ABSTRACT
Although dyslexia is characterized by a deficit in phonological representations, the nature of this deficit is debated. Previously, it was shown that adults with dyslexia respond differently to online manipulations of auditory feedback. In the present study, we found that individual differences in reading and reading-related skills within a group of 30 children (10–13 years old) with dyslexia were associated with the response to altered feedback. The fractional anisotropy of the arcuate fasciculus/superior longitudinal fasciculus was not directly related to the response to altered feedback. This study corroborates that speech perception-production communication is important for phonological representations and reading.
Introduction
Developmental dyslexia is characterized by persistent difficulties in accurate and fluent word reading and has a neurobiological basis (Lyon, Shaywitz, & Shaywitz, 2003). One of the main deficits in dyslexia is thought to be an impairment in the quality of phonological representations. These impaired phonological representations are often hypothesized to hinder the formation of fast, stable, and automatized connections between phonology and orthography (Boada & Pennington, Citation2006; Snowling, Citation1981; Sprugevica & Høien, Citation2003). A relatively direct way to measure phonological representations is by examining speech perception and production abilities. With respect to speech perception, individuals with dyslexia show weaker categorical perception of phonemes (Noordenbos & Serniclaes, Citation2015). and are also reported to have hyper-sensitive within-phoneme-category perception (Serniclaes, Van Heghe, Mousty, Carré, & Sprenger-Charolles, Citation2004). However, some researchers did not find perception deficits in dyslexia (Law, Vandermosten, Ghesquiere, & Wouters, Citation2014), or argued that a phonological deficit is secondary to a general auditory deficit (Hakvoort et al., Citation2016; Hakvoort, Van Der Leij, Maurits, Maassen, & Van Zuijen, Citation2015; Tallal, Miller, & Fitch, Citation1993). Others even questioned the existence of speech perception deficits and pointed to, for instance, attentional limitations in dyslexia (Ramus & Szenkovits, Citation2008). More recently, the phonological access hypothesis has been proposed, stating that individuals with dyslexia have adequate phonological representations but show difficulties in consciously accessing and manipulating these representations (Boets et al., Citation2013; Ramus & Szenkovits, Citation2008). In contrast to speech perception, speech production—a relatively direct way to measure phonological representations—has only received scant attention in dyslexia. However, the studies that have been conducted suggest that individuals with dyslexia show impairments in articulatory and oral motor skills (Elbro, Borstrom, & Petersen, Citation1998; Malek, Amiri, Hekmati, Pirzadeh, & Gholizadeh, Citation2013). In summary, many studies investigated phonological deficits in dyslexia by measuring performance on metacognitive tests (e.g. phonological awareness) or by examining speech perception, and a few by probing speech production. Most of these studies point to a deficit in phonological representations, although some do not find speech perception and/or production deficits, or hypothesize that the findings are better explained by limited access to phonological representations.
In a separate literature, however, work on neurocomputational models of speech motor control suggest that the quality of phonological representations hinges on the interaction between speech production (i.e. feed-forward) and speech perception (i.e. feedback) mechanisms (Tourville & Guenther, Citation2011). On a neural level, this interaction between speech production and sensory areas is hypothesized to be facilitated by a white matter tract that connects the involved temporo-parietal (for speech perception) and frontal (for speech production) areas: the arcuate fasciculus/superior longitudinal fasciculus. The present study, therefore, examined the nature of the phonological deficit in children with dyslexia, by directly probing dynamic interactions between speech perception and speech production mechanisms, using both behavioral (response to altered auditory feedback) and neuroimaging (fractional anisotropy, a measure of white matter organization, in the arcuate fasciculus/superior longitudinal fasciculus) measures. We will first describe what neurocomputational models of speech perception and production say about phonological representations. Then, it is explained how measuring the response to altered auditory feedback helps to measure phonological representations and what already has been found in adults with dyslexia. Third, the role of the arcuate fasciculus in speech feed-forward and feedback mechanisms are described. Finally, we will explain the role of these aspects in the current study.
Neurocomputational models of speech perception and speech production
According to neurocomputational models of speech production, a phonological representation is associated with a feed-forward and a feedback stream (Guenther, Ghost, & Tourville, Citation2006). The feed-forward stream maps the motor representations—hypothesized to be stored in the left ventral premotor cortex—of a phoneme onto the motor effectors, while feedback mechanisms (superior temporal and somatosensory/inferior parietal areas) monitor whether the output of the feed-forward trace matches the predicted auditory and somatosensory consequences (Guenther et al., Citation2006). Once a feedback monitoring mechanism detects a mismatch between the produced and intended speech production, a corrective signal is sent to the motor cortex to repair the mistake and potentially update the feed-forward representation of a phoneme (Houde & Nagarajan, Citation2011). As this feedback control is a slow and inefficient process, the feedback trace should largely disengage to optimize the computational costs of speech production once feed-forward commands are well-defined (Guenther et al., Citation2006). In addition, if corrective feedback signals are sent, they should be implemented slowly in the feed-forward system to avoid an unstable motor system (Houde & Nagarajan, Citation2011). These feedback mechanisms are critically involved in learning and maintaining speech abilities. For instance, deaf and hard-of-hearing children—for whom auditory feedback is not or only partially available—have significant difficulties in acquiring adequate speech production skills (Smith, Citation1975). Also in late adulthood, the quality of speech production is related to sensory feedback (Lane et al., Citation1997). Measuring the integrity and stability of speech feed-forward and feedback mechanisms in individuals with and without dyslexia may help to further understand the nature of the phonological deficit.
Altered auditory feedback and dyslexia
The interaction between speech feed-forward and feedback mechanisms is often measured by an online modification of the auditory feedback someone receives while speaking (Scheerer, Jacobson, & Jones, Citation2016). In these experiments, participants are usually asked to repeatedly produce a syllable, while being recorded. In some instances, the auditory feedback is modified in such a way that, for example, the frequency of the first formant is manipulated and fed back in real-time via headphones. As a result, participants hear the speech they produced, but the vowel does not sound exactly as intended. The changes in speech production in response to these manipulations reflect how speech perception is used to alter speech production. Although participants are usually not aware of the manipulation, they do typically respond by changing their speech in the opposite direction of the manipulation (Purcell & Munhall, Citation2006), but large individual differences exist (Lametti et al., 2012). Factors thought to influence these individual differences in the response to altered auditory feedback include: the strength of the manipulation (Niziolek & Guenther, Citation2013), the developmental phase of the participants (e.g. very young children do not adapt as strongly as adults; MacDonald, Johnson, Forsythe, Plante, & Munhall, Citation2012; Scheerer et al., Citation2016), and the shape of the participants’ vowel space (Niziolek, Nagarajan, & Houde, 2013).
Recently, an auditory feedback paradigm was used in Dutch adults with and without dyslexia to examine whether speech perception-production interactions are affected in people with dyslexia (Van Den Bunt et al., Citation2017). In that study, participants were asked to repeatedly produce the nonword /bɛp/ while the frequency of the first formant of the /ɛ/ sound was unaltered in the baseline phase, gradually manipulated to a 25% increase during the ramp phase, held at maximum (25%) during the hold phase, and again unaltered in the after-effect phase. It was found that adults with dyslexia showed a larger deviation from the baseline production in the ramp-up phase, and a weaker de-adaptation to the baseline in the after-effect phase than typically reading adults. These results were interpreted in light of the “perceptual magnet” theory (Feldman, Griffiths, & Morgan, Citation2009; Kuhl, Citation1991), which claims that a phonetic category prototype functions as a magnet that results in relatively poorer discriminability for neighboring stimuli close to the prototype and better discriminability for stimuli that are farther away from the prototype. With respect to the response to altered auditory feedback in dyslexia, a weaker magnet could increase the response to alterations in altered auditory feedback (when the percept deviates from the phonetic category prototype) and reduce the ability to reestablish the representations when feedback is back to normal (Van Den Bunt et al., Citation2017). Although these findings indicate that adults with dyslexia respond differently to altered auditory feedback—which might indicate an impairment in speech feed-forward and feedback mechanisms—several issues remain: 1) To what extent is a stronger response to altered auditory feedback characteristic of children, as it was found to be of adults, with dyslexia; 2) How does the response to altered auditory feedback relate to individual differences in reading and reading-related skills.
Regarding the first issue, participants with dyslexia of the previous study were university students, who—by definition—must have found ways to compensate for their reading deficit. Therefore, it is unclear whether children with dyslexia also show evidence for a weaker magnet associated with phonological representations by responding more strongly to altered auditory feedback. This is especially relevant, as feedback control is thought to be particularly important for the formation and establishment of phonological representations during childhood (Guenther et al., Citation2006). Regarding the second issue, an important follow-up question is how the response to altered auditory feedback relates to individual differences in reading and reading-related skills in children with dyslexia. Dyslexia is a heterogeneous disorder, and children with dyslexia differ in the severity and persistence of the disorder, as well as in the underlying cognitive deficits (e.g. phonological awareness, rapid naming). Administering altered auditory feedback to children from primary schools who participated in a dyslexia treatment training allows us to examine whether the severity and persistence of dyslexia is related to speech perception-production interaction. With respect to the associated cognitive deficits, the literature often distinguishes between phonological awareness—particularly associated with reading accuracy—and naming speed—particularly associated with reading fluency (Nelson, Citation2015). The precise role of these cognitive abilities is debated in a transparent orthography, in which the letter-sound couplings are highly consistent (Borgwaldt, Hellwig, & De Groot, Citation2005). A number of studies argue that in transparent orthographies the role of phonological awareness in reading development is relatively small (Georgiou, Parrila, & Papadopoulos, Citation2008; Share, Citation2008) and that its role further decreases over the course of development (De Jong & Van Der Leij, Citation2003). In contrast, rapid naming appears to be a stable long-term predictor of reading abilities in transparent orthographies (Furnes & Samuelsson, Citation2011). Relating the response to altered auditory feedback to individual differences across reading and reading-related abilities could shed more light on whether and how the interaction between speech perception and production is related to reading difficulties.
The neural basis of reading and the role of the arcuate fasciculus/superior longitudinal fasciculus
Fluent reading is often related to adequate functioning of two specialized left-hemisphere networks: The “dorsal” temporo-parietal network, which is classically related to phonological processing and articulation, and the “ventral” occipital-frontal network, which is involved in the mapping from visual representations of words onto meaning (Pugh et al., Citation2000). This former network is of particular importance for the interaction between speech perception and speech production. The temporo-parietal areas include the primary auditory cortex (i.e. Wernicke’s area)—an area crucially involved in the perception and processing of speech input (Geschwind, Citation1982). Hypoactivation of these temporo-parietal areas has often been reported in people with dyslexia (Shaywitz et al., Citation2002). The anterior part of this network includes the (pre)motor areas and left inferior frontal gyrus, and these areas are frequently reported to be involved in grapheme-to-phoneme correspondences and articulation (Long et al., Citation2016; Pugh et al., Citation2000).
These temporo-parietal and (inferior) frontal areas are interconnected by the arcuate fasciculus (AF), a white matter bundle adjacent (Schmahmann & Pandya, Citation2006) or part of (Kamali et al., 2014) the Superior Longitudinal Fasciculus (SLF), which makes the AF/SLF a logical choice as a tract to focus on in the context of the research questions of the current study. The AF/SLF is classically thought to be involved in the sensorimotor control of speech. For instance, conduction aphasia, characterized by difficulties in speech repetition while speech perception and production as such are intact, is often related to deficiencies in the fractional anisotropy in the AF/SLF (Catani & Mesulam, Citation2008; but see Bernal & Ardila, Citation2009). This is usually taken as evidence of impaired communication between the auditory cortex and speech motor areas. Importantly, an electrocorticography study showed that communication along the AF/SLF is indeed bidirectional (Matsumoto et al., Citation2004). Communication from motor and inferior frontal areas to (auditory) sensory areas in this way concurs with the proposed neurocomputational models of speech feed-forward and feedback mechanisms (Guenther et al., Citation2006), in which an afferent copy of the motor commands to the articulators is sent to sensory areas to compare intended speech with the produced speech.
Additionally, the AF/SLF is also one of the most frequently mentioned neural structures related to dyslexia (Andrews et al., Citation2010; Steinbrink et al., Citation2008; Vandermosten et al., Citation2012). Many studies have reported a reduction in fractional anisotropy in the left AF/SLF in people with dyslexia (Gullick & Booth, Citation2015; Langer et al., Citation2015; Vandermosten et al., Citation2012). However, others report a bilateral reduction of fractional anisotropy of the AF/SLF (Lebel et al., Citation2013; Steinbrink et al., Citation2008), or failed to find any difference between individuals with and without dyslexia (Andrews et al., Citation2010; Dougherty et al., Citation2007; Rollins et al., Citation2009). The observed group differences in the arcuate fasciculus are an indication that the AF/SLF is involved in reading and/or reading-related abilities. Vandermosten and colleagues (Citation2015) showed that individual differences in phonological skills correlated with the fractional anisotropy of several parts of the AF/SLF, suggesting that this could be an underlying mechanism of how the AF/SLF is related to reading skills. Relating the fractional anisotropy in the AF/SLF to the response to altered auditory feedback could clarify its role in speech perception/production interaction and consequently, in reading ability.
The present study
The primary purpose of the present study was to examine how individual differences in children with dyslexia are related to the response to altered auditory feedback. However, we first examined whether children with dyslexia, when compared to typically reading peers, showed deficiencies in speech perception-production interaction. Second, we examined whether and how individual differences in the severity (i.e. reading fluency) and persistence (i.e. response to intervention) of the reading deficit and underlying cognitive deficits (phonological awareness and rapid naming) in children with dyslexia were associated with speech perception-production interaction. Third, we examined whether and how deficiencies in this perception-production interaction were associated with differences in neuroanatomy, more specifically, to differences in the AF/SLF. We did so in the transparent orthography of Dutch, in which the relations between phonemes and graphemes are relatively straightforward.
The interaction between speech perception and production was measured using an altered auditory feedback paradigm, which was designed to elicit a response in all participants. Based on previous research in adults (Van Den Bunt et al., Citation2017), we hypothesized that children with dyslexia show a weaker perceptual magnet which results in a stronger response to altered auditory feedback and a weaker return to baseline when the feedback becomes unaltered again. More specifically, the following hypotheses were formulated: First, a stronger adaptation was expected for the children with dyslexia, compared with typically reading controls. Second, within the group of children with dyslexia only, we hypothesized that stronger adaptation and weaker de-adaptation is associated with the severity and persistence of the disorder (reading ability and response to intervention, respectively) and its associated cognitive deficits (i.e. phonological awareness and rapid naming). Specifically, lower reading ability and less response to intervention, as well as poorer rapid naming and phonological awareness skills were hypothesized to be associated with a stronger adaptation and weaker de-adaptation to altered auditory feedback. Third, the hypotheses for the role of the AF/SLF were harder to explicate. Conceptually, two relations in opposite directions can be envisioned between the fractional anisotropy in the AF/SLF and the response to altered auditory feedback. Higher functional anisotropy of the AF/SLF could facilitate the communication between speech perception and production areas and might therefore result in a stronger response to altered auditory feedback. However, adequate communication along the AF/SLF could also lead to more stable feed-forward commands and hence to more reluctance to change them as auditory feedback temporarily changes, resulting in weaker response to altered auditory feedback. In light of two earlier reported findings in people with dyslexia, that (1) dyslexia is characterized by a stronger response to altered feedback (Van Den Bunt et al., Citation2017), and (2) reports of a reduced fractional anisotropy of the AF/SLF in individuals with dyslexia (Lebel & Beaulieu, Citation2009; Vandermosten et al., Citation2012), we might expect that lower fractional anisotropy is associated with a stronger response to altered feedback.
Methods
Participants
Thirty children with dyslexia and 10 children without dyslexia were recruited to participate in an fMRI study about the neural underpinnings of response to dyslexia treatment. The data from three children with dyslexia were excluded from further analyses: in two cases, the software running the key experiment (altered auditory feedback) crashed; a third participant did not comply with the task instruction to speak within the scope of the microphone. The final sample thus consisted of 27, native Dutch, children with dyslexia (Mage = 12.31; SDage = 0.78) and 10 children with typical reading skills (Mage = 12.08; SDage = 0.76). Neuroimaging data were available for 24 participants with dyslexia and nine children with typical reading skills. Since the study primarily aimed to explain individual differences within dyslexia, the recruitment focused on children with an official diagnosis of dyslexia. To confirm the previous study with adults with and without dyslexia, a small number of typically reading individuals was also recruited. Children were recruited in several different ways: most children with dyslexia and typical readers already took part in a large longitudinal project on the evaluation of dyslexia treatment in collaboration with a dyslexia treatment provider in the Netherlands (Marant, Elst, The Netherlands) and were invited to an additional test session. Additionally, 15 children with dyslexia were approached via the same clinical partner, but did not participate in the larger study.
Finally, five typically reading children were recruited via flyers sent around to mainstream schools in the Netherlands. To be included in the typical reading group, children had to be in the age range of 10–13 years, attend a regular school, and no history of reading difficulties. All parents provided active informed consent for participation of their child in the current study, as well as access to the raw reading-related scores gathered before, during, and after the dyslexia treatment—in case of children with dyslexia. The children received a small monetary gift for their participation and travel expenses were reimbursed. The study was approved by the local medical ethical committee. Participant characteristics are provided in .
Table 1. Participant characteristics.
To be included in the group of dyslexic readers, participants had to have an official dyslexia diagnosis for which they underwent a standardized phonics-based treatment, available through the general health-care system in the Netherlands at the collaborating dyslexia treatment provider. This dyslexia treatment was only available to children in whom possible comorbid disorders were not present or sufficiently under control through drug medication. A further inclusion criterion for the dyslexia group was that the children had completed the dyslexia treatment program.
Diagnostic and intervention procedures were highly similar for all children and followed a nationally standardized protocol (Blomert, Citation2006). Every child that scored below the 10th percentile on reading measurements at three consecutive time-points in grade one and two was referred to a dyslexia center for an official diagnostic examination. The dyslexia diagnosis was based on a reading score of 1.5 standard deviations below average on standardized reading tests and 1.5 standard deviations below average on letter knowledge, phonological awareness or rapid naming (Blomert, Citation2006). If the child indeed was diagnosed with dyslexia, he/she was referred for a phonics-based dyslexia treatment. The treatment consists of 50 individual, 45-minute sessions and takes place at the school of the child. The first 12 sessions are aimed at establishing adequate letter-sound associations using primarily monosyllabic words (Tilanus, Segers, & Verhoeven, Citation2016). The remaining sessions are aimed at learning exception rules and speeded reading. During an intervention session, children first repeated the grapheme-phoneme association or exception rule of the week before, practiced the new rule, and practiced word reading fluency with word naming and repeated (text) reading exercises. They also received homework assignments for reading (four times 20 minutes a week) and spelling (two times 10 minutes a week). Reading was assessed before, during (after 12, 36 and 48 weeks), and after treatment.
Materials
Reading ability
The ability to read words was assessed with a standardized word reading test, the Een-Minuut-Test [One-Minute-Test] (Brus & Voeten, Citation1973). This test consisted of a list of 116 printed words of increasing difficulty for which participants were asked to read as many words out loud as possible in one minute, without making any errors. The score for word reading consisted of the total number correctly read words within the time limit.
Phonological awareness
Phonological awareness (PA) was measured using two subtests of the Dyslexie Screening Test [Dyslexia Screening Test] (Kort et al., Citation2005). The first subtest was phoneme deletion in which the child was asked to repeat a word while omitting a specific sound (e.g. say vlag [flag] without the /v/, answer lag [lay], most correct responses were nonwords). Maximum score was 16 correct items. The second subtest consisted of 11 spoonerisms (say “Harry Potter” but switch the first sounds; e.g. “Parry Hotter”). Having all items correct resulted in the maximum score of 11. The standardized scores of both subtests were averaged for further analyses.
Rapid automatized naming
Rapid automatized naming was measured using the letters and digit cards of the Continue Benoemen & Woorden Lezen [Continuous Naming and Word Reading] test (Van Den Bos & Lutje Spelberg, Citation2014). The participant was asked to name, as fast as possible, five 10-item rows with five unique items of either letters or digits. The total time in seconds for each card was used as the score for rapid automatized naming. The standardized scores of both subtests were averaged for further analyses.
Altered auditory feedback
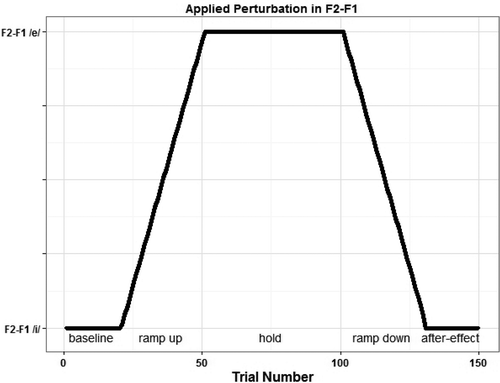
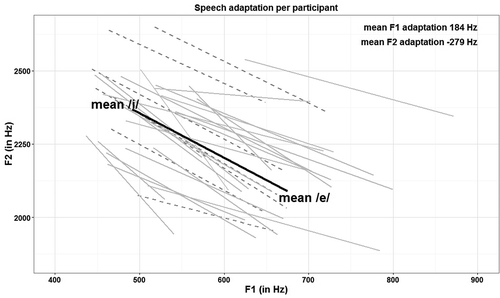
The Altered Auditory Feedback task was programmed using the Audapter software (Cai, Ghosh, Guenther, & Perkell, Citation2008; Tourville, Cai, & Guenther, Citation2013) and an external audio-card (Roland UA-25 EX, Hamamatsu, Japan). The audapter software allows to set formant adaptations for the first and second formants simultaneously. Speech productions were recorded at 48 kHz and downsampled to 16 kHz to reduce the computational load. Recording the speech signal and feeding it back occurred almost in realtime (< 11 ms). To obtain a response from all participants, we decided to tailor the adaptation to the participants’ individual vowel space. For this purpose, we first measured the /ɩ/ and the /ɛ/ vowel for each participant and then set the manipulation parameters for the altered feedback individually, resulting in a complete /ɩ/ to /ɛ/ change. Participants were first asked to say the word /bɩp/ 20 times, guided by a computer paced rhythm, once every three seconds. Then, similarly, the participant was asked to produce the word /bɛp/ 20 times. The last five /bɩp/ and /bɛp/ productions were used to determine the frequency of the first and second formants of both vowels in each participant. After this calculation, the parameters of the experiment were set individually in such a way that maximal perturbation meant a change from /bɩp/ to /bɛp/ in each participant. The baseline productions of the /ɩ/ and /ɛ/ vowels and the manipulation parameters are summarized in and displayed in . No significant differences in the baseline production and manipulation parameters of the experiment were found between groups.
Table 2. Overview of the baseline characteristics of the /ɩ/ and /ɛ/ production and the manipulation parameters, separately for children without and with dyslexia.
Figure 1. Speech adaptation parameters to change each participants /ɩ/ vowel into an /ɛ/ vowel under conditions of maximal perturbation. Light gray lines represent children with dyslexia; dark and dashed gray lines children with typical reading skills.
As illustrated in , the altered auditory feedback experiment itself consisted of 20 baseline trials in which the feedback to the participant was not manipulated (baseline), 30 trials in which the perturbation was gradually increased to maximum (ramp-up), 50 trials in which the perturbation was held at maximum (hold), 30 trials in which the perturbation was gradually decreased (ramp-down), and finally 20 trials in which the perturbation was back to normal (after-effect). On each trial, participants were instructed to say the word /bɩp/, while the fed back signal was strongly amplified to ensure that the participants heard their voice via the headphones, rather than via air- and bone conduction. The raw and manipulated signals were saved for analyses.
Diffusion-weighted imaging: data acquisition and preprocessing
A diffusion-weighted imaging (DWI) scan was made using a 3T MAGNETOM Trio PRISMAfit system (Siemens Healthcare, Erlangen, Germany). A three-multiband accelerated protocol with two shells was run to obtain these images (10 unweighted images; 30 direction shell at b = 1000; 60 direction shell at b = 3000, TE = 70ms; TR = 2360ms; voxel size = 2x2x2mm3). The resulting images were first preprocessed using the FSL Diffusion Toolbox (FMRIB’s Software Library; Woolrich et al., Citation2009). In short, raw dicom images were converted to a 4D nifty file, corrected for eddy currents (using eddy_correct), skull stripped (using bet), and a diffusion tensor model was fitted at each voxel. For three participants (one child with typical reading abilities, two children with dyslexia), the fractional anisotropy could not be estimated due to poor tensor fitting. The fractional anisotropy measures were registered to the 1 x 1 × 1 mm3 standard space included in the FSL toolbox using a nonlinear registration. Next, each brain was masked with the AF mask, using the diffusion tensor imaging tractography atlas from Catani and De Schotten (Citation2008) and thresholded at the default value of .2. The mean fractional anisotropy values in the left and right AF/SLF, and its subcomponents (anterior, posterior and long segments) were derived for each participant using fslstats.
Procedure
The child, together with the parent(s), was first invited to the dummy-scanner room in which the child could become acquainted with the MRI environment and the task to reduce anxiety and instruct them to lie as still as possible. After this, the child participated in the altered auditory feedback experiment while the parent signed or handed in the informed consent and filled in checklists for contraindications for participation in an MRI-study. Next, the child was placed in the real MRI-scanner for approximately 40 minutes. The MRI session started with anatomical T1 images, field map images, and functional scans to map reading and speech circuits (not reported in this paper). The DWI protocol was run last. The well-being of the child was systematically monitored before entering the dummy scanner, before and throughout the scanning session in the real MRI scanner, and after the scanning session to ensure the child was happy to continue. Parents were in the control room of the MRI scanner and were able to monitor the well-being of the child as well. Reading and reading-related scores were available for all participants from the longitudinal sample or were collected after the MRI session for the other participants.
Data analyses
For each participant, the frequency of the first and second formants of the raw and adapted signals during the altered auditory feedback task was manually determined by the first author using the following procedure. The produced formants were first plotted in two ways: first, using linear predictive coding (LPC; Rabiner & Schafer, Citation1978) in Matlab 2014a (The MathWorks Inc., Natick, MA, USA); second, using the default formant calculation implemented in the Audapter software (Cai et al., Citation2008; Tourville et al., Citation2013). Subsequently, the author indicated the position of both formants on the y-axis if both methods overlapped. If the methods did not overlap, the formant estimation of Audapter was used as default. Only if the formant estimation of Audapter was not stable, the LPC estimation was used. Because the amount of the applied F2–F1 manipulation was different for each individual, relative changes in adaptation were calculated by dividing the deviation from the mean during the baseline phase by the maximal perturbation for that participant and multiplied by 100. For instance, if someone’s F2–F1 difference between the /ɩ/ and /ɛ/ vowels was 500, and if his/her baseline F2–F1 for the /ɩ/ vowel was 1500, a /bɩp/ production with F2–F1 of 1550 counted as a 10% adaptation. These relative scores were entered into linear mixed-effect models using the lmer function of the lme4 package (Bates, Maechler, Bolker, & Walker, Citation2014) in R version 3.2.3 (R Development Core Team, 2015). For each model, the assumption of normally distributed residuals was checked (and confirmed) using the qqplot function. The phase of the experiment (baseline, ramp-up, hold, ramp-down, after-effect) and the trial numbers within these phases, plus their interaction, were entered as fixed factors in the null model. A maximal random effects structure was applied as suggested by Barr, Levy, Scheepers, & Tily (Citation2013). This means that at least random intercepts for participants as well as by-participant slope adjustments for phase and trial were entered in the models. The best model fit was determined by performing a likelihood ratio test using the ANOVA function of the stats package on subsequent models, starting from simply entering main effects and gradually moving to models with complex interactions. Moreover, a Benjamini and Hochberg correction for multiple significance testing was applied to the p-values to minimize the false discovery rate (Benjamini & Hochberg, Citation1995). First, the p-values were ranked in ascending order (so the smallest p-value has an i of 1, etc.). Then, the p-values were compared with the Benjamini–Hochberg critical value (i/m)*Q, where i is the rank, m the number of tests, and Q the false discovery rate of .05. The ANOVA tests on the models were only classified as significant if they passed this criterium. Satterthwaite approximations were used to estimate p-values within the model (Kuznetsova, Brockhoff, & Christensen, Citation2015). Total model fit was calculated using the MultiModel-Inference (MuMIn) package (Barton, Citation2018). The MUMIn package accepts the mixed effects model of the lme4 package as input and provides summary statistics on the model fit as output. It should be noted that estimating R2-values is not trivial for linear mixed effects models (Nakagawa & Schielzeth, Citation2013). The reported R2-values in this study represent the R2-values for the fixed effects only (also known as R2-marginal). Conditional R2-values provide the explained variance using both the fixed and random effects of the model and are not comparable to traditional R2-values as used in regression models and are, therefore, not reported in this study.
For all participants with dyslexia, the response to intervention was determined by calculating the growth slope during treatment using linear mixed-effects modeling: the score on word-reading was entered as dependent variable with time point during treatment as fixed factor. The random slope for each subject for this relation was used as response to intervention score.
The statistical analyses first explored the average response to the manipulation across all participants, then examined group differences between typically reading and dyslexia, and finally, individual differences within the dyslexia group only. All analyses were performed by separately entering trial number and the standardized measures of word-reading, response to intervention, phonological awareness, and rapid naming into the linear mixed-effects model, after which it was examined whether two-way or three-way interactions with phase and trial numbers significantly improved the model.
Finally, it was examined whether the fractional anisotropy of the AF/SLF differed between the typically reading children and children with dyslexia with a voxel-wise statistical analysis using Tract-Based Spatial Statistics (Smith et al., 2004, 2006) and using a t-test on the mean fractional anisotropy of the total, left, and right arcuate fasciculus. Next, it was examined whether the fractional anisotropy was related to individual differences in measures of word-reading, response to intervention, phonological awareness, and rapid naming, and whether and how the AF/SLF was related to the response to altered auditory feedback. For the reading-related measures that significantly correlated with the fractional anisotropy in the AF/SLF, additional linear mixed-effects models were run, in an exploratory manner, with both the behavioral measure and the AF/SLF measure.
Results
Response to altered auditory feedback in children with and without dyslexia
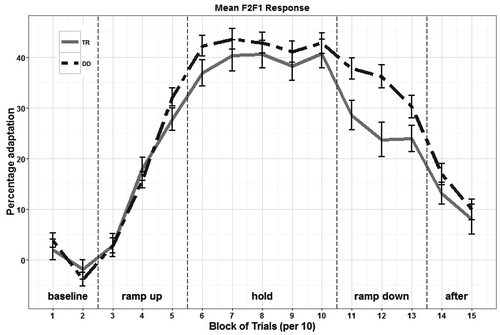
The first and second formants of 5700 /bɩp/ productions were estimated. In total, the formant calculation of 314 (5.51%) speech utterances failed due to an unstable production or estimation of one of the formants, mostly F2. On average, participants adapted their F2–F1 production with 41.41% in the direction opposite to the manipulation (range = 3.13%-91.52%). All participants changed their speech response in the direction opposite to the manipulation. The responses for both groups over the course of the experiment are depicted in . The null model, using participants from both groups, showed that during the ramp-up phase (β = 17.01, p < .001), the hold phase (β = 41.78, p < .001), the ramp-down phase (β = 32.25, p < .001), and the after-effect phase (β = 12.55, p < .001), participants showed significant opposing responses to the applied manipulation. Moreover, a main effect for trial (β = −3.60, p < .001) and interaction effects of the ramp-up phase with trial number (β = 13.59, p < .001) and during the after-effect phase with trial number (β = 3.85, p = .003) were found, indicating that the adaptation response increased as a function of the trial number within these phases. Next, the factor group (typically reading vs. dyslexia) was added to the linear mixed-effects model and compared with this null model (with a phase by trial interaction already in it). The model that was significantly better than the null model (χ2(5) = 13.06, p = .023, R2 = .229) was a model with a phase by trial and a phase by group interaction. The group of children with dyslexia showed a weaker return to baseline during the ramp-down phase than the typically reading children (β = 8.23, p = .004).
Is the response to altered auditory feedback related to individual differences in the severity and persistence of dyslexia?
Next, it was examined within the group of children with dyslexia only, whether and how scores of reading and response to intervention were related to the response to altered auditory feedback. With respect to the model for reading, a model with phase by trial and phase by reading score interactions was significantly better than the null model (χ2(5) = 14.10, p = .015 R2 = .231) and was not further improved by adding other interactions. In-line with the group differences between typical readers and the children with dyslexia, a higher reading score among children with dyslexia was associated with weaker adaptation during the ramp-up phase (β = −5.85, p = .042) and stronger de-adaptation during the ramp-down phase (β = −8.04, p < .001). No significant differences were found for the hold and after-effect phases; however, the results were in the same direction as during the ramp-up and ramp-down phases.
Regarding the relation between altered auditory feedback and the response to intervention, convergence could not be reached using the default optimizer. The optimx package was used to circumvent convergence issues (Nash, Varadhan, & Grothendieck, Citation2013). The best model for the response to intervention included a phase by trial and a phase by response-to-intervention interaction and this was significantly better than the null model (χ2(5) = 13.35, p = .020, R2 = .233). Adding interactions did not further improve the model. The only trend in the data was a weaker adaptation response during the hold phase as a function of response to intervention (β = −2.47, p = .070). A better response to treatment was thus associated with less adaptation as a response to altered feedback.
Is the response to altered auditory feedback related to individual differences in rapid naming and phonological awareness?
A model with phase by trial and phase by rapid naming was significantly better than the null model (χ2(5) = 20.37, p = .001, R2 = .226) and adding other interactions did not further improve the model. The direction of the effects was the same as that of reading and response to intervention: A better score (i.e. faster naming) on rapid naming was associated with a weaker deviation from baseline during the ramp-up phase (β = −5.01, p = .004) and a stronger de-adaptation to baseline during the ramp-down phase (β = −6.06, p < .001). No significant differences were found during the hold phase (β = −1.29, p = .413) and the after-effect phase (β = −2.77, p = .141).
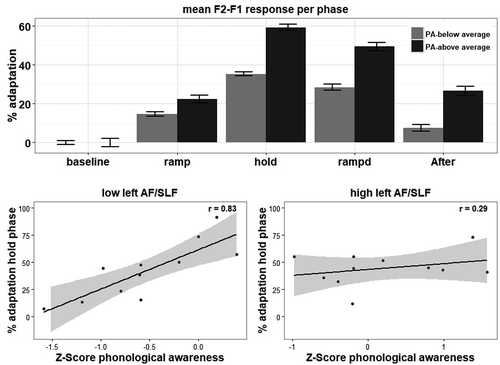
With respect to phonological awareness, a model with phase by trial and phase by phonological awareness score interactions was significantly better than the null model (χ2(5) = 73.28, p < .001, R2 = .251), and adding more interactions did not further improve the model. Remarkably, a higher score on phonological awareness was associated with a marginally stronger adaptation during the ramp-up phase (β = 3.60, p = .051), with a stronger response during the hold phase (β = 12.44, p < .001) and a weaker de-adaptation in the ramp-down (β = 9.51, p < .001) and after-effect phase (β = 8.65, p < .001). The responses per phase for the individuals with dyslexia are separately plotted for individuals with scores above and below average on phonological awareness in that group in the top panel of . Since this finding contradicts our hypothesis and also does not match the results of the relations between the response to altered auditory feedback and reading scores, we were particularly careful to assure that this finding was not driven by outliers, which was not the case.
Figure 4. Mean response to the altered auditory feedback per phase for the participants with dyslexia with a phonological awareness score below and above average (top panel). Error bars represent one standard error of the mean. The bottom panels show that this finding is mainly driven by participants with a low fractional anisotropy in the left AF/SLF (bottom left panel, r = .87, p = .001, 95% CI = .52-.97) and that this relation is not present for participants with a higher fractional anisotropy of the left AF/SLF (r = .29, p = .381, 95% CI = -.37-.76). Shaded areas represent 95% confidence interval.
The AF/SLF and its relations with reading(-related) measures and speech perception-production interaction
Voxel-wise statistical analysis did not reveal significant clusters of decreased or increased fractional anisotropy in children with compared to children without dyslexia. Also, the mean fractional anisotropy in children with dyslexia was not significantly different from that in typically reading peers for the AF/SLF as a whole (t(18.25) = .94, p = .360, d = .440), or for the left (t(15.46) = .93, p = .369, d = .471) and right AF/SLF (t(22.42) = .91, p = .370, d = .386) separately. Correlational analyses were performed to examine the relations between the fractional anisotropy of the AF/SLF and reading and reading-related measures, in children with dyslexia only. The resulting correlations are provided in . Measures of the AF/SLF as a whole, or the left or right AF/SLF separately only, correlated significantly with scores on both measures of phonological awareness. Reading and rapid automatized naming did not correlate with the fractional anisotropy of the AF/SLF.
Table 3. Correlations between the fractional anisotropy values of the AF/SLF and the reading-related measures.
Next, it was examined how the fractional anisotropy in the AF/SLF was related to the response to the altered auditory feedback manipulation, which was only the case for the fractional anisotropy in the left AF/SLF. Adding a phase by trial by left AF/SLF interaction to the null model significantly improved the model (χ2(10) = 23.45, p = .009, R2 = .250). The within-model approximations showed that a higher fractional anisotropy was related to stronger adaptation in the ramp-up and hold phases and weaker de-adaptation in the ramp-down and after-effect phases. Only the weaker de-adaptation during the ramp-down phase was significant (β = 5.21, p = .001). Since the fractional anisotropy in the left AF/SLF was significantly related to phonological awareness as well as to the response to altered auditory feedback, we examined, exploratively, whether the fractional anisotropy in the AF/SLF showed interaction effects on the relation between phonological awareness and response to altered feedback.
The model with a phase by left AF/SLF by phonological awareness interaction was significantly better than the model with a phase by trial and phase by phonological awareness model (χ2(10) = 117.55, p < .001, R2 = .358). The approximations within the model showed that, as in the previous analysis, a higher phonological awareness was associated with a stronger deviation from baseline during the ramp-up (β = 9.45, p < .001) and hold phases (β = 23.65, p < .001) and a weaker return to baseline during the ramp-down (β = 15.82, p < .001) and after-effect phases (β = 17.01, p < .001). Having a higher fractional anisotropy in the left AF/SLF was associated with a weaker deviation from baseline during the ramp (β = −5.52, p = .006) and hold phases (β = −14.19, p < .001). Also, a higher fractional anisotropy was associated with an adaptation response in the ramp-down (β = −7.73, p < .001) and after-effect (β = −8.17, p < .001) phases that was closer to baseline production. So, when controlling for the effects of phonological awareness, a higher fractional anisotropy was associated with less adaptation throughout the altered feedback experiment. Interestingly, we also found an interaction between score on phonological awareness and the fractional anisotropy of the left AF/SLF for the ramp-up (β = −4.55, p = .038), hold (β = −12.33, p < .001) and after-effect phase (β = −14.49, p < .001). This means that the opposing pattern for the relation between phonological awareness and response to altered feedback is mainly driven by participants with a low fractional anisotropy in the left AF/SLF. To illustrate the modulatory influence of the left AF/SLF on the relation between phonological awareness and response to altered feedback, we plotted the correlation between phonological awareness and response to altered feedback during the hold phase for participants with a relatively weaker fractional anisotropy (below median, n = 11) in the left AF/SLF and with a higher fractional anisotropy (above median, n = 11) in the left AF/SLF in the lower panels of .
Discussion
In the current study, it was first examined whether children with dyslexia responded differently to altered auditory feedback when compared with typically reading children. This group comparison showed that children with dyslexia adapted to the feedback manipulation to a similar extent, but did not de-adapt during the ramp-down phase as strongly as typically reading peers. Next, within the group of children with dyslexia only, it was examined how the response to altered auditory feedback related to individual differences in the severity and persistence of the reading difficulties and reading-related cognitive abilities. We found that more severe (word reading skills) and persistent (response to intervention) reading difficulties in children with dyslexia were associated with an impaired response to altered feedback. Similarly, lower rapid naming skills were associated with a stronger response to the alteration in auditory feedback. Contrary to our expectations, the relation between phonological awareness and the response to altered auditory feedback showed the opposite pattern. Better performance on the phonological awareness tasks was associated with a stronger response to altered feedback. Finally, we showed that the fractional anisotropy in the left AF/SLF was positively correlated with measures of phonological awareness and moderated the relation between phonological awareness and response to altered auditory feedback. The association of better phonological awareness with a stronger response to altered auditory feedback was driven by the children with a low fractional anisotropy of the left arcuate fasciculus.
As noted above, children with dyslexia showed a weaker de-adaptation in the ramp-down phase when compared with typically reading controls. Children with more severe and persistent reading difficulties and slower rapid naming abilities, within the group of children with dyslexia, showed a stronger adaptation during the ramp-up phase and a weaker de-adaptation during the ramp-down phase of the altered auditory feedback experiment. The response to altered auditory feedback is an integrated measure of speech feed-forward and feedback mechanisms (refer Guenther et al., Citation2006), but it does not reveal what specific aspects of these mechanisms are functioning awry when atypical responses are registered. These results seem in-line with the observed differences in response to altered auditory feedback between adults with and without dyslexia. In that study (Van Den Bunt et al., Citation2017), adults with dyslexia were also found to adapt more strongly in the ramp phase and to de-adapt to a weaker extent in the after-effect phase. The results of both these studies are in-line with the notion that dyslexia could be characterized by a weaker magnet that causes children with dyslexia to be moved away from the category prototype more easily (under conditions of altered feedback) and be attracted back to their baseline (when feedback is unaltered again) to a smaller extent. An important methodological difference with that study is that, in the study with adults, the amount of auditory alteration remained within a phoneme category (in Dutch), and since individuals with dyslexia are reported to exhibit better within-phoneme-category discrimination (Serniclaes et al., Citation2004), a stronger response to altered feedback could have been attributed to higher sensitivity to a within-phoneme-category change. The adaptation applied in the current study resulted in a complete vowel change, indicating that children with dyslexia showed this impaired response to altered feedback even when a phoneme category boundary was crossed. This renders the hypothesis that the impaired response to altered feedback results from better within-phoneme-category perception unlikely. Instead, we suggest that the results of the current study support the hypothesis that dyslexia is characterized by a weaker magnet. A weaker magnet might cause individuals with dyslexia to move away more easily from the prototype (non-significant at the group level in this study, but in the expected direction and evident in the individual differences analyses) and to be attracted back to the prototype when the feedback returns to normal to a smaller extent (significant in this study). In future studies, a purely perceptual measure of this magnet effect could be included to further corroborate this hypothesis. It should be noted that this weaker magnet could well have consequences for both the feedback and feed-forward traces of phonological representations. A weak magnet might cause the feedback system to send error signals for relatively small deviations from the category prototype. In turn, an active feedback system could hamper the establishment of stable and reliable feed-forward commands. This interpretation could relate to several earlier reported phenomena in dyslexia research. For instance, a weaker magnet could explain the increased within-phoneme-category perception in individuals with dyslexia, which is reported in studies on an allophonic mode of perception in dyslexia (Noordenbos, Segers, Serniclaes, Mitterer, & Verhoeven, Citation2012; Serniclaes et al., Citation2004). The earlier reported impairments in speech production in dyslexia (Catts, Citation1986, Citation1989; Foy & Mann, Citation2012) are also in-line with the hypothesis of a weaker magnet. Specifically, Houde and Nagarajan (Citation2011) suggested that if error signals from the feedback system are easily implemented in the forward stream, the motor control system becomes unstable. This might be the case in individuals with dyslexia.
The unexpected finding that will better phonological skills within the children with dyslexia was associated with a stronger response to altered auditory feedback should be considered within the context of the Dutch orthography and national treatment protocols. Phonological awareness has been reported to be less important for reading development in transparent orthographies, such as Dutch. Some studies indeed report no relation between phonological awareness and word-reading skills (e.g. Georgiou et al., Citation2008), or suggest that the relation decreases during reading development (De Jong & Van Der Leij, Citation2003). Similarly, in the current study, word reading skills, of the children with dyslexia, did not significantly correlate with phonological awareness (r = .22, p = .270). An opposite relation with the response to altered auditory feedback was, however, surprising. Although we should interpret these findings with caution, considering the small sample size, it is conceivable that relying on auditory feedback may help in developing phonological awareness skills, but may also cause a child to keep using the relatively slow phonological decoding route for word reading, rather than move toward building more efficient orthographic representations. This persistent use of the slower phonological decoding route possibly relates to the dyslexia treatment protocols that are implemented nation-wide in the Netherlands. These treatment protocols are largely based on efficacy studies in English, which is a fundamentally different language in terms of orthographic transparency (Borgwaldt et al., Citation2005) and, as a consequence, the treatment protocol puts a strong emphasis on mastering phonological awareness skills, before advancing to speeding up the reading process (Tilanus et al., Citation2016). As a result, some children receive extensive training in skills that allow them to perform better on phonological awareness measures, without a concomitant improvement in reading skills.
An important insight from the structural brain data that were included is that having a higher fractional anisotropy in the AF/SLF reduced the extent to which phonological skills were associated with a stronger adaptation response. Rephrased, the increased response to altered feedback for the children with high scores on phonological awareness is particularly apparent for the children with lower fractional anisotropy in the left AF/SLF. Possibly, some participants were aware of the (sub)phonemic structure of spoken language but an impaired communication between speech perception and production areas hinders the required feedback to update and stabilize feed-forward, motor, traces of phonological representations (Guenther et al., Citation2006). Alternatively, it is possible that the reading intervention has normalized the phonological awareness skills on behavioral level, but this intervention may not have had an impact on subtle neurobiological differences that might have led to the phonological differences. Future studies could obtain structural brain measures also prior to the reading intervention so that the effects of the intervention on neurobiological differences could be examined. Although the AF/SLF has been implicated in dyslexia and is hypothesized to relate to speech perception and/or production processes, follow-up studies should also include other white matter tracts. With respect to reading, whereas the AF/SLF is often associated with decoding, the inferior fronto-occipital fasciculus is hypothesized to underlie whole-word recognition (Yeatman, Rauschecker, & Wandell, Citation2013). It would be interesting to examine whether children with dyslexia with particular difficulties in pseudoword reading (i.e. decoding) show an even stronger deviation from their baseline under conditions of altered feedback and a slower return to their baseline when the feedback is back to normal.
Although the current study provides further insight into how the interaction between speech perception and speech production is involved in reading ability in children with dyslexia, it does not provide new evidence on the etiology of dyslexia. Future studies could examine whether measures of speech perception–production interactions are prospectively predictive of early reading development. It is important to note that several authors have proposed that the phonological deficit is secondary to an underlying general auditory deficit that affects the ability to acquire adequate phonological representations (Goswami et al., Citation2002; Hakvoort et al., Citation2016, Citation2015; Tallal, Citation1980). Examining the response to alterations in non-phonological forms of auditory information, such as amplitude or pitch, could further clarify the nature of the deficit in dyslexia. Moreover, it is a challenge to bring the current results in-line with the recently popular view that the phonological deficit in dyslexia is an impairment in the access to, rather than the quality of, phonological representations (Ramus & Szenkovits, Citation2008). If anything, the results of the current study seem to suggest that phonological representations are more easily accessed and modified in dyslexia, rather than the contrary. Future studies should aim to include measures of phonological access to further disentangle these different explanations.
Several methodological issues could also be addressed in future studies. First, the sample size of the individuals with dyslexia was small, and subtle effects might have been missed due to a lack of power. For instance, a bigger sample size might have been able to detect whether the speed, rather than the strength, of deviating from or returning to the baseline in the altered auditory feedback task, related to individual differences in reading and reading-related skills. Also, children within the dyslexia group differed slightly in age, reading experience, and time since the intervention and controlling more carefully for these factors might yield stronger results. Second, adequate phonological representations are particularly important for decoding and using a nonword reading task, rather than a word-reading task, might show stronger relationships between reading and the response to altered auditory feedback. Third, the rapid naming task relied primarily on fluency (under the assumption that accuracy was at ceiling), the phonological awareness tasks relied primarily on accuracy, and the reading task on a combination of accuracy and fluency (i.e. correctly read words per minute). Theoretically, it is possible that the type of task is somehow related to the response to altered auditory feedback. For instance, the task in the auditory feedback task is to produce a syllable. If anything, this is a task that puts more demand on accuracy (do I produce the syllable correctly?) than on speed and the response to altered feedback might be more strongly related to tasks that focus on accuracy as well. In future studies, it would be good to be consistent in task demands across tasks. Fourth, it is possible that the reading intervention has had an impact on the response to altered auditory feedback itself. It would be interesting to examine whether and how the response to altered auditory feedback is changed by reading intervention. Finally, since the current study is not a multi-modal imaging study, the brain mechanisms that underlie the response to altered auditory feedback could not be examined in full detail. Running altered auditory feedback paradigms in the MR scanner is not optimal considering the noise generated by the scanner, however, it is feasible to administer altered auditory feedback in the MEG scanner (e.g. Kort,Nagarajan, & Houde, 2014). This way, future studies could disentangle the contribution of different brain regions/networks to speech motor control.
This is the first study that shows how the interaction between speech perception and production is associated with individual differences in reading and reading-related measures in children with dyslexia. It was shown that the severity and persistence of reading difficulties, and deficits in rapid naming skills, in children with dyslexia were associated with a stronger response to altered auditory feedback. These findings can be seen as support for the notion of a weaker magnet in dyslexia, which might lead to a stronger adaptation during altered feedback and a weaker de-adaptation when feedback is back to normal. With respect to phonological awareness, we found that better phonological skills were associated with a stronger response to altered auditory feedback, particularly for children with a low fractional anisotropy in the left AF/SLF. This was attributed to the relative low importance of phonological awareness for reading in a transparent orthography, while relatively much effort is put in improving this awareness during treatment. Considering the importance of literacy skills for an individual’s academic and economic prospects as well as the societal costs associated with low literacy skills, there is probably no need to convince anyone of the relevance of research into the origins of differences in reading skill. Although the results reported in this study might not be directly applicable to improve reading skills in children with dyslexia, the main findings (a weaker magnet in children with dyslexia; individual differences within dyslexia are related to the response to altered auditory feedback) and the used methodology to measure phonological representations (measuring the response to altered feedback) can help the field to move forward which could ultimately result in advancements in dyslexia prevention, assessment and intervention.
Acknowledgments
We would like to thank the reviewers for their helpful suggestions to improve the manuscript, specifically for the suggested explanation of the model with the brain-behavior interaction. MAG was supported by an Innovational Research Incentives Scheme Veni grant (#275-89-017) from the Netherlands Organization for Scientific Research.
Additional information
Funding
References
- Andrews, J. S., Ben-Shachar, M., Yeatman, J. D., Flom, L. L., Luna, B., & Feldman, H. M. (2010). Reading performance correlates with white-matter properties in preterm and term children. Developmental Medicine and Child Neurology, 52(6), 94–100. doi:10.1111/j.1469-8749.2009.03456.x
- Barr, D. J., Levy, R., Scheepers, C., & Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. doi:10.1016/j.jml.2012.11.001
- Barton, K. (2018). MuMIn: Multi-model infererence. R package 1.40.4. Retrieved from https://CRAN.R-project.org/package=MuMIn
- Bates, D., Maechler, M., Bolker, B., & Walker, S. (2014). lme4: Linear mixed-effects models using Eigen and S4. Retrieved from http://cran.r-project.org/package=lme4
- Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal R Statistical Social, 57, 289–300.
- Bernal, B., & Ardila, A. (2009). The role of the arcuate fasciculus in conduction aphasia. Brain, 132, 2309–2316. doi:10.1093/brain/awp206
- Blomert, L. (2006). Protocol diagnostiek en behandeling [Protocol of diagnostics and treatment]. CVZ, Diemen, The Netherlands.
- Boada, R., & Pennington, B. F. (2006). Deficient implicit phonological representations in children with dyslexia. Journal of Experimental Child Psychology, 95(3), 153–193. doi:10.1016/j.jecp.2006.04.003
- Boets, B., Op De Beeck, H., Vandermosten, M., Scott, S. K., Céline, R., Mantini, D., … Wouters, J. (2013). Intact but less accessible phonetic representations in adults with dyslexia. Science, 342(6163), 1251–1254. doi:10.1126/science.1244333.Intact
- Borgwaldt, S. R., Hellwig, F. M., & De Groot, A. M. B. (2005). Onset entropy matters - Letter-to-phoneme mappings in seven languages. Reading and Writing, 18, 211–229. doi:10.1007/s11145-005-3001-9
- Brus, B. T., & Voeten, M. J. M. (1973). Een-Minuut Test [One-minute test. Nijmegen: Berkhout Testmateriaal.
- Cai, S., Ghosh, S. S., Guenther, F. H., & Perkell, J. (2008). A system for online dynamic perturbation of formant frequencies and results from perturbation of the Mandarin triphthong /iau/. Proceedings of the 8th Intl. Seminar on Speech Production (pp. 65–68), Strasbourg, France.
- Catani, M., & De Schotten, M. (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex, 44(8), 1105–1132. doi:10.1016/j.cortex.2008.05.004
- Catani, M., & Mesulam, M. (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex, 44(8), 953–961. doi:10.1016/j.cortex.2008.04.002
- Catts, H. W. (1986). Speech production/phonological deficits in reading-disordered children. Journal of Learning Disabilities, 19(8), 504–508. doi:10.1177/002221948601900813
- Catts, H. W. (1989). Speech production deficits in developmental dyslexia. Journal of Speech and Hearing Disorders, 54, 422–428. doi:10.1044/jshd.5403.422
- De Jong, P. F., & Van Der Leij, A. (2003). Developmental changes in the manifestation of a phonological deficit in dyslexic children learning to read a regular orthography. Journal of Educational Psychology, 95(1), 22–40. doi:10.1037/0022-0663.95.1.22
- Dougherty, R. F., Ben-Shachar, M., Deutsch, G. K., Hernandez, A., Fox, G. R., & Wandell, B. A. (2007). Temporal-callosal pathway diffusivity predicts phonological skills in children. Proceedings of the National Academy of Sciences of the United States of America, 104(20), 8556–8561. doi:10.1073/pnas.0608961104
- Elbro, C., Borstrom, I., & Petersen, D. (1998). Predicting dyslexia from kindergarten: The importance of distinctness of phonological representations of lexical items. Reading Research Quarterly, 33, 36–60. doi:10.1598/RRQ.33.1.3
- Feldman, N. H., Griffiths, T. L., & Morgan, J. L. (2009). The influence of categories on perception: Explaining the perceptual magnet effect as optimal statistical inference. Psychological Review, 116(4), 752–782. doi:10.1037/a0017196
- Foy, J. G., & Mann, V. A. (2012). Speech production deficits in early readers: Predictors of risk. Reading and Writing, 25, 799–830. doi:10.1007/s11145-011-9300-4
- Furnes, B., & Samuelsson, S. (2011). Phonological awareness and rapid automatized naming predicting early development in reading and spelling: results from a cross-linguistic longitudinal study. Learning and Individual Differences, 21(1), 85–95. doi:10.1016/j.lindif.2010.10.005
- Georgiou, G. K., Parrila, R., & Papadopoulos, T. C. (2008). Predictors of word decoding and reading fluency across languages varying in orthographic consistency. Journal of Educational Psychology, 100(3), 566–580. doi:10.1037/0022-0663.100.3.566
- Geschwind, N. (1982). Language and the brain. Scientific American, 226, 76–83. doi:10.1038/scientificamerican0472-76
- Goswami, U., Thomson, J., Richardson, U., Stainthorp, R., Hughes, D., Rosen, S., & Scott, S. K. (2002). Amplitude envelope onsets and developmental dyslexia: A new hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10911–10916. doi:10.1073/pnas.122368599
- Guenther, F. H., Ghosh, S. S., & Tourville, J. A. (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language, 96, 280–301. doi:10.1016/j.bandl.2005.06.001
- Gullick, M. M., & Booth, J. R. (2015). The direct segment of the arcuate fasciculus is predictive of longitudinal reading change. Developmental Cognitive Neuroscience, 13, 68–74. doi:10.1016/j.dcn.2015.05.002
- Hakvoort, B., De Bree, E., Van, D. L., Maassen, B., Van Setten, E., Maurits, N., & Van Zuijen, T. L. (2016). The role of categorical speech perception and phonological processing in familial risk children with and without dyslexia. Journal of Speech, Language, and Hearing Research, 59(6), 1448–1460. doi:10.1044/2016_JSLHR-L-15-0306
- Hakvoort, B., Van Der Leij, A., Maurits, N., Maassen, B., & Van Zuijen, T. L. (2015). Basic auditory processing is related to familial risk, not to reading fluency: An ERP study. Cortex, 63, 90–103. doi:10.1016/j.cortex.2014.08.013
- Houde, J. F., & Nagarajan, S. S. (2011). Speech production as state feedback control. Frontiers in Human Neuroscience, 5(October), 1–14. doi:10.3389/fnhum.2011.00082
- Kamali, A., Flanders, J., Brody, Hunter, J.V., Hasan, K.M. (2014). Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Structure and function, 219(1), 1–21. doi:10.1007/s00429-012-0498-y. Tracing
- Kort, W., Schittekatte, M., Van Den Bos, K. P., Vermeir, G., Lutje Spelberg, H. C., Verhaeghe, P., & Van Der Wild, S. (2005). DST-NL: Dyslexie screening test. Amsterdam, Nederland: Pearson.
- Kort, N.S., Nagarajan, S.S. & Houde, J.F. (2014). A bilateral cortical network responds to pitch perturbations in speech feedback. Neuroimage, 86 525–535. doi:10.1016/j.neuroimage.2013.09.042
- Kuhl, P. K. (1991). Human adults and human infants show a “perceptual magnet effect” for the prototypes of speech categories, monkeys do not. Perception & Psychophysics, 50(2), 93–107. doi:10.3758/BF03212211
- Kuznetsova, A., Brockhoff, P. B., & Christensen, R. H. B. (2015). lmerTest: Tests in linear mixed effects models. Retrieved from http://cran.r-project.org/package=lmerTest
- Lametti, D.R., Nasir, S.M., & Ostry D.J. (2012). Sensory preference in speech production revealed by simultaneous alteration of auditory and somatosensory feedback. The Journal of Neuroscience, 32(27), 9351–9358
- Lane, H., Wozniak, J., Matthies, M., Svirsky, M., Perkell, J., O’Connell, M., & Manzella, J. (1997). Changes in sound pressure and fundamental frequency contours following changes in hearing status. The Journal of the Acoustical Society of America, 101(4), 2244–2252. doi:10.1121/1.418245
- Langer, N., Peysakhovich, B., Zuk, J., Drottar, M., Sliva, D. D., Smith, S., … Gaab, N. (2015). White matter alterations in infants at risk for developmental dyslexia. Cerebral Cortex, bhv281. doi:10.1093/cercor/bhv281
- Law, J. M., Vandermosten, M., Ghesquiere, P., & Wouters, J. (2014). The relationship of phonological ability, speech perception, and auditory perception in adults with dyslexia. Frontiers in Human Neuroscience, 8(July), 1–12. doi:10.3389/fnhum.2014.00482
- Lebel, C., & Beaulieu, C. (2009). Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping, 30(11), 3563–3573. doi:10.1002/hbm.20779
- Lebel, C., Shaywitz, B., Holahan, J., Shaywitz, S., Marchione, K., & Beaulieu, C. (2013). Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain and Language, 125(2), 215–222. doi:10.1016/j.bandl.2012.10.009
- Long, M. A., Katlowitz, K. A., Svirsky, M. A., Clary, R. C., Byun, T. M. A., Majaj, N., … Greenlee, J. D. W. (2016). Functional segregation of cortical regions underlying speech timing and articulation. Neuron, 89(6), 1187–1193. doi:10.1016/j.neuron.2016.01.032
- Lyon, G. R., Shaywitz, S. E., & Shaywitz, B. A. (2003). Defining dyslexia, comorbidity, teachers’ knowledge of language and reading. Annals of Dyslexia. doi:10.1007/s11881-003-0001-9
- MacDonald, E. N., Johnson, E. K., Forsythe, J., Plante, P., & Munhall, K. G. (2012). Children’s development of self-regulation in speech production. Current Biology, 22(2), 113–117. doi:10.1016/j.cub.2011.11.052
- Malek, A., Amiri, S., Hekmati, I., Pirzadeh, J., & Gholizadeh, H. (2013). A comparative study on diadochokinetic skill of dyslexic, stuttering, and normal children. ISRN Pediatrics, 2013, 165193. doi:10.1155/2013/165193
- Matsumoto, R., Nair, D., LaPresto, E., Bingaman, W., Shibasaki, H., & Lüders, H. (2004). Functional connectivity in the human language system: A cortico-cortical evoked potential study. Brain, 127(10), 2316–2330. doi:10.1093/brain/awh246
- Nash, J. C., Varadhan, R., & Grothendieck, G. (2013). optimx. Retrieved from https://cran.r-project.org/web/packages/optimx/optimx.pdf
- Nakagawa, S., & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and evolution, 4(2), 133–142. doi:10.1111/j.2041-210x.2012.00261.x
- Nelson, J. M. (2015). Examination of the double-deficit hypothesis with adolescents and young adults with dyslexia. Annals of Dyslexia, 65(3), 159–177. doi:10.1007/s11881-015-0105-z
- Niziolek, C. A., &Guenther, F. H. (2013). Vowel category boundaries enhance cortical and behavioral responses to speech feedback alteration. The journal of Neuroscience, 33(29), 12090–12098
- Noordenbos, M. W., Segers, E., Serniclaes, W., Mitterer, H., & Verhoeven, L. (2012). Allophonic mode of speech perception in Dutch children at risk for dyslexia: A longitudinal study. Research in Developmental Disabilities, 33(5), 1469–1483. doi:10.1016/j.ridd.2012.03.021
- Noordenbos, M. W., & Serniclaes, W. (2015). The categorical perception deficit in dyslexia: A meta-analysis. Scientific Studies of Reading, 19(5), 340–359. doi:10.1080/10888438.2015.1052455
- Pugh, K. R., Mencl, W. E., Jenner, A. R., Katz, L., Frost, S. J., Lee, J. R., … Shaywitz, B. A. (2000). Functional neuroimaging studies of reading and reading disability (Developmental dyslexia). Mental Retardation and Developmental Disabilities, 6, 207–213. doi:10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P
- Purcell, D. W., & Munhall, K. G. (2006). Adaptive control of vowel formant frequency: Evidence from real-time formant manipulation. The Journal of the Acoustical Society of America, 120(2), 966–977. doi:10.1121/1.2217714
- Rabiner, L. R., & Schafer, R. W. (1978). Digital processing of speech signals. Pearson. Pearson Education Inc, Upper Saddle River, NJ
- R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
- Ramus, F., & Szenkovits, G. (2008). What phonological deficit? Quarterly Journal of Experimental Psychology (2006), 61(July2014), 129–141. doi:10.1590/S1516-80342007000400015
- Rollins, N., Vachha, B., Srinivasan, P., Chia, J., Pickering, J., Hughes, C. W., & Al, E. (2009). Simple developmental dyslexia in children: Alterations in diffusion-tensor metrics of white matter tracts at 3 T. Radiology, 251(3), 882–891. doi:10.1148/radiol.2513081346
- Scheerer, N. E., Jacobson, D. S., & Jones, J. A. (2016). Sensorimotor learning in children and adults: Exposure to frequency-altered auditory feedback during speech production. Neuroscience, 314, 106–115. doi:10.1016/j.neuroscience.2015.11.037
- Schmahmann, J., & Pandya, D. (2006). Fiber Pathways of the brain. Oxford University Press; 1 edition. New York.
- Serniclaes, W., Van Heghe, S., Mousty, P., Carré, R., & Sprenger-Charolles, L. (2004). Allophonic mode of speech perception in dyslexia. Journal of Experimental Child Psychology, 87(4), 336–361. doi:10.1016/j.jecp.2004.02.001
- Share, D. L. (2008). On the anglocentricities of current reading research and practice: The perils of overreliance on an “outlier” orthography. Psychological Bulletin, 134(4), 584–615. doi:10.1037/0033-2909.134.4.584
- Shaywitz, B. A., Shaywitz, S. E., Pugh, K. R., Mencl, W. E., Fulbright, R. K., Skudlarski, P., … Gore, J. C. (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry, 52(2), 101–110. doi:10.1016/S0006-3223(02)01365-3
- Smith, C. R. (1975). Residual hearing and speech production in deaf children. Journal of Speech and Hearing Research, 18, 795–811. doi:10.1044/jshr.1804.795
- Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens, T.E.J., Johansen-Berg, H…. & Matthews, P.M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23(S1), 208–219.
- Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens, T.E.J., Johansen-Berg, H…. Matthews, P.M., (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage, 31, 1487–1505.
- Snowling, M. J. (1981). Phonemic deficits in developmental dyslexia. Psychological Research, 43, 219–234. doi:10.1007/BF00309831
- Sprugevica, I., & Høien, T. (2003). Early phonological skills as a predictor of reading acquisition: A follow-up study from kindergarten to the middle of grade 2. Scandinavian Journal of Psychology, 44, 119–124. doi:10.1111/1467-9450.00329
- Steinbrink, C., Vogt, K., Kastrup, A., Müller, H. P., Juengling, F. D., Kassubek, J., & Riecker, A. (2008). The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia, 46(13), 3170–3178. doi:10.1016/j.neuropsychologia.2008.07.015
- Tallal, P. (1980). Auditory temporal perception, phonics, and reading disabilities in children. Brain and Language, 9(2), 182–198. doi:10.1121/1.2016007
- Tallal, P., Miller, S., & Fitch, R. H. (1993). Neurobiological basis of speech: A case for the preeminence of temporal processing. Annals of the New York Academy of Sciences, 682(1), 27–47. doi:10.1111/nyas.1993.682.issue-1
- Tilanus, E. A. T., Segers, E., & Verhoeven, L. (2016). Responsiveness to intervention in children with dyslexia. Dyslexia, 22(3), 214–232. doi:10.1002/dys.1533
- Tourville, J. A., Cai, S., & Guenther, F. H. (2013). Exploring auditory-motor interactions in normal and disordered speech. Proceedings of the 165th Meeting of the Acoustical Society of America, Montreal, Canada.
- Tourville, J. A., & Guenther, F. H. (2011). The DIVA model: A neural theory of speech acquisition and production. Language and Cognitive Processes, 26(7), 952–981. doi:10.1080/01690960903498424
- Van Den Bos, K. P., & Lutje Spelberg, H. C. (2014). CB en WL: Continu benoemen en woorden lezen [continuous naming and reading words]. Amsterdam, Nederland: Boom Test Uitgevers.
- Van Den Bunt, M. R., Groen, M. A., Ito, T., Francisco, A. A., Gracco, V. L., Pugh, K. R., & Verhoeven, L. (2017). Increased response to altered auditory feedback in dyslexia: A weaker sensorimotor magnet implied in the phonological deficit. Journal of Speech, Language, and Hearing Research, 60(3), 654–667. doi:10.1044/2016_JSLHR-L-16-0201
- Vandermosten, M., Boets, B., Poelmans, H., Sunaert, S., Wouters, J., & Ghesquière, P. (2012). A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain, 135(3), 935–948. doi:10.1093/brain/awr363
- Vandermosten, M., Vanderauwera, J., Theys, C., De Vos, A., Vanvooren, S., Sunaert, S., … Ghesquière, P. (2015). A DTI tractography study in pre-readers at risk for dyslexia. Developmental Cognitive Neuroscience, 14, 8–15. doi:10.1016/j.dcn.2015.05.006
- Woolrich, M. W., Jbabdi, S., Patenaude, B., Chappell, M., Makni, S., Behrens, T., … Smith, S. M. (2009). Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45(1), S173–S186. doi:10.1016/j.neuroimage.2008.10.055
- Yeatman, J. D., Rauschecker, A. M., & Wandell, B. A. (2013). Anatomy of the visual word form area: Adjacent cortical circuits and long-range white matter connections. Brain and Language, 125(2), 146–155. doi:10.1016/j.bandl.2012.04.010