Abstract
Endogenous overproduction of nitric oxide (NO) is believed to be a primary cause of refractory hypotension in septic shock. Under this condition, effectiveness of vasopressors is diminished due to hyporeactivity of blood vessels, a condition termed as vasoplesia. Effective reduction of NO levels should alleviate the condition. In this study, we investigated whether NO scavenging could modulate the endotoxin mediated vasoplesia in-vitro. Further, we explored whether NO scavenging in combination with a moderate NO synthase (NOS) inhibition would also be effective in modulating NO mediated vasoplesia. Experimental vasoplesia was produced invitro by incubating isolated rat thoracic aortic rings with lipopolysaccharide (LPS). Vessel rings were then treated with Nω-nitro- L-arginine methyl ester (L-NAME; a NOS inhibitor), human hemoglobin (Hb; a NO scavenger), or both L-NAME and Hb. Vascular reactivity was assessed by measuring vessel ring isometric tension changes to norepinephrine (NE) doses; the median effective doses (logEC50) of NE before and after each experimental treatment were compared. Following a 6-hour LPS treatment, vascular reactivity logEC50 values for NE were significantly increased compared with control vessel rings incubated without LPS. Treatment with either L-NAME alone or Hb alone significantly improved the vessel ring reactivity to NE. When both L-NAME and Hb were used concomitantly, vascular reactivity was also significantly improved. These results indicate that NO scavenging with Hb is as effective as NO synthesis inhibition with NAME in modulating the endotoxin induced vasoplesia. In conclusion, NO scavenging, alone or in combination with a moderate NOS inhibition, may render an alternative therapeutic approach to NOS synthesis inhibition in modulating the vasoplesia in septic shock.
INTRODUCTION
Refractory hypotension is a major clinical challenge in the treatment of septic shock. One factor contributing to this problem is “vasoplesia”, the condition of diminished vascular responsiveness to pressor agents such as norepinphrine. The pathogenesis of this unique vascular abnormality is not well understood. One plausible mechanism could be vascular smooth muscle intoxication with endogenous nitric oxide (NO), a potent vasodilator. In sepsis or endotoxemia, inducible NO synthase (iNOS) present in immune cells, vascular smooth muscle cells and other cells, is activated producing high levels of NO Citation[1-5]. Indeed, elevated nitrogen oxide levels have been observed in patients with sepsis Citation[6-7]. This elevated NO synthesis could lead to accumulation of toxic levels of NO in tissues and blood causing widespread vasodilation and systemic hypotension. To correct this condition, systemic NO levels must be reduced. NO synthesis inhibition using L-arginine analogs and similar inhibitors has been a popular approach to treatment of sepsis mediated hypotension Citation[8-11]. However, NO synthesis inhibition has not yielded a clear beneficial outcome but, rather, shown to be detrimental in some cases Citation[12-16]. As an alternative approach, NO scavenging with natural heme based NO scavengers, such as hemoglobin (Hb), could be used to reduce blood NO levels. In this study, we investigated whether NO scavenging with Hb could modulate the endotoxin mediated experimental vasoplesia in-vitro. We also explored whether NO scavenging in combination with a moderate NOS inhibition would render additional benefit in modulating NO mediated vasoplesia.
MATERIALS AND METHODS
Rat Thoracic Aortic Rings
Male Sprague-Dawley rats (250–350g body weight) were anesthetized with sodium pentobarbital (50–75mg/Kg, IP). Through a midline incision, the heart and lungs were removed en bloc. After a wash in modified Krebs buffer (in mM: NaCl, 118; KCl, 4.8; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 24; glucose, 11; and disodium EDTA, 0.03; pH 7.4), the thoracic aorta was carefully excised and prepared as 2–3mm vessel ring segments. The vessel rings were placed in a 25ml tissue bath and mounted between two opposing stainless hooks; one was secured to a fixed tissue holder while the other connected to a tension transducer (Grass Model FT03) via a 3–0 silk suture. Tension changes were recorded on a Model 7 Grass Polygraph (Quincy, MA). The vessel ring was bathed in a Krebs buffer maintained at 37°C. The buffer was continuously oxygenated by bubbling 95%O2–5%CO2gas.
Drugs and Chemicals
Hemoglobin (Hb) has been known to have an extremely high affinity for NO Citation[[17]]. In this study, acellular free Hb was used as a NO scavenger as its biochemical properties are well characterized. Purified human hemoglobin reconstituted in Ringer's Lactate (Hb; 7±0.5gHb/dl) was obtained from Hemosol, Inc. (Toronto, Canada) and was stored at −80°C until use.
E. coli lipopolysaccharide (055:B5 3120–25) (LPS) was purchased from Difco Laboratories (Detroit, MI). A commercial pharmaceutical preparation of norepinephrine (NE; levartrenol bitartarate, Winthrop Laboratories, New York, NY) was used. Acetylcholine chloride (Ach), Nω-nitro-L-argine methyl ester (L-NAME), and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Data Representation and Statistical Analysis
Tension changes following an experimental treatment are represented as percent of pretreatment values. For dose response experiments, responses were calculated as percent of the maximum response. Mean response values are plotted against dose and the logmedian effective dose (logEC50) was obtained by a non-linear regression (sigmoidal curve) using Prism GraphPad software (San Diego, CA). Unless otherwise indicated, all values are represented as mean ± 1 standard deviation. Statistical significance between two means was tested by unpaired Student's t-tests. When comparing means of three or more groups, ANOVA and Newman-Keules tests were used. Mean differences are considered significant if P<0.05.
Experimental Protocol
The responsiveness of vessel rings were first assessed by treating with 50nM norepinephrine (NE). The integrity of the endothelium was, then, assessed by treating the vessel rings with 33μM Ach. Vessels rings showing less than 10% relaxation were considered deficient of functional endothelium and excluded. Unless otherwise indicated, the vessel rings were allowed to relax for 1 hour at 2 grams of imposed tension before experimental procedures were initiated. For dose-response tests, test agents were added cumulatively to the bathing buffer and changes in tension were recorded.
Induction of Vasoplesia in Isolated Aortic Rings
Pairs of aortic rings obtained from adjacent regions of the aorta of the same animal were used in these experiments. After tests of vessel integrity and equilibration, a NE dose response relationship was obtained. The vessel rings were then washed three time with a fresh Krebs buffer and re-equilibrated. One of the vessel rings was randomly chosen and treated with LPS (140μg/mL) and the other with the same volume of buffer. The vessel ring pairs were incubated for 3 or 6 hours. The buffer treated vessels served as matching time controls. After the respective incubation period, the NE dose response was repeated to assess effect of LPS on vascular reactivity.
NO Synthesis Inhibition
The vessel rings were subjected to an initial NE dose response test and LPS treatments as described above. After 6 hours of incubation with LPS, one of the two vessel rings were treated with 600μM Nω-nitro-L-argine methyl ester(NAME). The other vessel ring was treated with equal volume of Krebs buffer and served as a control. The NE dose response test was then repeated for each vessel ring. Incubation time of 6 hours was chosen because it was the minimum time necessary to develop vasoplesia that can be assessed by the NE dose response test.
NO Scavenging
The aortic rings were prepared and the initial NE dose response test performed as described above. After a buffer wash, each vessel ring was incubated with LPS (140μg/mL) for 6 hours. At the conclusion of the incubation period, one of the two aortic rings were treated with a 50μL of human Hb solution (7g Hb/dl) and the other with an equal volume of buffer solution. Ten minutes later, the NE dose response test was repeated for both vessel rings.
NO Scavenging with Concomitant NO Synthesis Inhibition
Another group of aortic rings were subjected to an initial NE test and LPS incubation as above. After 6 hours of incubation, both L-NAME (600μM) and Hb (2μM) were added to one of the vessel rings. The other vessel ring was treated with equal volume of buffer solution. The NE dose response test was then repeated and the changes in tension recorded.
RESULTS
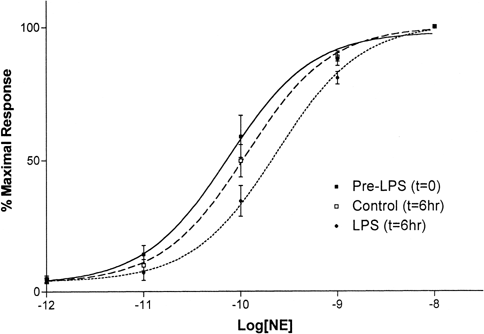
In-Vitro Induction of Vasoplesia
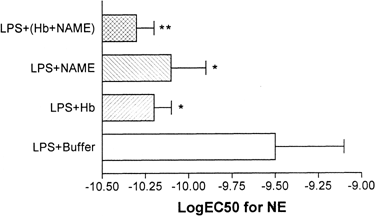
Initial attempts to induce vasoplesia by incubating aortic ring segments in tissue culture plates with LPS were not successful. The vessel rings were placed in tissue culture media or rat serum without oxygenation. After a 3–6 hour incubation, none of the vessel rings, either with or without LPS, responded to NE. Reasoning that lack of oxygenation might have caused the failure, subsequent experiments were conducted in an oxygenated tissue bath kept at 37°C. Following incubation with LPS, vessel rings did show diminished response to NE but the extent of hyporeactivity varied with duration of LPS incubation time. For vessel rings incubated with LPS for 3 hours or less, the NE dose response characteristics were not noticably different from that of LPS untreated control vessel rings. Vessel rings incubated with LPS for 6 hours, however, showed a significantly diminished vessel ring reactivity to NE; the mean LogEC50 values for LPS vessel rings and corresponding controls were −9.64±0.08M and −9.65±0.07M respectively (P<0.02, N=8 each) (Figure-). Increasing the LPS dose from 140μg/ml to 300μg/ml did not notably affect vasoplesia induction time.
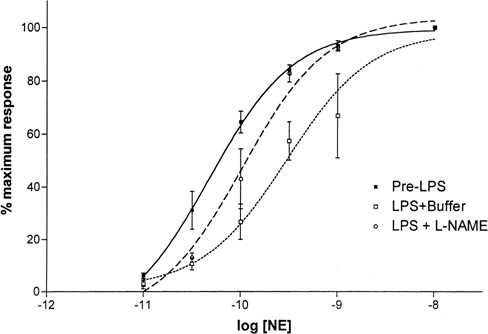
NO Synthesis Inhibition
For vessel rings incubated with LPS for 6 hours, treatment with 600μM NAME enhanced subsequent vascular reactivity to NE as indicated by the shifting of NE dose response curves toward the left (Figure-). The mean logEC50 value after NAME treatment was significantly lower than pre-NAME value: −10.1±0.2M and −9.5±0.2M, respectively (P<0.01).
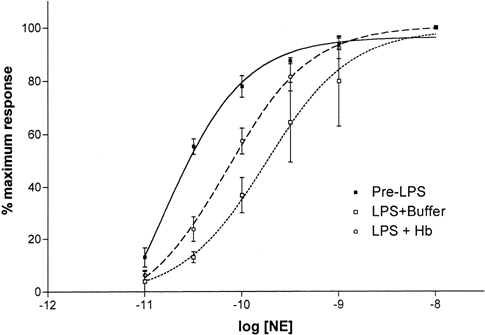
NO Scavenging with Hb
Addition of 2μM Hb to vessel rings incubated with LPS for 6 hours caused enhanced responsiveness to NE. Hb treatment caused NE dose response curves to shift back toward the pre-LPS curves (Figure-). The mean logEC50 for NE dose response curves after Hb treatment and was significantly lower than pre-Hb value; −10.2±0.1M and −9.76±0.21M, respectively (P<0.01, N=5 each).
NO Scavenging with Concomitant NO Synthesis Inhibition
Treatment of vessel rings with both 600μM NAME and 2μM Hb also caused significant enhancement in post-LPS vascular responsiveness to NE (Figure-). The mean LogEC50 value after the combined NAME and Hb treatments was −10.3±0.1M while that of pretreatment value was −9.5±0.2M (P<0.001, N=5 each; Figure-). However, when compared with NAME alone or Hb alone, there was no additional improvement.
Figure 4. Effect of combined NO synthesis inhibition and NO scavenging on NE dose responses of vessel rings treated with LPS for 6 hours. Treatment with both NAME and Hb shifted the NE dose-response curve back toward the left. Values are mean±SD (N=5 each).
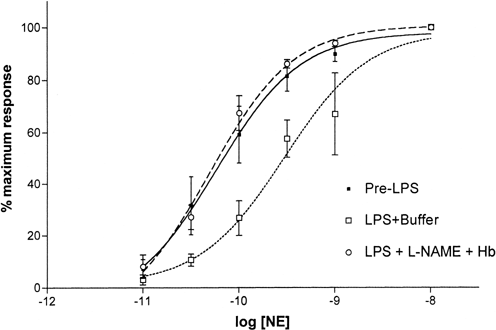
Figure 5. Improved vascular reactivity of LPS treated vessel rings following treatment with Hb, NAME, or both. When compared with control vessel rings (LPS+buffer), vessel rings treated with Hb, NAME or Hb+NAME showed a significantly lower logEC50 values. Values are mean±SD (N=4-6 each). *P<0.05, **P<0.01 compared with control.
DISCUSSION
This study was initiated to investigate 1) whether the endotoxin mediated vascular hyporeactivity to a vasopressor norepinephrine could be modulated with NO scavenging, and 2) whether NO scavenging with concomitant NO synthesis inhibition would further enhance post-LPS vascular reactivity to NE.
The reason why initial attempts to induce vasoplesia by incubating vessel rings in tissue culture plates failed is not completely clear; hypoxic conditions that might have been existed could have a been factor. The vessel rings were incubated in the culture medium or rat serum for over 3 hours without oxygenation. Like most other mammalian muscle cells, vascular smooth muscle cells depend on oxidative phosphorylation as a primary energy source; an inadequate supply of oxygen may have contributed to their subfunctional responses. In addition, oxygen may also play an important role in development of LPS mediated vasoplesia since molecular oxygen is required for NO synthesis from L-arginine. Further, oxygen could participate in forming toxic free radicals, mediating inflammatory responses and cellular damage that may precede LPS induced vasoplesia. The subsequent success in induction of vasoplesia using an oxygenated tissue bath appears to support this concept. Considering that hypotension or vasoplesia usually does not occur until 3–5 hours after LPS infusion, the finding that satisfactory induction of vasoplesia requires at least 6 hour incubation with LPS is not surprising. This result is consistent with a recent report that iNOS mRNA expression and plasma NO levels in rat reached peak 2–4 hours after LPS administration in-vivo Citation[[18]].
That NAME reversed the hyporeactivity of LPS treated vessel rings to NE supports the hypothesis that NO is involved in the pathophysiology. As shown in this study, NAME is a potent NO synthesis inhibitor. This type of NO synthesis inhibitor, however, is not selective; NAME and other NOS inhibitors of this class inhibit both cNOS and iNOS Citation[[5]]. Overactivation of iNOS appears to be a primary reason for the elevated NO levels in sepsis and endotoxemia; cNOS activity is considered necessary for normal vascular control and should be preserved Citation[[19]]. Thus, while inhibition of iNOS is considered desirable, abolition of cNOS function as well could lead to further hemodynamic complications. Indeed, despite improved blood pressure and vascular resistance, NO synthesis inhibition lead to depressed cardiac function and no improvement in mortality Citation[13-16]. In this study, low dose (600μM) NAME was used to spare some cNOS and iNOS functions.
Recently, more selective inhibitors for iNOS such as S-methylisothiourea (SMT) Citation[[22]] and aminoguanidine Citation[[23]] have been identified and tested. For example, SMT is known to be at least 20 times more potent as iNOS inhibitor than Ng-monomethyl-L-arginine and other similar less specific inhibitors Citation[[22]]. Inducible NOS specific inhibitors would ameliorate hemodynamic complications associated NOS inhibition. However, no clear hemodynamic benefit have been observed with such agents Citation[[24]]. In addition, whether the iNOS activity should be completely abolished can be debated. In response to infection and sepsis, the immune cells (neutrophils, monocytes, macrophages, etc) turn on iNOS and produce large amounts of NO as part of host defense mechanisms. Complete blockage of iNOS function with inhibitors, regardless of specificity for iNOS, may prove to be detrimental to sepsis recovery and eventual survival.
Certain heme proteins such as Hb are known to have an extremely high affinity for NO; ferrous Hb affinity for NO is known to be over 1,000 times that for carbon monoxide Citation[[17]]. Thus, it is plausible that Hb and other similar heme proteins could serve as effective NO scavengers in NO mediated vasoplesia. NO scavenging should lead to lower blood and tissue NO levels which, in turn, could downregulate the guanylyl cyclase mediated vascular relaxation pathway. Indeed, Hb treatment reversed vascular ring hyporesponsiveness to NE supporting the hypothesis. OxyHb scavenging of NO in endotoxemic rats has been confirmed; the electron spin resonance signal for nitrosyl Hb was detected following Hb infusion in the endotoxemic animals Citation[20-21]. Of note, Hb's ability to scavenge NO depends on the heme iron oxidation state and the nature of ligand; while oxyHb (Fe+2) and deoxyHb (Fe+2) are excellent NO scavengers, nitrosyl Hb(Fe+2) and ferric Hb (Fe+3) derivatives, in general, are not.
NO synthesis inhibition and NO scavenging are two distinct mechanisms: NO synthesis inhibition prevents new NO synthesis while NO scavenging inactivates existing NO without directly affecting NO synthesis mechanisms. Thus, the combined effects of NO synthesis inhibition and scavenging expected to be complementary. That is, if NAME and Hb were used concomitantly, the overall effect would be greater than NAME alone or Hb alone. Surprisingly, although significant enhancement in vascular reactivity to NE was observed, concomitant treatment of LPS incubated vessel rings with NAME and Hb produced no clear additional improvement compared with each agent alone. One possible explanation may be when Hb alone is used there is no impairment in the level of NO synthesis; intracellularly produced NO diffuses out into the bathing buffer down the concentration gradient where it reacts with Hb. When both NAME and Hb are administered together, however, little or no NO are produced to react with Hb; consequently, Hb produces little or no additional effect beyond that of NAME. In-vivo responses may be different, however, because in sepsis or endotoxemia NO is produced not only by the vascular endothelial cells but also by macrophages and other cells. In fact, in studies with endotoxemic rats, concomitant infusion of NAME and Hb did produce further improvements in blood pressure Citation[[21]] indicating possible enhancement of vascular responsiveness.
The NO scavenging approach was initially conceived on the premise that, in sepsis, some level of iNOS activity must be preserved for proper immune function and survival. NO scavenging with Hb should allow removal of excess NO without directly affecting iNOS or cNOS function, thus, complications associated with NO synthesis inhibition could be avoided. How much of iNOS function should be preserved and how to achieve such an optimal functional level is the question. Further studies are clearly needed to answer these questions.
In summary, whether NO scavenging could modulate endotoxin mediated vasoplesia was investigated in the isolated rat thoracic aorta. Results showed that NO scavenging with Hb was as effective as NOS inhibition with NAME in modulating vasoplesia. Concomitant NO scavenging with a moderate dose of NAME was also effective but did not produce any clear additional benefit in this in-vitro vascular model. In conclusion, NO scavenging, alone or in combination with a moderate NO synthesis inhibition, may render a more desirable therapeutic outcome in the treatment of septic shock as it may better preserve the essential NOS functions.
REFERENCES
- Palmer R M. The discovery of nitric oxide in the vessel wall: a unifying concept in the pathogenesis of sepsis. Arch Surg 1993; 128: 396–401
- Kilbourn R G, Griffith O W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Instit 1992; 84: 827–831
- Lorente J A, Landin L, Renes E, de Pablo R, Jorge P, Rodena E, Liste D. Role of nitric oxide in the hemodynamic changes of sepsis. Critical Care Med 1993; 21: 759–767
- Parrilo J E. Pathogenic mechanisms of septic shock. New Engl J Med 1993; 328: 1471–1477
- Cobb J P, Danner R L. Nitric oxide and septic shock. JAMA 1996; 275: 1992–1996
- Ochoa J B, Udekwu A O, Billiar T R, Curran R D, Cerra F B, Simmons R L, Peitzman A B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg 1991; 214: 621–626
- Evans T, Carpenter A, Kinderman H. Evidence of increased nitric oxide production in patients with the sepsis syndrome. Cir Shock 1993; 41: 77–81
- Hotchkiss R S, Karl I E, Parker J L, Adams H R. Inhibition of NO synthesis in septic shock. Lancet 1992; 339: 434–435
- Cobb J P, Cunnion R E, Danner R L. Nitric oxide as a target for therapy in septic shock. Crit Care Med 1993; 21: 1261–1263
- Meyer J, Traber L D, Sharon N, Lentz C W, Makazawa H, Herndon D N, Noda H, Traber D L. Reversal of hyperdynamic responses to continuous endotoxin administration by inhibition of NO synthesis. J Appl Physiol 1992; 73: 324–328
- Fox G A, Paterson N AM, MacCormack D G. Effect of inhibition of NO synthase on vascular reactivity in a rat model of hyperdynamic sepsis. Am. J Physiol 1994; 267: H1377–H1382
- Nava E, Palmer R MJ, Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial?. Lancet 1991; 338: 1555–1557
- Cobb J P, Natanson C, Hoffman W D, Lodato R F, Banks S, Koev C A, Solomon M A, Elin R J, Hosseini J M, Danner R L. Nw-amino-L-arginine, an inhibitor of nitric oxide synthesis, raises vascular resistance but increases mortality rates in awake canines challenged with endotoxin. J Exp Med 1992; 176: 1175–1182
- Tiao G, Rafferty J, Ogle C, Fischer J E, Hasselgren P. Detrimental effect of nitric oxide synthase inhibition during endotoxemia may be caused by high levels of tumor necrosis factor and interleukin-6. Surgery 1994; 116: 332–338
- Spain D A, Wilson M A, Bar-Natan M F, Garrison R N. Nitric oxide synthesis inhibition aggravates intestinal microvascular vasoconstriction and hypoperfusion of bacteremia. J Trauma 1994; 36: 720–725
- Avontuur J AM, Tutein Nolthenius R P, Bodegom J W, Bruining H A. Prolonged inhibition of nitric oxide synthesis in severe septic shock: A clinical study. Crit Care Med 1998; 26: 660–667
- Gibson Q H, Roughton F JW. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957; 136: 507–526
- Sade K, Schwarz D, Wolman Y, et al. Time course of lipopolysaccharide induced nitric oxide synthase mRNA expression in rat glomeruli. J Lab Clin Med 1999; 134: 471–477
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB Journal 1992; 6: 3051–3064
- Kim H W, Hughes J K, Breiding P, Greenburg A G. Nitric oxide scavenging: an alternative therapeutic approach to nitric oxide synthesis inhibition in nitric oxide-mediated hypotension of sepsis. Surg Forum 1994; 45: 67–69
- Kim H W, Breiding P, Greenburg A G. Enhanced modulation of hypotension in endotoxemia by concomitant nitric oxide synthesis inhibition and nitric oxide scavenging. Art. Cells, Blood Subs., and Immob. Biotech. 1997; 25: 153–162
- Szabo C, Southan G J, Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1994; 91: 12472–12476
- Scott J A, Machoun M, McCormack D G. Inducible nitric oxide synthase and vascular reactivity in rat thoracic aorta: effect of aminoguanidine. Am. J. Physiol. 1996; 80: 271–277
- Brooke M, Hinder F, McGuire R, Traber L D, Traber D L. Selecitve inhibition of inducible nitric oxide synthase: effects of hemodynamics and regional blood flow in healthy and septic sheep. Crit. Care Med. 1999; 27: 162–157