 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The quality of language that children hear in their environment is associated with the development of language-related brain regions, in turn promoting vocabulary knowledge. Although informative, it remains unknown how these environmental influences alter the structure of neural tissue and subsequent vocabulary outcomes. The current study uses magnetic resonance elastography (MRE) to examine how children’s language environments underlie brain tissue mechanical properties, characterized as brain tissue stiffness and damping ratio, and promote vocabulary knowledge. Twenty-five children, ages 5–7, had their audio and video recorded while engaging in a play session with their parents. Children also completed the Picture Vocabulary Task (from NIH Toolbox) and participated in an MRI, where MRE and anatomical images were acquired. Higher quality input was associated with greater stiffness in the bilateral inferior frontal gyrus and right superior temporal gyrus, whereas greater vocabulary knowledge was associated with lower damping ratio in the right inferior frontal gyrus. These findings suggest changes in neural tissue composition are sensitive to malleable aspects of the environment, whereas tissue organization is more strongly associated with vocabulary outcome. Notably, these associations were independent of maternal education, suggesting more proximal measures of a child’s environment may be the source of differences in neural tissue structure underlying variability in vocabulary outcomes.
Introduction
Environmental and biological factors shape children’s vocabulary development (McLaughlin & Gabard-Durnam, Citation2022; Raizada, Richards, Meltzoff, & Kuhl, Citation2008). Differences in the environment, such as the quantity and quality of maternal input (defined here as more complex utterances and more diverse words) are known to impact children’s vocabulary knowledge (Hart & Risley, Citation1995). Quality of maternal input has been shown to be more strongly associated with children’s vocabulary development than the sheer quantity of input (Hirsh-Pasek et al., Citation2015; Masek, Ramirez, McMillan, Hirsh-Pasek, & Golinkoff, Citation2021; Pan, Rowe, Singer, & Snow, Citation2005; Rowe, Meredith, Citation2012; Zhang, Citation2020). Hearing more decontextualized language (e.g. talk of past and future), more complex syntax, and experiencing more conversational turn taking in early childhood are all associated with more positive language outcomes (Hadley et al., Citation2017; Romeo et al., Citation2018; Rowe, Meredith, Citation2012; Silvey, Demir-Lira, Goldin-Meadow, & Raudenbush, Citation2021). Variations in the input children hear from their mother impact brain development and are consistently shown to account for differences in vocabulary based on socioeconomic status (Fernald & Weisleder, Citation2011; Luo, Masek, Alper, & Hirsh-Pasek, Citation2022; Luo et al., Citation2021; Weisleder & Fernald, Citation2013). Yet how the quality of input children hear relates to the structure and integrity of neural tissue during early brain development, and in turn vocabulary, remains unknown.
It is well accepted that language skills support school readiness and are the strongest predictors of later academic success (Morgan, Farkas, Hillemeier, Hammer, & Maczuga, Citation2015; Pace, Alper, Burchinal, Golinkoff, & Hirsh-Pasek, Citation2019). Vocabulary knowledge has been shown to be especially foundational for later academic success, with several studies documenting the link between early vocabulary and later reading achievement (Morgan et al., Citation2015; Network, Citation2007; Storch & Whitehurst, Citation2002), the development of code-related skills (such as print knowledge and phonological awareness; Mitchell & Brady, Citation2013), and the ability to learn new words (Maguire et al., Citation2018). Vocabulary knowledge has also been shown to be highly susceptible to differences in the home language environment. Hearing more decontextualized language (e.g. talk of past and future), more complex syntax, the use of more questions, and experiencing more conversational turn taking in early childhood are all associated with more positive gains in vocabulary knowledge (Hirsh-Pasek et al., Citation2015; Luo et al., Citation2022; Rowe, Citation2013; Silvey et al., Citation2021). While both the quantity and quality of maternal input have gained notoriety for their role in the “vocabulary gap” between children from higher and lower SES homes, significant variability within SES exists in both the input they hear and their vocabulary outcomes (Alper et al., Citation2021; Hirsh-Pasek et al., Citation2015; Masek et al., Citation2021; Pan, Rowe, Singer, & Snow, Citation2005; Schwab & Lew-Williams, Citation2016). The input children hear is a more direct measure of children’s environments, is more modifiable than SES (Alper et al., Citation2021), and is consistently shown to account for SES-based differences in vocabulary (Luo et al., Citation2021; Weisleder & Fernald, Citation2013). Thus, the focus of research studies investigating individual differences in vocabulary outcome should consider the quality of input children hear, above and beyond the influence of SES.
These early differences in the home language environment are not only thought to shape children’s vocabulary outcomes but also their underlying neural architecture. The social gating hypothesis was one of the earliest theories providing evidence of the neural correlates underlying the association between maternal input and vocabulary outcome (Kuhl, Citation2007). Under this theory, infants require language input to discriminate and learn native speech sounds, a relationship which is predictive of later vocabulary outcome (Newman, Rowe, & Bernstein Ratner, Citation2016; Rivera-Gaxiola, Klarman, Garcia-Sierra, & Kuhl, Citation2005; Singh, Steven Reznick, & Xuehua, Citation2012; Wang, Seidl, & Cristia, Citation2021) and subserved by differences in activation of auditory brain regions (Kuhl, Citation2004, Citation2007, Citation2010; Kuhl, Ramírez, Bosseler, Lin, & Imada, Citation2014; Rivera-Gaxiola, Silva-Pereyra, & Kuhl, Citation2005). More complex, interactive features of the language input have also been shown to relate to neural development. For instance, children who experience more conversational turn-taking with their parents have been shown to have greater left perisylvian cortical surface area (Merz, Maskus, Melvin, He, & Noble, Citation2019), greater functional activity in the left inferior frontal gyrus (Romeo et al., Citation2018), and stronger, more coherent white matter connectivity in the left arcuate fasciculus connecting the left inferior frontal gyrus with the left posterior superior temporal gyrus (Romeo et al., Citation2018). Differences in brain development have also been associated with vocabulary outcomes. The pace of vocabulary development is predictive of connectivity in the bilateral arcuate fasciculus (Su et al., Citation2018) and cortical thickness in the left supramarginal gyrus, which is connected to the inferior frontal gyrus and the ventral premotor cortex via the superior longitudinal fasciculi (Asaridou, Demir-Lira, Goldin-Meadow, & Small, Citation2017). Neural activation in bilateral cortical regions in the temporal lobe (superior temporal gyri and middle temporal gyri) has also been associated with more successful word learning (Chandrasekaran, Kraus, & Wong, Citation2012; Wong & brain, Citation2007).
Despite a wealth of research investigating the association between maternal input and brain development, maternal input and language outcome, and language outcome and brain development, only three studies to date have investigated the association between all three components. In the same sample of 4- to 6-year-old children, Romeo and colleagues reported that children who experience more conversational turn-taking with their parents had better general language abilities (composite language scores were created by averaging the PPVT-4 standard score and the CELF-5 CLS subtests), and this relationship was mediated by greater functional activity (Romeo et al., Citation2018) and stronger, more coherent white matter connectivity (Romeo et al., Citation2018) in language-related regions. Building on these groundbreaking studies, Merz et al. (Citation2019) found that language input was associated with reading skills via left perisylvian surface area in a sample of 5- to 9-year-olds. Although these studies are foundational to our understanding of neural pathways by which language experiences shape language outcomes, the current study builds on them by assessing how language experiences shape brain tissue integrity and subsequent vocabulary development.
In this study, we use magnetic resonance elastography (MRE) to assess brain tissue integrity through its mechanical properties (Muthupillai et al., Citation1995), which provide sensitive metrics of brain development related to functional outcomes (Johnson & Telzer, Citation2018; McIlvain, Schwarb, Cohen, Telzer, & Johnson, Citation2018). MRE is a noninvasive phase contrast-based MRI technique that produces quantitative maps of brain mechanical properties including viscoelastic shear stiffness and damping ratio. These measures describe the makeup and organization of the glial matrix and are thought to reflect brain health (Hiscox, Schwarb, McGarry, & Johnson, Citation2021; Sack, Jöhrens, Würfel, & Braun, Citation2013). Specifically, stiffness is considered to reflect tissue composition, including myelin content, neuron density, and glial matrix integrity, all of which change regionally during development (Guo et al., Citation2019). Brain tissue stiffness is strongly affected by aging and neurodegeneration (Hiscox et al., Citation2021; Murphy, Huston, & Ehman, Citation2019) but has been shown to be modified by environmental inputs, such as engagement in exercise (Sandroff, Johnson, & Motl, Citation2017; Schwarb et al., Citation2017). Damping ratio describes the relative viscous-to-elastic component of the tissue and is thought to reflect the microstructural organization of the tissue (Johnson et al., Citation2013; Testu et al., Citation2017). Damping ratio has been associated with cognitive performance, as damping ratio of the hippocampus is related to memory outcomes (Daugherty, Schwarb, McGarry, Johnson, & Cohen, Citation2020; Hiscox et al., Citation2020; Schwarb et al., Citation2017; Schwarb, Johnson, McGarry, & Cohen, Citation2016; Schwarb, Nail, & Schumacher, Citation2015), and damping ratio of frontal cortex regions relating to fluid intelligence and rule-learning (Johnson et al., Citation2018; Schwarb et al., Citation2019). In neurotypical adult populations, higher stiffness and lower damping ratio are generally associated with greater brain health and stronger performance on functional tasks. In the pediatric population, mechanical property development has been less well-studied (Johnson et al., Citation2018). It is expected that maturation does affect brain properties, both globally and regionally, and that improved mechanical properties will relate brain function similarly as in an adult population, but more work is required to understand these concepts definitively.
MRE offers an innovative, noninvasive approach to understanding the neural tissue microstructure associated with maternal language input and vocabulary outcome. We utilize this approach in the current study to investigate a) whether the quality of maternal language input relates to stiffness or damping ratio and b) if differences in these neural measures are associated with vocabulary outcome in children ages 5–7 years. We focus on three regions-of-interest (ROIs) implicated in language processing: the inferior frontal gyrus (IFG), the superior temporal gyri (STG), and the supramarginal gyrus (SMG). The findings from the current study seek to inform our understanding of the neural structure underlying known associations between language input and vocabulary outcome.
Methods
Participants
Twenty-five children, ages 5–7 years old (Mage = 6.5, SDage = 1.03, number of females = 14) from the mid-Atlantic region of the United States participated in the current study. All participants were right-handed, monolingual English speakers, with no history of neurological disorder or developmental delay based on parental self-report. Parents also reported no familial history of reading or language delay or disorder. Maternal education was collected based on self-report and was categorized into the following five categories: Did not complete high school (N = 3), Some college but no degree/certification (N = 5), Associate Degree (N = 3), Bachelor Degree (N = 9), and Graduate Degree (N = 5). Information related to children’s age, race, ethnicity, vocabulary knowledge, phonological processing ability, and home experiences across maternal education levels can be found in . The study was approved by the Institutional Review Board at the University of Delaware and was in compliance with the Declaration of Helsinki. Parental consent and child assent were obtained for all individuals who participated in the current study.
Table 1. Demographic information for the current sample across levels of Maternal Education. In addition to measures of age, ethnicity, and race, the current study collected four additional measures of the home environment: 1) Quality of Neighborhood: on a scale of 1–5, parents rated their satisfaction with their neighborhood, local schools, safety, amenities, and home. 2) Number of Books in the home. 3) The number of social services utilized out of the following list: income tax credit, SNAP, WIC, government housing, school lunch, and welfare cash. 4) Generational Experiences: on a scale of 1–4, parents rated whether they had stable finances, food to eat, a stable caregiver, and grew up in a safe neighborhood during their own childhood. Children also completed the CTOPP as a measure of their phonological processing.
Using the cohen.ES function from the pwr package (Cohen, Citation1988) of R (RStudio Team, Citation2020), we calculated that an effect size of .5 was appropriate to reliably detect large effects across all brain-behavior correlations. Using this information, we calculated the sample size necessary to execute correlation analyses in the current study using the pwr.r.test function. With a significance level at 0.05, power at 80%, and estimated effect size (r) of 0.5, we calculated that a sample size of 22.6 was necessary to detect reliable one-tail effects. Therefore, although the current sample was small, we have sufficient power to identify large effects in all subsequent analyses.
Maternal language input
Mothers and children completed a 15–20 minute semi-structured play session in the lab to measure maternal language input. This play session was similar to the Three Box Task utilized in Hirsh-Pasek et al. (Citation2015). In this task, children and mothers are presented with three boxes, each containing a different toy. The three toys selected for the current study were age-appropriate and chosen to elicit conversation between mothers and children. The toys included a pair of dinosaur puppets, a fishing game with fish of different colors, and a magnetic Lego activity that included numbers for counting. Mothers and children were instructed to open the boxes in the order which they were presented (i.e. Box 1, then Box 2, then Box 3). The order of the boxes was counterbalanced across children. The researcher left the room during this time and interactions were recorded using a Sony mini HD 1080p camera. Trained research assistants transcribed the video footage collected during the play session using the Systematic Analysis of Language Transcripts (SALT) software program. The first author transcribed 20% of the same transcripts to establish reliability across transcriptions. There was high agreement among raters in their coding of the variables of interest (R = 99.90, p < .001).
SALT generates the number of total words, number of different words, and mean length utterance for both children and mothers. To limit the number of comparisons, the current study focused on quality, rather than quantity of maternal input. Quality has been shown to be more predictive of child outcomes (Hirsh-Pasek et al., Citation2015; Masek et al., Citation2021; Pan et al., Citation2005; Rowe, Meredith, Citation2012; Zhang, Citation2020). Both mean length of utterance and number of different words produced by parents are common measures of quality examined in studies of language input and are automatically generated by SALT. SALT also generates the number of utterances produced by both the child and adult. Since children’s own language use is shown to influence their mothers’ subsequent language input (Huttenlocher, Waterfall, Vasilyeva, Vevea, & Hedges, Citation2010) and to ensure that higher quality interactions were not solely attributed to greater input by the mother or child, number of mother and child utterances were included as covariates in the current study (similar to Romeo et al., Citation2018).
Vocabulary
Vocabulary knowledge was measured using the Picture Vocabulary Task (PVT) within the NIH Toolbox. On an iPad, children were shown four pictures on a single screen while simultaneously hearing a word. They were then instructed to select the picture that best matches the word they heard. Children were permitted as much time as necessary to provide their responses. Items in this task are calibrated to an item response theory (IRT) model (Revicki & Cella, Citation1997), allowing for an individually tailored assessment wherein correct responses led to more difficult items, and incorrect responses led to easier items. This IRT is used to score the assessment. A theta score is calculated for each participant based on their overall ability or performance on the assessment. From this theta score, an Uncorrected Standard Score and Age-Corrected Standard Score are computed. Uncorrected Standard Scores representing the participant’s performance in comparison to the census-matched US population and are most useful when trying to identify individual performance independent of age, gender, or other demographic factors. These Uncorrected Standard Scores were used as the primary outcome measure.
Imaging
Image data was acquired at the University of Delaware using a Siemens 3-Tesla Prisma MRI scanner and 64-channel head RF-receiver coil. The protocol included MRE and anatomical images. Prior to MRE, whole-head, high resolution structural images, including a T1-weighted, magnetization-prepared rapid gradient-echo (MPRAGE) anatomical volume (TR/TI/TE = 2500/1070/2.9 ms, voxel resolution = 1.0 mm isotropic, FOV = 256 × 256 mm2, 176 sagittal slices) were collected. The MRE data were acquired using a single-shot 2D-EPI sequence at 2.5 mm isotropic resolution. Vibrations were delivered to the posterior of the skull at 50 Hz using a pneumatic actuator system with a passive pillow driver (Resoundant, Inc.; Rochester, MN). The MRE sequence uses motion encoding gradients to image displacement of tissue into the phase of the MRI image through synchronization with applied motion, and four time points are acquired by changing the synchronization over one period of vibration. Additional imaging parameters include: FOV = 240 × 240 mm2, matrix size 96 × 96, 48 slices, TR/TE 6720/69 ms, GRAPPA R = 3. The MRE scan time was 3 minutes and 35 seconds, which has previously been shown to be suitable for scanning children (Chaze, Citation2019). Participants watched an age-appropriate movie during the MRI scan to help avoid excessive motion.
Image processing
In an effort to promote the reproducibility of our neuroimaging data, all anatomical data were first converted using HeuDiConv (https://github.com/nipy/heudiconv, Halchenko et al., Citation2021) and then organized to cohere with the Brain Imaging Data Structure (BIDS; Gorgolewski et al., Citation2016). The T1-weighted (T1w) image was corrected for intensity non-uniformity (INU) within fMRIPrep 1.3.1 (Esteban et al., Citation2019). The T1w-reference was then skull-stripped and brain surfaces were reconstructed using Freesurfer. Brain tissue segmentation of cerebrospinal fluid (CSF), white matter (WM), and gray matter (GM) was performed on the brain-extracted T1w images.
The MRE complex displacement fields were calculated from the phase images after unwrapping with FSL PRELUDE (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, Citation2012) and temporal Fourier filtering. A nonlinear inversion algorithm (NLI) (McGarry et al., Citation2012) was used to calculate maps of shear stiffness and damping ratio from the MRE displacement fields. NLI uses displacement images to create whole-brain mechanical property maps of the complex viscoelastic shear modulus, G* = G’+iG”, where G’ is the storage modulus and G” is the loss modulus. The viscoelastic shear stiffness, µ, is calculated as µ = 2|G*|2/(G’+|G*|) (Manduca et al., Citation2001) and damping ratio, ξ, is calculated as ξ = G”/2 G’ (McGarry & Van Houten, Citation2008). Data quality was confirmed by calculating octahedral shear strain signal-to-noise ratio (OSS-SNR) (McGarry et al., Citation2011), which is an MRE-specific measure of SNR.
Average mechanical property values in regions-of-interest (ROIs) were determined, including the inferior frontal gyrus (IFG), the superior temporal gyrus (STG), and the superior marginal gyrus (SMG; ), which were identified with the Harvard Oxford Cortical Atlas. All standard space ROIs were registered to the subject-specific, corrected MPRAGE anatomical scans using a FSL FNIRT nonlinear registration (Andersson et al., Citation2008) and were subsequently registered to MRE native space using a linear affine transformation with 6 degrees of freedom in FSL FLIRT (Jenkinson, Bannister, Brady, & Smith, Citation2002). Hemispheric masks were used to decouple the left and right components of each ROI. Regional mechanical properties were extracted by using each unilateral ROI mask as spatial information during property estimation with NLI through soft prior regularization (SPR; McGarry et al., Citation2013). Cerebrospinal fluid (CSF) was segmented from the 2 mm MNI standard-space template using FSL FAST (Zhang, Brady, & Smith, Citation2001). Any pixel with greater than 25% CSF was excluded from analysis, and the remaining region was spatially averaged to obtain one average stiffness and one average damping ratio value for each subject in each ROI.
Figure 1. Regions included in the current analysis: inferior frontal gyrus (IFG), superior temporal gyrus (STG), and superior marginal gyrus (SMG). a) ROIs mapped on a single subject’s T1 scan alongside their corresponding whole brain b) stiffness map and c) damping ratio map. Stiffness and damping ratio were extracted for each unilateral ROI.
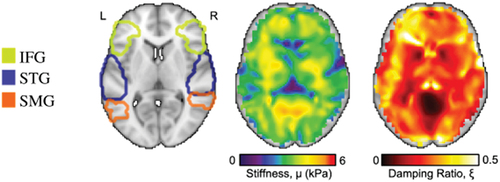
After image processing, one participant was identified as an outlier as their stiffness values across nearly all ROIs were 1.5 standard deviations above the Inter-Quartile Range, while damping ratio values across nearly all ROIs were 1.5 standard deviations below. Their data was removed from subsequent analyses, resulting in a total of 24 participants (Mage = 6.4, SDage = 1.02, number of females = 13).
Results
Association between brain viscoelasticity and language input
Children on average heard 649.75 total words from their mother during the play session (SD = 343.62). Children heard an average of 208.29 different words (NDW; SD = 65.41), and mothers produced an average mean length utterance of 4.57 words (MLU; SD = 0.53) during the play session. Since the maternal mean length of utterance and number of different words were not highly correlated within this sample (R = −0.05, p = .83), we examined them independently. Both of these values were z-scored in subsequent analyses. Neither the quantity (R = 0.13, p = .567), NDW (R = −0.08, p = .73), or MLU (R = 0.08, p = .73) of maternal language input was associated with maternal education in the current sample.
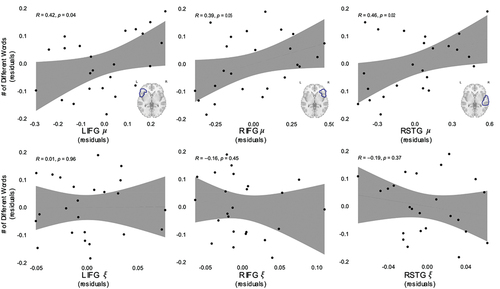
Correlation analyses were conducted to investigate the association between NDW produced by the mother and viscoelastic measures of stiffness and damping ratio within each ROI. Age, gender, maternal education, and the number of utterances produced by both the child and adult were included as covariates. illustrates partial Pearson’s correlations between ROI stiffness and NDW, which were significant in the Left Inferior Frontal Gyrus (LIFG; rpartial = 0.47, p = .04), Right Inferior Frontal Gyrus (RIFG; rpartial = 0.44, p = .05), and Right Superior Temporal Gyrus (rpartial = 0.48, p = .03). Correlations between damping ratio and NDW were not significant in these same regions (LIFG; rpartial = −0.03, p = .90; RIFG; rpartial = −0.15, p = .52; RSTG; rpartial = −0.27, p = .24; ). These correlations did not withstand Holm correction and no other regions exhibited significant correlation with NDW in either stiffness or damping ratio (see Supplementary Table 1 for all comparisons). No associations between MLU and viscoelastic properties were significant (see Supplementary Table 1 for all comparisons).
Figure 2. Number of different words spoken by mothers is positively associated with stiffness (µ) in the LIFG, RIFG, and RSTG gyrus. Damping ratio () of the same regions was not associated with maternal language input. All values plotted are residuals from partial correlations accounting for age, maternal education, and the number of utterances produced by both the child and adult.
Association between brain viscoelasticity and vocabulary outcome
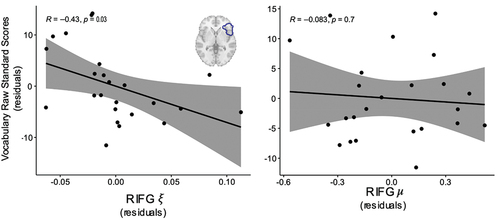
All children performed well on the picture vocabulary test (Uncorrected Standard Score; M = 70.58, SD = 7.25). An Age-Corrected Standard Score of 100 (SD = 15) is considered average, with participants in the current study performing similarly to this standard (M = 104.29, SD = 16.58). Vocabulary scores were moderately correlated with NDW (rpartial = 0.39, p = .05), but not with NTW (rpartial = −0.10, p = .64) or MLU (rpartial = 0.001, p = .99), when controlling for age, gender, maternal education, and total number of utterances produced by both the parents and child. Similar to the previous analysis, we examined the partial correlation between viscoelastic measures of stiffness and damping ratio for each ROI and Uncorrected Standard Vocabulary scores, with age and maternal education included as covariates. Interestingly, damping ratio in the RIFG was significantly related to child vocabulary knowledge (rpartial = −0.44, p = .04; ), with a lower damping ratio being associated with improved vocabulary. Stiffness in this same region had no association with vocabulary (rpartial = −0.08, p = .72; ). These correlations did not withstand Holm correction (seen in Supplementary Table 1), and no other regions exhibited significant correlation with vocabulary knowledge in either stiffness or damping ratio (see Supplementary Table 2 for all comparisons).
Figure 3. Damping ratio () in the RIFG is negatively associated with child vocabulary outcomes when controlling for age and maternal education. Stiffness (µ) was not associated with child vocabulary outcomes. All values plotted are residuals from partial correlations accounting for age and maternal education.
Association between viscoelastic measures of stiffness and damping ratio
Due to the inverse association between viscoelastic measures of stiffness and damping ratio with measures of language input and vocabulary, we sought to examine the association between the two viscoelastic measures within each ROI. We conducted six pairwise Pearson’s correlations between stiffness and damping ratio for each ROI, controlling for age and maternal education. Apart from the LSMG (rpartial = −0.48, p = .03), stiffness and damping ratio were not correlated in any ROI (see Supplementary Table 1).
Discussion
Differences in the quality of maternal language input children are exposed to has been shown to have a considerable effect on their subsequent vocabulary knowledge. Studies have indicated that differences in brain function and structure underlie this association; however, the current study is the first to identify how viscoelastic neural tissue properties contribute to this relationship. Brain mechanical properties, including both stiffness and damping ratio, change regionally during development (McIlvain et al., Citation2018) and are highly sensitive to brain health and function. Using MRE, we found that higher quality input, measured as number of different words, was associated with greater stiffness in three ROIs (right and left IFG and right STG). The RIFG was also a significant predictor of a child’s vocabulary: lower damping ratio in this region was associated with greater vocabulary knowledge. Notably, these associations were independent of maternal education, echoing past findings, which advocate for the use of more direct measures of a child’s language environment (Pace, Luo, Hirsh-Pasek, & Golinkoff, Citation2017; Romeo et al., Citation2018).
The LIFG has been identified as an important region for language processing in several neuroimaging studies. Functional connectivity (Jasińska et al., Citation2021; King, Camacho, Montez, Humphreys, & Gotlib, Citation2021), activation (Friederici, Citation2011; Romeo et al., Citation2018), cortical thickness and surface area (Brito, Citation2017; Merz et al., Citation2019), and connectivity of white matter tracts (Asaridou et al., Citation2017; Romeo et al., Citation2018) in the LIFG are all associated with processing of linguistic information. In the current study, we expand on these past findings by providing the first evidence of an association between brain stiffness and vocabulary knowledge in the LIFG using MRE. MRE offers a sensitive assessment of brain microstructural integrity, reflecting important aspects of brain health as MRE measures are thought to capture myelin composition, neuronal density, and glial matrix organization. In contrast, volumetric measures also provide a method to assess tissue structure, however large-scale volume changes are likely less sensitive than MRE measures to tissue microstructure. For example, several studies have measured memory and cognitive function, and have found correlations between task performance and damping ratio, despite lack of findings with volumetric measures (Hiscox et al., Citation2020; Schwarb et al., Citation2016). Sensitive metrics of brain microstructural health can be critical in detecting neuromechanical deficits resulting in reduced function in atypically developing populations.
With the exception of the LIFG, all significant associations in the current study were driven by differences in the right hemisphere. While less reported, the RIFG and RSTG have also been implicated in language. Evidence from clinical populations has indicated that the right fronto-temporal network might be utilized when impairments in left-sided functions exist (Chapman, Max, Gamino, McGlothlin, & Cliff, Citation2003; Fernandez et al., Citation2004; Rota et al., Citation2009; Weiller et al., Citation1995). Additionally, neural activation in bilateral regions of the temporal lobe have been associated with more successful word learning (Chandrasekaran et al., Citation2012; Wong & brain, Citation2007). Specifically, higher-level language learning engages the right hemisphere; thus, successful language learning is contingent upon coordination within and between both hemispheres (Qi & Legault, Citation2020). The current findings add evidence to this view as the neural tissue integrity of bilateral language-related brain regions may underlie past associations between structural and functional differences in these same regions and language outcomes.
Many previous studies have focused on associations between socioeconomic status (SES) and language; however, SES is a broadly defined construct, composed of a range of correlated environmental factors, which differentially exert their influence on brain and language development. One of these factors is maternal language input, which offers a more proximal measure of a child’s language development, while the outcome – vocabulary knowledge – can be considered as a more distal measure. The findings in this work provide correlational evidence suggesting tissue stiffness is sensitive to more malleable aspects of the environment, whereas brain tissue damping ratio is more strongly associated with outcome measures, such as vocabulary. It is particularly interesting that we observed correlations between input and stiffness and vocabulary and damping ratio in the same RIFG region, suggesting that these two measures capture different aspects of underlying tissue structure that are uniquely affected by the environment or affect outcome. Given that both our study and past longitudinal studies have shown how variability in the language input predicts differences in later vocabulary knowledge (Rowe, Citation2013; Rowe, Meredith, Citation2012), it is plausible that changes in stiffness may later manifest in tissue damping ratio supporting vocabulary, though in our sample the two measures are not significantly correlated in the RIFG, and we are limited in our ability to draw causal conclusions due to the correlational nature of the analyses. Although mediation analyses would better address this question, we do not have sufficient power with our sample size. Despite this limitation, our findings still have clear practical implications. Substantial intervention efforts have been made to increase the amount of language parents address to their children (e.g. quantity, mean length of utterance), but our findings speak to the efficacy of programs, which encourage variation in the way parents talk with their children (e.g. number of different words; Leech & Rowe, Citation2020; Leech, Wei, Harring, & Rowe, Citation2018; Romeo et al., Citation2018).
Previous MRE studies of healthy adult subjects have indicated that higher stiffness and lower damping ratio are correlated with improved performance on functional tasks. The current findings agree that greater stiffness and lower damping ratio are correlated with better language input and output. However, there have been only a very limited number of publications, which analyze how brain mechanical properties change between childhood and adulthood, and these studies are not comprehensive. Brain tissue properties change regionally, but subtly, as the brain matures (McIlvain et al., Citation2018; Ozkaya et al., Citation2021; Yeung, Jugé, Hatt, & Bilston, Citation2019). There is only one previous study examining neural correlations of behavior with MRE in the pediatric brain, which indicated maturational imbalance in regional mechanical properties related to a cognitive assessment of risk-taking behavior (McIlvain et al., Citation2020). The current study is also the first recorded use of MRE in a sample of typically developing children all under the age of 8 years old. The current study is the first to provide evidence of the development of brain mechanical properties in children of such a young age, and the role these neural differences have upon language development. Although our findings shed light on tissue brain health in a typically developing population, additional research is needed to establish the trajectory of brain stiffness and damping ratio across the lifespan. Future research should continue to examine viscoelastic neural properties in the context of development and the role proximal aspects of a child’s language environment have upon subsequent brain and language development.
Many of the neural regions highlighted as significant in the current study are known to correspond with reading development. Although families reported no history of reading or language delay or disorder in the current study, it should not be overlooked that differences in viscoelastic neural properties may underlie known SES-related neural differences associated with reading development (Merz, Maskus, Melvin, He, & Noble, Citation2020; Noble, Wolmetz, Ochs, Farah, & McCandliss, Citation2006; Romeo et al., Citation2018). While the current study collected measures of reading on the Test of Word Reading Efficiency (TOWRE-2), only 13 children were able to complete this measure due to their age and reading level. Despite the lack of reading measures in the current study, based on the reciprocal hypothesis (Nagy, Townsend, Lesaux, & Schmitt, Citation2012; Stanovich & Cunningham, Citation1993; Wagner & Meros, Citation2010), early differences in vocabulary knowledge likely influence later reading comprehension, and vice versa. Therefore, we speculate that early differences in neural tissue underlying vocabulary outcome may have a trickle-down effect on subsequent reading development. Another limitation of the current study relates to the fact that no observed correlations remained statistically significant following Holm corrections for multiple comparisons, indicating our results should be interpreted with caution. While our power analysis indicates we have a sufficient sample size to reveal large effects, these preliminary findings need to be replicated in a larger sample in the future.
Mounting empirical evidence has indicated that the quantity and quality of language children hear in the home, as well as their brain structure and function, vary on the basis of SES (i.e. surface area, cortical thickness, gray matter volume; for review, see Brito & Noble, Citation2014; Hackman & Farah, Citation2009; Tomalski et al., Citation2013); however, our findings build on recent evidence that significant variability within and across SES exists (Alper et al., Citation2021; Masek et al., Citation2021; Hirsh-Pasek et al., Citation2015; Song, Spier & Tamis-LeMonda, Citation2014; Hurtado, Marchman & Fernald, Citation2008). Studies of representationally biased convenience samples may uncover “core” neural patterns that are not truly characteristic of all children across vocabulary ability or SES. It is important to identify these individual differences, as individual variability in the language children hear in the home may influence brain microstructural health, resulting in reduced neural function in language-related regions.
Understanding how MRE measures relate to language input and performance at an early age can provide critical information toward identifying the need for early intervention strategies to lessen this neurodevelopmental gap. Specifically, in the current study, we found that the number of different words children heard, but not the mean length of mothers’ utterances, was associated with increased stiffness. Thus, targeting word diversity, rather than complexity of utterances, may foster neurodevelopment in brain regions associated with vocabulary outcomes.
Supplemental Material
Download MS Word (17.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
Data and analysis scripts are publicly available at: https://github.com/juliagoolia28/manuscripts/blob/master/mre_vocab/readme.md
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Alper, R. M., Beiting, M., Luo, R., Jaen, J., Peel, M., Levi, O., Robinson, C & Hirsh-Pasek, K. (2021). Change the things you can: Modifiable parent characteristics predict high-quality early language interaction within socioeconomic status. Journal of Speech, Language, and Hearing Research, 64(6), 1992–2004. doi:10.1044/2021_JSLHR-20-00412
- Andersson, J. L. R., Smith, S. M., & Jenkinson, M. (2008). FNIRT - FMRIB; non-linear image registration tool. In Fourteenth Annual Meeting of the Organization for Human Brain Mapping, Melbourne, Australia (p. 496).
- Asaridou, S. S., Demir-Lira, Ö. E., Goldin-Meadow, S., & Small, S. L. (2017). The pace of vocabulary growth during preschool predicts cortical structure at school age. Neuropsychologia, 98, 13–23. doi:10.1016/j.neuropsychologia.2016.05.018
- Brito, N. H., & Noble, K. G. (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8(SEP), 276. doi:10.3389/fnins.2014.00276
- Brito, N. H. (2017). Influence of the home linguistic environment on early language development. Policy Insights from the Behavioral and Brain Sciences, 4(2), 155–162. doi:10.1177/2372732217720699
- Champely, S., Ekstrom, C., Dalgaard, P., Gill, J., Weibelzahl, S., Anandkumar, A., and De Rosario, M. H. (2018). Package ‘pwr’. R package version, 1(2).
- Chandrasekaran, B., Kraus, N., & Wong, P. C. M. (2012). Human inferior colliculus activity relates to individual differences in spoken language learning. Journal of Neurophysiology, 107(5), 1325–1336. doi:10.1152/JN.00923.2011
- Chapman, S. B., Max, J. E., Gamino, J. F., McGlothlin, J. H., & Cliff, S. N. (2003). Discourse plasticity in children after stroke: Age at injury and lesion effects. Pediatric Neurology, 29(1), 34–41. doi:10.1016/S0887-8994(03)00012-2
- Chaze, C. A., McIlvain, G., Smith, D. R., Villermaux, G. M., Delgorio, P. L., Wright, H. G., and Johnson, C. L. (2019). Altered brain tissue viscoelasticity in pediatric cerebral palsy measured by magnetic resonance elastography. NeuroImage: Clinical, 22, 101750
- Daugherty, A. M., Schwarb, H. D., McGarry, M. D. J., Johnson, C. L., & Cohen, N. J. (2020). MR elastography of human hippocampal subfields: CA3-Dentate gyrus viscoelasticity predicts relational memory accuracy. Journal of Cognitive Neuroscience, 32(9), 1704. doi:10.1162/JOCN_A_01574
- Esteban, O., Markiewicz, C. J., Blair, R. W., Moodie, C. A., Isik, A. I., Erramuzpe, A., Kent, J. D, Goncalves, M., DuPre, E., Snyder, M. Oya, H. & Gorgolewski, K. J. (2019). FMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16(1), 111. doi:10.1038/S41592-018-0235-4
- Fernald, A., & Weisleder, A. (2011). Early language experience is vital to developing fluency in understanding. Handbook of Early Literacy Research, 3, 3–19.
- Fernandez, B., Cardebat, D., Demonet, J. F., Joseph, P. A., Mazaux, J. M., Barat, M., & Allard, M. (2004). Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke, 35(9), 2171–2176. doi:10.1161/01.STR.0000139323.76769.B0
- Friederici, A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. doi:10.1152/PHYSREV.00006.2011/SUPPL_FILE/DESCRIPTIONS2.DOCX
- Gorgolewski, K. J., Auer, T., Calhoun, V. D., Craddock, R. C., Das, S., Duff, E. P., Flandin, G., Ghosh, S.S., Glatard, T., Halchenko, Y.O. Handwerker, D.A & Poldrack, R. A. (2016). The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Scientific Data 2016 3:1, 3(1), 1–9. doi:10.1038/sdata.2016.44
- Guo, J., Bertalan, G., Meierhofer, D., Klein, C., biomaterialia, S. S.-A., Steiner, B., Wang, S., da Silva, R.V., Infante-Duarte, C., Koch, S, and Sack, I. (2019). Brain maturation is associated with increasing tissue stiffness and decreasing tissue fluidity. Acta Biomaterialia, 99, 433–442. 2019
- Hackman, D. A., & Farah, M. J. (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. doi:10.1016/j.tics.2008.11.003
- Hadley, P. A., Rispoli, M., Holt, J. K., Papastratakos, T., Hsu, N., Kubalanza, M., & Mckenna, M. M. (2017). Input subject diversity enhances early grammatical growth: evidence from a parent-implemented intervention. Language Learning and Development, 13(1), 54–79. doi:10.1080/15475441.2016.1193020
- Halchenko, Y., Goncalves, M., Castello, M. V. D. O., Ghosh, S., Salo, T., Hanke, M., Meyer, K. (2021). nipy/heudiconv:. doi:10.5281/ZENODO.5557588
- Hart, B., & Risley, T. R. (1995). Meaningful differences in the everyday experience of young American children. Baltimore , USA: P.H. Brookes.
- Hirsh-Pasek, K., Adamson, L. B., Bakeman, R., Owen, M. T., Golinkoff, R. M., Pace, A., Yust, P.K. & Suma, K. (2015). The contribution of early communication quality to low-income children’s language success. Psychological Science, 26(7), 1071–1083. doi:10.1177/0956797615581493
- Hiscox, L. V., Johnson, C. L., McGarry, M. D. J., Schwarb, H., van Beek, E. J. R., Roberts, N., & Starr, J. M. (2020). Hippocampal viscoelasticity and episodic memory performance in healthy older adults examined with magnetic resonance elastography. Brain Imaging and Behavior, 14(1), 175–185. doi:10.1007/S11682-018-9988-8/FIGURES/2
- Hiscox, L. V., Schwarb, H., McGarry, M. D. J., & Johnson, C. L. (2021). Aging brain mechanics: Progress and promise of magnetic resonance elastography. NeuroImage, 232, 117889. doi:10.1016/J.NEUROIMAGE.2021.117889
- Hurtado, N., Marchman, V. A., & Fernald, A. (2008). Does input influence uptake? Links between maternal talk, processing speed and vocabulary size in Spanish‐learning children. Developmental science, 11(6), F31–F39.
- Huttenlocher, J., Waterfall, H., Vasilyeva, M., Vevea, J., & Hedges, L. V. (2010). Sources of variability in children’s language growth. Cognitive Psychology, 61(4), 343–365. doi:10.1016/j.cogpsych.2010.08.002
- Jasińska, K. K., Shuai, L., Lau, A. N. L., Frost, S., Landi, N., & Pugh, K. R. (2021). Functional connectivity in the developing language network in 4-year-old children predicts future reading ability. Developmental Science, 24(2), e13041. doi:10.1111/DESC.13041
- Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. doi:10.1016/S1053-8119(02)91132-8
- Jenkinson, M., Beckmann, C. F., Behrens, T. E. J. J., Woolrich, M. W., & Smith, S. M. (2012). Fsl. NeuroImage, 62(2), 782–790. doi:10.1016/j.neuroimage.2011.09.015
- Johnson, C. L., McGarry, M. D. J., Gharibans, A. A., Weaver, J. B., Paulsen, K. D., Wang, H., Georgiadis, J. G. (2013). Local mechanical properties of white matter structures in the human brain. NeuroImage, 79, 145–152. doi:10.1016/J.NEUROIMAGE.2013.04.089
- Johnson, C., & Telzer, E. (2018). Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain. Developmental Cognitive Neuroscience, 33, 176–181. doi:10.1016/j.dcn.2017.08.010
- Johnson, C. L., Schwarb, H., Horecka, K. M., McGarry, M. D. J., Hillman, C. H., Kramer, A. F., Cohen, N.J. & Barbey, A. K. (2018). Double dissociation of structure-function relationships in memory and fluid intelligence observed with magnetic resonance elastography. NeuroImage, 171, 99. doi:10.1016/J.NEUROIMAGE.2018.01.007
- King, L. S., Camacho, M. C., Montez, D. F., Humphreys, K. L., & Gotlib, I. H. (2021). Naturalistic language input is associated with resting-state functional connectivity in infancy. The Journal of Neuroscience, 41(3), 424–434. doi:10.1523/JNEUROSCI.0779-20.2020
- Kuhl, P. K. (2004). Early language acquisition: Cracking the speech code. Nature Reviews Neuroscience, 5(11), 831–841. doi:10.1038/nrn1533
- Kuhl, P. K. (2007). Is speech learning “gated” by the social brain? Developmental Science, 10(1), 110–120. doi:10.1111/j.1467-7687.2007.00572.x
- Kuhl, P. K. (2010). Brain mechanisms in early language acquisition. Neuron, 67(5), 713–727. doi:10.1016/j.neuron.2010.08.038
- Kuhl, P. K., Ramírez, R. R., Bosseler, A., Lin, J. F. L., & Imada, T. (2014). Infants’ brain responses to speech suggest Analysis by Synthesis. Proceedings of the National Academy of Sciences of the United States of America, 111(31), 11238–11245. doi:10.1073/pnas.1410963111
- Leech, K., Wei, R., Harring, J. R., & Rowe, M. L. (2018). A brief parent-focused intervention to improve preschoolers’ conversational skills and school readiness. Developmental Psychology, 54(1), 15–28. doi:10.1037/dev0000411
- Leech, K. A., & Rowe, M. L. (2020). An intervention to increase conversational turns between parents and young children. Journal of Child Language. doi:10.1017/S0305000920000252
- Luo, R., Pace, A., Levine, D., Iglesias, A., de Villiers, J., Golinkoff, R. M., Wilson, M.S & Hirsh-Pasek, K. (2021). Home literacy environment and existing knowledge mediate the link between socioeconomic status and language learning skills in dual language learners. Early Childhood Research Quarterly, 55, 1–14. doi:10.1016/j.ecresq.2020.10.007
- Luo, R., Masek, L. R., Alper, R. M., & Hirsh-Pasek, K. (2022). Maternal question use and child language outcomes: The moderating role of children’s vocabulary skills and socioeconomic status. Early Childhood Research Quarterly, 59, 109–120. doi:10.1016/J.ECRESQ.2021.11.007
- Maguire, M. J., Schneider, J. M., Middleton, A. E., Ralph, Y., Lopez, M., Ackerman, R. A., & Abel, A. D. (2018). Vocabulary knowledge mediates the link between socioeconomic status and word learning in grade school. Journal of Experimental Child Psychology, 166, 679–695. doi:10.1016/j.jecp.2017.10.003
- Manduca, A., Oliphant, T. E., Dresner, M. A., Mahowald, J. L., Kruse, S. A., Amromin, E., Felmlee, J.P., Greenleaf, J.F. & Ehman, R. L. (2001). Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Medical Image Analysis, 5(4), 237–254. doi:10.1016/S1361-8415(00)00039-6
- Masek, L. R., Ramirez, A. G., McMillan, B. T. M., Hirsh-Pasek, K., & Golinkoff, R. M. (2021). Beyond counting words: A paradigm shift for the study of language acquisition. Child Development Perspectives, 15(4), 274–280. doi:10.1111/CDEP.12425
- McGarry, M. D. J., & Van Houten, E. E. W. (2008). Use of a Rayleigh damping model in elastography. Medical and Biological Engineering and Computing, 46(8), 759–766. doi:10.1007/s11517-008-0356-5
- McGarry, M. D. J., Van Houten, E. E. W., Perrĩez, P. R., Pattison, A. J., Weaver, J. B., & Paulsen, K. D. (2011). An octahedral shear strain-based measure of SNR for 3D MR elastography. Physics in Medicine and Biology, 56(13), 153–164. doi:10.1088/0031-9155/56/13/N02
- McGarry, M. D. J., Houten, E. E. W. V., Johnson, C. L., Georgiadis, J. G., Sutton, B. P., Weaver, J. B., & Paulsen, K. D. (2012). Multiresolution MR elastography using nonlinear inversion. Medical Physics, 39(10), 6388–6396. doi:10.1118/1.4754649
- McGarry, M., Johnson, C. L., Sutton, B. P., Van Houten, E. E., Georgiadis, J. G., Weaver, J. B., & Paulsen, K. D. (2013). Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE transactions on medical imaging, 32(10), 1901–1909.
- McIlvain, G., Schwarb, H., Cohen, N. J., Telzer, E. H., & Johnson, C. L. (2018). Mechanical properties of the in vivo adolescent human brain. Developmental Cognitive Neuroscience, 34, 27–33. doi:10.1016/j.dcn.2018.06.001
- McIlvain, G., Clements, R. G., Magoon, E. M., Spielberg, J. M., Telzer, E. H., & Johnson, C. L. (2020). Viscoelasticity of reward and control systems in adolescent risk taking. NeuroImage, 215, 116850. doi:10.1016/J.NEUROIMAGE.2020.116850
- McLaughlin, K., & Gabard-Durnam, L. (2022 Experience-driven plasticity and the emergence of psychopathology: Amechanistic framework integrating development and the environment into the Research). Journal of psychopathology and clinical science,131(6): 575–587. doi:10.1037/abn0000598 .
- Merz, E. C., Maskus, E. A., Melvin, S. A., He, X., & Noble, K. G. (2019). Socioeconomic disparities in language input are associated with children’s language related brain structure and reading skills. Child Development, cdev.13239. doi:10.1111/cdev.13239
- Merz, E. C., Maskus, E. A., Melvin, S. A., He, X., & Noble, K. G. (2020). Socioeconomic disparities in language input are associated with children’s language-related brain structure and reading skills. Child Development, 91(3), 846–860. doi:10.1111/cdev.13239
- Mitchell, A. M., & Brady, S. A. (2013). The effect of vocabulary knowledge on novel word identification. Annals of Dyslexia, 63(3–4), 201–216. doi:10.1007/S11881-013-0080-1
- Morgan, P. L., Farkas, G., Hillemeier, M. M., Hammer, C. S., & Maczuga, S. (2015). 24-month-old children with larger oral vocabularies display greater academic and behavioral functioning at kindergarten entry. Child Development, 86(5), 1351–1370. doi:10.1111/cdev.12398
- Murphy, M. C., Huston, J., & Ehman, R. L. (2019). MR elastography of the brain and its application in neurological diseases. NeuroImage, 187, 176–183. doi:10.1016/J.NEUROIMAGE.2017.10.008
- Muthupillai, R., Lomas, D., Rossman, P. J., Greenleaf, J. F., Manduca, A., & Ehman, R. L. (1995). Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269 5232 1854–1857.
- Nagy, W., Townsend, D., Lesaux, N., & Schmitt, N. (2012). Words as tools: Learning academic vocabulary as language acquisition. Reading Research Quarterly, 47(1), 91–108. doi:10.1002/RRQ.011
- Network, N. E. C. C. R.; NICHD Early Child Care Research. (2007). Age of entry to kindergarten and children’s academic achievement and socioemotional development. Early Education and Development, 18(2), 337–368.
- Newman, R., Rowe, M. L., & Bernstein Ratner, N. (2016). Input and uptake at 7 months predicts toddler vocabulary: The role of child-directed speech and infant processing skills in language development. Journal of Child Language, 43(5), 1158–1173. doi:10.1017/S0305000915000446
- Noble, K. G., Wolmetz, M. E., Ochs, L. G., Farah, M. J., & McCandliss, B. D. (2006). Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science, 9(6), 642–654. doi:10.1111/j.1467-7687.2006.00542.x
- Ozkaya, E., Fabris, G., Macruz, F., Suar, Z. M., Abderezaei, J., Su, B., Laksari, K., Wu, L., Camarillo, D.B., Pauly, K.B. & Kurt, M. (2021). Viscoelasticity of children and adolescent brains through MR elastography. Journal of the Mechanical Behavior of Biomedical Materials, 115, 104229. doi:10.1016/J.JMBBM.2020.104229
- Pace, A., Luo, R., Hirsh-Pasek, K., & Golinkoff, R. M. (2017 Identifying pathways between socioeconomic status and language development). Annual Review of Linguistics 3 285–308 . doi:10.1146/annurev-linguistics-011516-034226
- Pace, A., Alper, R., Burchinal, M. R., Golinkoff, R. M., & Hirsh-Pasek, K. (2019). Measuring success: Within and cross-domain predictors of academic and social trajectories in elementary school. Early Childhood Research Quarterly, 46, 112–125. doi:10.1016/j.ecresq.2018.04.001
- Pan, B. A., Rowe, M. L., Singer, J. D., & Snow, C. E. (2005). Maternal correlates of growth in toddler vocabulary production in low-income families. Child Development, 76(4), 763–782. doi:10.1111/1467-8624.00498-i1
- Qi, Z., & Legault, J. (2020). Neural hemispheric organization in successful adult language learning: Is left always right? Psychology of Learning and Motivation 72 119–163 .
- Raizada, R. D. S., Richards, T. L., Meltzoff, A., & Kuhl, P. K. (2008). Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage, 40(3), 1392–1401. doi:10.1016/j.neuroimage.2008.01.021
- Revicki, D. A., & Cella, D. F. (1997). Health status assessment for the twenty-first century: Item response theory, item banking and computer adaptive testing. Quality of Life Research, 6(6) 6:6, 595–600. 10.1023/A:1018420418455.
- Rivera-Gaxiola, M., Silva-Pereyra, J., & Kuhl, P. K. (2005). Brain potentials to native and non-native speech contrasts in 7- and 11 -month-old American infants. Developmental Science, 8(2), 162–172. doi:10.1111/J.1467-7687.2005.00403.X
- Rivera-Gaxiola, M., Klarman, L., Garcia-Sierra, A., & Kuhl, P. K. (2005). Neural patterns to speech and vocabulary growth in American infants. NeuroReport, 16(5), 495–498. doi:10.1097/00001756-200504040-00015
- Romeo, R. R., Leonard, J. A., Robinson, S. T., West, M. R., Mackey, A. P., Rowe, M. L., & Gabrieli, J. D. E. (2018). Beyond the 30-million-word gap: Children’s conversational exposure is associated with language-related brain function. Psychological Science, 29(5), 700–710. doi:10.1177/0956797617742725. https://pubmed.ncbi.nlm.nih.gov/29442613
- Romeo, R. R., Segaran, J., Leonard, J. A., Robinson, S. T., West, M. R., Mackey, A. P., Yendiki, A., Rowe, M.L. & Gabrieli, J. D. E. (2018). Language exposure relates to structural neural connectivity in childhood. Journal of Neuroscience, 38(36), 7870–7877. doi:10.1523/JNEUROSCI.0484-18.2018
- Romeo, R. R., Christodoulou, J. A., Halverson, K. K., Murtagh, J., Cyr, A. B., Schimmel, C., Chang, P., Hook, P.E & Gabrieli, J. D. E. (2018). Socioeconomic status and reading disability: Neuroanatomy and plasticity in response to intervention. Cerebral Cortex, 28(7), 2297–2312. doi:10.1093/cercor/bhx131
- Rota, G., Sitaram, R., Veit, R., Erb, M., Weiskopf, N., Dogil, G., & Birbaumer, N. (2009). Self-regulation of regional cortical activity using real-time fMRI: The right inferior frontal gyrus and linguistic processing. Human Brain Mapping, 30(5), 1605–1614. doi:10.1002/HBM.20621
- Rowe, M. L. (2013). Decontextualized language input and preschoolers’ vocabulary development. Semin Speech Lang, 34(4), 260–266. doi:10.1055/s-0033-1353444
- Rowe, Meredith, L. (2012). A Longitudinal Investigation of the role of quantity and quality of child-directed speech in vocabulary development. Child Development, 83(5), 1762–1774. doi:10.1111/j.1467-8624.2012.01805.x
- RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com
- Sack, I., Jöhrens, K., Würfel, J., & Braun, J. (2013). Structure-sensitive elastography: On the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter, 9(24), 5672–5680. doi:10.1039/C3SM50552A
- Sandroff, B. M., Johnson, C. L., & Motl, R. W. (2017). Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: A novel application of magnetic resonance elastography. Neuroradiology, 59(1), 61–67. doi:10.1007/S00234-016-1767-X
- Schwab, J. F., & Lew-Williams, C. (2016). Language learning, socioeconomic status, and child-directed speech. Wiley Interdisciplinary Reviews: Cognitive Science, 7(4), 264–275. doi:10.1002/wcs.1393
- Schwarb, H., Nail, J., & Schumacher, E. H. (2015). Working memory training improves visual short-term memory capacity. Psychological Research, 80(1), 128–148. doi:10.1007/s00426-015-0648-y
- Schwarb, H., Johnson, C. L., McGarry, M. D. J., & Cohen, N. J. (2016). Medial temporal lobe viscoelasticity and relational memory performance. NeuroImage, 132, 534. doi:10.1016/J.NEUROIMAGE.2016.02.059
- Schwarb, H., Johnson, C. L., Daugherty, A. M., Hillman, C. H., Kramer, A. F., Cohen, N. J., & Barbey, A. K. (2017). Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. NeuroImage, 153, 179–188. doi:10.1016/J.NEUROIMAGE.2017.03.061
- Schwarb, H., Johnson, C. L., Dulas, M. R., McGarry, M. D. J., Holtrop, J. L., Watson, P. D., Wang, J.X., Voss, J.L., Sutton, B.P & Cohen, N. J. (2019). Structural and functional MRI evidence for distinct medial temporal and prefrontal roles in context-dependent relational memory. Journal of Cognitive Neuroscience, 31(12), 1857–1872. doi:10.1162/JOCN_A_01454
- Silvey, C., Demir-Lira, Ö. E., Goldin-Meadow, S., & Raudenbush, S. W. (2021). Effects of time-varying parent input on children’s language outcomes differ for vocabulary and syntax. Psychological Science, 32(4), 536–548. doi:10.1177/0956797620970559
- Singh, L., Steven Reznick, J., & Xuehua, L. (2012). Infant word segmentation and childhood vocabulary development: A longitudinal analysis. Developmental Science, 15(4), 482–495. doi:10.1111/j.1467-7687.2012.01141.x
- Song, L., Spier, E. T., & Tamis-Lemonda, C. S. (2014). Reciprocal influences between maternal language and children's language and cognitive development in low-income families. Journal of child language, 41(2), 305–326.
- Stanovich, K. E., & Cunningham, A. E. (1993). Where does knowledge come from? Specific associations between print exposure and information acquisition. Journal of Educational Psychology, 85(2), 211–229. doi:10.1037/0022-0663.85.2.211
- Storch, S. A., & Whitehurst, G. J. (2002). Oral language and code-related precursors to reading: Evidence from a longitudinal structural model. Developmental Psychology, 38(6), 934–947. doi:10.1037/0012-1649.38.6.934
- Su, M., Thiebaut de Schotten, M., Zhao, J., Song, S., Zhou, W., Gong, G., McBride, C., Ramus, F. & Shu, H. (2018). Vocabulary growth rate from preschool to school age years is reflected in the connectivity of the arcuate fasciculus in 14‐year‐old children. Wiley Online Library, 21(5), 12647. doi:10.1111/desc.12647
- Testu, J., McGarry, M. D. J., Dittmann, F., Weaver, J. B., Paulsen, K. D., Sack, I., & Van Houten, E. E. W. (2017). Viscoelastic power law parameters of in vivo human brain estimated by MR elastography. Journal of the Mechanical Behavior of Biomedical Materials, 74, 333–341. doi:10.1016/J.JMBBM.2017.06.027
- Tomalski, P., Moore, D. G., Ribeiro, H., Axelsson, E. L., Murphy, E., Karmiloff‐Smith, A., Johnson, M.H & Kushnerenko, E. (2013). Socioeconomic status and functional brain development–associations in early infancy. Developmental Science, 16(5), 676–687. doi:10.1111/desc.12079
- Wagner, R. K., & Meros, D. (2010). Vocabulary and reading comprehension: Direct, indirect, and reciprocal influences. Focus on Exceptional Children, 2010.
- Wang, Y., Seidl, A., & Cristia, A. (2021). Infant speech perception and cognitive skills as predictors of later vocabulary. Infant Behavior and Development, 62. doi:10.1016/j.infbeh.2020.101524
- Weiller, C., Isensee, C., Rijntjes, M., Huber, W., Müller, S., Bier, D., Dutschka, K, Woods, R.P., Noth, J & Diener, H. C. (1995). Recovery from wernicke’s aphasia: A positron emission tomographic study. Annals of Neurology, 37(6), 723–732. doi:10.1002/ANA.410370605
- Weisleder, A., & Fernald, A. (2013). Talking to children matters: Early language experience strengthens processing and builds vocabulary. Psychological Science, 24(11), 2143–2152. doi:10.1177/0956797613488145
- Wong, P., & brain, T. P.-H. (2007). Neural characteristics of successful and less successful speech and word learning in adults. Wiley Online Library, 28(10) 2007, undefined, 995–1006. 10.1002/hbm.20330.
- Yeung, J., Jugé, L., Hatt, A., & Bilston, L. E. (2019). Paediatric brain tissue properties measured with magnetic resonance elastography. Biomechanics and Modeling in Mechanobiology, 18(5), 1497–1505. doi:10.1007/S10237-019-01157-X
- Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. doi:10.1109/42.906424
- Zhang, Y. (2020). Quality matters more than quantity: Parent–child communication and adolescents’ academic performance. Frontiers in Psychology, 11, 1203. doi:10.3389/fpsyg.2020.01203
