Abstract
We investigated the effect of a perfluorocarbon emulsion (FC) added to the University of Wisconsin (UW) solution on hypothermic (4°C, 12–72h) preservation of rat small bowel grafts. The FC was 90%w/v perfluorooctylbromide, 2%w/v egg yolk phospholipids and 1.4%w/v mixed fluorocarbon-hydrocarbon molecular dowels. Four groups were defined: [1] UW flush and UW storage; [2] UW flush and FC storage; [3] flush with FC diluted 2 times with UW (FU) and FU storage; [4] FU flush and storage in oxygenated FU. Preservation was estimated with a histological score based on villus epithelium adhesion, on villus sloughing and on crypt cell adhesion to the basal membrane. Antioxidant potential was estimated by measurement of total thiol functions (SH) and activities of glutathione-peroxidase (GSH-P), superoxide dismutase (SOD) and catalase. FC in flush improved preservation during the first 24h (p<0.01). Storage in FC appeared superior to UW for the first 24h (p<0.01). Oxygenation (100% O2) of the storage medium yielded superior results at 12h and 24h (p<0.01 and p<0.001 versus group [1] respectively). After 72h, SOD and catalase activities increased in groups [3] and [4], and SOD decreased in group [1] (p<0.05). SH progressively decreased in group [1] (p<0.05) and GSH-P increased at 24 and 48h in groups [3] and [4] (p<0.01). The increase of O2 in the perfusion flush or storage medium ameliorated the preservation status and protected the antioxidant potential of the small bowel.
INTRODUCTION
Quality of long term intestinal preservation remains disappointing, necessitating transplantation of the graft as soon as possible. Cold preservation beyond 12 hours is accompanied by a dramatic decrease of recipient survival Citation[1-2]. Although hypothermia drastically reduces oxygen consumption, a certain degree of metabolic activity (mainly anaerobic glycolysis) remains as attested by the decrease of cellular energy charge during cold storage Citation[[3]]. Increasing oxygenation during preservation has the potential to fulfill the remaining metabolic demand of the organ, thus suppressing the ischemic component of the lesions caused during hypothermia. Perflucorcarbons are organic liquids, biologically inert with high oxygen carrying capacity. They can be emulsified with phospholipids for intravascular use, and fluorocabon emulsions are currently evaluated in phase II and III clinical trials as hemoglobin substitute Citation[[4]]. We developped a new perfluorocarbon-egg yolk phospholipid emulsion with high stability and effective particle size control, using fluorocarbon-hydrocarbon diblocks as stabilizers Citation[5-6]. We previously reported the stability of the physico-chemical properties of this emulsion when diluted with the University of Wisconsin solution (UW) and demonstrated its absence of cytotoxicity on human endothelial cells in culture Citation[[7]]. Based on these encouraging results, we investigated the effect of adding the emulsion to UW for small intestine preservation.
MATERIALS AND METHODS
Organ Harvesting
Inbred rats from LEW (RT11) strain, weighing 200–300 g were purchased from Charles River LTD, UK. They were cared for according to the Home Office Guidelines in the UK. After anesthesia with halothane and heparin injection (100 U/100 g), the animal was exsanguinated. The whole intestine was then immediately flushed through the aorta with the preservation fluid. The intestinal lumen was then rinsed with an antibiotic solution (streptomycin 5 mg% and penicillin 200,000 U/L) followed by the same preservation solution as used for storage. The organ was stored at 4°C, oxygenated by either room air or by bubbling with pure oxygen in the defined storage medium.
Perfluorocarbon Emulsion
The concentrated perfluorocarbon emulsion (FC) consisted of perfluorooctylbromide (90% w/v, 47% v/v) emulsified with egg yolk phospholipids (2% w/v) and stabilized with mixed fluorocarbon-hydrocarbon molecular dowels (F6H10; 1.4% w/v) in phosphate buffer (pH 6.9; average particle size 0.22 μm; viscosity 20 cp at a shear rate of 0.73 s−1). This emuslsion was stable after sterilization and after dilution with UW Citation[[7]].
Experimental Groups
Duration of preservation extended from 12 to 72 hours. Four groups were defined depending on the type of vascular and luminal flush, and storage medium: [1] UW/UW : UW flush and storage in UW; [2] UW/FC : UW flush and FC storage; [3] FU/FU : flush with FC diluted 2 times with UW (FU) and storage in FU; [4] FU/FU+O2 : flush with FU and storage in oxygenated FU.
Endpoints
Histology
A direct correlation exists for small bowel histology between microscopic morphology and duration of preservation, which permitted others Citation[[8]] and us to ascribe a histology score. Histological grading was performed using a villus score (0–4) based on the adhesion of the villus epithelium to the lamina propria and on villus sloughing, and a crypt score (0–4) based on the adhesion of crypt cells to the basal membrane ().
Table I. Histological Score Used to Describe the Morphological Lesions of the Intestinal Graft After Preservation
Biochemical Measurements
After weighing, tissues were homogenized at +4°C in 0.05 M phosphate buffer, 0.025 mM NaEDTA, pH 6.4. The supernatants (15 minutes centrifugation at 27,000 g; 4°C) were directly used for spectrophotometric measurements. The total thiol functions (−SH) were measured by Ellmann's technique with 5,5′-dithio-bis-(2-nitrobenzoic acid). Catalase (Cat) enzymatic activity was measured by following the consumption of added hydrogen peroxide at 240 nm. Superoxide dismutase (SOD) and glutathione peroxidase (GSH-P) activities were assessed respectively, by the inhibition of cytochrome C reduction by xanthine oxidase Citation[[9]], and by the consumption of NADPH at 340 nm in the presence of GSH and GSH reductase. The results are expressed in U/g tissue protein for Cat, SOD and GSH-P and in μmoles/g crude tissue for −SH. The time evolution is expressed in percentage of control values. For technical reasons, no data were obtained for time 12 h and for all times for group 2.
Statistical analysis was carried out using a two-tailed student's t-test or ANOVA when appropriate.
RESULTS
Histology
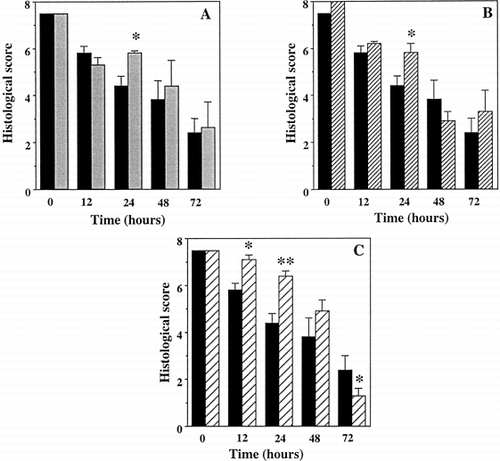
Increasing histological lesions were observed during preservation whatever type of flush or storage (), except for group 4 where histology did not show significant changes for the first 12 hours versus controls (0h preservation)
Table II. Evolution of Histological Score (0–8) During 72 h Preservation at +4°C Results Are Expressed as Mean ± SEM; n = 5 in Each Group; for Each Time Point, 5 Histological Samples Were Blindly Assessed
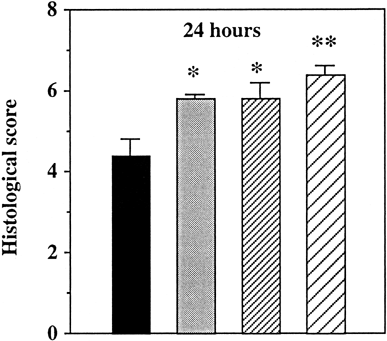
Addition of FC in the vascular flush significantly improved preservation during the first 24 h (p < 0.05) ( and ). The efficacy of intestinal storage in the pure emulsion appeared superior to UW storage at 24 h (p < 0.05 versus group 1) (B). Oxygenation of the storage medium with 100% O2 yielded even superior results at the 12 h (NS versus time 0h; p < 0.05 versus group 1) and 24 h (p < 0.001 versus group 1) time points (C). We observed a similar trend at 48 h but it did not reach statistical significance (p = 0.14 versus group 1) (C). As preservation was extended, differences among the first three groups progressively reached non significance. Surprisingly, the histological score of group 4 after 72 hours was worse than group 1 (p < 0.05) (C).
Antioxidant Potential Status
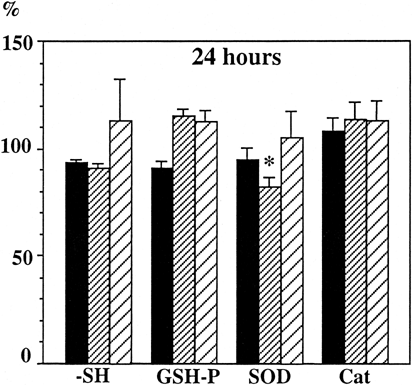
The indicates that no decrease in antioxidant potential was observed after 24 h preservation for all groups, except a slight decrease for SOD in group 3. However, a progressive decrease of -SH fucntions was observed in group 1, statistically significant after 72 h (p < 0.05 versus 0 h). Thiol functions are involved in the catalytic cycle of many enzymes, but, in our assays, this decrease of total −SH did not affect GSH peroxidase since its activity increased with time, particularly after 72 h preservation, what would implicate that the −SH functions buried within the core of enzymatic proteins were not affected Citation[[10]].
DISCUSSION
As we observed in our previous study employing endothelial cells Citation[[7]], the addition of a perfluorocarbon emulsion in the preservation flush or storage medium appeared devoid of deleterious effects. On the contrary, the fluorocarbon-containing medium gave better histological score after 24 hours of preservation than the UW solution. The same improvement was also seen as pure perfluorocarbon emulsion was used as storage medium instead of UW. On increasing oxygenation of the storage solution by 100% oxygen bubbling, the preservation status of the graft improved significantly during the first 24 hours of storage such that after 12 hours of preservation, the histology score was not significantly different from non preserved controls.
These observations suggest that the improved preservation status of the grafts for the first 24 hours (and probably 48 hours) can be directly linked to the increase oxygenation during storage. The beneficial effect of oxygenation in hypothermia has already been reported in cold storage or continuous perfusion models Citation[11-14], but also in double layer perservation models for pancreas Citation[[15]], heart Citation[[16]] and small bowel Citation[[17]]. As for the pancreas, the thin width of the bowel allows for the diffusion of oxygen through the entire wall as opposed to low surface volume ratio organs as liver Citation[[18]]. The cellular mechanisms explaining this positive effects of oxygenation are beginning to be understood. Oxygen disponibility appears to induce a return of the oxidative phosphorylations (as suggested by higher ATP tissue levels during cavitary two-layer preservation with oxygen bubbling compared to values without oxygen) Citation[16-17], furnishing energy for the persistent metabolic activity and for the reparations of hypothermia-induced lesions. More recently, ATP concentration of ischemically damaged pancreas was shown to increase during cavitary two-layer preservation parallely of tissue pO2 increase Citation[[19]].
For longer preservation periods, the beneficial effect of perfluorocarbon addition and oxygenation observed in this study, disappeared. After 72 hours of preservation, the histology score of oxygenated perfluorocarbon emulsion was even worse than the other groups. This effect might result from more rapid substrate depletion, inadequate substrate furniture by UW (small bowel uses glutamine as respiratory fuel, source of carbon and nitrogen) Citation[[20]] or hypothermia induced inhibition of enzymatic pathways in presence of high levels of oxygen.
In conclusion, these results establish that perfluorocarbon emulsions are without specific cytotoxicity. The increase of oxygen concentration in the perfusion flush or storage medium proved to ameliorate the preservation status and to better protect the antioxidant potential of the small bowel during a 24 to 48 hours period. A better definition of the cellular mechanisms involved is needed to extend the preservations in these conditions.
ACKNOWLEDGMENTS
This work was supported by the Commission of the European Communities (BMH I—CT 94–1249) and by the Fund for Medical Research—Belgium (grant 3.4581.96). A. DeRoover is a research fellow of the Fund for Scientific Research—Belgium.
REFERENCES
- Kokudo Y, Furuay T, Takeyoshi T, Nakamura K, Zhang S, Murase N, Todo S. Comparison of University of Wisconsin, Euro-Collins and lactated Ringer's solutions in rat small bowel preservation for orthotopic small bowel transplantation. Transplant Proc 1994; 26: 1492–1493
- Muller A R, Nalesnik M, Platz K P, Langrehr J M, Hoffman R A, Schraut WH. Evaluation of preservation conditions and various solutions for small bowel preservation. Transplantation 1994; 57: 649–655
- Reckendorfer H, Burgmann H, Sperlich M, Spieckermann PG. Small intestine metabolism during hypothermic storage using different protecting solutions. Tranplant Proc 1992; 24: 1094
- Spahn D R, van Brempt R, Theilmeier G, Reibold J P, Welte M, Heinzerling H, Birck K M, Keipert P E, Messmer K. Perflubron emulsion delays blood transfusions in orthopedic surgery. European Perflubron Emulsion Study Group. Anesthesiology 1999; 91: 1195–208
- Riess J G, Cornélus C, Follana R, Krafft M P, Mahé A M, Postel M, Zarif L. Novel fluorocarbon-based injectable oxygen carrying formulations with long-term room-temperature storage stability. Adv Exp Med Biol 1994; 345: 227–237
- Cornélus C, Krafft M P, Ries J G. Improved control over particles sizes and stability of concentrated fluorocarbon emulsions by using mixed fluorocarbon/hydrocarbon molecular dowels. Art Cells, Blood Subst, Immob Biotech 1994; 22: 1183–1191
- Mathy-Hartert M, Krafft M P, Deby C, Deby-Dupont G, Meurisse M, Lamy M, Riess JG. Effects of perfluorocarbon emulsions on cultured human endothelial cells. Art Cells, Blood Subst, Immob Biotech 1997; 25: 563–575
- Park P O, Haglund U, Bulkley G B. The sequence of development of intes- tinal tissue injury after strangulation ischemia and reperfusion. Surgery 1990; 107: 574–580
- Fridovich I. Handbook of methods for radical research. Greenwald RA, CRC Press, Inc, Boca Raton, FA 1986; 121–122
- Levine R L, Mosoni L, Berlett B S, Stadtman E R. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci USA 1996; 93: 15036–15040
- Eyal Z, Manax W, Bloch J H, Lillehei R C. Successful in vitro preservation of the small bowel including maintenance of mucosal integrity with chlorpromazine, hypothermia, and hyperbaric oxygenation. Surgery 1965; 57: 25
- Ku Y, Fukumoto T, Samizo T, Maekawa Y, Nishida T, Shiki H, Tominaga M, Kuroda Y, Saitoh Y. Effect of cold aerobic perfusion on nonparenchymal cell viability. Transplant Int 1994; 7(suppl 1)S175–S180
- Kamada N, Calne R Y, Wight DGD, Lines J G. Orthotopic rat liver transplantation after long term preservation by continuous perfusion with fluorocarbon emulsion. Transplantation 1980; 30: 43–48
- Segel L D, Minten JMO, Schweighardt F K. Fluorochemical emulsion APE-LM substantially improves cardiac preservation. Am J Phys 1992; 263: H730–H739
- Kuroda Y, Morita A, Fujino Y, Tanioka Y, Ku Y, Saitoh Y. Successful extended preservation of ischemically damaged pancreas by the two-layer (University of Wisconsin solution/perfluorochemical) cold storage method. Transplantation 1993; 56: 1087–1090
- Kuroda Y, Kawamura T, Tanioka Y, Morita A, Hiraoka K, Matsumoto S, Kim Y, Fujino Y, Suzuki Y, Ku Y, Saitoh Y. Heart preservation using a cavitary two-layer (University of Wisconsin solution/perfluorochemical) cold storage method. Transplantation 1995; 59: 699–701
- Kuroda Y, Sakai T, Suzuki Y, Tanioka Y, Matsumoto S, Kim Y, Fujita H, Hamano M, Hasegawa Y, Ku Y, Saitoh Y. Small bowel preservation using a cavitary two-layer (University of Wisconsin solution/perfluorochemical) cold storage method. Transplantation 1996; 61: 370–373
- Sumimoto R, Jamieson N V, Kamada N. Attempted application of the two-layer storage method to liver preservation. Transplantation 1990; 49: 1027–1028
- Matsumoto R, Kuroda Y, Hamano M, Kim Y, Suzuki Y, Ku Y, Saitoh Y. Direct evidence of pancreatic tissue oxygenation during preservation by the two-layer method. Transplantation 1996; 62: 1667–1670
- Souba W W, Klimberg S, Plumpley D A, Salloum R M, Flynn T C, Bland K I, Copelant E M. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res 1990; 48: 383–391