypic and Molecular Identification Techniques. Res. J. Microbiol. 2011, 6(9), 678. DOI: 10.3923/jm.2011.678.692.
- Farah, Z.; Streiff, T.; Bachmann, M. R. Preparation and Consumer Acceptability Tests of Fermented Camel Milk in Kenya. J. Dairy Res. 1990, 57(2), 281–283. DOI: 10.1017/S002202990002690X.
- Jans, C.; Bugnard, J.; Njage, P. M. K.; Lacroix, C.; Meile, L. Lactic Acid Bacteria Diversity of African Raw and Fermented Camel Milk Products Reveals a Highly Competitive, Potentially Health-Threatening Predominant Microflora. LWT 2012, 47(2), 371–379. DOI: 10.1016/j.lwt.2012.01.034.
- Shori, A. B. Comparative Study of Chemical Composition, Isolation and Identification of Micro-Flora in Traditional Fermented Camel Milk Products: Gariss, Suusac, and Shubat. J. Saudi Soc. Agric. Sci. 2012, 11(2), 79–88. DOI: 10.1016/j.jssas.2011.12.001.
- Miyamoto, T.; Gichuru, S. G.; Akimoto, T.; Nakae, T. Identification and Properties of Lactic Acid Bacteria Isolated from Traditional Fermented Beverages in East Africa. Jpn. J. Zootech. Sci 1986, 57(3), 265–276. DOI: 10.2508/chikusan.57.265.
- Digo, C.; Kamau-Mbuthia, E.; Matofari, J.; Ng’etich, W. Potential Probiotics from Traditional Fermented Milk, Mursik of Kenya. Int. J. Nutr. Metab. 2017, 10(9), 75–81. DOI: 10.5897/IJNAM2016.0203.
- Digo, C. A. Isolation and Characterization of Lactic Acid Bacteria and Their Inhibitory/Safety Potential in Traditionally Fermented Milk, Mursik in Kenya; Egerton University: Njoro Kenya, 2015.
- Nieminen, M. T.; Novak-Frazer, L.; Collins, R.; Dawsey, S. P.; Dawsey, S. M.; Abnet, C. C.; White, R. E.; Freedman, N. D.; Mwachiro, M.; Bowyer, P. Alcohol and Acetaldehyde in African Fermented Milk Mursik—A Possible Etiologic Factor for High Incidence of Esophageal Cancer in Western Kenya. Cancer Epidemiol. Biomarkers Prev. 2013, 22(1), 69–75. DOI: 10.1158/1055-9965.EPI-12-0908.
- Jespersen, L. Occurrence and Taxonomic Characteristics of Strains of Saccharomyces cerevisiae Predominant in African Indigenous Fermented Foods and Beverages. FEMS Yeast Res. 2003, 3(2), 191–200. DOI: 10.1016/S1567-1356(02)00185-X.
- Nyambane, B.; Thari, W. M.; Wangoh, J.; Njage, P. M. Lactic Acid Bacteria and Yeasts Involved in the Fermentation of Amabere Amaruranu, a Kenyan Fermented Milk. Food Sci. Nutr. 2014, 2(6), 692–699. DOI: 10.1002/fsn3.162.
- Katiku, M. M.; Matofari, J. W.; Nduko, J. M. Preliminary Evaluation of Probiotic Properties and Safety Profile of Lactiplantibacillus plantarum Isolated from Spontaneously Fermented Milk, Amabere Amaruranu. Heliyon. 2022, 8(8), e10342. DOI: 10.1016/j.heliyon.2022.e10342.
- Sichangi, M.; Nduko, J.; Matofari, J. Molecular Investigation of Potential Lactic Acid Bacteria Starter Culture Organisms/Probiotics in the Kenyan Spontaneously Fermented Milk, Amabere Amaruranu. Pak. J. Nutr. 2020, 19(3), 132–145. DOI: 10.3923/pjn.2020.132.145.
- Kimonye, J.; Robinson, R. Iria Ri Matii: A Traditional Fermented Milk from Kenya. Dairy Ind. Int. 1991, 56(2), 34–35.
- Nout, M. Process Development and Preservation of Busaa, a Kenyan Traditional Opaque Maize Beer. Chemische Mikrobiol. Technologie Lebensmittel 6 1980, 175, 182.
- Kadere, T. T. Baseline Survey, Biochemical, Microbial, and Technological Studies on “Mnazi’’. 2012.
- Wambugu, J. N. Characterization of Lactic Acid Bacteria Isolated from Coconut Wine (Mnazi) for Probiotic Potential; JKUAT, 2015.
- Njoki, W. J.; Boga, H. I.; Kutima, P.; Maina, M. J.; Kadere, T. T. Probiotic Potential of Lactic Acid Bacteria Isolated from Coconut (Cocos Nucifera) Wine (Mnazi) in Kenya. Int. J. Life Sci. Res. 2015, 3(1), 113–120.
- Kadere, T. Chemical Composition and Preservation of Mnazi and Its Distillate (Pyuwa). Eur. J. Nutr. Food Saf. 2021, 13(1), 46–58. DOI: 10.9734/ejnfs/2021/v13i130347.
- Kadere, T.; Oniang’o, R.; Kutima, P.; Muhoho, S. Traditional Tapping and Distillation Methods of Coconut Wine (Mnazi) As Practised in the Coastal Region of Kenya. Afr. J. Food Agric. Nutr. Dev. 2004, 4(1). DOI: 10.4314/ajfand.v4i1.19153.
- Wahome, J. N. Microbiological and Chemical Characterisation of the Traditional Manufacture of Muratina Wine; University of Nairobi: Nairobi, Kenya, 2003.
- Papas, R. K.; Sidle, J. E.; Wamalwa, E. S.; Okumu, T. O.; Bryant, K. L.; Goulet, J. L.; Maisto, S. A.; Braithwaite, R. S.; Justice, A. C. Estimating Alcohol Content of Traditional Brew in Western Kenya Using Culturally Relevant Methods: The Case for Cost Over Volume. Aids Behav. 2010, 14(4), 836–844. DOI: 10.1007/s10461-008-9492-z.
- Carey, K.; Kinney, J.; Eckman, M.; Nassar, A.; Mehta, K. Chang’aa Culture and Process: Detecting Contamination in a Killer Brew. Procedia Eng. 2015, 107, 395–402. DOI: 10.1016/j.proeng.2015.06.097.
- Mutisya, D.; Willis, J. Budget Drinking: Alcohol Consumption in Two Kenyan Towns. J. East. Afr. Stud. 2009, 3(1), 55–73. DOI: 10.1080/17531050802682770.
- Liu, L.; She, X.; Chen, X.; Qian, Y.; Tao, Y.; Li, Y.; Guo, S.; Xiang, W.; Liu, G.; Rao, Y. Microbiota Succession and Chemical Composition Involved in the Radish Fermentation Process in Different Containers. Front. Microbiol. 2020, 11, 445. DOI: 10.3389/fmicb.2020.00445.
- Groenenboom, A. E.; Shindano, J.; Cheepa, N.; Smid, E. J.; Schoustra, S. E. Microbial Population Dynamics During Traditional Production of Mabisi, a Spontaneous Fermented Milk Product from Zambia: A Field Trial. World J. Microbiol. Biotechnol. 2020, 36(12), 1–14. DOI: 10.1007/s11274-020-02957-5.
- Kalui, C. M.; Mathara, J. M.; Kutima, P. M. Probiotic Potential of Spontaneously Fermented Cereal Based Foods–A Review. Afr. J. Biotechnol. 2010, 9(17), 2490–2498.
- Holzapfel, W. Use of Starter Cultures in Fermentation on a Household Scale. Food Control 1997, 8(5–6), 241–258. DOI: 10.1016/S0956-7135(97)00017-0.
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two Faces of Fermented Foods—The Benefits and Threats of Its Consumption. Front. Microbiol. 2022, 13, 845166. DOI: 10.3389/fmicb.2022.845166.
- Scarsella, E.; Zecconi, A.; Cintio, M.; Stefanon, B. Characterization of Microbiome on Feces, Blood and Milk in Dairy Cows with Different Milk Leucocyte Pattern. Animals. 2021, 11(5), 1463. DOI: 10.3390/ani11051463.
- Cui, J.; Shi, C.; Xia, P.; Ning, K.; Xiang, H.; Xie, Q. Fermented Deer Blood Ameliorates Intense Exercise-Induced Fatigue via Modulating Small Intestine Microbiota and Metabolites in Mice. Nutrients. 2021, 13(5), 1543. DOI: 10.3390/nu13051543.
- Kurwinjila, R. Low Cost Optimization of the Flavor, Consistency and Keeping Quality of Fermented Milk with Particular Reference to Consumer Acceptability in Kenya; University of Nairobi: Nairobi, Kenya, 1980.
- Kurwijila, R. Maintenance of Ractivated, Lyophilised, Mixed Type Lactic Cultures by Subculturing and by Cold Storage. Indian J. Dairy Sci. 1983.
- van Hijum, S. A.; Vaughan, E. E.; Vogel, R. F. Application of State-Of-Art Sequencing Technologies to Indigenous Food Fermentations. Curr. Opin. Biotechnol. 2013, 24(2), 178–186. DOI: 10.1016/j.copbio.2012.08.004.
- Johansen, P. G.; Owusu-Kwarteng, J.; Parkouda, C.; Padonou, S. W.; Jespersen, L. Occurrence and Importance of Yeasts in Indigenous Fermented Food and Beverages Produced in Sub-Saharan Africa. Front. Microbiol. 2019, 10, 1789. DOI: 10.3389/fmicb.2019.01789.
- Loretan, T. The Diversity and Technological Properties of Yeasts from Indigenous Traditional South African Fermented Milks; University of the Free State: Bloemfontein, South Africa, 1999.
- Fleet, G. Yeasts in Dairy Products. J. Appl. Bacteriol. 1990, 68(3), 199–211. DOI: 10.1111/j.1365-2672.1990.tb02566.x.
- Gadaga, T.; Mutukumira, A.; Narvhus, J. Enumeration and Identification of Yeasts Isolated from Zimbabwean Traditional Fermented Milk. Int. Dairy J. 2000, 10(7), 459–466. DOI: 10.1016/S0958-6946(00)00070-4.
- Network, F. A. Management of Trees Used in Mursik (Fermented Milk) Production in Trans-Nzoia District, Kenya. J. Ethnobiol. 2000, 20(1), 75–91.
- Orech, F.; Schwarz, J. Ethno-Phytotherapeutic Remedies Used in Meat, Milk, and Blood Products by the Maasai People of Kenya. South African J. Bot. 2017, 108, 278–280. DOI: 10.1016/j.sajb.2016.10.026.
- Kurwijila, L. R.; Lore, T. A.; Omore, A. O. Technologies for Enhancing Value Addition, Quality and Safety of Milk Produced by Smallholder Farmers in Africa, 2005.
- Ashenafi, M. Effect of Container Smoking and Incubation Temperature on the Microbiological and Some Biochemical Qualities of Fermenting Ergo, a Traditional Ethiopian Sour Milk. Int. Dairy J. 1996, 6(1), 95–104. DOI: 10.1016/0958-6946(94)00037-9.
- Wanjala, N. W.; Matofari, J. W.; Nduko, J. M. Antimicrobial Effect of Smoking Milk Handling containers’ Inner Surfaces As a Preservation Method in Pastoral Systems in Kenya. Pastoralism 2016, 6(1), 1–7. DOI: 10.1186/s13570-016-0064-y.
- Chelule, P.; Mokoena, M.; Gqaleni, N. Advantages of Traditional Lactic Acid Bacteria Fermentation of Food in Africa. Cur. Res. Technol. Educat. Topics Appl. Microbiol. Microbial Biotechnol. 2010, 2, 1160–1167.
- Abdul Hakim, B. N.; Xuan, N. J.; Oslan, S. N. H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions As Functional Food in Dietetics and the Food Industry. Foods 2023, 12(15), 2850. DOI: 10.3390/foods12152850.
- Sanya, A. C.; Madode, Y. E.; Schoustra, S. E.; Smid, E. J.; Linnemann, A. R. Technological Variations, Microbial Diversity and Quality Characteristics of Maize Ogi Used for Akpan Production in Benin. Food Res. Int. 2023, 170, 113038. DOI: 10.1016/j.foodres.2023.113038.
- Sikombe, T. W.; Moonga, H. B.; Schoustra, S. E.; Shindano, J.; Stieger, M.; Smid, E. J.; Linnemann, A. R. Sensory Characteristics and Consumer Acceptability of Four Variants of Mabisi, a Traditionally Fermented Zambian Dairy Product. LWT 2023, 188, 115410. DOI: 10.1016/j.lwt.2023.115410.
- Chaudhari, A.; Dwivedi, M. K. The Concept of Probiotics, Prebiotics, Postbiotics, Synbiotics, Nutribiotics, and Pharmabiotics. In Probiotics in the Prevention and Management of Human Diseases; Elsevier: Amsterdam, Netherlands, 2022; pp. 1–11.
- Palma, M. L.; Zamith-Miranda, D.; Martins, F. S.; Bozza, F. A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E. T.; Douradinha, B. Probiotic Saccharomyces cerevisiae Strains As Biotherapeutic Tools: Is There Room for Improvement? Appl. Microbiol. Biotechnol. 2015, 99(16), 6563–6570. DOI: 10.1007/s00253-015-6776-x.
- Wolfe, B. E.; Dutton, R. J. Fermented Foods As Experimentally Tractable Microbial Ecosystems. Cell. 2015, 161(1), 49–55. DOI: 10.1016/j.cell.2015.02.034.
- Tamang, J. P.; Lama, S. Probiotic Properties of Yeasts in Traditional Fermented Foods and Beverages. J. Appl. Microbiol. 2022, 132(5), 3533–3542. DOI: 10.1111/jam.15467.
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and Potentially Probiotic Yeasts—Characteristics and Food Application. Foods 2021, 10(6), 1306. DOI: 10.3390/foods10061306.
- Wu, F.; Xie, X.; Du, T.; Jiang, X.; Miao, W.; Wang, T. Lactococcus lactis, a Bacterium with Probiotic Functions and Pathogenicity. World J. Microbiol. Biotechnol. 2023, 39(12), 325. DOI: 10.1007/s11274-023-03771-5.
- Wels, M.; Siezen, R.; Van Hijum, S.; Kelly, W. J.; Bachmann, H. Comparative Genome Analysis of Lactococcus lactis Indicates Niche Adaptation and Resolves Genotype/Phenotype Disparity. Front. Microbiol. 2019, 10, 4. DOI: 10.3389/fmicb.2019.00004.
- Wouters, J. T.; Ayad, E. H.; Hugenholtz, J.; Smit, G. Microbes from Raw Milk for Fermented Dairy Products. Int. Dairy J. 2002, 12(2–3), 91–109. DOI: 10.1016/S0958-6946(01)00151-0.
- Fernández, M.; Hudson, J. A.; Korpela, R.; de Los Reyes-Gavilán, C. G. Impact on Human Health of Microorganisms Present in Fermented Dairy Products: An Overview. Biomed Res. Int. 2015, 2015, 1–13. DOI: 10.1155/2015/412714.
- Enujiugha, V. N.; Badejo, A. A. Probiotic Potentials of Cereal-Based Beverages. Crit. Rev. Food Sci. Nutr. 2017, 57(4), 790–804. DOI: 10.1080/10408398.2014.930018.
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 1000517. DOI: 10.3389/fnut.2022.1000517.
- Gibson, G. R.; Hutkins, R.; Sanders, M. E.; Prescott, S. L.; Reimer, R. A.; Salminen, S. J.; Scott, K.; Stanton, C.; Swanson, K. S.; Cani, P. D. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14(8), 491–502. DOI: 10.1038/nrgastro.2017.75.
- Holzapfel, W. H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and Important Features of Probiotic Microorganisms in Food and Nutrition. Am. J. Clin. Nutr. 2001, 73(2), 365s–373s. DOI: 10.1093/ajcn/73.2.365s.
- Pandey, K. R.; Naik, S. R.; Vakil, B. V. Probiotics, Prebiotics and Synbiotics-A Review. J. Food Sci. Technol. 2015, 52(12), 7577–7587. DOI: 10.1007/s13197-015-1921-1.
- Saarela, M.; Mogensen, G.; Fonden, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic Bacteria: Safety, Functional and Technological Properties. J. Biotechnol. 2000, 84(3), 197–215. DOI: 10.1016/S0168-1656(00)00375-8.
- Deepak, T. S.; Jayadeep, P. A. Prospects of Maize (Corn) Wet Milling By-Products As a Source of Functional Food Ingredients and Nutraceuticals. Food Technol. Biotechnol. 2022, 60(1), 109–120. DOI: 10.17113/ftb.60.01.22.7340.
- Nyyssölä, A.; Ellilä, S.; Nordlund, E.; Poutanen, K. Reduction of FODMAP Content by Bioprocessing. Trends Food Sci. Technol. 2020, 99, 257–272. DOI: 10.1016/j.tifs.2020.03.004.
- Gao, Z.; Daliri, E. B.-M.; Wang, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Inhibitory Effect of Lactic Acid Bacteria on Foodborne Pathogens: A Review. J. Food Prot. 2019, 82(3), 441–453. DOI: 10.4315/0362-028X.JFP-18-303.
- MacDonald, R.; Reitmeier, C. Understanding Food Systems: Agriculture, Food Science, and Nutrition in the United States; Academic Press: Cambridge, Massachusetts, USA, 2017.
- Byakika, S.; Mukisa, I. M.; Mugabi, R.; Muyanja, C. Antimicrobial Activity of Lactic Acid Bacteria Starters Against Acid Tolerant, Antibiotic Resistant, and Potentially Virulent E. Coli Isolated from a Fermented Sorghum-Millet Beverage. Int. J. Microbiol. 2019, 2019, 1–10. DOI: 10.1155/2019/2013539.
- Oyelana, O.; Coker, A. Microbial Contamination at Different Stages of Production of Ogi in Mowe: A Rural Community, Southwest, Nigeria. Bacteriol. J. 2012, 2(1), 1–11. DOI: 10.3923/bj.2012.1.11.
- Oyedeji, A. B.; Green, E.; Jeff-Agboola, Y. A.; Olanbiwoninu, A. A.; Areo, E.; Martins, I. E.; El-Imam, A. M.; Adebo, O. A. Presence of Pathogenic Microorganisms in Fermented Foods. In Indigenous Fermented Foods for the Tropics; Elsevier: Amsterdam, Netherlands, 2023; pp. 519–537.
- Byakika, S.; Mukisa, I. M.; Byaruhanga, Y. B.; Male, D.; Muyanja, C. Influence of Food Safety Knowledge, Attitudes and Practices of Processors on Microbiological Quality of Commercially Produced Traditional Fermented Cereal Beverages, a Case of Obushera in Kampala. Food Control 2019, 100, 212–219. DOI: 10.1016/j.foodcont.2019.01.024.
- Kroll, R.; Patchett, R. Induced Acid Tolerance in Listeria Monocytogenes. Lett Appl. Microbiol. 1992, 14(5), 224–227. DOI: 10.1111/j.1472-765X.1992.tb00691.x.
- Inatsu, Y.; Bari, M.; Kawasaki, S.; Isshiki, K. Survival of Escherichia coli O157: H7, Salmonella Enteritidis, Staphylococcus Aureus, and Listeria Monocytogenes in Kimchi. J. Food Prot. 2004, 67(7), 1–1497. DOI: 10.4315/0362-028X-67.7.1497.
- Tosun, H.; Seckin, A. K.; Gönül, Ş. A. Acid Adaptation Effect on Survival of Escherichia coli O157: H7 in Fermented Milk Products. Turk. J. Vet. Anim. Sci. 2007, 31(1), 61–66.
- Wafula, E. N.; Muhonja, C. N.; Kuja, J. O.; Owaga, E. E.; Makonde, H. M.; Mathara, J. M.; Kimani, V. W.; Ren, Z. Lactic Acid Bacteria from African Fermented Cereal-Based Products: Potential Biological Control Agents for Mycotoxins in Kenya. J. Toxicol. 2022, 2022, 1–17. DOI: 10.1155/2022/2397767.
- Omara, T.; Kiprop, A. K.; Wangila, P.; Wacoo, A. P.; Kagoya, S.; Nteziyaremye, P.; Peter Odero, M.; Kiwanuka Nakiguli, C.; Baker Obakiro, S.; Patarata, L. The Scourge of Aflatoxins in Kenya: A 60-Year Review (1960 to 2020). J. Food Qual. 2021, 2021, 1–31. DOI: 10.1155/2021/8899839.
- Becker‐Algeri, T. A.; Castagnaro, D.; de Bortoli, K.; de Souza, C.; Drunkler, D. A.; Badiale‐Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81(3), R544–R552. DOI: 10.1111/1750-3841.13204.
- El-Sayed, A. S.; Ibrahim, H.; Farag, M. A. Detection of Potential Microbial Contaminants and Their Toxins in Fermented Dairy Products: A Comprehensive Review. Food Anal. Methods 2022, 15(7), 1880–1898. DOI: 10.1007/s12161-022-02253-y.
- Owusu-Kwarteng, J.; Akabanda, F.; Agyei, D.; Jespersen, L. Microbial Safety of Milk Production and Fermented Dairy Products in Africa. Microorganisms 2020, 8(5), 752. DOI: 10.3390/microorganisms8050752.
- Byakika, S.; Mukisa, I. M.; Wacoo, A. P.; Kort, R.; Byaruhanga, Y. B.; Muyanja, C. Potential Application of Lactic Acid Starters in the Reduction of Aflatoxin Contamination in Fermented Sorghum-Millet Beverages. Int. J. Food Contam. 2019, 6(1), 1–8. DOI: 10.1186/s40550-019-0074-9.
- Shetty, P. H.; Jespersen, L. Saccharomyces cerevisiae and Lactic Acid Bacteria as Potential Mycotoxin Decontaminating Agents. Trends Food Sci. Technol. 2006, 17(2), 48–55. DOI: 10.1016/j.tifs.2005.10.004.
- Authority, E. F. S. Scientific Opinion on Establishing Food-Based Dietary Guidelines. Efsa J. 2010, 8(3), 1460.
- Mohajan, H. Food and Nutrition Scenario of Kenya, 2014.
- Dietz, A.; Foeken, D.; Soeters, S.; Klaver, W.; Akinyoade, A.; Leliveld, A.; Smits, H.; van’t Wout, M. Agricultural Dynamics and Food Security Trends in Kenya. Agric. Dynam. Food Secur. Trends Kenya 2014, (4).
- Nduko, J. M.; Matofari, J. W.; Nandi, Z. O.; Sichangi, M. B. Spontaneously Fermented Kenyan Milk Products: A Review of the Current State and Future Perspectives, 2016.
- Maundu, P.; Achigan-Dako, E.; Morimoto, Y. Biodiversity of African Vegetables. In African indigenous vegetables in urban agriculture; Routledge: London, 2009; pp. 97–136.
- Shibia, M.; Rahman, S.; Chidmi, B. Consumer Demand for Meat in Kenya: An Examination of the Linear Approximate Almost Ideal Demand System, 2017.
- Muunda, E.; Mtimet, N.; Bett, E.; Wanyoike, F.; Alonso, S. Milk Purchase and Consumption Patterns in Peri-Urban Low-Income Households in Kenya. Front. Sustain. Food Syst. 2023, 7, 54. DOI: 10.3389/fsufs.2023.1084067.
- Bergevoet, R.; Engelen, A. v The Kenyan Meat Sector Opportunities for Dutch Agribusiness; LEI Wageningen UR: Den Haag, Netherlands, 2014.
- Vila‐Real, C. P. D. M.; Pimenta‐Martins, A. S.; Kunyanga, C. N.; Mbugua, S. K.; Katina, K.; Maina, N. H.; Gomes, A. M. P.; Pinto, E. C. B. Nutritional Intake and Food Sources in an Adult Urban Kenyan Population. Nutr. Bull. 2022, 47(4), 423–437. DOI: 10.1111/nbu.12582.
- Blackmore, E.; Guarin, A.; Vorley, W.; Alonso, S.; Grace, D. Kenya’s Informal Milk Markets and the Regulation–Reality Gap. Dev. Policy Rev. 2022, 40(3), e12581. DOI: 10.1111/dpr.12581.
- KNBS. Enhanced Food Balance Sheets for Kenya. 2023. https://www.knbs.or.ke/download-category/enhanced-food-balance-sheets-for-kenya/.
- ArcGISOnline. Food Balance Sheet: Kenya Crop Conditions Bullettin. https://www.arcgis.com/apps/dashboards/270b617893a141d9aed87c33c9685017.
- Bebe, B. O.; Rademaker, C.; van der Lee, J.; Kilelu, C.; Tonui, C. Sustainable Growth of the Kenyan Dairy Sector: A Quick Scan of Robustness, Reliability and Resilience: Executive Summary; Wageningen Livestock Research: Wageningen, Netherlands, 2017.
- Wairimu, E.; Mburu, J.; Gachuiri, C. K.; Ndambi, A. Characterization of Dairy Innovations in Selected Milksheds in Kenya Using a Categorical Principal Component Analysis. Trop. Anim. Health Prod. 2021, 53(2), 1–12. DOI: 10.1007/s11250-021-02596-4.
- Migose, S. A. Addressing Variation in Smallholder Farming Systems to Improve Dairy Development in Kenya; Wageningen University and Research: Wageningen, Netherlands, 2020.
- Rademaker, C. J.; Bebe, B. O.; Van Der Lee, J.; Kilelu, C.; Tonui, C. Sustainable Growth of the Kenyan Dairy Sector: A Quick Scan of Robustness, Reliability and Resilience; Wageningen University & Research, Wageningen Livestock Research: Wageningen, Netherlands, 2016.
- Alonso, S.; Angel, M. D.; Muunda, E.; Kilonzi, E.; Palloni, G.; Grace, D.; Leroy, J. L. Consumer Demand for Milk and the Informal Dairy Sector Amidst COVID-19 in Nairobi, Kenya. Curr. Dev. Nutr. 2023, 7(4), 100058. DOI: 10.1016/j.cdnut.2023.100058.
- Oselu, S.; Ebere, R.; Arimi, J. M.; Amante, E. Camels, Camel Milk, and Camel Milk Product Situation in Kenya in Relation to the World. Int. J. Food Sci. 2022, 2022, 1–15. DOI: 10.1155/2022/1237423.
- FAOSTAT, F. Food and Agriculture Organization Corporate Statistical Database. FAO Online Database; 2016. http://www.fao.org/faostat/en/#data (accessed Feb. 5, 2019).
- Njarui, D. M.; Gatheru, M.; Wambua, J. M.; Nguluu, S. N.; Mwangi, D. M.; Keya, G. A. Consumption Patterns and Preference of Milk and Milk Products Among Rural and Urban Consumers in Semi-Arid Kenya. Ecol. Food Nutr. 2011, 50(3), 240–262. DOI: 10.1080/03670244.2011.568908.
- Knoll, N.; Kuhnt, K.; Kyallo, F. M.; Kiage-Mokua, B. N.; Jahreis, G. High Content of Long-Chain N-3 Polyunsaturated Fatty Acids in Red Blood Cells of Kenyan Maasai Despite Low Dietary Intake. Lipids Health Dis. 2011, 10(1), 1–9. DOI: 10.1186/1476-511X-10-141.
- Lindell, J. Maasai Herding and Milking Strategies, 2013.
- Mtimet, N.; Karugia, J. T. Consumer Perception of Milk Safety in Kenya, 2020.
- Mathara, J.; Miyamoto, T.; Koaze, H. Production of Traditional Fermented Milk in Kenya (A Review); Shizuoka Prefectural University Publication: Shizuoka, Japan, 1995; pp 257–264.
- Muinde, A. Popular Milk, Mursik Now in Shops. Jun. 14, 2011, 2016.
- Kamau, G. G. Investigation on Entrepreneurial Viability of Indigenous Innovations in Kenya. A Survey of Baringo and Nakuru Counties on Mursik Milk, 2018.
- Akweya, B.; Gitao, C.; Okoth, M. The Acceptability of Camel Milk and Milk Products from North Eastern Province in Some Urban Areas of Kenya. Afr. J. Food Sci. 2012, 6(19), 465–473.
- Ogolla, J. A.; Dede, C.; Okoth, M. W.; Hensel, O.; Sturm, B. Strategies and Technologies for Camel Milk Preservation and Utilization of Non-Marketed Milk in Arid and Semi-Arid Areas. East African Agric. For. J. 2017, 82(2–4), 144–167. DOI: 10.1080/00128325.2017.1363686.
- Macauley, H.; Ramadjita, T. Cereal Crops: Rice, Maize, Millet, Sorghum, Wheat, 2015.
- Rapando, P. L.; Serrem, C. A.; Serem, D. J. Effect of Soy Fortification on the Quality of Mkarango a Traditional Kenyan Fermented Maize Meal Snack. Food Sci. Nutr. 2020, 8(9), 5007–5016. DOI: 10.1002/fsn3.1798.
- Tarus, C. Maize Crisis: A Position Paper on Strategies for Addressing Challenges Facing Maize Farming in Kenya. East Afr. Scholars Publisher 2019, 2(3), 149–158.
- TheAfricaReport. Kenya 2022: The Electoral Politics of Maize-Meal Shortages. https://www.theafricareport.com/228263/kenya-2022-the-electoral-politics-of-maize-meal-shortages/.
- Orr, A.; Weltzien, E.; Rattunde, F. Research and Development for Sorghum and Millets in Sub-Saharan Africa: What Have We Learned? Outlook Agric. 2022, 51(4), 435–447. DOI: 10.1177/00307270221133127.
- Handschuch, C.; Wollni, M. Improved Production Systems for Traditional Food Crops: The Case of Finger Millet in Western Kenya. Food Secur. 2016, 8(4), 783–797. DOI: 10.1007/s12571-016-0577-7.
- Adavachi, W. M. The Role of Fermented Maize-Based Products on Nutrition Status and Morbidity of Children 6-59 Months Old in Western Kenya; University of Nairobi: Nairobi, Kenya, 2017.
- Achi, O. K.; Asamudo, N. U. Cereal-Based Fermented Foods of Africa As Functional Foods. In Bioactive Molecules in Food, Mérillon, J.-M. and Ramawat, K.G., Eds.; Springer International Publishing, 2019; pp. 1527–1558.
