Abstract
We studied the effects of hemoglobin-vesicles modified with PEG (PEG-HbV), a type of liposome-encapsulated hemoglobin (LEH), on human platelet functions in vitro. The effect of a low concentration of PEG-HbV (Hb; 5.8 mg/dl) was assessed by examining an agonist-induced aggregation response, and that of relatively high concentrations of PEG-HbV (Hb; 0.29, 1 and 2 g/dl) by measuring the release of RANTES (Regulated upon activation, normal T-cell expressed and presumably secreted) from platelets, which is regarded as a marker of platelet activation. The preincubation of platelets with PEG-HbV at 5.8 mg/dl of Hb did not affect platelet aggregation induced by collagen, thrombin and ristocetin. The pretreatment of platelet-rich plasma (PRP) with PEG-HbV at concentrations up to 2 g/dl of Hb had no aberrant effects on the collagen-induced RANTES release. Furthermore, the collagen-induced release of RANTES from PRP was not affected by longer incubation with PEG-HbV at 2 g/dl of Hb. The basal levels of RANTES from PRP were unchanged in the presence of PEG-HbV. These results suggest that PEG-HbV, at the concentrations studied, have no aberrant effects on platelet functions in the presence of plasma.
INTRODUCTION
Hemoglobin vesicles (HbV) or liposome-encapsulated hemoglobin (LEH) are candidates for red cell substitutes Citation[1-4]. To improve the dispersion of the vesicles, polyethyleneglycol (PEG)-modified HbV (PEG-HbV) have been developed as safe and effective blood substitutes Citation[[5]].Surface modification with PEG, also known as steric stabilization, has the beneficial effect of decreasing interactions of the lipid bilayer with blood proteins, decreasing self-aggregation of liposomes, and increasing circulation half-life in vivo Citation[6-8]. In practice, the PEG modification of HbV suppressed inter-vesicular aggregation and offer prompt flow in vessels in comparison with the unmodified HbVCitation[[5]].
The biocompatibility of LEH is important to the clinical use of these materials. Transient thrombocytopenia is one of the most significant hematologic effects observed after infusion of LEH in rodents Citation[9-11]. Exchange transfusion with unmodified HbV in anesthetized rats also resulted in a slightly decreased platelet count in the circulaton, although the change was insignificantCitation[[12]]. During LEH-induced thrombocytopenia, transient sequestration of platelets occurred in the lungs and liver Citation[[13]]. These effects were not limited to LEH and were also observed for administration of negatively charged liposomes Citation[14-15]. The transient reduction in platelet counts caused by these liposomes was also associated with sequestration of platelets and liposomes in the lung and liver Citation[14-15]. In order to explain this phenomenon, the interaction of liposomes with platelets have been well studied in vitro Citation[13-16]. However, there is little information about effects of LEH including HbV or PEG-HbV on human platelet functions.
Platelet activation is apparently necessary to prevent bleeding in vivo, however, a nonphysiological activation leads to initiation and modulation of inflammatory responses because platelets contain an array of potent proinflammatory substances. RANTES (Regulated upon activation, normal T-cell expressed and presumably secreted), one of the C-C chemokines, is a useful marker for platelet activation as it is stored in α-granules of platelets and was shown to be released after stimulation Citation[[17]]. Furthermore, RANTES exerts a variety of effects on white blood cells and is supposed to be involved in inflammatory reactions as well as allergic responses Citation[[17]]. In this study, therefore, we examined the biocompatibility of PEG-HbV by estimating their effects on agonist-induced platelet aggregation response and RANTES release from platelets in vitro.
MATERIALS AND METHODS
PEG-HbV
Hemoglobin vesicles modified with PEG (PEG-HbV) suspended in phosphate buffered saline were prepared as previously described Citation[[5]]. The encapsulated carbonylhemoglobin contained pyridoxal 5′-phosphate (PLP) at a molar ratio of [Hb]/[PLP] = 1/1 as an allosteric effector and 5mM DL-homocysteine. The lipid bilayer was composed of Presome PPG-I [a mixture of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylecholine (DPPC), cholesterol, 1,2-dipalmitoyl-sn-glycero-3-phosphatidylglycerol (DPPG) at a molar ratio of 5:5:1]. For the PEG surface modification, 1,2 - distearyl - sn - glycero - 3 - phosphatidylethanoleamine - N - [poly(ethylene glycol) 5,000] was used. The Hb concentration of the PEG-HbV solution was adjusted to 10 g/dl. The particle size of PEG-HbV was nearly 230±81 nm in diameter.
Preparation of Platelet Suspension
Apheresis platelet concentrates was centrifuged at 140g for 15 min at 22°C to eliminate residual erythrocytes from the resulting platelet rich plasma (PRP). The PRP was acidified to pH 6.5 with citric acid. For preparation of washed platelets, 1mM of prostaglandin E1 (PGE1; Sigma Chemical) was added to the PRP which was then centrifuged at 1000g for 10 min at 22°C. The pellet was gently suspended in HEPES Tyrode' buffer (pH6.7) containing PGE1 (except the second time) and washed twice. Finally platelets were resuspended in pH7.4 HEPES Tyrode' buffer containing 1mM Ca++and Mg++. In some experiments, platelets suspended in AB plasma collected by centrifugation (1000g, 10 min, 22°C) of the acidified PRP were used. Fresh PRP was obtained from citrated venous blood of unselected healthy donors by centifugation (140g, 15 min, 22°C).
Measurement of Platelet Aggregation
A platelet suspension (5×105/ml) was preincubated with PEG-HbV (Hb; 5.8 mg/dl) at 37°C for 3 min on an aggregometer (PA-200, Kowa, Tokyo, Japan). Then various concentrations of collagen (NYCOMED ARZNEIMITTEL GMBH, Germany), thrombin (Calbiochem-Novabiochem Co., Darmstadt, Germany) or ristocetin (Biopool, Ventura, CA, USA) were added to the mixture and incubated at 37°C for 5 min for the measurement of platelet aggregation. The experiment on thrombin-induced platelet aggregation was performed with washed platelets. Other experiments were performed with platelets suspended in AB plasma.
Assay of Mediator Release
The platelet mediator release reaction was initiated as described by Santos et al. Citation[[28]] with slight modification. Briefly, a platelet suspension (washed platelets; 2×108/ml, PRP; 1.6×108/ml) was incubated with PEG-HbV (Hb concentration; 0.3, 1 and 2 g/dl) at 37°C for 10 or 60 min prior to activation with collagen (5 μg/ml for washed platelets; 1 and 2μg/ml for PRP) at 37°C for 5 min in a microcentrifuge tube. After incubation, the tube was centrifuged at 10,000g for 1 min. Cell-free supernatant was transferred into another tube and kept at 4°C until measurement of release. The levels of RANTES were measured with an enzyme-linked immunosorbent assay (ELISA) kit (Amersham Pharmacia Biotech, Buckinghamshire, England) in duplicate, according to the manufacturer' recommendations.
Statistical Analysis
For statistical analysis, Wilcoxon' signed-rank test was used. Ap value < 0.05 was considered to indicate significant differences.
RESULTS
Effect of PEG-HbV on Agonist-Induced Platelet Aggregation
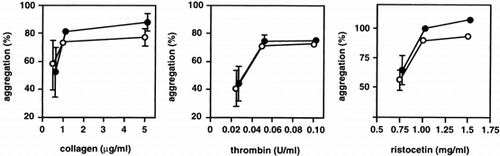
We first examined the effect of PEG-HbV on platelet aggregation induced by agonists. Platelets were preincubated for 3 min with 0.06% of PEG-HbV (Hb; 5.8 mg/dl). Platelets were stimulated at various concentrations with the agonists collagen, thrombin, and ristocetin. There was no significant difference in the aggregation responses of platelets with and without PEG-HbV treatment at any concentration of agonist (). Although only low concentrations of PEG-HbV were used because of turbidity, these results indicated that small amounts of the PEG-HbV solution did not affect platelet aggregation in response to agonists at the PEG-HbV concentrations employed.
Figure 1. Effect of PEG-HbV on agonist-induced platelet aggregation. Platelets were treated without (open circle) or with (closed circle) 5.8 mg/dl (Hb) of PEG-HbV at 37°C for 3 min on an aggregometer prior to addition of various concentrations of collagen, thrombin or ristocetin. Values are means ± SE of three donors. Error bars are not shown when smaller than the symbols.
Effect of PEG-HbV on Collagen-Induced Platelet Mediator Release
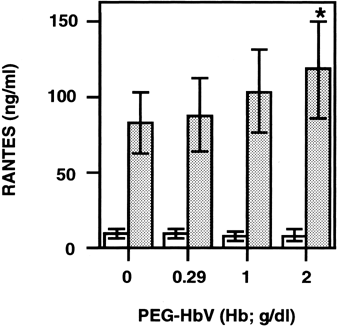
We then examined the effect of relatively high concentrations of PEG-HbV on spontaneous and collagen-induced RANTES release from platelets. Various concentrations of PEG-HbV were preincubated with washed platelets, and the mixture was stimulated with collagen. Then the levels of RANTES in the supernatant of the mixture were measured. The stimulation of washed platelets with collagen caused a marked increase in the levels of RANTES in the cell-free releasates. The collagen-induced RANTES release was gradually increased by preincubation in varying amounts of PEG-HbV solution. Preincubation of washed platelets with 20% PEG-HbV, equivalent to 2 g/dl of Hb, resulted in a significant enhancement compared to that without PEG-HbV (). Spontaneous RANTES release was unaffected during incubation of washed platelets with PEG-HbV alone.
Figure 2. Effect of PEG-HbV on collagen-induced RANTES release from washed platelets. Washed platelets were incubated with various concentrations of PEG-HbV at 37°C for 10 min and then stimulated without (open column) or with (hatched column) collagen at 37°C for 5 min. The levels of RANTES in the supernatant of the mixture were measured by ELISA. Values are means ± SE of five donors. *p < 0.05, in comparison with control (no PEG-HbV)
We then examined whether the enhancement of RANTES release with PEG-HbV preincubation could occur in the case of PRP. As shown in , the levels of RANTES in the cell-free releasates from PRP stimulated with 1μg/ml of collagen tended to decrease depending upon the amount of PEG-HbV, but the change was insignificant (A). With 2 μg/ml of collagen stimulation, levels of RANTES in the supernatant of PRP preincubated with various concentrations of PEG-HbV were similar to control (B). In addition, prolongation of the preincubation time of platelets with 20% PEG-HbV from 10 to 60 min also had no effect on RANTES release (). Without collagen stimulation, the spontaneous release of RANTES induced by PEG-HbV was unaffected either with 10 or 60 min of preincubation time.
Figure 3. Effect of PEG-HbV on collagen-induced RANTES release from PRP. Platelets were incubated with various concentrations of PEG-HbV at 37°C for 10 min and then stimulated without (open column) or with (hatched column) collagen for 5 min at 37°C. The concentration of collagen used was 1 μg/ml in (A) and 2 μg/ml in (B). Values are means ± SE of five donors (A) or three donors (B).
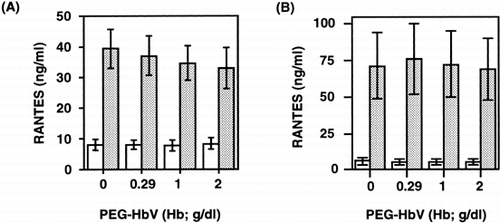
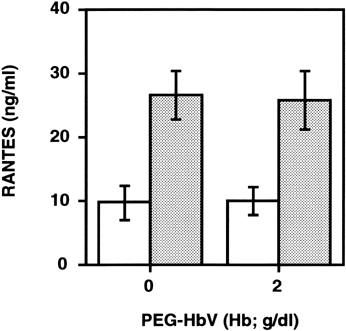
Figure 4. Effect of longer incubation of PRP with PEG-HbV on collagen-induced RANTES release. Platelets were incubated with or without 2 g/dl (Hb) of PEG-HbV at 37°C for 60 min, and then stimulated without (open column) or with 1 μg/ml of collagen (hatched column). Values are means ± SE of five donors.
DISCUSSION
The effects of both empty and hemoglobin-containing phospholipid vesicles on platelets have been studied because transient thrombocytopenia occurred after the administration of these compounds in rodents Citation[9-11], Citation[14-15]. Unmodified HbV also slightly decreased the platelet count in rats Citation[[12]]. It is known that negatively charged liposomes bind to rat platelets resulting in platelet-liposome microaggregation in the presence of plasma without platelet agonists. The platelet-liposome interaction has been explained as follows; negatively charged liposomes incubated with PRP for 15 min at 37°C become coated with serum complement factor C3b. These C3b-coated liposomes then bound to platelets via the CR1 receptor. Trapping of platelet-liposome microaggregates of significant size within the microvasculature of the lung and liver offered a possible explanation for the rapid removal of negatively charged liposomes from blood circulation Citation[[16]]. In contrast, this microaggregation was not observed in the case of human platelets, because human platelets lack CR1 receptors. In addition, another study showed that although a transient thrombocytopenia ocurred in rats, the thrombin-induced platelet aggregation in whole blood as measured by impedance aggregometor was not affected by LEH Citation[[13]]. Thus, our results showing that PEG-HbV did not affect agonist-induced human platelet aggregation are in line with the reports described above, although we used only low concentrations of PEG-HbV because measurement was by aggregometor with turbidmetry.
While the interaction between liposomes and platelets has been demonstrated, little is known about the effects of liposome or LEH on human platelet release. Within platelets, α-granules are the major store for inflammatory mediators including RANTES Citation[[17]]. RANTES has diverse inflammatory effects; it mediates histamine release from basophils Citation[[19]] and exocytosis of eosinophil cationic protein Citation[[20]], and enhances the generation of radical oxygen products from eosinophils Citation[[21]]. It is powerful chemoattractant for T cells, basophils, and eosinophils Citation[[18]], Citation[[21]]. Thus an aberrant RANTES release caused by inappropriate activation of platelets could result in inflammatory reactions as well as allergic responses. In this regard, we investigated whether PEG-HbV affects platelet RANTES release with or without collagen stimulation. The preincubation of washed platelets with 20% PEG-HbV for 10 min at 37°C resulted in a significant enhancement in the collagen-induced release of RANTES (). The mechanism of this enhancement is not clear at present. However, such an effect did not occur in the case of PRP that reflects a more physiological state, suggesting that PEG-HbV have no adverse effect on platelet function in the presence of plasma ().
Recent studies have demonstrated that LEH can activate the complement system of rats, pigs and humans Citation[23-24]. In rats, short term toxicity studies of LEH revealed hyperventilation, hypotension and hypertension, bradycardia and tachycardia, decreased cardiac index, leukocytosis, thrombocytopenia, and elevation of plasma thromboxane B2 Citation[9-11]. According to recent in vivo and in vitro studies, complement activation could be a major underlying mechanism of these changes Citation[24-25]. It was shown that the soluble-C5b-9 level was elevated in serum after incubation with LEH for 60 min at 37°C Citation[[23]]. Because C5b-9 increases platelet prothrombinase activity and causes platelet a-granule release Citation[[26]], longer incubation of PRP with PEG-HbV might cause an enhancement of RANTES release from platelets via C5b-9 formation. On the other hand, a previous study showed that PEG modification inhibited C1q binding to liposome and subsequent complement activation Citation[[27]]. In view of this, we examined the effects of a longer incubation of PRP with PEG-HbV on platelet RANTES release, but found that there was no enhancement of the release. This result suggests that PEG-HbV do not induce complement activation. Alternatively, even if PEG-HbV could activate complement, the resultant soluble-C5b-9 level in PRP was not high enough to modify the release response of platelets after incubation with PEG-HbV.
In conclusion, the present study demonstrated that PEG-HbV did not affect agonist-induced platelet aggregation nor RANTES release in the presence of plasma. These results are of value for estimating the biocompatibility of PEG-HbV for blood cells. Further study is required to elucidate whether the administration of PEG-HbV has an aberrant effect on platelet functions in vivo.
ACKNOWLEDGMENTS
We express our gratitude to the late Dr. Sadayoshi Sekiguchi, the ex-director of Hokkaido Red Cross Blood Center, for his support of this study. This work was supported in part by a Health Science Research Grant (Artificial Blood Project) from the Ministry of Health and Welfare, Japan.
REFERENCES
- Djordjevich L, Miller I F. Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp. Hematol 1980; 8: 584–592
- Hunt C A, Burnette R R, MacGregor R D, Strubble A F, Lau D T, Taylor N, Kiwada H. Synthesis and evaluation of a prototypal artificial red cell. Science 1985; 230: 1165–1168
- Rudolph A S. Encapsulation of hemoglobin in liposome. Blood Substitutes: Physiolosical Basis of efficacy. Birkhauser, Boston, 90–104
- Tsuchida E, Takeoka S. Stabilized hemoglobin vesicles. Artificial Red Cells, E. Tsuchida. Wiley, New York, 35–64
- Sakai H, Takeoka S, Park S I, Kose T, Nishide H, Izumi Y, Yoshizu A, Kobayashi K, Tsuchida E. Surface modification of hemogrobin vesicles with poly (ethylene glycol) and effects on aggregation, viscosity, and blood flow during 90% exchange transfusion in anestheized rats. Bioconjugate Chem 1997; 8: 23–30
- Lasic D D, Martin F J, Gabizon A, Huang S K, Papahadjopoulos D. Sterically stabilized liposomes: A hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta 1990; 1070: 187–192
- Yoshioka H. Surface modification of haemoglobin-containing liposomes with polyethylene glycol prevents liposome aggregation in blood plasma. Biomaterials 1991; 12: 861–864
- Woodle M C, Matthey K K, Newman M S, Hidayat J E, Collins L R, Redemann C, Martin F J, Papahadjopoulos D. Versatility in lipid compositions showing prolonged circulation with sterically stabilized liposomes. Biochim Biophys Acta 1992; 1105: 193–200
- Rabinovici R, Rudolph A S, Feuerstein G. Characterization of hemodynamic, hematologic, and biochemical responses to administration of liposome-encapsulated hemoglobin in the conscious, freely moving rat. Circ Shock 1989; 29: 115–132
- Rabinovici R, Rudolph A S, Yue T, Feuerstein G. Biological responses to liposome-encapsulated hemoglobin (LEH) are improved by a PAF antagonist. Circ Shock 1990; 31: 431–445
- Rabinovici R, Rudolph A S, Feuerstein G. Improved biological properties of synthetic distearoyl phosphatidyl choline-based liposome in the conscious rat. Circ Shock 1990; 30: 207–219
- Izumi Y, Sakai H, Hamada K, Takeoka S, Yamahata T, Kato R, Nishide H, Tsuchida E, Kobayashi K. Physiologic responses to exchange transfusion with hemoglobin vesicles as an artificial oxgen carrier in anesthetized rats: changes in mean arterial pressuer and renal cortical tissue oxygen tension. Crit Care Med 1996; 24: 1869–1873
- Phillips W T, Klipper R, Fresne D, Rudolph A S, Javors M, Goins B. Platelet reactivity with liposome-encapsulated hemoglobin in the rat. Exp Hematol 1997; 25: 1347–1356
- Reinish L W, Bally M B, Loughrey H C, Cullis P R. Interactions of liposomes and platelets. Thromb Haemost 1988; 60: 518–523
- Doerschuk C M, Gie R P, Bally M B, Cullis P R, Reinish L W. Platelet distribution in rabbits following infusion of liposomes. Thromb Haemost 1989; 30: 392–396
- Loughrey H C, Bally M B, Reinish L W, Cullis P R. The binding of phosphastidylglycerol (PG) liposomes to rat platelets is mediated by complement. Thromb Haemost 1990; 64: 172–176
- Klinger M HF. Platelets and inflammation. Anat Embryol 1997; 196: 1–11
- Baggiolini M, Dahinden C A. CC chemokines in allergic inflammation. Immunol Today 1994; 15: 127–133
- Kuna P, Reddigari S R, Schall T J, Rucinski D, Viksman M Y, Kaplan A P. RANTES, a monocyte and T lymphocyte chemotactic cytokine release histamine from human basophils. J Immunol 1992; 149: 636–642
- Rot A, Krieger M, Brunner T, Bischoff S C, Schall T J, Dahinden C A. RANTES and macrophage inflammatory protein-1α induce migration and activation of normal human eosinophil granulocytes. J Exp Med 1992; 176: 1489–1495
- Chihara J, Hayashi N, Kakazu T, Yamamoto T, Kurachi D, Nakajima S. RANTES augments radical oxygen products from eosinophils. Int Arch Allergy Immunol 1994; 104(Suppl 1)52–53
- Murphy W J, Taub D D, Anver M, Conlon K, Oppenheim J J, Kelvin D J., Longo D L. Human RANTES induces the migration of human T lymphocytes into the peripheral tissues of mice with severe combined immune deficiency. Eur J Immunol 1994; 24: 1823–1827
- Szebeni J, Wassef N M, Hartman K R, Rudolph A S, Alving C R. Complement activation in vitro by the red cell substitute, liposome-encapsulated hemoglobin: mechanism of activation and inhibition by soluble complement receptor type 1. Transfusion 1997; 37: 150–159
- Szebeni J, Alving C R. Complement-mediated acute effects of liposome-encapsulated hemoglobin. Art Cells Blood Subs and Immob Biotech 1999; 27: 23–41
- Goins B, Phillips W T, Klipper R, Rudolph A S. Role of complement in rats injected with liposome-encapsulated hemoglobin. J Surg Res 1997; 68: 99–105
- Wiedmer T, Esmon C T, Sims P J. On the mechanism by which complement C5b-9 increase platelet prothrombinase activity. J Biol Chem 1986; 261: 14587–14592
- Bradley A J, Devine D V, Ansell S M, Janzen J, Brooks D E. Inhibition of liposome-induced complement activation by incorporated Poly (Ethylene Glycol)-Lipids. Arch Biochem Biophys 1998; 357: 185–194
- Santos M T, Valles J, Marcus A J, Safier L B, Broekman M J, Islam N, Ullman H L, Eiroa A M, Azner J. Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes: a new approach to platelet activation and recruitment. J Clin Invest 1991; 87: 571–580