Abstract
Electron paramagnetic resonance (EPR) spectroscopy was used to compare the rates of autoxidation at 37°C of acellular and liposome-encapsulated hemoglobin (LEH) crosslinked between alpha chains with bis (3,5-dibromosalicyl) fumarate (ααHb). This method avoids the difficulties inherent in using conventional ultraviolet-visible (UV-vis) spectroscopy caused by the high turbidity of liposome suspensions. Rate constants of 0.039/h and 0.065/h were obtained for the ααHb and LEH samples, respectively. Similar oxidation measurements with ααHb using UV-vis spectroscopy gave a rate constant comparable to that obtained with EPR spectroscopy. Indirect measurement of the oxidation kinetics of LEH utilizing extraction of ααHb with chloroform from partially oxidized LEH samples was unreliable because the amount of extractable hemoglobin was inversely proportional to the degree of oxidation. EPR measurements showed a shift in the g value and substantial enhancement in the intensity of the bis-histidine low-spin B complex for the encapsulated hemoglobin, indicating a perturbation of this low-spin complex. We suggest that lipid-associated perturbations are responsible for the enhancement of the oxidation observed with the LEH samples compared to the unencapsulated material.
INTRODUCTION
Liposome encapsulation constitutes a flexible method of packaging macromolecules in a vehicle engineered for targeted delivery of therapeutic compounds. Common goals of this technology include increased stability of therapeutic agents after injection, increased pharmacokinetic half-lives, and decreased clinical side effects. Potential applications include improved adjuvancy, controlled delivery of drugs, enhancement of protein absorption, and transport of bioengineered oxygen-carrying molecules in the circulation Citation[1-4].
Stability of the active compounds inside the lipid vesicles is central to their success. One problem of particular concern with proteins is the degree to which their macromolecular structure is destabilized by interaction with the hydrophobic portions of encapsulating membranes. In addition to functional degradation, such changes may lead to intraliposomal protein adherence and errors in the quantitative assays of the encapsulated compound. This interferes with quality control measurements during production and with attempts to estimate potential therapeutic efficacy.
The use of liposome encapsulation to limit the toxicities of hemoglobin-based red blood cell substitutes Citation[5-7] serves as a prototypical example of an application that embodies these goals and problems. Normally, hemoglobin injected into the bloodstream immediately begins to oxidize, altering its oxygen-binding capability Citation[[8]]. This decreases its oxygen-carrying capacity while at the same time producing toxic-free radical intermediates and exchangeable ferrichemes that can participate in tissue-damaging peroxidative reactions Citation[9-11]. In theory, trapping hemoglobin in gas-permeable unilamellar lipid vesicles would be expected to limit its exposure to sensitive tissues, allow it to better retain its therapeutic activity, and slow down its degradation. However, methemoglobin (metHb) accumulation continues to occur inside liposomes due to autooxidation of the ferrous hemes of hemoglobin Citation[[9]], Citation[[12]], oxidation of the oxyhemes by vascular nitric oxide Citation[[13]], and peroxidative interactions with the phopholipids of the liposome membrane bilayer Citation[14-15]. Although oxidation of membrane phospholipids by hemoglobin can be diminished by inclusion of the membrane-bound antioxidant α-tocopherol, in these LEH formulations Citation[[16]] unalleviated accumulation of metHb still occurs.
Measurements of oxidized hemoglobin concentrations and functional degradation rates are necessary for determining the postadministration efficacy of these LEH preparations. Overall hemoglobin functionality, in terms of ligand binding, can be easily measured because its active centers possess sensitive chromophores that are directly observable by a variety of spectral techniques. However, accurate direct measurements of LEH oxidation are almost impossible to carry out using conventional spectroscopic methods such as UV-vis spectroscopy because of the high turbidity of the liposome suspensions. Indirect measurements are plagued by the potential for residual hemoglobin trapping in partially dissolved membrane fragments associated with chemical extraction procedures. The magnitude of measurement error associated with chemical extraction is currently unknown.
In this study, we used EPR spectroscopy to compare the autooxidation rates of liposome-encapsulated and unencapsulated human hemoglobin crosslinked between alpha chains with bis (3,5-dibromosalicyl) fumarate (ααHb). EPR has three distinct advantages over UV-vis spectroscopy: it is not affected by sample turbidity, it can be used with concentrated hemoglobin samples, and it can distinguish between high- and low-spin forms of ferric heme Citation[[17]]. This method provided quantitative measurements of hemoglobin oxidation rates that were compared with parallel measurements obtained using conventional chloroform extraction procedures and UV-vis spectroscopy. EPR measurements of LEH oxidation also provided evidence for hemoglobin–membrane interactions, which appear to influence the rate of autooxidation.
MATERIALS AND METHODS
ααHb in Ringer's acetate, pH 7.50, was manufactured under aseptic conditions in the Blood Research Detachment, Walter Reed Army Institute of Research Citation[[18]] and was used both as free hemoglobin and in the preparation of LEH at the Center for Bio/Molecular Science and Engineering, Naval Research Laboratory, as previously described Citation[[19]]. Liposome formulation consisted of diastearoylphosphatidylcholine (DSPC), dimyristoylphosphatidylglycerol (DMPG), cholesterol, and α-tocopherol (vitamin E). α-Tocopherol was added to reduce methemoglobin formation and interactions between hemoglobin and the lipid bilayer Citation[[16]].
EPR Measurements
The intra-liposomal ααHb concentration of 6.3 g/dl was determined from the total Hb concentration of the LEH suspension measured with a CIBA-Corning 855 Blood Gas Analyzer (Medfield, MA), divided by the LEH spun hematocrit. LEH and 6.3 g/dl acellular ααHb samples were incubated at 37°C in 4-mm OD EPR quartz tubes (Wilmad Glass Co., Buena, NJ). Oxidation reactions of sample pairs were quenched by submersion of EPR tubes in liquid nitrogen at periodic intervals from time 0 to 168 h. Breakage during freezing was prevented by inserting thin polyethylene tubes, sealed at the bottom, into the EPR tubes containing the samples. The tubes were stored in liquid nitrogen until EPR spectral measurements were made.
The relative concentrations of oxidized hemoglobin in each sample were determined from EPR spectra acquired at 7.0 K using an IBM ER-200D-SRC spectrometer with 100-kHz modulation. A liquid transfer Helitran cryogenic unit (model LTD-3-100, Air Products Inc., Allentown, PA), in conjunction with an APD-E temperature controller, was used to regulate the EPR cavity temperature. Relative levels of total oxidized hemoglobin were determined by the double integration of the EPR first-derivative spectra in the region between 1000 and 3800 Gauss for different samples at each selected time.
UV-Vis Measurements
Acellular ααHb
Samples (6.3 g/dL) of ααHb were incubated in EPR quartz tubes as described above. At each selected time, individual ααHb samples were removed from their respective EPR tubes and diluted to a final concentration of 0.1 g/dL in 0.1 M potassium phosphate buffer, pH 7.20, in 1.0-cm pathlength glass cuvettes. Visible spectra were acquired from 500 to 650 nm in 2-nm steps using a Hewlett Packard 8450A diode array spectrophotometer. MetHb formation was determined by least-squares multicomponent analysis of the spectral matrix for each data series Citation[[12]]. Singular value decomposition of the data sets was used to verify the number of spectral components changing over time Citation[[20]]. An extended incubation for 336 h was also performed when complete oxidation of the 168-h sample was not achieved.
Liposome-Encapsulated ααHb
These measurements were indirect using chloroform extraction because the turbidity of the samples would not allow direct measurements using UV-vis spectroscopy. LEH samples were incubated in EPR tubes for different time intervals. In addition to a 168-h sample, a 336-h sample was prepared as with the acellular ααHb. At the end of each incubation, the LEH liposome membranes were extracted into chloroform, releasing encapsulated hemoglobin. Chloroform was added to incubated LEH in a ratio of 3:4 and vortexed for 1 min. The vortexed samples were centrifuged for 10 min at 4000 rpm in a Beckman GPR Centrifuge. Hemoglobin in the upper aqueous layer was then pipetted off, and 100 μL of this ααHb extract was added to 2.90 of 0.1 M potassium phosphate buffer, pH 7.20. Visible spectra were acquired and amounts of methemoglobin determined using least-squares multicomponent analysis curve fits as described above. Total hemoglobin in aqueous extracts was determined using the reference photometrically determined hemiglobincyanide method recommended by the International Committee for Standardization in Hematology (ICSH) Citation[[21]].
RESULTS
EPR Measurements
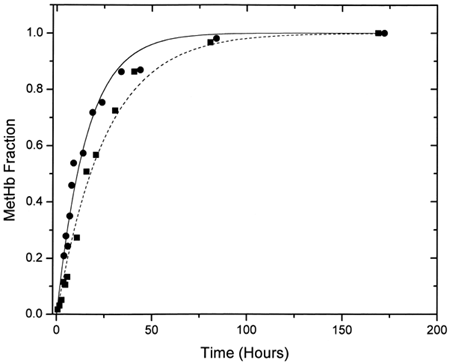
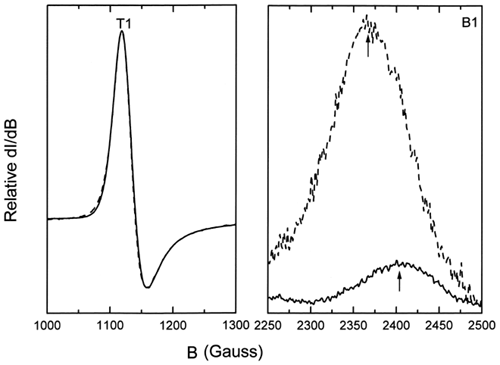
shows a typical EPR spectrum obtained for metααHb. Five distinct states (T, R, A, B, and C) Citation[[17]] of the oxidized chains are observable. These are a) the dominant high-spin tetragonal water complex (T1 and T2) with g⊥ = 6.0 and g= 2.0 respectively, b) the minor high-spin rhombic complex (R) with g = 4.3, and c) the minor low-spin complexes (A, B, and C) with g values ranging from 1.63 to 2.98. A is the low-spin hydroxide complex, B is the low-spin bis-histidine complex with a water molecule still in the heme pocket, whereas C is the more stable low-spin bis-histidine complex without any water molecule in the pocket Citation[[17]]. The relative intensities of the methemoglobin high-spin tetragonal complex were obtained from double integration of the EPR first-derivative spectra between 1000 and 3800 Gauss. Spectral intensities from 1000 to 1300 Gauss were easily observed to increase with increasing degree of autooxidation (). The rate constants for the oxidation reactions were determined using the first-order exponential model given by the expression:where m is the methemoglobin formed at any given time t, and k the experimental rate constant. This model was found to fit our experimental data adequately. The obtained rate constants indicated a higher rate of oxidation for the encapsulated ααHb (kLEH = 0.065 ± 0.007 h−1) when compared to the free ααHb (kααHb = 0.039 ± 0.002 h−1, see ). Data for both samples were normalized relative to the value of metHb formed at 168 h, since our parallel UV-vis measurements showed a leveling off in the metHb formation at this time. Comparison of the low-spin complexes for the acellular ααHb and the LEH showed a shift in the g value and substantial enhancement of the intensity of the B complex for the LEH (), indicating a perturbation of the low-spin B complex. This perturbation we believe to be the result of the interaction between the formed metHb and the liposomes.
Figure 1. Typical EPR spectra of oxidized hemoglobin. The lower curve is magnified 8.3 times (upper curve) to show all the peaks in the region between 500 and 4500 Gauss.
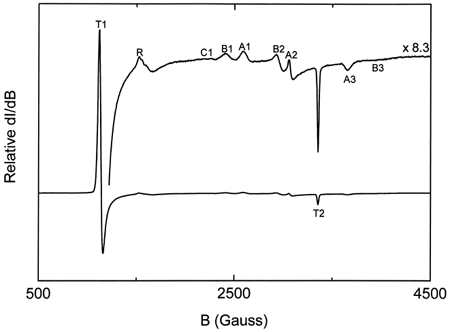
Figure 2. Relative intensities of the EPR high-spin tetragonal metHb complex spectra for ααHb autooxidation at different times.
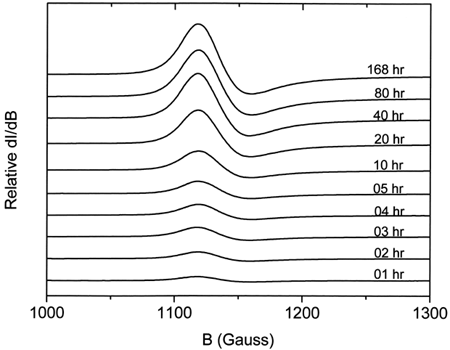
UV-Vis Measurements
Acellular ααHb
Visible spectra for ααHb were acquired at the selected times during the oxidation reaction. Measurements were for up to 168 h, with an extra data point obtained at 332 h in order to confirm the leveling off observed at the 168th hour. In addition to this leveling off, we observed that not all the hemoglobin was oxidized at this point. Rather, a steady-state oxidation of about 80% of the total Hb was obtained. Singular value decomposition (SVD) analysis of the acquired spectral matrix suggested three major (changing) components. However, reliable least-squares fits to the data curves were only obtained with a four-component spectral calibration set consisting of oxyHb, aquometHb, deoxyHb, and low-spin azometHb. shows the variation of these different Hb species with time. As expected, changes in oxyHb and aquometHb were reciprocal, while deoxyHb content exhibited very little variation (around 1%). The fourth component, the low-spin azometHb-like component, fluctuated around 3% throughout the course of the autooxidation. The variations observed with the azometHb matched the pattern observed for the third component of the SVD analysis (), suggesting that the third component was indeed an azometHb-like low-spin metHb species, possibly with an anion bound to the Fe3+ in the heme pocket. Substituting a hemichrome or ferrylHb spectrum for the azometHb species in the parent data set produced comparatively poorer multicomponent fits to the data sets.
Figure 5. Plot of the variation of the amounts of the different hemoglobin species with time during ααHb autooxidation as determined by multicomponent fits of their UV-vis spectra. Fitted species are oxy (▪), met (•), deoxy (□), and azomet-like (♦) hemoglobin.
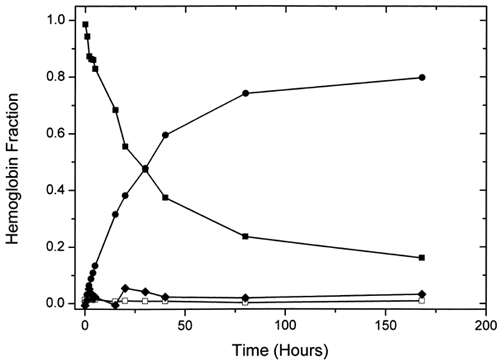
Figure 6. Comparison of the variation of the azometHb-like component with time from SVD (solid line) and multicomponent (dashed line) analysis.
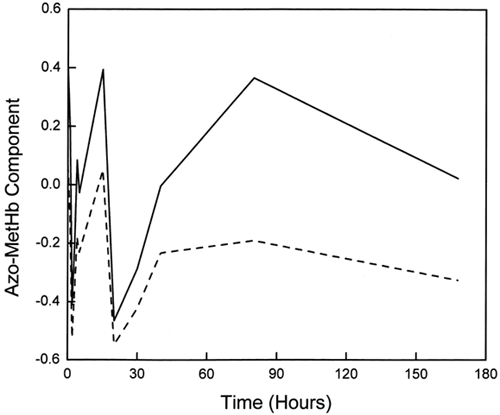
The total methemoglobin produced at any given time was determined by the sum of the amounts of aquometHb and the low-spin azometHb-like component. A first-order exponential curve fit gave a rate constant of 0.033 ± 0.002 h−1 with this method.
Liposome-Encapsulated ααHb
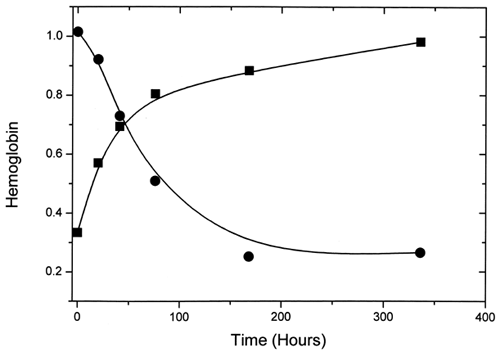
Hemoglobin from LEH was obtained by extraction with chloroform. Here, the measured total hemoglobin values were found to decrease as amounts of methemoglobin in the extracts increased (). A decrease in value from 1.01 g/dL at 33% methemoglobin to 0.26 g/dL at 98% oxidation was observed. This suggests that, as the amount of methemoglobin formed in the LEH increases, proportionally less of it may be extracted into the aqueous media. In the presence of lipid membranes, methemoglobin is often readily converted to low-spin hemichromes, which could be difficult to dislodge from membrane fragments into the aqueous phase. These results imply that standard extraction procedures like the one used here may introduce significant errors in the direction of underestimating liposome-encapsulated methemoglobin formation. With the assumption that most of the unextracted hemoglobin was methemoglobin, we calculated a value of 97% for the methemoglobin formed by 168 h. This would mean that virtually all the hemoglobin in the LEH was converted to methemoglobin by 168 h, in contrast to the case with the acellular hemoglobin.
DISCUSSION
Hemoglobin spontaneously undergoes autooxidation resulting in the formation of methemoglobin, a nonoxygen-transporting form of hemoglobin Citation[9-10]. In the vasculature, this oxidation of acellular hemoglobin is further exacerbated because of its interaction with other plasma moieties Citation[[13]]. In the development of alternative oxygen carriers for transfusion, LEHs have been produced to reduce the interaction of hemoglobin with plasma components Citation[5-7]. However, the absence of reliable methods to measure the effects of encapsulation on the integrity of the contained hemoglobins have made it difficult to estimate the potential oxygen-carrying capacity of these materials.
EPR spectroscopy has previously been used quantitatively to study the reaction of hydrogen peroxide with methemoglobin Citation[[22]]. In this study we used this technique quantitatively to determine for the first time the effect of liposome encapsulation on the autooxidation of ααHb. With this method it is possible to make measurements at the normal (high) hemoglobin concentrations used in animal and human studies Citation[[8]], Citation[23-25], while avoiding the turbidity problems associated with UV-vis spectroscopic measurements of LEH suspensions, since EPR spectroscopy only “sees” methemoglobin in the samples.
These results demonstrate that liposome encapsulation increases the rate of autooxidation of ααHb at 37°C, although it is likely that this increase would have been much greater had α-tocopherol been excluded from the liposome formulation. Previous studies Citation[[16]] have shown that membrane-bound antioxidants like α-tocopherol reduce metHb formation and lipid membrane–hemoglobin interaction.
The similarity of rate constants for the oxidation of unencapsulated ααHb measured by standard optical and EPR spectral techniques (0.033 and 0.039 h−1, respectively) demonstrates the reliability of applying EPR spectroscopy to this type of quantitative kinetic measurement. As more automated and user-friendly EPR spectrophotometers become available, these types of measurements could become routine.
An unexpected result of this study was that the acellular ααHb did not fully autooxidize, instead achieving a steady-state equilibrium well below 100% metHb by 168 h of incubation. The maximum amount of oxidation observed was 83%, was sustained to at least 336 h, and was not concentration-dependent for up to a ten-fold dilution (results not shown). It may be that, in the absence of superoxide dismutase or other reactive compounds, the buildup of superoxide in the incubation medium and subsequent re-reduction of the metHb simply limited the extent of the reaction by creating an oxidation–reduction equilibrium. Alternatively, given the preponderance of acetate in the hemoglobin solution (7:1 molar ratio of acetate:heme), it is also plausible that during the course of ααHb autooxidation sufficient acetaldehyde was produced by electron transfer reactions (possibly mediated by superoxide) to contribute to the maintenance of this redox plateau.
Extraction of the lipid bilayer into chloroform was attempted to see whether a simple extraction procedure with UV-vis spectroscopy could provide comparable data on LEH oxidation kinetics. This method proved to be unreliable because the amounts of total hemoglobin extracted into the aqueous medium were inversely proportional to the amounts of oxidized liposomal ααHb produced. This result may be attributed to the tighter membrane binding of hemichromes and other low-spin methemoglobin species formed, preventing their release from the membrane during the extraction procedure. It is also possible that denaturation of hemoglobin in LEH increases with the amount of methemoglobin present during extraction, since methemoglobin is more susceptible to denaturation than reduced forms of hemoglobin.
A shift in the g value and substantial enhancement of the intensity of the water containing bis-histidine low-spin B complex of the encapsulated ααHb () suggest a perturbation of the low-spin B complex due to interactions between the ααHb and the liposome membrane. This perturbation may be responsible for enhanced autooxidation of membrane-bound hemoglobin, leading to an increase in the overall rate and magnitude of ααHb oxidation inside the liposomes.
In conclusion, we used EPR spectroscopy to determine that the rate of oxidation of encapsulated ααHb is higher than that of acellular ααHb with the particular liposome formulation used in this study. With EPR spectroscopy it was also possible to observe a pertubation of the low-spin B complex of the LEH. We attributed the enhanced oxidation rate to this perturbation. Our results on the use of chloroform extraction with UV-vis spectroscopy for the measurement of formed methemoglobin in LEH suggested that this methodology may be inaccurate because the amounts of total hemoglobin extracted into aqueous solution were found to decrease as the amounts of oxidized liposomal ααHb increased.
ACKNOWLEDGMENTS
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official, nor do they reflect the views of the Department of the Army or the Department of Defense (AR 360-5). This is a U.S. Government work. There is no copyright. The use of registered trade names in this article is solely for the identification of products and does not imply endorsement of the products or claims made for them.
REFERENCES
- Wearley L. L. Recent Progress in Protein and Peptide Delivery by Noninvasive Routes. Crit. Rev. Ther. Drug Carrier Syst. 1991; 8(4)331–394
- Weiner A. L. Liposomes for Protein Delivery: Selecting Manufacture and Development Processes. Immunomethods 1994; 4(3)201–209
- Gluck R. Liposomal Presentation of Antigens for Human Vaccines. Pharm. Biotechnol. 1995; 6: 325–345
- Patel G. B., Sprott G. D. Archeobacterial Ether Lipid Liposomes (Archaeosomes) as Novel Vaccine and Drug Delivery Systems. Crit. Rev. Biotechnol. 1999; 19(4)317–357
- Rudolph A. S. Encapsulation of Hemoglobin in Liposomes. Blood Substitutes: Physiological Basis of Efficacy, R. M. Winslow, K. D. Vandegriff, M. Intaglietta. Birkhauser, Boston 1995; 90–104
- Bessinger R. L., Farmer M. C., Gossage J. L. Liposome Encapsulated Hemoglobin as a Red Cell Surrogate. Trans. Am. Soc. Artif. Intern. Organs 1986; 32: 58–63
- Rudolph A. S., Sulpizio T., Heible P., Macdonald V. W., Chavez M., Feuerstein G. Liposome Encapsulation Attenuates Hemoglobin-Induced Vasoconstriction in Rabbit Arterial Segments. J. Appl. Physiol. 1997; 82(6)1826–1835
- Hess J. R., Macdonald V. W., Brinkley W. W. Systemic and Pulmonary Hypertension after Resuscitation with Cell-Free Hemoglobin. J. Appl. Physiol. 1993; 74(4)1769–1778
- Abugo O. O., Rifkind J. M. Oxidation of Hemoglobin and the Enhancement Produced by Nitroblue Tetrazolium. J. Biol. Chem. 1994; 269: 24845–24853
- Balla J., Jacob H. S., Balla G., Nath K., Eaton J. W., Vercellotti G. M. Endothelial-Cell Heme Uptake from Heme Proteins: Induction of Sensitization and Desensitization to Oxidant Damage. Proc. Nat. Acad. Sci. U.S.A. 1993; 90(20)9285–9289
- Signorini C., Ferrali M., Ciccoli L., Sugherini L., Magnani A., Comporti M. Iron Release, Membrane Protein Oxidation and Erythrocyte Aging. FEBS Lett. 1995; 362: 165–170
- Macdonald V. M. Measuring Relative Rates of Hemoglobin Oxidation and Denaturation. Methods Enzymol. 1994; 231: 480–490
- Eich R. F., Li T., Lemon D. D., Doherty D. H., Curry S. R., Aitken J. F., Mathews A. J., Johnson K. A., Smith R. D., Phillips G. N., Jr., Olson J. S. Mechanism of NO-induced oxidation of Myoglobin and Hemoglobin. Biochemistry 1996; 35(22)6976–6983
- Szebeni J., Toth K. Lipid Peroxidation in Hemoglobin Containing Liposomes: Effects of Membrane Lipid Composition and Cholesterol Content. Biochim. Biophys. Acta 1986; 857: 139–145
- Itabe H., Kobayashi T., Inoue K. Generation of Toxic Phospholipids During Oxyhemoglobin Induced Peroxidation of Phosphatidylcholines. Biochim. Biophys. Acta 1988; 961: 13–21
- Stratton L. P., Rudolph A. S., Knoll W. K., Farmer M. C. The Reduction of Methemoglobin Levels by Antioxidants. Hemoglobin 1988; 12: 353–368
- Levy A, Kuppusamy P., Rifkind J. M. Multiple Heme Pocket Subconformations of Methemoglobin Associated with Distal Histidine Interactions. Biochemistry 1990; 29: 9311–9316
- Highsmith F. A., Driscoll C. M., Chung B. C., Chavez M. D., Macdonald V. W., Manning J. M., Lippert L. E., Berger R. L., Hess J. R. An Improved Process for the Production of Sterile Modified Hemoglobin Solutions. Biologicals 1997; 25: 257–268
- Rudolph A. S., Klipper R. W., Goins B., Phillips W. T. In Vivo Biodistribution of a Radiolabeled Blood Substitute: 99MTc-Labeled Liposome Encapsulated Hemoglobin in an Anesthetized Rabbit. Proc. Nat. Acad. Sci. U.S.A. 1991; 88: 10976–10980
- Shrager R. I. Chemical Transitions Measured by Spectra and Resolved Using Singular Value Decomposition. Chemomet. Intel. Lab. Syst. 1986; 1: 59–70
- ICSH. Recommendations for Reference Method for Hemoglobinometry in Human Blood (ICSH Standard EP 6/2: 1977) and Specifications for International Hemiglobincyanide Reference Preparation (ICSH Standard EP 6/3: 1977). J. Clin. Pathol. 1978; 31: 139–143
- Svistunenko D. A., Patel R. P., Wilson M. T. An EPR Investigation of Human Methemoglobin Oxidation by Hydrogen Peroxide: Methods to Quantify All Paramagnetic Species Observed in the Reaction. Free Radical Res. 1996; 24(4)269–280
- Keipert P. E., Gonzales A., Gomez C. L., Macdonald V. W., Hess J. R., Winslow R. M. Acute Changes in Systemic Blood Pressure and Urine Output Following Exchange Transfusion with Diaspirin-Crosslinked Hemoglobin Solution in Conscious Rats. Transfusion 1993; 33: 701–708
- Lee R., Neya K., Svizzero T. A., Vlahakes G. J. Limitations of the Efficacy of Hemoglobin-Based Oxygen-Carrying Solutions. J. Appl. Physiol. 1995; 79: 236–242
- Kasper S. M., Walter M., Grune F., Bischoff A., Erasmi H., Buzello W. Effects of a Hemoglobin-Based Oxygen Carrier (HBOC-201) on Hemodynamics and Oxygen Transport in Patients Undergoing Preoperative Hemodilution for Elective Abdominal Aortic Surgery. Anesth. Analg. 1996; 83: 921–927