Abstract
Immobilization of bovine serum albumin (BSA) on oxidized cellulose (OC) containing carboxylic groups, a biocompatible and bioresorbable polymer, was investigated in water and different buffer solutions (pH 2–7) at 5°C. The maximum amounts of BSA loaded on OC in pH 2, 3, 4, 6, and 7 buffer media were 9.82, 10.52, 8.86, 9.16, 6.05, and 2.69% (w/w), respectively. In water, the corresponding value was 9.82%. The release study was performed on powder, pellet and suspension (in castor and sesame oils) dosage forms of OC-BSA immobilization products prepared in water and pH 2 buffer, in pH 7.4 phosphate buffer at 37°C. The results revealed the release of BSA to be the fastest from oil suspensions, intermediate from powders, and slowest from pellets. In conclusion, the results presented suggest that OC has the potential to be used as an immobilizing matrix for BSA and other proteins in water and pH 2–4 buffer solutions.
INTRODUCTION
In recent years, immobilization technology involving covalent and/or noncovalent fixation of drugs, enzymes, and proteins on soluble or insoluble polymeric carriers has received considerable interest Citation[[1]]. It offers potential in drug delivery, including drug targeting. Proteins immobilized on organic or inorganic polymer supports frequently exhibit prolonged half-life, enhanced resistance to degradation, and decreased immunogenicity. Further, immobilized drugs/proteins can be compressed into a pellet and used as an implant, or formulated as a suspension and administered by injection. Cellulose and its derivatives such as cellulose acetates, cellulose nitrates, cellulose triacetate, and diethylaminoethylcellulose (DEAE-cellulose) have been extensively used as immobilizing matrices for a variety of agents, including proteins, whole cells, and enzymes Citation[[1]]. However, these polymers are not biodegradable and, hence, are not suitable for use as an injectable or implantable carrier system.
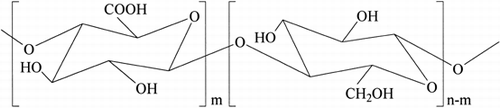
6-Carboxycellulose, commonly referred to as oxidized cellulose (OC, ), is a biocompatible, bioresorbable material Citation[[2]]. It degrades in-vivo to produce glucose and two or three carbon fragment units Citation[3-4]. The degradation rate, in general, increases with an increase in oxidation level and a decrease in degree of polymerization Citation[[2]]. Currently, OC containing 16–24% carboxylic content is commercially available for use in humans to stop bleeding during surgery Citation[[5]] and to prevent the formation and reformation of adhesions after surgery Citation[6-7]. OC has also been found to form a complex with a variety of substances containing amine groups, including drugs (e.g., kanamycin sulfate Citation[[8]], gentamycin Citation[9-10], hydroxythiamine Citation[[11]], methotrexate Citation[[11]], and adrenaline Citation[[12]]), enzymes (e.g., trypsin Citation[[13]], proteinase Citation[14-15], and cytokines Citation[[16]]), and amino acids Citation[17-18]. These studies show ionic interactions to be the driving force for complexation of these agents with OC.
In this study, using bovine serum albumin (BSA) as a model protein, we have found that the interaction of BSA on OC is pH dependent, increasing with decreasing pH of the immobilization medium. This suggests that not only ionic interactions but also hydrophobic forces appear to play an important role in the immobilization of BSA on OC. Since the long-term goal of this research is the development of an OC-based biodegradable implantable or injectable delivery system for proteins, the release of BSA from powder, oil suspension, and tablet dosage forms of OC-BSA immobilization products was also studied, and the results are included in this paper.
MATERIALS AND METHODS
Materials
Oxidized cellulose (carboxylic content 16%), sodium stearyl fumerate (PRUV®), and sesame oil were received from Eastman Chemical Co. (Kingsport, Tennessee, USA), Penwest Company (formerly Mendell Co. Inc., Patterson, New York, USA), and Croda Inc. (New York, NY, USA), respectively. BSA (A4503, Lot No. 129F0136) and castor oil were purchased from Sigma chemical Co. (St. Louis, Missouri, USA) and Ruger Chemicals (Hillside, New Jersey, USA), respectively. The Coomassie blue concentrate was obtained from Bio-Rad Laboratories (Hercules, California, USA). All other chemicals were analytical grade.
Immobilization of BSA on OC
Stock solutions of BSA in water and aqueous buffer solutions (pH 2 and 3: 0.01M citrate buffer; pH 4 and 6: 0.01M acetate buffer; and pH 7: 0.01M phosphate buffer), corresponding to BSA concentration of 1 mg/ml, were prepared. To a 10 ml BSA stock solution, 100 mg of OC was added and the mixture was stirred at 5°C for different time periods using a magnetic stirrer. The reaction suspension was then centrifuged for 10 minutes at 2000 r.p.m. Hundred microliters of the supernatant was removed and analyzed immediately for BSA by the Bradford test (vide infra). The residue was collected, freeze-dried, and stored in a freezer (∼ 5°C) until used. All immobilization reactions were conducted in triplicate. Control solutions containing BSA only were prepared and subjected to identical conditions prior to analysis.
Determination of BSA Loading
The amount of BSA bound to OC was determined by the Bradford method Citation[[19]] using Coomassie blue as a reagent. The latter was prepared by diluting one part of the Coomassie blue concentrate with four parts of distilled water. For BSA analysis, 2.5 ml of the Coomassie reagent and 100 μl of the BSA solution were taken in a stoppered quartz cell (1 cm width) and mixed. At the end of one minute, absorbance measurements were made at 595 nm using an ultraviolet-visible spectrophotometer (Shimadzu UV 2100U, Shimadzu Corp., Kyoto, Japan). The amount of BSA bound to OC was determined from the difference in BSA concentrations before and after treating the solution with OC. Control BSA solutions (i.e., without OC), containing the same amount of BSA as was used in the reaction sample, were also prepared and analyzed as described above. All assays were performed in triplicate.
Preparation of OC-BSA Pellets
OC-BSA immobilization products prepared in water and 0.01M citrate buffer (pH 2.0) were used in the study. They were compressed, with and without 0.5% PRUV®, on a Carver press using a 1/3 inch die and flat-faced punches. Each pellet weighed 30 mg, and showed a hardness value of 6–7 kp as measured on a Schlenniger 2E/106 hardness tester.
Release Studies
The release of BSA was studied from powder, pellet (with and without 0.5% PRUV®), and oil suspension dosage forms of OC-BSA prepared in water and pH 2 buffer solution. Oils used in the study were castor oil and sesame oil. An accurately weighed amount of the test sample was suspended in a 10 ml phosphate buffer solution (pH 7.4) containing 10% (v/v) ethanol using a shaker at 37°C. Hundred microliters of the supernatant were withdrawn at 4, 10, and 24 hours and then every 24 hours for 6 days, and analyzed immediately for BSA by the Bradford method Citation[[19]] described above. The control used in the study was a pH 7.4 phosphate buffer solution containing the same amount of BSA as was present in the test sample and 10% (v/v) ethanol. This solution was stored at 37°C and analyzed at the same time intervals as was used for the test samples.
RESULTS AND DISCUSSION
Immobilization of BSA on OC
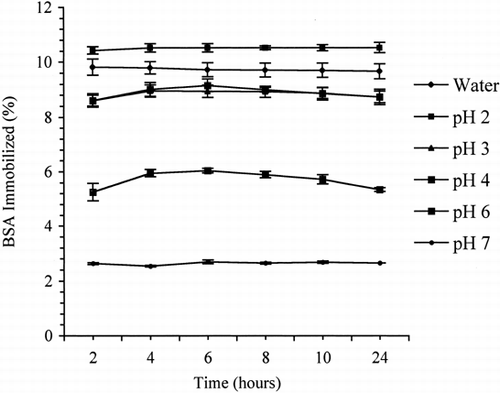
The binding of BSA to OC as a function of time in water and different pH buffer solutions is shown in . As is evident, the immobilization of BSA on OC was complete in about two hours in water, about four hours in pH 2 and 3 buffer solutions, and about six hours in pH 4, 6, and 7 buffer media. No loss of BSA was observed in control solutions, suggesting that BSA is stable under experimental conditions used. The maximum loading of BSA on OC achieved in water and different pH buffer solutions are presented in .
Table I. Amounts of BSA Loaded on OC
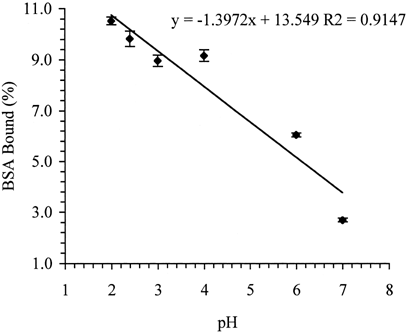
As is evident from , the immobilization of BSA on OC increases linearly with a decrease in the pH of the immobilization medium (R2 = 0.9147). The high loading of BSA seen in water can attributed to the pH effect because OC being a weak polyacid (pKa 3.5 and 4.0) Citation[[22]] partially dissociates in water and produces a suspension with a pH of about 2.4.
The isoelectric point of BSA is 4.8–5.0 Citation[[23]]. Thus, in pH 2 buffer and water, it appears that the immobilization of BSA on OC takes place via both physical adsorption (i.e., hydrogen bonding, hydrophobic interactions, and/or van der Waal forces) and ionic interaction Citation[[1]], Citation[[20]], the former being the predominant mechanism. At higher pH conditions, however, OC-COOH progressively ionizes to OC-COO−, while BSA converts from a fully protonated form, (NH3+-BSA-COOH), to a zwitterion (NH3+-BSA-COO−) and subsequently to NH3+-BSA-COO−. Thus, as the pH increases, more and more ionic interaction between OC and BSA dominates, causing a progressive increase in the formation the water-soluble OC-BSA complex, and consequently, a decrease in the yield of the insoluble OC-BSA immobilization product. At or above pH 7.0, OC and BSA predominantly exist as OC-COO− and NH3-BSA-COO−, respectively, and hence, the yield of OC-BSA is significantly decreased.
Release Studies
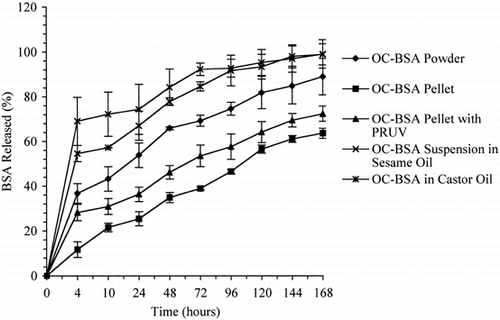
The release profiles of BSA from powder, pellet and suspension dosage forms of OC-BSA, prepared in water, are shown in . All samples, except for the pellets that had no PRUV®, exhibited an initial fast release (“burst effect”). In the first four hours, the amounts of BSA released from OC-BSA suspensions in sesame oil and castor oil were about 69% and 55%, respectively, from OC-BSA powder about 37%, and from OC-BSA pellet containing 0.05% PRUV® about 28%. After four hours, all samples released BSA slowly. After six days, the 100% of BSA were released from castor and sesame oil suspensions of OC-BSA. The powder and pellet, with and without PRUV®, forms, in contrast, released only 90%, 64%, and 72% of BSA, respectively, over the same time period.
Figure 4. Release profiles of BSA from powder, pellet, and oil suspension dosage forms of OC-BSA prepared in water (n=3).
shows the release profiles of BSA from OC-BSA prepared in pH 2.0 buffer solution. As is evident, both castor oil and sesame oil suspensions of OC-BSA exhibited similar release profiles. OC-BSA pellets with and without PRUV® also showed no significant difference in their release patterns. After four hours, the amounts of BSA released from OC-BSA suspensions, powder, and pellets were 49%, 28%, and 11%, and After six days, about 84%, 69%, and 59%, respectively.
Figure 5. Release profiles of BSA from powder, pellet, and oil suspension dosage forms of OC-BSA prepared in pH 2.0 buffer solution (n=3).
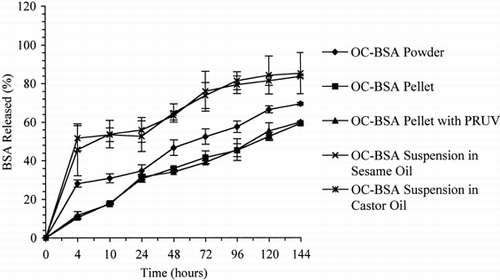
The above results show that (i) irrespective of whether OC-BSA was prepared in water or pH 2.0 buffer, the release of BSA was the fastest from oil suspensions, intermediate from the powder forms, and the slowest from the pellet formulations, and (ii) OC-BSA prepared in water released BSA at a faster rate than that made in pH 2.0 buffer solution. The faster release of BSA from the sesame oil and castor oil suspensions compared to that from the respective OC-BSA powders may be due to the plasticization effect of oils on OC. The difference in the release properties of OC-BSA prepared in water versus that made in pH 2 buffer could be due to different proportions of interaction forces present between OC and BSA.
CONCLUSION
The results presented above show that the immobilization of BSA on OC is favored at low pH conditions and in water. The release of BSA was the fastest (84–100%) from OC-BSA suspension in oils (castor and sesame oils), intermediate (69–90%) from OC-BSA powders, and the slowest (59–72%) from OC-BSA pellets, over six days, suggesting that the pellet form is useful as a sustained-release delivery system whereas powder and suspension forms may serve as a pulse-release delivery system. The presence of PRUV® in the pellets did not change the release profile of BSA.
Further work to (i) characterize the secondary structure of BSA in the insoluble fraction of the immobilization product and (ii) isolate and identify the soluble fraction of OC-BSA complex by freeze drying, is in progress. Immobilization of other proteins, such as lysozyme, on OC is also being investigated. Lysozyme Citation[[23]] has an isoelectric point of ca. 11. Therefore, unlike BSA, it is expected to show higher affinity to OC at a physiological pH. The successful demonstration of binding of various proteins on OC may allow its use not only as a carrier for proteins in the design of a biodegradable delivery system but also as an adjuvant in the development a solid vaccine.
REFERENCES
- Protein Immobilization. R. F. Taylor. Marcel Dekker, New York 1991; 14
- Ashton W. H.. Oxidized cellulose product and method for preparation of the same. US Patent 3364200, 1968
- Dimitrijevich S. D., Tatarko M., Gracy R. W., Wise G. E., Oakford L. X., Linsky C. B., Kamp L. In-vivo degradation of oxidized regenerated cellulose. Carbohydr. Res. 1990; 198: 331–341
- Dimitrijevich S. D., Tatarko M., Gracy R. W., Linsky C. B., Olsen C. Biodegradation of oxidized regenerated cellulose. Carbohydr. Res. 1990; 195: 247–256
- Johnson & Johnson. Surgicel® Absorbable Hemostate, PC-0506. Johnson & Johnson Patient Care, Inc. 1989
- Cohen S. M., Franklin R. R., Haney A. F., Malinak L. R., Patton G. W., Rock J. A., Rosenberg M., Webster B. W., Yuzpe A. A. Prevention of postsurgical adhesions by Interceed (TC7), an absorbable adhesion barrier: a prospective, randomized multicenter clinical study. Fertility and Sterilty 1989; 51: 933–938
- Ethicon. Interceed® Absorbable Adhesion Barrier, AP0635. Ethicon, Inc., Johnson & Johnson Patient Company. 1992
- Dol‘berg E. B., Shuteeva L. N., Yasnitskii B. G., Obolentseva G. V., Khadzhai Y. I., Furmanov Y. A. Interaction of oxidized cellulose with medicinal compound. III. Synthesis and biological properties of the reaction product of oxidized cellulose with kanamycin sulfate. Khim. -Farm. Zh. 1974; 8: 23
- Firsov A. A., Nazarov A. D., Fomina I. P. Biodegradable implants containing gentamicin: drug release and pharmacokinetics. Drug Dev. Ind. Pharm. 1987; 13: 1651–74
- Kaputskii F. N., Bychkovskii P. M., Yurkshtovich T. L., Burtin S. M., Korolik E. V., Buslov D. K. Study of photrin sorption by monocarboxycellulose. Colloid J. 1995; 57: 42–45
- Zimatkina T., Yurkshtovich T., Zimatkin R. F., Kaputsky F. Antitumor activity of hydroxythiamine and methotrexate immobilized on monocarboxycellulose. Polish J. Pharmacol. 1996; 48: 163–169
- Balakleevskii A. I., Gubkina N. I., Tkachev S. V., Khaniia R. L., Kalinaki M. I., Kotsyurva VN. Adrenaline complex with monocarboxycellulose with prolonged action. USSR SU Patent 1266541, 1986
- Kaputskii F. N., Alinovskaya V. A., Yurkshtovich T. L. Effect of the type of an ionic group in the composition of cellulose on immobilization of trypsin. Vesti Akad. Navuk BSSR, Ser. Khim. Navuk 1989; 27–31
- Alinovskaya V. A., Kaputskii F. N., Yurkshtovich T. L., Talapin V. M., Stel‘makh V. A.. Polyhydroglucuronic acid for proteinase immobilization. USSR SU Patent 1406161, 1988
- Alinovskaya V. A., Yurkshtovich T. L., Kaputskii F. N. Ser. Khim. Navuk 1989; 3: 27–31
- Vosika G. J., Cornelius D. A.. Immobilized Cytokines. US Patent 316,992, 1989
- Belaya A. V., Yurshtovich T. L., Kaputskii F. N., Kosterova R. I. Effect of solution pH on the sorption of bipolar ions by oxidized cellulose. Vesti Akad. Navuk BSSR, Ser. Khim. Navuk 1984; 4–7
- Belaya A. V., Yurshtovich T. L., Kaputskii F. N., Pavlyuchenko G. M. Effect of structural characteristics of oxidized cellulose on the sorption of amino acids. Zh. Prikl. Khim.(Leningard) 1985; 58: 2079–2083
- Bradford M. M. A rapid and sensitive method for the quantitation of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976; 72: 248–254
- Andrade J. D. Surface and interfacial aspects of biomedical polymers. J. D. Andreade. Plenum, New York 1985; 14: 1–80, Protein Adsorption
- Mazur A., Harrow B. Textbook of Biochemistry. 10th edition, Saunders, Philadelphia 1971
- Zhu L. Design and Examination of Oxidized cellulose as an Amine Drug Carrier for Implantable Drug Delivery. Ph.D. Thesis, The University of Iowa, Iowa City, IA 2000
- Stryer L. Biochemistry. Third edition, Freeman, New York 1988; 203, Freeman