SUMMARY
The purpose of this study was to evaluate osteochondral lesions of the knee, treated intraoperatively with low-power laser stimulation, and assess results at 24 weeks. Surgery was performed under general anesthesia on six rabbits; a bilateral osteochondral lesion was created in the femoral medial condyles with a drill. All of the left lesions underwent immediate stimulation using the diode Ga-Al-As laser (780nm), whereas the right knees were left untreated as control group. After 24 weeks, the explants from the femoral condyles, either treated employing laser energy or left untreated, were examined histomorphometrically. Results obtained on the lased condyles showed good cell morphology and a regular aspect of the repaired osteocartilaginous tissue.
INTRODUCTION
In 1976, Mitchell and Shepard Citation[[1]] described healing of the osteocartilaginous defect by means of hyaline cartilage transforming into dense connective tissue in one year. Fibrocartilage lacks both durability and many of the mechanical properties characterizing the hyaline cartilage, which normally covers articular surfaces Citation[[2]]. Considering the positive experimental results obtained with “in vitro” Citation[3-4] and “in vivo” Citation[[5]] laser stimulation, the aim of the present study is to investigate the histologic findings of osteochondral lesions treated intraoperatively with low-power Ga-Al-As diode laser, and evaluate their healing process after 24 weeks.
MATERIALS AND METHODS
European and Italian Laws on animal experimentation were strictly followed during the entire study and the animal experimental protocol was approved by the Scientific Committee of Rizzoli Orthopaedic Institute and responsible public authorities, as requested by the Italian Law according to the EC rules (Law by Decree, 27 January, 1992 n. 116). The Department of Experimental Surgery / I.O.R. acts in accordance with the Statement of Compliance and is authorized by the National Institutes of Health (N. AS 5424-01).
The active material of the diode laser device was Ga-Al-As (M3000, produced by SIMED s.r.l. and distributed by TEAM LASER s.r.l.—Padua, Italy) configured in MOCVD, with a wavelength of 780 nm. and an output power of 2,500 mW. The device was in the isolated class 1, type B and in the laser class III B. All the instructions for a safe use of the device were strictly followed.
Surgery was performed on six New Zealand male rabbits (3.7±0.1 kg body weight, aged 25±0.2 weeks) under sterile conditions and general anesthesia; the same number of animals used for a previous study Citation[[5]] was chosen to allow for comparison of results. An anteromedial arthrotomy of each knee was performed with the joint positioned in maximum flexion, and an osteochondral lesion with a 2.5 mm diameter and a 2 mm depth was drilled on the medial area of each femoral condyle. All of the left lesions underwent immediate intraoperative stimulation with the Ga-Al-As laser using the following parameters: 300 Joules/cm2, 1 Watt, 300 Hertz, 10 minutes; the spot size of the laser irradiation was 0.5cm2. The parameters selected, were the same that had been chosen for a previous experiment Citation[[3]], Citation[[5]].
After bringing the laser point to a perpendicular position of 1cm from the osteochondral lesion site, and waiting until the selected density of energy for stimulation had been reached, laser stimulation was performed on the target tissue. After laser treatment, the surgical wounds were closed in layers of suture.
The untreated right limbs underwent the same surgical procedure and served as a control group.
Immediately after surgery and for 2 days thereafter, all the animals were treated with antibiotics (Flumequine-Flumexil, ATI s.r.l., Ozzano E.-BO, Italy) and analgesics (Orudis-Ketoprofene, Rhône-Poulenc-Rorer, Milan, Italy).
Rabbits were allowed to move freely in the cage without any joint immobilization. 24 weeks after surgery and after induction of general anesthesia, the animals were euthanized (Tanax, Hoechst AG, Frankfurt a M., Germany) for the extraction of the femurs.
The part of the condyle including the surgery site was prepared for histologic evaluation. Half of the explanted condyles were fixed in paraformaldehyde and embedded in methacrylate. The remaining condyles were decalcified and embedded in paraffin.
Methacrylate-embedded condyles were cut (diamond saw microtome, Leica 1600, Ernst Leitz, Wetzlar, Germany) to include the defective and normal articular cartilage and at the same regular interval to allow for comparison. 30 μm-thick sagittal sections from each specimen were obtained from the center of the repair site, stained with Toluidine Blue and then examined by a blinded investigator. Each section was analyzed using a Zeiss Axioscope microscope (Carl Zeiss, Oberkochen, Germany) connected to an image analyzer (Kontron S300, v.2, Kontron Elektronik, Munchen, Germany), to evaluate defect healing, surface regularity and cell morphology. Paraffin-embedded samples were cut from the center of the repair site (microtome HM340E, Zeiss); 6 μm-thick sagittal sections obtained from each specimen were stained with Safranin-O and Picrosirius Red dye and observed with a polarized microscope, to highlight collagen and glycosaminoglycanes. Each lased specimen (Lased Group) was compared with the untreated contralateral sample (Not lased group).
In addition, cell density was calculated for all specimens along the border between the repaired cartilage and the cartilage adjacent to the surgically created defect. An area measuring 0.11 × 0.11 mm2, positioned at the same height and distance from the two cartilage regions, was assessed by calculating the number of cartilaginous cells observed with an image analyzer (Kontron S300, v.2, Kontron Elektronik, Munchen, Germany). The average cell density resulting from the examined areas is expressed in cells/mm2 Citation[[6]].
A statistical evaluation of data was performed using SPSS/PC+Statistic TM 4.0 software package (SPSS Inc., Chicago, Illinois, USA). The Student' t-test was done to compare data; significance level was set at p<0.05.
RESULTS
All of the animals were in good general conditions at the established experimental times. At sacrifice, all rabbits weighed 4 ± 0.1 Kg (weight gain in all of the animals).
General Observations
No evidence of dislocation of the knee joint was observed.
Comparison between the sites treated intraoperatively with laser stimulation and the untreated sites, revealed that in the lased group the surgical wounds experienced better and faster healing and resolution of knee articulation swelling occurred faster. At sacrifice, no evidence of joint infection was registered in all knees.
Histologic Results
Results of histomorphometric evaluations are summarized in the grading scale shown in the modified Citation[[5]]. Each of the histologic and morphometric characteristics was given a score, previously found to be reliable Citation[7-8]. A score was assigned to cell morphology, matrix staining, defect filling and surface regularity, with “perfect” repair being equal to 12.
Table I. Scoring System for Histologic and Morphometric Results (Maximum = 12 Points). Samples (n.6 per Group) Were Prepared 24 Weeks After Surgery
At 24 weeks, histomorphometric analysis of the lased specimens revealed a complete healing of the osteochondral lesion. All of the samples showed a more regular defect filling with bony tissue growth, which means a better overall structural integrity().
Figure 1. Osteochondral defect at 24 weeks after laser treatment (toluidine blue). Magnification: ×10. Complete healing of the osteochondral defect surgically created; the arrow shows the border between repaired tissue (right) and adjacent tissue (left). The repaired tissue has an overall good structural integrity with a smooth and regular articular surface.
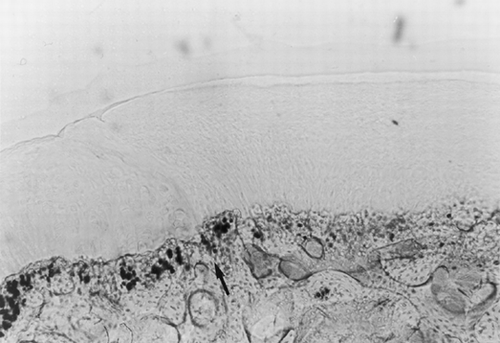
The articular surface turned out to be smooth and regular; fibrous tissue, fibrocartilage and hyaline-like cartilage could be observed in variable percentages in the reconstituted cartilaginous layer. In all of the specimens, cartilaginous cells from the repaired area appeared arranged in a palisade structure and partially integrated with the cartilage of the area adjacent to the lesion.
The border between the repaired tissue and the adjacent cartilaginous and bony tissues is well depicted (see arrow on ). Comparisons of the area cell density were performed between the cartilage repaired after laser treatment and the cartilage adjacent to the defect: results are reported in .
Table II. Mean Cell Density ± SD of the Lased Specimens. Each Sample Is the Result of the Comparison Between the Cartilage Repaired Using the Laser and the Cartilage Adjacent to the Lesion
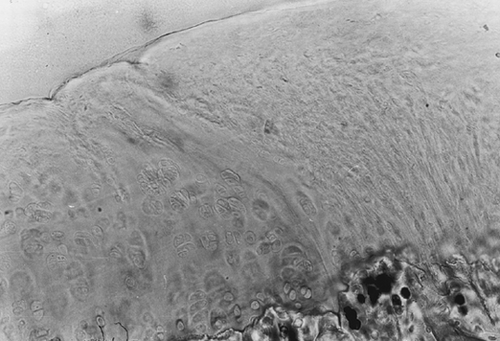
The mean value of the cartilaginous cell number for the two groups showed remarkable difference only for one specimen. Matrix staining of the reconstituted cartilage (Safranin-O and Picrosirius Red) revealed the presence of proteoglycans and collagen, and was more intense in the deepest layers and less intense in the superficial layers ().
Figure 2. Osteochondral defect at 24 weeks after laser treatment (safranin-0 and picrosirius red). Magnification: ×20. Cartilaginous cells appear arranged in a palisade structure; matrix staining of the reconstituted cartilage is more intense for both proteoglycans and collagen in the deepest layers.
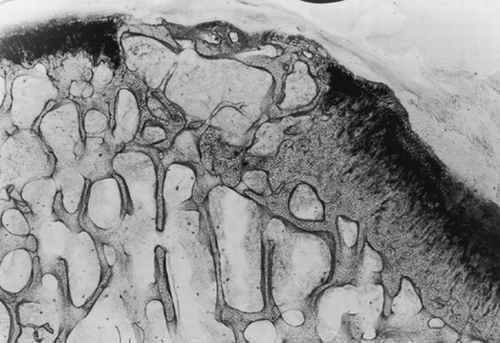
Histomorphometric analysis of the control samples (not lased) revealed that after 24 weeks, the defect left to spontaneous healing presented no signs of cartilaginous regeneration. An irregular growth of the reconstituted bony tissue was observed beyond the articular profile. On the articular surface, the lesion presented a layer of irregular fibrous tissue, where there were neither chondrocytes nor the components typical of cartilage. In fact, matrix staining turned out to be negative and that did not allow for assessment of cell density also in these samples ().
Figure 3. Osteochondral defect at 24 weeks without laser treatment (toluidine blue). Magnification: ×2.5. The repaired tissue appears disorganized and reveals an irregular growth of the reconstituted bony tissue, a thin layer of fibrous tissue on the articular surface and the absence of chondrocytes.
DISCUSSION
According to the results, the lased condyles demonstrated a better repair of the osteochondral defect in comparison with untreated condyles (p<0.001). After 24 weeks, the overall mean result of 7.3 points obtained for the lased specimens was higher than the value obtained for the same samples after 12 weeks, i.e. 6.7 points Citation[[5]]. On the contrary, at 24 weeks, the overall mean score of the untreated samples, equal to 1 point, turned out to be lower than the score obtained for the same samples but at 12 weeks, i.e. 3.8 points Citation[[5]]. Also the difference in cell density between the cartilage from the repaired area and the cartilage adjacent to the defect, which was registered for both lased and untreated samples, revealed that the effect of the intraoperative laser stimulation confirmed the previous results obtained in the repair of an osteochondral defect surgically created.
In accordance with the parameters suggested by other authors for evaluating animal bone maturity Citation[[6]], the rabbits belonging to this experimental group (3.7±0.1 kg body weight, aged 25±0.2 weeks) could be classified between adolescent and adult rabbits at surgery. In spite of that, the better lesion repair and cell density value registered in the adjacent cartilage of lased samples versus untreated samples (), both confirmed that the effect of laser stimulation could allow for comparison with results achieved by other Authors in young rabbits Citation[[6]].
As already described in a previous paper Citation[[5]], the combination of the advantages deriving from subchondral bone exposure Citation[[9]], the ascertained effects and results of laser stimulation of the bone Citation[10-12], and the choice of the laser device and of the safest parameters Citation[[5]] to achieve the desired goal Citation[[13]], enabled us to achieve this result also at 24 weeks after surgery.
Concerning the assessment of bone maturity, age (49±0.2 weeks) and weight (4±0.1 Kg) at sacrifice of the rabbits used for this research, classified these animals as adults. Results obtained demonstrated that thanks to the laser stimulation Citation[[5]], the better initial healing conditions were not negatively affected by the maturation-depend decline, as already described for the repair of osteocartilaginous lesions in rabbit Citation[[6]].
The selected and used low-power laser parameters ensured good results for both “in vitro” stimulation of cartilaginous cells Citation[[3]] and “in vivo” stimulation of a surgically created lesion in a rabbit at 12 Citation[[5]] and 24 weeks. Another experimental study will be conducted by our group to verify results one year after repair of an osteocartilaginous lesion, and assess relevant mechanical properties after intraoperative laser stimulation.
The results of the researches on low-power laser, are “sometimes” difficult to compare Citation[[14]]; subsequently, the protocol we used for studying laser stimulation of osteochondral defects could represent a useful starting point for comparison with other similar studies.
It is the Authors' opinion that laser treatment of osteochondral lesions can be particularly effective and, in the near future, good and safe experimental results could be available to transfer data into clinical practice.
Durability over time of cartilage repair in osteochondral lesions achieved by means of a non-invasive therapy, such as low-power laser stimulation, may have positive consequences in terms of clinical relevance.
ACKNOWLEDGMENTS
Financial support for this research was partially given by the Italian Ministry of Health (Progetto Finalizzato: ingegneria tessutale in ortopedia), by the Rizzoli Orthopaedic Institute and by the University of Bologna, Bologna, Italy. We thank Patrizio Di Denia and Claudio Dal Fiume (Dept. of Experimental Surgery) for their technical assistance.
REFERENCES
- Mitchell N., Shepard N. The resurfacing of adult rabbit articular cartilage in intrarticular fractures in rabbits. J Bone Joint Surg Am 1976; 58: 230–233
- Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopedics Jun 1997; 20(6)525–538
- Morrone G, Guzzardella G A, Torricelli P, Fini M, Giardino R. In vitro experimental research of rabbit chondrocytes biostimulation with diode laser Ga-Al-As: a preliminary study. Art. Cells, Blood Subs., and Immob. Biotech. 1998; 26(4)437–439
- Morrone G, Guzzardella G A, Tigani D, Torricelli P, Fini M, Giardino R. Biostimulation of human chondrocytes with Ga-Al-As diode laser: in vitro research. Art. Cells, Blood Subs., and Immob. Biotech. 2000; 28(2)193–201
- Morrone G, Guzzardella G A, Torricelli P, Rocca M, Tigani D, Barbanti G Brodano, Fini M, Giardino R. Osteochondral lesion repair of the knee in the rabbit after low-power diode Ga-Al-As laser biostimulation: an experimental study. Art. Cells, Blood Subs., and Immob. Biotech. 2000; 28(4)
- Wei X, Messner K. Maturation-depend durability of spontaneous cartilage repair in rabbit knee joint. J Biomed Mater Res, Sept 15 1999; 46(4)539–48
- Moran M E, Kim H KW, Salter R B. Biological resurfacing of full-thickness defects in patellar articular cartilage in rabbit. J Bone Joint Surg (Br) 1992; 74-B: 659–67
- O'Driscoll S W, Keeley F W, Salter R B. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion: an experimental investigation in the rabbit. J Bone Joint Surg (Am) 1986; 68-A: 1017–35
- Newman A P. Articular cartilage repair. American Journal of sports Medicine 1998; 26(2)309–324
- Luger E J, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 1998; 22: 97–102
- Tang X M, Chai B P. Effects of CO2 laser irradiation on experimental fracture healing: a transmission electron microscopic study. Lasers Surg Med 1986; 6: 346–352
- Trelles M A, Mayayo E. Bone fracture consolidates faster with low-power laser. Lasers Surg Med 1987; 7: 36–45
- Gorisch W, Boergen K P. Heat-induced contraction of blood vessels. Lasers Surg Med 1982; 2: 1–13
- Basford J R. Low intensity laser therapy: still not an established clinical tool. Lasers Surg Med 1995; 16: 331–342