Abstract
Infusion of hemoglobin-based oxygen-carrying solutions (HBOCs) produce an immediate rise in blood pressure with most solutions, both in animals and humans, as a result of systemic and pulmonary vasoconstriction. Autoregulation of the O2 supply by the microvasculature has been proposed as a phenomenon involved in the vasoconstriction elicited by HBOCs. Nevertheless, little is known about the ability of various HBOCs to induce constriction in the microcirculation according to their specific physicochemical properties (viscosity, molecular weight, P50, etc.). This study was therefore designed to assess the effects of three HBOCs, that is, bis(3,5-dibromosalicyl) fumarate–crosslinked hemoglobin (αα-Hb), dextran-benzene-tetracarboxylate–conjugated hemoglobin (Hb-Dex-BTC) ando-raffinose–oligomerized hemoglobin (o- raffinose-Hb), on the vascular tone of rat mesenteric arterioles (diameter, 15–25 μm) viewed microscopically in moderate hemodilution conditions. The effects of HBOCs were compared to those elicited by a reference solution of hydroxyethyl starch (HES-200) infused in the same conditions. In each experimental group, a fall in arteriolar diameter was observed 2 min and 5 min after infusion of the solution. The maximum changes were observed in Hb-Dex-BTC and o-raffinose-Hb groups, in which diameter decreased from 6.9 ± 0.5% and 5.2 ± 0.7%, respectively, 2 min after infusion. The changes in arteriolar diameter induced by Hb-Dex-BTC and o-raffinose-Hb were significantly higher than those elicited by HES-200 and αα-Hb. In conclusion, our data indicate that moderate hemodilution with HBOCs induces instantaneous constriction in rat mesenteric arterioles, with amplitudes depending on both pharmacological and physicochemical properties of the hemoglobin solution infused.
INTRODUCTION
Hemoglobin-based oxygen-carrying solutions (HBOCs) are currently under clinical investigation as a means to reduce the viral and/or immunological risks associated with transfusion of red blood cells (RBCs) and the need in allogeneic blood Citation[[1]]. These solutions are prepared with hemoglobin (Hb) extracted from RBCs and modified according to various chemical or genetic methods Citation[1-2]. Infusion of HBOCs produces an immediate rise in blood pressure with most solutions, both in animals and humans, as a result of systemic and pulmonary vasoconstriction Citation[[2]]. Though the physiological pathways involved in this vasoconstrictive action may be numerous, particular attention has been drawn to the ability of cell-free Hb to scavenge the endothelium-derived relaxing factor nitric oxide (NO) in arterial and venous rings in vitro Citation[3-4]. However, the putative correlation between the inhibition of the NO vasodilatory function by cell-free Hb and the hypertension elicited by HBOCs has not been clearly established in vivo Citation[5-6]. Recently, autoregulation of the O2 supply has been proposed as an additional phenomenon involved in the microvascular constriction elicited by HBOCs Citation[7-8]. According to the theory of autoregulation, the increase in the fraction of dissolved O2 into plasma following intravenous infusion of cell-free Hb would lead to an oversupply of O2 to the tissues, which would trigger blood flow regulation through vasoconstriction. Moreover, Nolte et al. demonstrated, through the hamster dorsal skin-fold chamber preparation, that vasoconstriction of <40-μm arterioles was responsible for the very short-term (0–2 min) pressor action of DCLHb™ in isovolemic or hypervolemic conditions Citation[[9]].
In other respects, several authors have shown that the chemical modification applied to Hb in the design of HBOCs (and, consequently, the physicochemical properties of the solutions) influenced the vasoconstrictive properties of these solutions at the macrohemodynamic level Citation[10-11]. In addition, in hemodilution settings, which are future clinical applications for HBOCs, Tsai et al. indicated that maintaining the viscosity of blood (mixed with a substitutive fluid) in normal values is an important factor in determining the density of functional capillaries Citation[[12]]. Thus, viscosity of HBOCs may be a crucial parameter for the efficacy of these potential blood substitutes. At the time when several HBOCs may be used in clinical settings as red blood cell (RBC) substitutes, investigation on the effects of such fluids in the microvasculature is obviously required, since one of their primary functions should be oxygenation of anoxic or ischemied tissues Citation[[1]]. This study was therefore designed to assess the short-term effects of three HBOCs, prepared according to various chemical processes and exhibiting different physicochemical properties, on the vascular tone of rat mesenteric arterioles viewed microscopically.
MATERIAL AND METHODS
Test Solutions
HES-200, used as a reference solution, was obtained from Biosedra (Louviers, France) and contains 6 g/dL of low-molecular-weight hydroxyethyl starch in saline (commercial product: Elohès® 6%). Hb-Dex-BTC solution (Pasteur-Mérieux sérums & vaccins, Marcy l'Etoile, France) contains 8.5 g/dL of human Hb prepared from outdated RBCs and conjugated to a macromolecular allosteric effector, dextran- benzene-tetracarboxylate, as previously described Citation[[13]]; the solution was suspended in Tyrode medium, pasteurized, and frozen at −20°C without preservatives. αα-Hb solution (U.S. Army; gift from Dr. A. Alayash, C.B.E.R., Bethesda, MD) contains 8.2 g/dL of heat-treated human Hb obtained from outdated RBCs, stabilized by cross-linking between the two α subunits with bis (3,5-dibromosalicyl) fumarate, suspended in Ringer's lactate and frozen at −80°C Citation[[14]]. o-Raffinose-Hb solution contains 10 g/dL of pasteurized solution of human Hb prepared from outdated RBCs, cross-linked internally with raffinose and polymerized to form o-raffinose polyhemoglobin Citation[[15]]; the solution was suspended in Ringer's lactate and was frozen at −80°C without preservatives. o-Raffinose-Hb was generously provided by Hemosol, Inc. (Toronto, ON). The main physicochemical properties of the solutions are presented in .
Table 1. Physicochemical Parameters of Hemoglobin-Based Oxygen Carriers and HES 200
Animals and Surgery
The animal protocol was approved by the Animal Protection Bureau of the French Ministry for Fishing, Agriculture, and Food, and the experiments were condcted in accordance with the Guiding principles for Research Involving Animals. The animals were fed ad libitum before the experiments. At the end of the experiments, the rats were sacrificed by an excess dose of pentobarbital.
Thirteen male Wistar rats (Depré Breeding center, St. Doulchard, France) weighing 387 ± 28 g were anesthetized by intramuscular injection of 200 mg/kg body weight of thiopental sodium (Nesdonal, Laboratoire Specia, France). The left jugular vein was canulated with a polyethylene tube (internal diameter 0.8 mm; Merck-Clévenot, France) for injection of test solutions. After a median laparotomy, an intestinal loop was placed on the table of an inverted microscope (Axiovert 10, Zeiss, France; magnification ×100) and the microvasculature of a mesenteric window was observed. The mesentery was heated with a lamp throughout the experiments and was wet with saline periodically.
Arteriolar Diameter Measurements
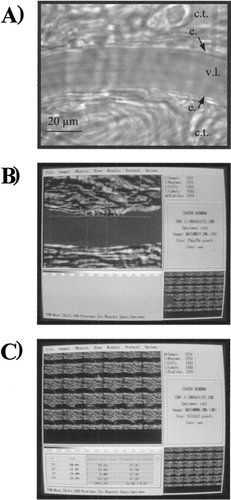
Microscopic images of one mesenteric arteriole (diameter, 15–25 μm) were recorded and analyzed as previously described Citation[[16]]. Briefly, images were collected by a videotape recorder through a video color CCD camera (DXC 107P, Sony, France). Videotaped images of arterioles were analyzed off-line using an analog-to-digital converter (Starwise, Imstar, France) and appropriate software (Morphostar, version 5.5k, Imstar, France). These images could be displayed on a high-resolution video monitor (Trinitron color video monitor, PVM 144 2QM, Sony, France) and stored in a personal computer with an acquisition rate of 15 images per min. In order to limit the variability in the arteriole inner diameter determination, the same operator performed all the calculations. For the measurements, cursors were aligned by the operator with the vessel edges using a digitized tablet, and Morphostar software was used to calculate the arteriolar diameter in micrometers (b and c). In each experiment, the process of arteriolar diameter determination was applied to each digitized image, in basal conditions and for the 5 min following IV infusion of the test solutions.
Figure 1. A View of a rat mesenteric arteriole in basal conditions. c.t.: connective tissue; e.: endothelium; v.l.: vascular lumen. B) View of a digitized image of a mesenteric arteriole during the early step of determination of the diameter with Morphostar software. The arteriolar lumen is colored in red and the cursors used to calculate the arteriolar diameter are aligned with the vessel edges. C) View of 30 digitized images of a mesenteric arteriole during the last step of calculation of the diameter. Examples of the results of calculation are given in the table in the lefthand corner of the picture.
Treatment Groups
The rats were randomly assigned to one of the treatment groups: HES-200 (n = 3), Hb-Dex-BTC (n = 3), αα-Hb (n = 4), and o-raffinose-Hb (n = 3). The volume of each solution infused represented a fixed amount of 10% of the rat's total blood volume, estimated as 6.5% of the total body weight Citation[[17]], in order to achieve moderate hemodilution. After a 1-min baseline period of recording, the solutions were infused through the venous tube at the rate of 1 mL/min. The total doses infused ranged from 55 to 65 mg/kg b.w. The images of arterioles were collected continuously for the 5 min following injection.
Statistical Analysis
In spite of the number of animals involved in the study, a statistical analysis was performed on diameter values since the standard deviations were very small. Therefore, pre- and postinfusion diameters for each group of treatment were compared using nonparametric Mann-Whitney tests. Comparison between the treatment groups were made using one-way ANOVA (Statview®, Abacus Concepts, USA), with the assumption that the values were normally distributed. A P value less than 0.05 was considered significant.
RESULTS
Images of arterioles are presented in . The values of preinfusion arteriolar diameters were 16.56 ± 0.16 μm, 19.52 ± 0.12 μm, 16.06 ± 0.15 μm, and 19.32 ± 0.13 μm in HES-200, Hb-Dex-BTC, αα-Hb, and o-raffinose-Hb groups, respectively; the baseline values of diameter were not significantly different between the experimental groups. For each experiment, the standard deviation calculated on the 15 values collected during the 1-min baseline period was approximately one percent of the inner diameter, indicating the good reproducibility of those measurements.
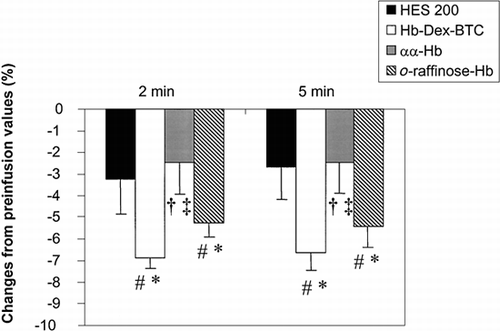
Variations of the inner diameter after infusion of the test solutions are presented in . In each experimental group, a fall in arteriolar diameter was observed 2 and 5 min after infusion of the solution. The maximum changes were observed in Hb-Dex-BTC and o-raffinose-Hb groups, in which diameter decreased from 6.9 ± 0.5% and 5.2 ± 0.7%, respectively, 2 min after infusion. In each experimental group, no significant difference was found between the diameter values measured 2 and 5 min after infusion of the solutions.
Figure 2. Variations of the inner diameter of rat mesenteric arterioles following intravenous infusion of HES 200 (n = 3), Hb-Dex-BTC (n = 3), αα-Hb (n = 4), or o-raffinose-Hb (n = 3). #: P < 0.05, postinfusion vs. preinfusion; *: P < 0.05, solution vs. HES 200; †: P < 0.05, solution vs. Hb-Dex-BTC; ‡: P < 0.05, solution vs. o-raffinose-Hb.
DISCUSSION
This study is, to our best knowledge, the first to investigate the effects of three clinically relevant HBOCs (i.e., cross-linked, oligomerized, and conjugated Hb), on the rat intact mesenteric microvasculature. The rat mesentery was chosen because it represents a common model for analysis of the microvasculature by intravital microscopy Citation[[18]]. In this model, we assessed the action of these HBOCs on arteriolar tone after mild hemodilution leading to a moderate decrease in the viscosity of blood mixed with the solution. One limitation in this study is the lack of blood pressure monitoring in parallel with arteriolar diameter measurements; nevertheless, each HBOC tested has proved to induce immediate blood pressure increase in various species, including rats Citation[[6]], Citation[9-11], Citation[[15]]. Conversely, HES-200 was chosen as a reference solution because this substitutive fluid has been used for some time in clinical practice and it has little action on hemodynamics Citation[[10]]. In addition, HES-200 has physicochemical characteristics (molecular weight, viscosity) close to those of the HBOCs tested. Another question that may be addressed concerns the small amplitude of variations of arteriolar diameter, since it exhibited a maximal decrease of ∼7% from baseline. These results are nevertheless consistent with those observed previously in the same experimental model when neurotransmitters such as acetylcholine were injected Citation[[16]]. However, higher variations may have been expected in larger arterioles dedicated to flow controlling Citation[[19]]. Thus, in a study conducted in conscious hamsters undergoing moderate hypervolemia (10% of total blood volume), Nolte et al. found that DCLHb™ (the commercial analog of αα-Hb) produced a ∼20% decrease of arteriolar diameter in striated skin muscle that lasted only 2 min Citation[[19]]. In cat pial arterioles, Asano et al. reported that the decrease of arteriolar diameter following infusion of sebacyl cross-linked Hb was <10% Citation[[20]]. The differences in the amplitude and duration of diameter changes in comparison with those observed in our model may be attributed to various differences in the design of the experiments (animal species, anesthesia, localization and diameter of arterioles, chemical modification applied to Hb). In that respect, our results do not seem contradictory to those of others; rather, they emphasize the need to compare clinically the various HBOCs in the same experimental model in order to understand the role of microvasculature in their pressor effect.
The most interesting finding in this study is the difference of action on the arteriolar tone between the various HBOCs tested, αα-Hb producing a similar reduction of diameter to that of the reference solution (HES-200), whereas Hb-Dex-BTC and o-raffinose-Hb induced a significantly larger vasoconstriction. Various physiological mechanisms and physicochemical parameters may account for these differences but an exhaustive list of them and to what extent each is involved remains hard to establish. However, in these experiments three main factors may be discussed: 1) the viscosity of blood mixed with solutions, 2) the oxygenation capacity of the Hb solutions, and 3) the molecular weight of Hb molecules.
1. According to Poiseuille-Hagen formula, decreasing blood viscosity elicited by hemodilution induces a decrease in the vessel resistance and, under the principle of autoregulation, would lead to redistribution of blood flow through vasoconstriction in response to the improvement of O2 supply to tissues Citation[[19]]. This phenomenon would thus explain the decrease in arteriolar diameter observed with all solutions, including HES-200. In other respects, the decrease in blood viscosity after infusion of the test solutions has been reported to be dependent on the intrinsic viscosity of the solutions Citation[[10]], Citation[[21]]. We can therefore expect that the decrease in arteriolar diameter related to the sole decrease in blood viscosity occurred with the following pattern: αα-Hb > o-raffinose-Hb > Hb-Dex-BTC ≈ HES-200 (). Since the changes in arteriolar tone did not follow the same order, alternative mechanisms must be proposed to account for the vasoconstriction observed. One alternative may involve the influence of the solutions on the rheological behavior of RBCs. We observed transitory episodes of stasis in all rats infused with Hb-Dex-BTC, which may be explained by the hyperaggregating properties of this solution, as reported previously by Menu et al. in vitro Citation[[21]]. This requires further investigation in order to quantify the impact of HBOCs on rheological parameters in vivo, since it may lead to microvascular disorders such as thrombosis or decreased blood flow in postcapillary venules Citation[[22]].
2. As proposed by Intaglietta et al., autoregulation may be involved in microvascular vasoconstriction, independently to hemodilution per se. The authors demonstrated that the increase in O2 supply to tissues, due to the increase in the fraction of dissolved O2 into plasma, led to vasoconstriction, which aims to adjust oxygen delivery to metabolic need Citation[[7]]. In this study, in absence of in situ PO2 measurements, no correlation between the oxygenation capacity of the HBOCs (i.e., their P50 or oxygen half-saturation pressure of Hb), and their effect on arteriolar diameter may be proposed. The oxygen capacity of blood (P50 ≈ 26–28 Torr in normal conditions) is however likely to be altered after mixing blood to Hb solutions, as proposed by Page et al. Citation[[16]]. Despite large differences in their P50 values, Hb-Dex-BTC and o-raffinose-Hb (23 and 34 Torr, respectively) had similar effects on arteriolar diameter. Moreover, these effects were significantly different from those induced by αα-Hb, which possesses a P50 value of 29 Torr. This suggests that additional mechanisms are likely to affect the microvascular response to HBOC infusion.
3. Among these mechanisms is the influence of both the molecular weight of Hb molecules and the dose infused. In these experiments, this latter is unlikely to account for the differences in the arteriolar tone response, since the doses injected were very close from one Hb solution to another. Conversely, since the three Hb solutions possess different molecular weights, the action of each HBOC on the vascular tone may have been specific and this may explain, in part, the differences in the response of mesenteric arterioles. Molecular weight of Hb molecules has indeed been proved to be responsible for the pharmacological properties of HBOCs by virtue of interactions of cell-free Hb with endothelial factors such as nitric oxide and endothelin-1 Citation[[2]], Citation[4-6]. Although many pathways have been proposed to account for the vasoconstrictive action of Hb solutions, the endothelial penetration of cell-free Hb has not been studied in the microvasculature. However, Faivre-Fiorina et al. provided evidence that a high-molecular-weight HBOC (namely Hb-Dex-BTC) would be able to penetrate rapidly into aortic endothelial cells in guinea pigs, through mechanisms likely to involve endocytosis or transendothelial transport vesicles Citation[[23]]. Further investigation is required to clarify whether similar mechanisms may occur at the microvascular level and, if so, to what extent they affect arteriolar tone.
In conclusion, within the limitations of the experimental procedure, our data indicate that moderate hemodilution with HBOCs induces instantaneous constriction in mesenteric arterioles through various mechanisms related to both pharmacological and physicochemical properties of the Hb solutions.
ACKNOWLEDGMENTS
This study was supported in part by the Association Recherche et Transfusion (Paris, France) and by the Fondation pour la Recherche Médicale (Paris, France).
REFERENCES
- Winslow R. M. New Transfusion Strategies: Red Cell Substitutes. Annu. Rev. Med. 1999; 50: 337–353
- Alayash A. I. Hemoglobin-Based Blood Substitutes: Oxygen Carriers, Pressor Agents, or Oxidants?. Nat. Biotechnol. 1999; 17: 545–549
- Hart J. L., Ledvina M. A., Muldoon S. M. Actions of Diaspirin Cross-Linked Hemoglobin on Isolated Rat and Dog Vessels. J. Lab. Clin. Med. 1997; 129: 356–363
- Nakai K., Ohta T., Sakuma I., Akama K., Kobayashi Y., Tokuyama S., Kitabatake A., Nakazato Y., Takahashi T. A., Sadayoshi S. Inhibition of Endothelium-Dependent Relaxation by Hemoglobin in Rabbit Aortic Strips: Comparison Between Acellular Hemoglobin Derivatives and Cellular Hemoglobins. J. Cardiovasc. Pharmacol. 1996; 28: 115–123
- Doherty D. H., Doyle M. P., Curry S. R., Vali R. J., Fattor T. J., Olson J. S., Lemon D. D. Rate of Reaction with Nitric Oxide Determines the Hypertensive Effect of Cell-free Hemoglobin. Nature Biotechnol. 1998; 16: 672–676
- Rohlfs R. J., Bruner E., Chiu A., Gonzales A., Gonzales M. L., Magde D., Magde M. D., Jr., Vandegriff K. D., Winslow R. M. Arterial Blood Pressure Responses to Cell-Free Hemoglobin Solutions and the Reaction with Nitric Oxide. J. Biol. Chem. 1998; 273: 12128–12134
- Intaglietta M., Johnson P. C., Winslow R. M. Microvascular and Tissue Oxygen Distribution. Cardiovasc. Res. 1996; 32: 632–643
- Page T. C., Light W. R., Hellums J. D. Prediction of Microcirculatory Oxygen Transport by Erythrocyte/Hemoglobin Solution Mixtures. Microvasc. Res. 1998; 56: 113–126
- Nolte D., Botzlar A., Pickelmann S., Bouskela E., Messmer K. Effetcs of Diaspirin-Cross-Linked Hemoglobin (DCLHb™) on the Microcirculation of Striated Skin Muscle in the Hamster: A Study on Safety and Toxicity. J. Lab. Clin. Med. 1997; 130: 314–327
- Caron A., Menu P., Faivre-Fiorina B., Labrude P., Alayash A. I., Vigneron C. Cardiovascular and Hemorheological Effects of Three Chemically-Modified Human Hemoglobin Solutions in Hemodiluted Rabbits. J. Appl. Physiol. 1999; 86: 541–548
- Winslow R. M., Gonzales A., Gonzales M. L., Magde M., McCarthy M., Rohlfs R. J., Vandegriff. K. D. Vascular Resistance and the Efficacy of Red Cell Substitutes in a Rat Hemorrhage Model. J. Appl. Physiol. 1998; 85: 993–1003
- Tsai A. G., Friesenecker B., McCarthy M., Sakai H., Intaglietta M. Plasma Viscosity Regulates Capillary Perfusion During Extreme Hemodilution in Hamster Skinfold Model. Am. J. Physiol. 1998; 275: 2170–2180
- Prouchayret F., Fasan G., Grandgeorge M., Vigneron C., Menu P., Dellacherie E. A Potential Blood Substitute from Carboxylic Dextran and Oxyhemoglobin. I. Preparation, Purification and Characterisation. Blood Substitutes and Oxygen Carriers, T. M.S. Chang. Marcel Dekker Inc., New York 1993; 144–147
- Winslow R. M., Chapmann K. W. Pilot Scale Preparation of Hemoglobin Solutions. Methods in Enzymology. Hemoglobins: Biochemical and Analytical Methods, J. Everse, K. D. Vandegriff, R. M. Winslow. Academic Press, Orlando 1994; 3–16
- Adamson J. G., Moore C. Hemolink, an o-Raffinose–Crosslinked Hemoglobin-Based Oxygen Carrier. Blood Substitutes: Principles, Methods, Products and Clinical Trials, T. M.S. Chang. Karger Landes Systems, Basel 1998; 62–81
- Belougne E., Aguejouf O., Doutremepuich F., Doutremepuich C. Action of Neurotransmitters: Acetylcholine, Adrenaline and Serotonin on Arterial Thrombosis Induced by a Laser Beam. Thromb. Res. 1996; 84: 189–198
- Mitruka B. M., Rawnsley H. M. Sample Collection, Preparation, and Preservation. Clinical Biochemical and Hematological References Values in Normal Experimental Animals, B. M. Mitruka, H. M. Rawney. Masson Publishing Inc., New York 1977; 21–39
- Tsai A. G., Friesenecker B., Mazzoni M. C., Kerger H., Buerk D. G., Johnson P. C., Intaglietta M. Microvascular and Tissue Oxygen Gradients in the Rat Mesentery. Proc. Natl. Acad. Sci. USA 1998; 95: 6590–6595
- Nathan A. T., Singer M. The Oxygen Trail: Tissue Oxygenation. Brit. Med. Bull. 1999; 55: 96–108
- Asano Y., Koehler R. C., Ulatowski J. A., Traystman R. J., Bucci E. Effect of Cross-Linked Hemoglobin on Endothelial-Dependent Dilation in Cat Pial Arterioles. Am. J. Physiol. 1998; 44: H1313–H1321
- Menu P., Longrois D., Faivre B., Donner M., Labrude P., Stoltz J.-F., Vigneron C. Rheological Behaviour of Red Blood Cells Suspended in Hemoglobin Solutions. In Vitro Study Comparing Dextran-Benzene-Tetra-Carboxylate Hemoglobin and Plasma Expanders. Transfusion Sci. 1999; 20: 5–16
- Mchedlishvili G., Gobejishvili L., Mamaladze A., Momtselidze N., Varazashvili M. Microcirculatory Stasis Induced by Hemorrheological Disorders: Further Evidence. Microcirculation 1999; 6: 97–106
- Faivre-Fiorina B., Caron A., Fassot C., Fries I., Labrude P., Vigneron C. Exchange Transfusion with a Hemoglobin Solution in Guinea Pig: Evidence for the Presence of Hemoglobin Inside Aortic Endothelial Cells. Am. J. Physiol. 1999; 276: 766–H770