Abstract
Worldwide, great efforts are being made to develop a clinically useful artificial oxygen carrier. Toxicological and immunological compatibility is generally tested using animal experiments but inflammatory parameters in particular show large species-specific differences. Therefore, we developed an in vitro system using human components to establish a compatibility profile of unknown compounds. The test system comprises induction of hemolysis, activation of complement (C3a), induction/suppression of cytokine production, influence on cell proliferation, direct toxicity on peripheral leukocytes, and phagocytosis of the material under test and of microbes. The test system will be described, along with results of various perfluorocarbon emulsions. When testing lecithin-based perfluorodecalin (PFD) emulsions, and comparing them to Pluronic-based PFD emulsions, we could show that Pluronic-based emulsions were virtually untoxic to peripheral human leukocytes. They neither inhibited cell proliferation nor caused any hemolysis, but caused mild to moderate inhibition of endotoxin-induced cytokine production. At the same time, lecithin-based PFD emulsion caused substantial cytotoxicity in phagocytic cells like monocytes (60–100% after 24 h incubation) and granulocytes (10–20% after 24 h incubation). They also suppressed endotoxin-induced cytokine production in monocytes to more than 98% and inhibited cell proliferation of an endothelial (ECV 304) and a monocytic cell line (MonoMac6) to more than 95%.
INTRODUCTION
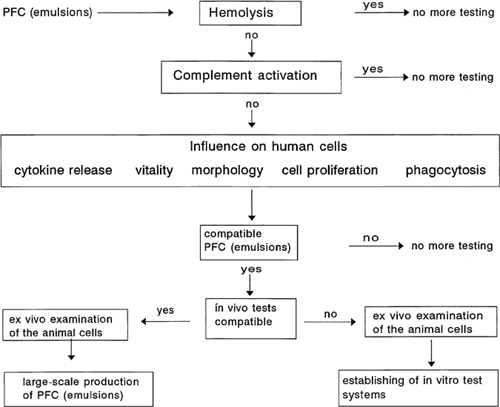
During and after the development of artificial oxygen carriers designed for potential use in humans, and following analysis of their physicochemical properties, we see the importance of determining their biocompatibility profile in vitro before they may be tested in animals or humans. On the one hand, many extensive animal experiments could be saved; on the other hand, using tests based on human cells may provide a better predictive value for the judgement of in vivo compatibility in humans. It is possible that a substance can be shown to be intolerable in the animal model but be without major problems for use in humans. Much more likely, and abundantly evident from the literature, are situations where extensive experiments with rodents seemed to indicate compatibility and beneficial effects, whereas ensuing human trials ended in severe disappointment. Therefore we developed a multitask test system exploring the interference of test substances with vital functions on the cellular level (). We gathered information on direct toxic effects as well as influences on several cellular functions.
To estimate direct cytotoxicity, data will show the degree of hemolysis induced by different perfluorocarbon emulsions (PFC emulsions) and their effects on the survival of peripheral human leukocytes. To determine the rate of dead leukocytes, we used flow cytometry. We prefer this method because many more cells can be counted in relation to light microscopy. Furthermore, a discrimination between monocytes, lymphocytes, and granulocytes is possible, and for each population the rate of dead cells can be measured separately. In addition to cytotoxicity, the influence of perfluorocarbon emulsions on cell proliferation is determined. We use only human cell lines, and especially cells that will be first in contact with drugs for intravenous application. Therefore, data will be presented with an endothelial cell line (ECV 304) and a monocytic cell line (MonoMac6). The cytokine release is an important cellular function. We focused our tests on interleukin-1β and interleukin-6, because these are pleiotropic factors that are released by activated monocytes, neutrophils, and several lymphocytes. They play a key role in defense against infectious diseases, tissue damage, and inflammation. Massive induction of these cytokines, or massive suppression of stimulan-induced cytokines, would be reason for caution. The biocompatibility profile is completed by testing complement activation and phagocytosis. These data will be shown elsewhere.
We investigated various perfluorodecalin emulsions. Perfluorodecalin (PFD) possesses a high solubility for oxygen and carbon dioxide. PFD was developed to be used as red cell substitute Citation[[1]], Citation[[3]]. PFD alone is not soluble in water and must be emulsified for intravenous application. The block polymer Pluronic and lecithin are widely used as emulsifiers. The PFD/lecithin emulsion, the PFD/Pluronic emulsion and, in addition, perfluorodecalin and the emulsifiers alone, were tested. Lecithin is a common component of several pharmaceutical preparations (e.g., lipid solutions for intravenous application).
MATERIALS AND METHODS
Peripheral leukocytes, serum, and erythrocytes were obtained from healthy volunteer donors.
Cell Lines
ECV 304 cells are human umbilical cord venous endothelial cells Citation[[4]]. ECV 304 cells were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The cells were cultured with Medium 199 (Gibco, Eggenstein, Germany) supplemented with 20% heat-inactivated fetal calf serum (Roth, Karlsruhe). MonoMac6 are human acute monocytic leukemia cells Citation[[5]]. They were also purchased from the German collection. The culture medium for MonoMac6 contained: RMPI 1640 (Gibco) supplemented with 10% heat-inactivated fetal calf serum (Myoclone Super Plus, Gibco) and 10 mL HEPES buffer (Gibco) 2.5 mL nonessential amino acids (Gibco), 3.5 mL L-glutamin (Gibco), and 4.5 mL OPI (Sigma, Steinheim, Germany).
Test Substances
Ringer's lactate (Baxter, Unterschleissheim, Germany) perfluorodecalin (PFD; Fluka # 77264); PFD emulsion with lecithin, containing 18.5% (w/v) perfluorodecalin and 1.5% (w/v) perfluorodimorpholinopropane (FDMP, Eup0002), emulsified with 2.5% (w/v) lecithin (Lipoid E100®, Lipoid KG) in Ringer's lactate, homogenized and steam sterilized; initial average droplet size: 130–239 nm; PFD emulsion with Pluronic, containing 18.5% (w/v) perfluorodecalin and 1.5% (w/v) perfluorodimorpholinopropane (FDMP, Eup 0002), emulsified with 4% (w/v) Pluronic PE 6800® (BASF, Ludwigshafen, Germany) in Ringer's lactate, homogenized and steam sterilized; initial average droplet size: 290–346 nm; lecithin-emulsion, containing 2.5% (w/v) lecithin (Lipoid E100, Lipoid KG) in Ringer's lactate; initial average droplet size: 222 nm; 4% (w/v) Pluronic PE 6800® (BASF, Ludwigshafen, Germany) in Ringer's lactate.
Sterility Control
For all test substances, controls of sterility were employed each time before use. The substances were plated onto blood/agar plates and incubated at 37°C, 5 vol.-% CO2 in humid atmosphere. After 24 and 48 h, the plates were controlled for bacterial growth. None of the substances used in the tests showed bacterial growth.
Hemolysis Assay
A 20% (w/v) erythrocyte suspension was prepared with Ringer's lactate. To determine the hemolytic activity of the test substances, 50 μL of the erythrocyte suspension was incubated together with 100 μL of the test substance plus 100 μL of serum or isotonic saline. Erythrocyte suspension, together with isotonic saline, was used as negative control; erythrocyte suspension with distilled water was used as positive control. The samples were incubated for 1 h at 37°C in a water bath, then the samples were centrifuged for 2 min at 2.000g (Heraeus Instruments, Stuttgart, Germany) and the optical density of the supernatants were measured spectrophotometrically (SLT Labinstruments, Crailsheim, Germany). A test substance induced hemolysis when the following quotient was greater than 2:where ODt + R.L./s + e is the optical density of the supernatant consisting of test substance (t) plus Ringer's lactate or serum plus erythrocytes (e); ODt + R.L./s is the optical density of the supernatant consisting of test substance plus Ringer's lactate or serum; and ODR.L./s + e is the optical density of the supernatant consisting of Ringer's lactate or serum plus erythrocytes.
Cytokine Assay
Mononuclear leukocytes were prepared with gradient separation (Lympho- flot separation medium, Biotest, Dreieich, Germany) using human blood (buffy coat from routine blood donations). The mononuclear leukocytes were used with a final concentration of 1 × 106 cells/mL and maintained with 5 vol.-% fetal calf serum (Roth, Germany) and RPMI (1640, Gibco, Eggenstein, Germany). One hundred micro liters of the cell suspension were incubated for 4 h at 37°C, 5 vol.-% CO2, humid atmosphere, together with 50 μL of the test substance or the controls. Then lipopolysaccharide (Salmonella typhimurium, Difco, Augsburg, Germany) was added to a final concentration of 10 ng/mL and the cell suspension was incubated for another 20 h. Thereafter, the interleukin-1β and interleukin-6} concentrations of the supernatant were measured using the ELISA technique (IL1β Quantikine, DPC, Bad Nauheim, Germany; IL6, Pharmingen, Hamburg, Germany). The degree of suppression or induction of interleukin-1β (IL1β) or interleukin-6 (IL6) production was determined by the following quotients:where [IL1β (or IL6)]MNL + test compounds + LPS equals concentration of IL1β (IL6) after incubation of mononuclear leukocytes (MNL) with various test compounds plus lipopolysaccharide (LPS); and [IL1β(or IL6)]MNL + LPS equals concentration of IL1β (IL6) after incubation of mononuclear leukocytes (MNL) with LPS.
Cell Proliferation Assay
For this assay, 96-well culture plates (Falcon, Becton, Dickinson, Heidelberg, Germany) were used. Each well contained 5 × 104 cells (ECV 304 or MonoMac6) in a volume of 100 μL. To each well were added 50 μL of a test substance or the control (culture medium). The test substances and the controls were placed symmetrically and alternately to avoid position effects on the microtiter plate. The culture plates were incubated for 24 h at 37°C, 5 vol.-% CO2, humid atmosphere. Then 20 μL of 3H-thymidine (37 MBq/mL, Amersham) was added to each well. After another 24 h of incubation at 37°C, the culture plates were harvested using an automated harvester. As the ECV 304 are adherent cells, cultures were washed and trypsinized (trypsin from Biochrom, Berlin, Germany) before harvesting. Cell proliferation was determined by scintillation counting (Betaplate, LKB Wallac/Pharmacia, Finland). The relative 3H-thymidine incorporation was calculated as follows:where tc is the test compound and cm is the culture medium.
Toxicity Assay
For peripheral leukocyte separation, anticoagulated (sodium-Heparin, 10 I.E./mL) blood was incubated at ambient temperature for 1 h on a density gradient (Ficoll 1077, Biochrom, Berlin, Germany) without centrifugation. Thereafter, the supernatant was obtained. For each sample, 75 μL leukocyte suspension was incubated together with 50 μL test substance at 37°C, 5 vol.-% CO2, humid atmosphere. Samples were obtained after 0, 2, 4, 6, 8, 13, 17, and 24 h. The cells were stained with CD45 FITC (panleukocyte marker, green fluorescence, Becton Dickinson, Heidelberg, Germany), CD14 PE (monocyte marker, orange fluorescence, Becton Dickinson), and propidium iodide (Sigma, Steinheim, Germany, DNA staining, determination of dead cells). The flow cytometric analyses were performed on a flow cytometer from Coulter (Coulter Epics XL, Coulter, Krefeld, Germany). Gates were set for the various leukocyte populations; 10,000 cells were acquired; data were analysed using XL1 and XL2 software (Coulter).
RESULTS
Hemolysis Assay
The results of the hemolysis tests are shown in . Only lecithin and the lecithin/perfluorodecaline emulsion cause a weak positive hemolysis in the presence of isotonic saline. In the presence of serum, none of the test compounds caused a hemolysis.
Table 1. Hemolysis Assay
Induction and Suppression of the Interleukin-1β Production of Mononuclear Leukocytes
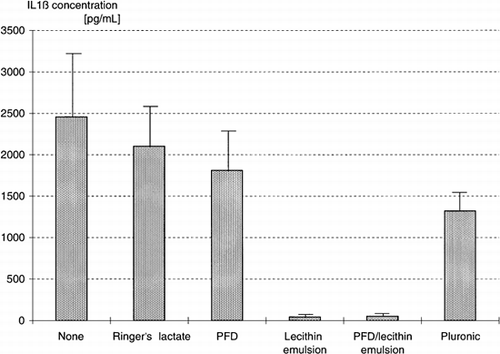
Lipopolysaccharide (LPS) is a strong stimulus for mononuclear leukocytes (MNL). After incubation of MNL with LPS, they produce (among others) interleukin-1β and interleukin-6. The results of our tests are depicted in . We used Ringer's lactate as control, because it is also a component of the PFC emulsions. The incubation of MNL with Ringer's lactate resulted in a mild decrease of interleukin-1β concentration. The incubation with perfluorodecalin (PFD) induced an average suppression of 26%, a decrease that is not significant. In contrast, lecithin and the PFD/lecithin emulsion suppressed the interleukin-1β concentration to 98%. Finally, Pluronic caused a suppression of 46%. None of the test compounds induced interleukin-1β by itself.
Induction and Suppression of the Interleukin-6 Production of Mononuclear Leukocytes
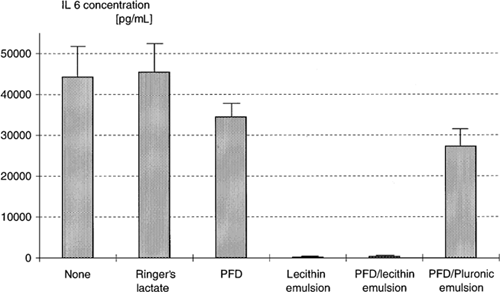
The determination of the interleukin-6 concentrations after incubation of mononuclear leukocytes with PFC emulsions and LPS showed similar results () in comparison to the interleukin-1β tests. With PFD, the interleukin-6 production of mononuclear leukocytes is reduced by 22%. The PFD/Pluronic emulsion caused an average decrease of 38%. As with interleukin-1β, production of interleukin-6 was abolished by lecithin and the lecithin/PFD emulsion. In these supernatants, the means are 157 pg/mL (lecithin) and 325 pg/mL (lecithin/PFD emulsion). In contrast, the supernatants of mononuclear leukocytes cultured only with culture medium and stimulated with LPS contained an average of 44,239 pg/mL. None of the test compounds induced interleukin-6 production by itself.
Cell Proliferation Tests with ECV 304 and MonoMac6
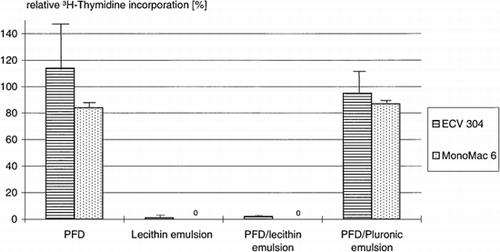
The two human cell lines showed analogous behavior. The uptake of 3H-thymidine by the endothelial cells is not reduced in the presence of PFD and the PFD/Pluronic emulsion (, ). In the case of MonoMac6, the 3H-thymidine incorporation is slightly reduced. In contrast, the 3H-thymidine uptake by endothelial and monocytic cells in the presence of lecithin and lecithin/PFD emulsion was nearly zero.
Table 2. Relative 3H-thymidine Incorporation of ECV 304 and MonoMac 6 After Incubation with Various Test Compounds (tc)
Cell Toxicity
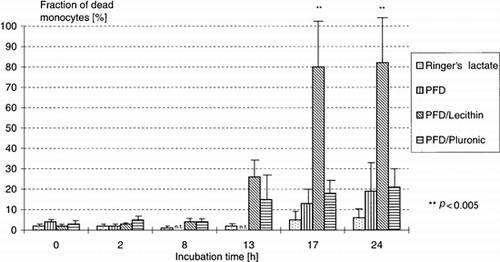
The results of the cell toxicity assays are shown in . The measured values for a incubation time of 2, 4, 6, and 8 h were within the controls. Ringer's lactate was again used as negative control, because the emulsions also contain it. The in vitro incubation of peripheral leukocytes itself leads to a slight increase in the rate of dead cells. After a 24-h incubation of leukocytes with PFD or PFD/Pluronic emulsion, about 20% dead monocytes and approximately 12% dead granulocytes were found. The lecithin/PFD emulsion was especially toxic for monocytes (p < 0.005) and after 24 h the values for lymphocytes were also increasing. depicts the fraction of dead monocytes depending on incubation time and test substance.
Table 3. Toxicity of Perfluordecalin (PFD) and Various PFD Emulsions on Peripheral Human Leucocytes
DISCUSSION
In our tests, perfluorodecalin alone did not cause hemolysis, but did cause a slight suppression of interleukin-1β and interleukin-6 production and a low toxicity versus monocytes and granulocytes. PFD had only mild effects on cell proliferation of endothelial (ECV 304) and monocytic cells (MonoMac6).
Pluronic-based PFD emulsion caused mild to moderate inhibition of endotoxin-induced cytokine production but showed no significant increase in toxicity compared to PFD. The influence of PFD /Pluronic emulsion on cell proliferation is low and comparable to PFD.
PFD-emulsion with lecithin as emulsifier suppressed the interleukin-1β/6 release of LPS-stimulated monocytes to more than 98%. The lecithin-based PFD emulsion showed considerable toxicity for phagocytic cells, especially monocytes. Accordingly, the cell proliferation of endothelial and monocytic cells was also nearly abolished.
In conclusion, PFD alone is generally untoxic. As PFD is not soluble in water, it must be emulsified for intravenous application. We tested two emulsions, using either lecithin (2.5% w/v) or Pluronic (4% w/v) as emulsifier. The lecitihin/ PFD emulsion showed considerable toxicity versus monocytes and inhibition of important cellular functions (cytokine release and cell proliferation). This toxicity cannot be attributed to PFD but to the emulsifier or to the interactive effects of the entire PFD emulsion. In some of the tests we also used lecitihin as 2.5% (w/v) emulsion in Ringer's lactate without other components. Lecithin alone suppressed the cytokine release and the cell proliferation to the same extent as the PFD/lecithin emulsion. These results seem to show that lecithin was responsible for the immunosuppressive effects. Edwards et al. Citation[[6]] studied the effect of a similar PFD/lecithin emulsion on neutrophil chemoluminescence. The generation of a superoxide anion radical is another important neutrophil function. Edwards et al. Citation[[6]] showed a dose-dependant decrease in PMA-induced neutrophil chemoluminescence. In contrast to our experiments, this effect could not be attributed to lecithin. Other authors Citation[7-8] reported trombocytopenia in healthy adults after administration of low doses (∼1 mL/kg body weight) of PFC emulsions containing egg yolk lecithin.
The PFD/Pluronic PE 6800 emulsion tested showed, among other things, mild to moderate inhibition of endotoxin-induced cytokine production. The effects and properties of Fluosol DA®, also a PFD emulsion containing Pluronic F68 and perfluorotripropylamine, have been studied for years. Preclinical and clinical studies Citation[[9]] have been undertaken to evaluate the properties and the benefits for patients with acute blood loss Citation[[10]], myocardial infarction Citation[[11]], and those undergoing percutanous transluminal coronary angioplasty (PTCA) Citation[[12]], and so on. In animal experiments with dogs Citation[[13]], it was shown that Fluosol is able to reduce the infarct size after reperfusion. The authors demonstrated in a following study Citation[[14]] that the infusion of Fluosol resulted in a reduction in neutrophil concentration after 12–24 h, a suppression of neutrophil chemotaxis, and lysozyme release. In this way, Fluosol prevented, at least in part, the so-called myocardial reperfusion injury. These immunsuppressive effects of Fluosol can also be detrimental. Mice given Fluosol and endotoxin showed lower survival rates Citation[[15]].
The immunosuppressive effects, especially of the lecithin-based PFD emulsion we tested, might be beneficial if the emulsion is used in acute ischemia (e.g., myocardial infarction) to diminish the postischemic inflammatory damage. But it can have an unwanted immunosuppressive effect when used in high doses in combination with immunodeficiency or sepsis of the recipient.
In conclusion, our system includes a differentiated pattern of biocompatibility assays, which in total provide an in vitro biocompatibility profile of the tested substances. Thus, it can be a useful tool in preclinical development of any oxygen carriers and other drugs for intravenous application.
REFERENCES
- Remy B., Deby Dupont G., Lamy M. Red Blood Cell Substitutes: Fluorocarbon Emulsions and Haemoglobin Solutions. Br. Med. Bull. 1999; 55: 277–298
- Habler O., Kleen M., Messmer K. Artificial Oxygen Carriers. Alternatives to Homologous Blood Transfusion?. Zentralbl. Chir. 1999; 124: 260–270
- Spence R. K., Norcross E. D., Costabile J., McCoy S., Cernaianu A. C., Alexander J. B., Pello M. J., Atabek U., Camishion R. C. Perfluorocarbons as Blood Substitutes: The Early Years. Experience with Fluosol DA-20% in the 1980s. Art. Cells Blood Subst. Immob. Biotechnol. 1994; 22: 955–963
- Takahashi K., Wasaki Y., Hata J., Mukai K., Goto T. Spontaneous Transformation and Immortalization of Human Endothelial Cells. In Vitro 1990; 25: 265–274
- Ziegler-Heitbrock H. W.L., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a Human Cell Line (Mono Mac 6) with Characteristics of Mature Monocytes. Int. J. Cancer 1988; 41: 456–461
- Edwards C. M., Lowe K. C., Röhlke W., Geister U., Reuter P., Meinert H. Effects of a Novel Perfluorocarbon Emulsion on Neutrophil Chemiluminescence in Human Whole Blood In Vitro. Art. Cells Blood Subst. Immob. Biotech. 1997; 25: 255–260
- Keipert P. E. Use of Oxygent, a Perfluorochemical-Based Oxygen Carrier, as an Alternative to Intraoperative Blood Transfusion. Art. Cells Blood Subst. Immob. Biotechnol. 1995; 23: 381–394
- Kaufman R. J. The Results of a Phase I Clinical Trial of a 40% V/V Emulsion of HM (Oxyfluor). Art. Cells Blood Subst. Immobil. Biotechnol. 1994; 22: A112
- Ohyanagi H., Saitoh Y. Development and Clinical Application of Perfluorochemical Artificial Blood. Int. J. Art. Organs 1986; 9: 363–368
- Gould S. A., Rosen A. L., Sehgal L. R., Sehgal H. L., Langdale L. A., Krause L. M., Rice C. L., Chamberlin W. H., Moss G. S. Fluosol-DA as a Red-Cell Substitute in Acute Anemia. N. Engl. J. Med. 1986; 314: 1653–1656
- Wall T. C., Califf R. M., Blankenship J., Talley J. D., Tannerbaum M., Schwaiger M., Gacioch G., Cohen M. D., Sauz M., Leimberger J. D. Intravenous Fluosol in the Treatment of Acute Myocardial Infarction. Circulation 1994; 90: 114–126
- Jaffe C. C., Wohlgelernter D., Cabin H., Bowman L., Dechelbaum L., Remetz M., Cleman M. Preservation of Left Ventricular Ejection Fraction During Percutaneous Transluminal Coronoary Angioplasty by Distal Transcatheter Coronary Perfusion of Oxygenated Fluosol DA 20%. Am. Heart J. 1988; 6: 1156–1164
- Bajaj A. K., Cobb M. A., Virmani R., Gay J. C., Light R. T., Forman M. B. Limitation of Myocardial Reperfusion Injury by Intravenous Perfluorochemicals: Role of Neutrophil Activation. Circulation 1989; 79: 645–656
- Forman M. B., Pitarys C. J., II, Vildibill H. D., Lambert T. L., Ingram D. A., Virmani R., Murray J. J. Pharmacologic Perturbation of Neutrophils by Fluosol Results in a Sustained Reduction in Infarct Size in the Canine Model of Reperfusion. J. Am. Coll. Cardiol. 1992; 19: 205–216
- Ohyanagi H. Perfluorochemical Emulsions as Blood Substitutes: Clinical Data and New Applications. Artificial Red Cells, E. Tsuchida. John Wiley and Sons, Chicester 1995; 199–226