Abstract
Polyacrylic acid nanoparticles were successfully synthesized using a reverse microemulsion polymerization process. They had a narrow size range, averaging ∼50 nm, and were stable in buffer. The particles were isolated and lyophilized in dry powder form, and were redispersible as individual particles in buffer. The drug timolol maleate was loaded into the nanoparticles from aqueous drug solutions and, when the drug-loaded particles were dispersed in a phosphate buffer solution, the drug slowly released over several hours from the nanoparticles.
INTRODUCTION
Bioadhesion and, more specifically, mucoadhesion is an important feature of topical sustained-release dosage forms, providing some control over the site and duration of drug release Citation[1-10]. Polyacrylic acid (PAA) is one of the best mucoadhesive polymers; it has also been found to enhance drug absorption and bioavailability across mucosae Citation[11-14].
For ocular drug delivery, one of the major problems is to provide and maintain an adequate concentration of the drug in the precorneal area over a duration of up to several hours. Topical dropwise administration of opthalmic drugs in aqueous solutions usually results in extensive drug loss, mainly due to tear fluid and eyelid dynamics, which cause rapid removal of the solution from the eye Citation[[15]]. Only 1–3% of the administered dose penetrates the cornea and reaches intraocular tissues Citation[[16]]. The addition of suitable polymers to increase the viscosity of liquid drug formulations is a common method for increasing contact time, and hence drug bioavailability, through the mucosae Citation[[17]]. The capacity of mucoadhesive polymers to increase viscosity, as well as to adhere to mucin-epithelial surfaces, has been utilized to design drug delivery systems for application to mucous-rich environments, including that of the precorneal region Citation[18-19]. Recently, Thermes et al. Citation[[20]] have shown that the mucoadhesive polymer PAA modified the kinetic release profiles of timolol in the albino rabbit eye, and resulted in the highest timolol concentrations in the iris and ciliary body at later sampling times, compared to equal viscosity solutions of polyvinyl alcohol. Davies et al. Citation[[21]] also reported the effect of a PAA solution on the bioavailability of pilocarpine by measuring the relative miotic response intensities produced in the rabbit eye. It was observed that addition of the PAA solution produced a significant increase (P < 0.05) in drug bioavailability as compared to the effect of adding an equal viscosity solution of polyvinyl alcohol, which is not a mucoadhesive polymer, or buffer (PBS). Microspheres of cross-linked polyacrylic acid (Carbopol(®907) were found to retain approximately 25% of the instilled dose of pilocarpine on the ocular surface Citation[[22]]. Another mucoadhesive PAA derivative, Polycarbophil®, has been shown to improve ocular penetration of the antibiotic drug gentamicin in the pigmented rabbit Citation[[23]].
In studies relating to the effect of particle size in ophthalmic delivery systems, a three-fold increase postinstillation of the drug indomethacin in the cornea, aqueous humor and iris–ciliary body was observed only when the drug was released from submicron particles of poly(ε-caprolactone), compared to when it was released from microparticulate carriers Citation[24-25]. It was concluded that the main factor responsible for the favorable corneal transport of indomethacin was the small size and high surface area of the nanoparticulate carriers Citation[[25]].
The previous studies with PAA mucoadhesive carriers and other nanoparticulate delivery systems suggest that a polymer like PAA with well-established mucoadhesive properties and in subcolloidal, nanoparticulate form should have a strong potential as a carrier for opthalmic drug delivery. A recent article by Kriwet, Walter, and Kissel Citation[[26]] described the preparation of lightly crosslinked PAA microparticles and nanoparticles using an inverse emulsion technique. This system is also referred to as a reverse emulsion, and is not a reverse microemulsion. Nevertheless, Kriwet et al. were able to obtain nanoparticles having diameters in the range of 80–150 nm with a relatively narrow size distribution. The particles also showed excellent bioadhesive properties in vitro on excised tissue surfaces Citation[[26]].
To date, no one has prepared nanoparticles of polyacrylic acid in a reverse microemulsion. Such reverse microemulsion solutions are rendered thermodynamically stable by a surfactant. The water droplets in the water-in-oil reverse microemulsion are sometimes referred to as “water pools.” Hydrophilic monomers can be dissolved and subsequently polymerized in the stabilized aqueous pools of a reverse microemulsion Citation[[27]]. Since the sizes of reverse micellar water droplets in a reverse microemulsion are of the order of several tens of nanometers in diameter and they are highly monodisperse, preparations of nanoparticles in a reverse microemulsion system should produce highly monodisperse nanoparticles. The aim of the present study was to utilize the reverse microemulsion polymerization technique to prepare monodisperse, bioadhesive nanoparticles of PAA for use as mucosal drug delivery carriers. In this article we present the synthesis, isolation, and characterization of such nanoparticles, and the loading and in vitro release of an ophthalmic drug from the particles. Bioadhesive properties of the particles are currently being evaluated.
MATERIALS AND METHODS
Materials
Acrylic acid (AA) was purchased from Aldrich Chemical Company, Inc., Milwaukee, WI, and was used after distillation under reduced pressure. Span 80 (sorbitan monooleate), Tween 80 (polyoxyethylene-sorbitan monooleate), timolol hydrogen maleate salt, and ammonium persulfate (Sigma ultra) were procured from Sigma Chemical Co. (St. Louis, MO) and used without further purification. Formvar 15/95 resin powder was purchased from Electron Microscopy Sciences (Ft. Washington, PA). Phosphotungstic acid, hydrate, crystal was from Ted Pella (Redding, CA). The buffer components sodium phosphate (dibasic, anhydrous), sodium phosphate (monobasic, monohydrate crystal) were purchased from J.T. Baker (New Jersey). Hexanes (HPLC grade) were from Fisher Scientific (New Jersey). Waters used were deionized and distilled.
Methods
Preparation of the PAA Nanoparticles
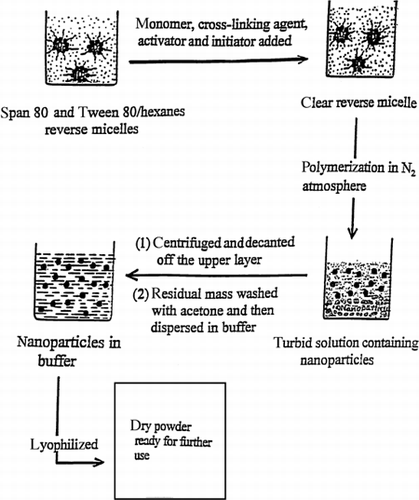
PAA nanoparticles were prepared by a reverse microemulsion polymerization process using N, N′-methylene-bis-acrylamide (MBA) as a cross-linking agent (to prevent dissolution of the particles during drug loading, storage and release), ammonium persulphate (APS) as the initiator, and N, N, N′,N′-tetramethylethylene- diamine (TEMED) as activator (). The surfactants, Span 80, and Tween 80 were dissolved in hexanes at concentrations of 0.08 and 0.02 M, respectively. In a typical experiment, 400 μL of freshly distilled AA, 20 μL of TEMED solution (20 vol.-%), 20 μL MBA solution (0.05 g/mL), and 100 μL of 2.5% ammonium persulfate solution as initiator were added to 90 mL of the above reverse microemulsion solution. The solution was homogeneous and transparent. Polymerization was carried out under a nitrogen atmosphere at 40°C for 6–8 h with continuous stirring. The above method produced turbid solutions containing PAA nanoparticles. After the polymerization, the reaction mixture was transferred to centrifuge tubes and allowed to settle overnight. The next day the tubes were centrifuged for 0.5 h and the supernatant was decanted. The white mass left in the centrifuge tube was washed twice with 10 mL of acetone. The residual mass was air-dried for a couple of minutes and then dispersed in 10 mL of distilled water and sonicated for 10 min. A stable bluish dispersion of PAA nanoparticles in water was obtained. This dispersion was diluted for measurement of the particle size by dynamic laser light scattering.
Particle Size Measurement
Dynamic laser light scattering measurements were used to determine the size of the nanoparticles. We used a Brookhaven 9000 instrument with BI200SM goniometer having an air-cooled argon ion laser (488 nm) as the light source. The time dependence of the intensity autocorrelation function of the scattered intensity was derived by using a 252-channel digital correlator. The size of the nanoparticles was determined from diffusion of particles using the Stokes-Einstein equation. All measurements were carried out either in distilled water or in phosphate buffers.
Visualization of the Nanoparticles with Transmission Electron Microscopy (TEM)
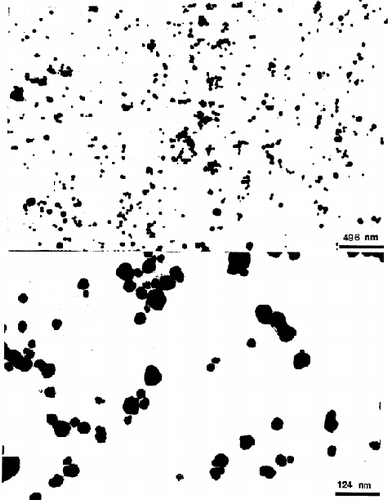
The morphological examination of the nanoparticles was performed using a Philips 410 transmission electron microscope, following staining with phosphotungstic acid solution (0.2% w/v). Suspensions were dispersed in the staining solution for 30 min at room temperature, placed on a copper-rhodium grid (300 mesh) covered with Formvar, dried, and observed by TEM.
Stability of the PAA Nanoparticles
Approximately 30 mg of nanoparticles suspended in 4 mL of phosphate buffer (50 mM, pH 7.0) were added to each of the three scintillation vials of 20-mL size. These vials were separately stored in the refrigerator (∼4°C), at room temperature (∼20–23°C), and in an incubator maintained at 37°C. The light bluish solutions were checked by the naked eye every week for any aggregated and/or floating material.
Lyophilization of the PAA Nanoparticles
About 100 mg of the particles in 8 mL of phosphate buffer (50 mM, pH 7.0) were lyophilized in a Labconco Freeze Dry System/Freezone 4.5. The lyophilized powder was redispersed in water and particle sizes were again measured using dynamic laser light scattering.
Drug-Loading into the PAA Nanoparticles
Timolol maleate (TM) (formula weight = 432.5), an antiglaucoma agent, was chosen as the drug for loading in the nanoparticles. TM is a beta blocker and is commonly prescribed for initial and maintenance therapy to glaucoma patients. Twenty five mg of dry PAA nanoparticles were added to 100–500 μL of a TM solution (5 mg/mL) and left to stand overnight at room temperature (21–23°C). The next day, the solution was diluted to a volume of 5 mL with water, and the mixture was allowed to stand for another 24 h. The solution was ultra-filtered through a 100-kD cutoff filter (a polyethersulfone membrane from Millipore) to separate the nanoparticles from the free TM. The amount of drug loaded into the particles was determined by depletion of TM from the solution, based on ultraviolet assay of the filtrate at 295 nm.
In Vitro Release Experiments
We designed a simple dialysis experiment to measure drug delivery kinetics from the nanoparticles. In this experiment, the particles were suspended in water or phosphate buffer inside a closed dialysis membrane tube, and the appearance of the drug in the surrounding water or buffer solution was measured as a function of time. It is clear that this experiment in no way mimics the real situation of drug particles on the surface of the eye, but it does permit comparison of many different parameters, such as drug loading levels, drug compositions, particulate sizes, and particulate compositions. In this experiment, 50 mg of nanoparticles containing 2.0 mg of TM were added to 5 mL water (or phosphate buffer) in a closed cellulose membrane tubing (Spectrapor No. 2, cutoff 12–14 kD, diameter 16 mm, Spectrum Laboratories, Inc., Laguna Hills, CA) and dialysed against 60 mL of deionized water (or 50 mM phosphate buffer) at pH 7.4. Aliquots of 0.6 mL of the dialysis solution were withdrawn at appropriate intervals, absorbance was measured for each at 295 nm, and the withdrawn samples were placed back into the dialysis solution. The entire experiment was carried out at room temperature (21–23°C), while the dialysis solution was stirred slowly at constant speed. As controls, 5 mL of a TM solution in water (0.4 mg/mL) (or in phosphate buffer) was dialyzed under similar conditions against 60 mL of deionized water (or 50 mM phosphate buffer, pH 7.4).
RESULTS AND DISCUSSION
Preparation of Nanoparticles
Unlike emulsion or inverse emulsion polymerization, which involve initial reaction mixtures consisting of two separate phases, our reverse microemulsion polymerization procedure starts with an initially homogeneous solution of the reaction mixture. Since ammonium persulphate is a water-soluble initiator, it can be expected that the initiation of AA polymerization takes place within the small water droplets (waterpools) in the reverse microemulsion. The polymerization reaction and formation of nanoparticles both take place within these aqueous droplets by a primary growth process. The size of the nanoparticles formed initially approaches that of the aqueous droplet. Due to interdroplet interactions, they sometimes coalesce and form larger particles. As the waterpools in which the polymerization reaction proceeds are highly monodisperse and small in size, the method can produce very small nanoparticles with a narrow particle size distribution.
Estimating Particle Size and Size Distribution
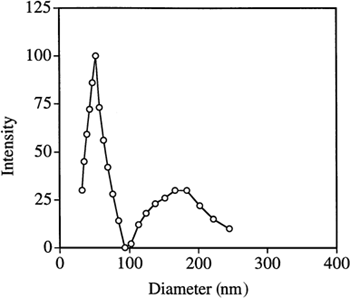
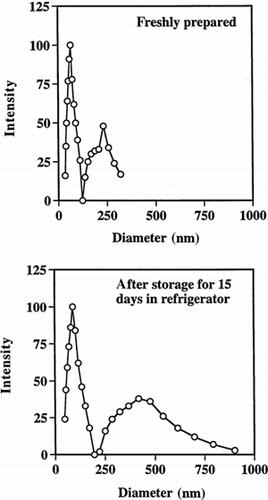
Typical dynamic laser light scattering data showing the sizes of the PAA particles in water are shown in . The results are summarized in . It can be seen that the particle size distribution is bimodal; mean diameters of the smaller particles are 50–75 nm and of the larger ones are 150–200 nm. The total mass fraction (estimated by the average intensity) of the smaller particles is approximately 60–80% of the total particulate mass. The bimodal size distribution could indicate that nucleation and growth of the particles occurred by two different mechanisms, or it may simply reflect some aggregation of the small particles. For example, it is possible that AA and MBA partition to varying extents in both water and hexane, leading to some polymerization in both phases. The particles formed in the waterpools should be small in size and highly monodisperse. However, the particles obtained are much bigger than the waterpool size (∼10 nm), which could be due to aggregation and/or coalescence of the polymerizing reverse micellar droplets Citation[[28]]. shows two representative transmission micrographs (TEM) of the PAA nanoparticles. The photos show that most of the particles are highly monodisperse and very small in size (∼50 nm).
Table 1. Polyacrylic Acid Particle Sizes in Water as Measured by Quasielastic Laser Light Scattering
Lyophilization and Stability of the Particles
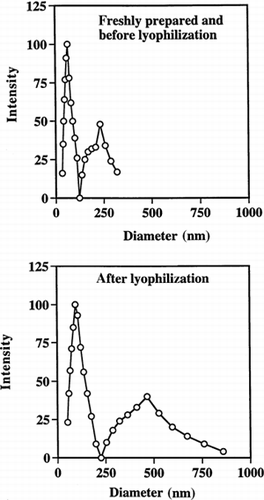
Since PAA particles are highly adhesive in nature, it was important to verify that the particles could be lyophilized and then redispersed in water. We also checked their stability at three temperatures (4°C, R.T., and 37°C) while suspended in phosphate buffer (50 mM, pH 7). After lyophilization and resuspension in buffer, the mean diameters of the particles increased from 50–75 nm to ≈100 nm, and from 150–200 nm to ≈470 nm for the smaller- and bigger-size particle populations, respectively (). Polydispersity of both the smaller and larger particle size distributions also increased (). Similar tendencies were seen on storage of the particles. After 15 days storage at 4°C they grew to almost double their original size and also demonstrated increased polydispersity (). After 2 weeks storage at 37°C, some particles separated out as a white mass floating on top of the solution. In contrast, at 4°C and room temperature, the particle suspension remained as a clear bluish solution. The aggregation seen at 37°C is probably due to self-adhesion of some of the particles, and this process would be expected to be faster at higher temperatures.
Drug-Loading and Release
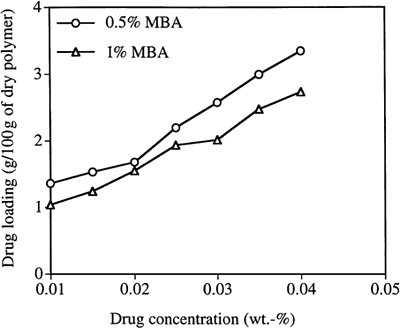
The drug loading, based on dry polymer weight, is plotted as a function of loading solution concentration in . It can be seen that the loading of drug increases almost linearly as the concentration of the drug in the loading solution increases. As could be expected, the loading is lower for the particles with higher cross-link density. It is reasonable that particles with higher cross-link density will show reduced loading of drug due to their smaller pore sizes and smaller volume fraction of “free” water available to solvate the drug within the particle.
Figure 6. Loading timolol maleate (TM) into PAA nanoparticles as a function of TM concentration in the loading solution. (MBA is the crosslinker.)
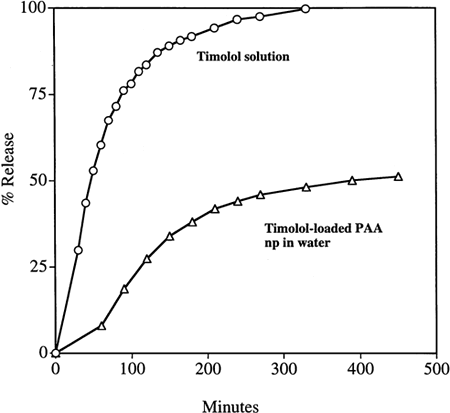
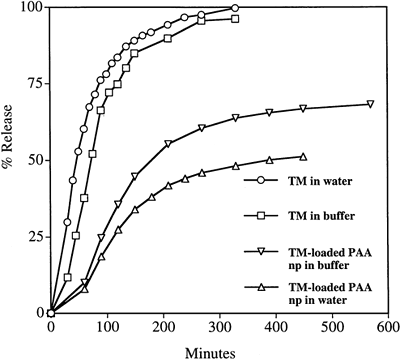
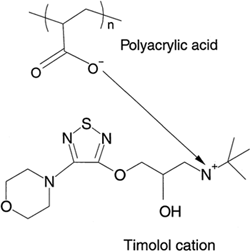
The in vitro kinetic release profiles of TM from the particles were compared with dialysis of a simple TM solution as a control, dialyzing against either deionized water or phosphate buffer (50 mM, pH 7.4). The results are shown in and . It is evident from the figures that the release of TM into either water or phosphate buffer was considerably slower from the PAA nanoparticles than from a simple TM solution of the same total mass concentration. A release of 50% of the drug from the nanoparticles occurred over 5 h; in contrast, all the TM was dialyzed out of the simple TM solution in 3 h. Total release from the particles was not obtained even after 24 h when dialyzed against deionized water (). It is possible that, in the experiments with deionized water as the medium, some of the TM is ionically entrapped within the polyanionic PAA matrix, with the TM maleate counter ion being replaced by the PAA carboxyl ion. Indeed, using phosphate buffer as the dialysis solution, the release of TM from the nanoparticle solution was faster than when dialyzed against deionized water. This supports the hypothesis that the drug cation may be binding with the anionic acrylate carboxyl groups when the particles are suspended in deionized water (). When dialyzed against buffer (instead of water), sodium ions in the buffer enter the dialysis bag and replace the timolol cation, facilitating faster release.
CONCLUSIONS
Lightly crosslinked PAA nanoparticles (50–250 nm) have been synthesized using a reverse microemulsion technique. The nanoparticles are stable when stored at 4°C or room temperature, but tend to aggregate at 37°C. The antiglaucoma agent TM was loaded into the nanoparticles and the drug was gradually dialyzed from the nanoparticles into deionized water or phosphate buffer, compared to very rapid dialysis from a simple TM solution. TM also released faster from the particles into phosphate buffer than into deionized water, indicating the importance of ion exchange to the release process. These bioadhesive particles could be utilized as carriers for drug delivery to the eye and to other mucosal surfaces, such as the nose, mouth, throat, and vagina.
ACKNOWLEDGMENTS
The authors thank Alcon Laboratories, Inc., Ft. Worth, Texas, for providing financial assistance to carry out this research. We would also like to thank Pedro Verdugo for use of the photon correlation spectrophotometer in his lab, and Monica Orellana for helping us with it. We gratefully acknowledge Audrey Wass for helping with the TEM experiments.
REFERENCES
- Peppas N. A., Sahlin J. J. Hydrogels: Mucoadhesive and Bioadhesive Materials–-A Review. Biomaterials 1996; 17: 1553–1561
- Ahuja A., Khar R. K., Ali J. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1997; 23: 489–515
- Tur K. M., Chang H. S. Evaluation of Possible Mechanism(s) of Bioadhesion. Int. J. Pharm. 1998; 1: 61–74
- BlancoFuente H., Anguianolgea S., OteroEspinar F. J., BlancoMendez J. In Vitro Bioadhesion of Carbopol Hydrogels. Int. J. Pharm. 1996; 142: 169–174
- Jones D. S., Woolfson A. D., Brown A. F. Textural, Viscoelastic and Mucoadhesive Properties of Pharmaceutical Gels Composed of Cellulose Polymers. Int. J. Pharm. 1997; 15: 223–233
- Genta I., Conti B., Perugini P., Pavanetto F., Spadaro A., Puglisi G. Bioadhesive Microspheres for Opthalmic Administration of Acyclovir. J. Pharm. Pharmacol. 1997; 49: 737–742
- Gu J. M., Robinson J. R., Leung S. H.S. Binding of Acrylic Polymers to Mucin/Epithelial Surface: Structure Property Relationship. Crit. Rev. Therap. Drug Carrier Sys. 1988; 5: 21–67
- Park H., Robinson J. R. Mechanism of Mucoadhesion of Polyacrylic Acid Hydrogels. Pharm. Res. 1987; 4: 457
- Thermes F., Grove J., Rozier A., Plazonnet B., Constancis A., Bunel C., Vairon J. P. Mucoadhesion of Copolymers and Mixtures Containing Polyacrylic Acid. Pharm. Res. 1992; 9: 1563–1567
- Shojaei A. H., Li X. L. Mechanisms of Buccal Mucoadhesion of Novel Copolymers of Acrylic Acid and Polyethylene Glycol Monomethylether Monomethacrylate. J. Controlled Release 1997; 47: 151–161
- Luessen H. L., Leeuw B. J., Perard D., Lehr C. M., Boer A. G., Verhoef J. C., Junginger H. E. Mucoadhesive Polymers in Peroral Peptide Drug Delivery. I. Influence of Mucoadhesive Excipents on the Proteolytic Activity of Intestinal Enzymes. Eur. J. Pharm. Sci. 1996; 4: 117–128
- Lueben H. L., Borchard G., Verhoef J. C., Lehr C. M., Boer A. G., Junginger H. E. Mucoadhesive Polymers in Peroral Peptide Drug Delivery. II. Carbomer® and Polycarbophil® are Potent Inhibitors of the Intestinal Proteolytic Enzyme Trypsin. Pharm. Res. 1995; 12: 1293–1298
- Lueben H. L., Rentel C. O., Kotze A. F., Lehr C. M., Boer A. G., Verhoef J. C., Junginger H. E. Mucoadhesive Polymers in Peroral Peptide Drug Delivery. IV. Polycarbophil® and Chitosan are Potent Enhancers of Peptide Transport Across Intestinal Mucosae In Vitro. J. Controlled Release 1997; 45: 15–23
- Lueben H. L., Bohner V., Perard D., Langguth P., Verhoef J. C., Boer A. G., Merkle H. P., Junginger H. E. Mucoadhesive Polymers in Peroral Peptide Drug Delivery. V. Effect of Poly(Acrylates) on Enzymatic Degradation of Peptide Drugs by Intestinal Brush Border Membrane Vesicles. Int. J. Pharm. 1996; 141: 117–128
- Lee V. H.L., Robinson J. R. Mechanistic and Quantitative Evaluation of Precorneal Pilocarpine Disposition in Albino Rabbits. J. Pharm. Sci. 1979; 68: 673–684
- Patton T. F., Robinson J. R. Quantitative Precorneal Disposition of Topical Applied Pilocarpine Nitrate in Rabit Eyes. J. Pharm. Sci. 1976; 65: 1295–1301
- Hui H. W., Robinson J. R. Ocular Delivery of Progesterone Using a Bioadhesive Polymer. Int. J. Pharm. 1985; 26: 203–213
- Gurny R., Ibrahim H., Aebi A., Buri P., Wilson C. G., Washington N., Edman P., Camber O. Design and Evaluation of Controlled Release Systems for the Eye. J. Controlled Release 1987; 6: 367–373
- Saetone M. F., Chetoni P., Torracca M. T., Burgalassi S., Giannaccini B. Evaluation of Muco-Adhesive Properties and In Vivo Activity of Opthalmic Vehicles Based on Hyaluronic Acid. Int. J. Pharm. 1989; 51: 203–212
- Thermes F., Rozier A., Plazonnet B., Grove J. Bioadhesion: The Effect of Poly(Acrylic Acid) on the Ocular Bioavailability of Timolol. Int. J. Pharm. 1992; 81: 59–65
- Davies N. M., Farr S. J., Hadgraft J., Kellaway I. W. Evaluation of Mucoadhesive Polymers in Ocular Drug Delivery. I. Viscous Solutions. Pharm. Res. 1991; 8: 1039–1043
- Durrani A. M., Farr S. J., Kellaway I. W. Precorneal Clearance of Mucoadhesive Microspheres from the Rabbit Eye. J. Pharm. Pharmcol. 1994; 47: 581–584
- Lehr C. M., Lee Y. H., Lee V. H. Improved Ocular Penetration of Gentamicin by Mucoadhesive Polymer Polycarbophil® in the Pigmented Rabbit. Invest. Opthalmol. Vis. Sci. 1994; 35: 2809–2814
- Calvo P., Alonso M. J., Vila-Jato J. L., Robinson J. R. Improved Occular Bioavailability of Indomethacin by Novel Ocular Drug Carriers. J. Phar. Pharmacol. 1996; 48: 1147–1152
- Calvo P., Vila-Jato J. L., Alonso M. J. Comparative In Vitro Evaluation of Several Colloidal Systems, Nanoparticles, Nanocapsules, and Nanoemulsions, as Ocular Drug Carriers. J. Pharm. Sci. 1996; 85: 530–536
- Kriwet B., Walter E., Kissel T. Synthesis of Bioadhesive Poly(Acrylic Acid) Nano- and Microparticles Using an Inverse Emulsion Polymerization Method for the Entrapment of Hydrophilic Drug Candidates. J. Controlled Release 1998; 56: 149–158
- Candau F., Leong Y. S., Fitch R. M. Kinetic Study of the Polymerisation of Acrylamide in Inverse Microemulsion. J. Polym. Sci.: Polymer Chem. Ed. 1985; 23: 193–214
- Clint J. H., Collins I. R., Williams J. A., Robinson B. H., Towey T. F.P., Cajean P., Khan-Lodhi A. Synthesis and Characterisation of Colloidal Metal and Semiconductor Particles Prepared in Microemulsions. Faraday Discuss. 1993; 95: 219